Abstract
Deafness is the most common curable childhood handicap. It is a well recognised fact that unidentified hearing impairment can adversely affect optimal speech and language development and therefore academic, social and emotional development. Universal neonatal hearing screening programmes are implemented in many developed countries. However it is still in its early stage in India. The incidence of hearing impairment in India is 1–6 per thousand newborns screened (Paediatrics 19:155–165, 1998; Indian J Paediatr 74(6):545–549, 2007; Status of Disability in India, pp 172–185 2000). To determine the incidence of permanent hearing loss of moderate to evere variety in neonates taking care in a tertiary care rural based hospital in Gujarat. It was a non randomised observational study done for duration of 3 years. All neonates born in Shri Krishna Hospital underwent screening using two stage protocols with DPOAE test and final confirmation done with BERA. Total 2534 neonates were screened out of them 52 failed and 2482 (97.94 %) neonates passed in the 1st DPOAE test with 2.05 % refer rate. Total 7 (2 per 1000) neonates were detected with hearing impairment. 10 % neonates had one or other high risk factor. Out of high risk neonates, 1.8 % were diagnosed with hearing impairment in high risk group. Overall the follow-up rate was 72.7 %. Hospital based universal hearing screening of new born before discharge is feasible at a rural based tertiary care centre. Non specialist staff is invaluable in achieving a satisfactory referral rate with two stage hearing screening protocol. However, more efficacious tracking and follow up system is needed to improve the follow up rate for diagnosis.
Keywords: Universal neonatal hearing screening, OAE-BERA, Congenital deafness, Impaired hearing
Introduction
The incidence of hearing impairment in India is 1–6 per thousand newborns screened [1, 2, 3]. It is well recognised that unidentified hearing impairment can adversely affect optimal speech and language development, and consequently the acquisition of literacy skill, academic, social, and emotional development are adversely affected. There is evidence showing that neonates with hearing impairment whose identification and remediation took place prior to 6 months of age have been enabled to perform significantly better on vocabulary, communication, intelligence, social skills and behaviour that was necessary for success in their later lives [4]. The ultimate goal is to improve access to education and vocational rehabilitation services, and to generate awareness amongst the masses. Over 5 % of the world’s population has disabling hearing loss, of which 10 % (32 million) are children. The majority of these children live in middle and low income countries. Congenital causes lead to hearing loss being present at or acquired soon after birth. These can be caused by hereditary or non-hereditary genetic factors or by certain complications that occur during pregnancy and childbirth. Early detection and intervention remains the key factor in minimising the impact of hearing loss on a child’s development and educational achievements. The World Health Organisation has quoted that in infants and children with hearing loss, early identification and management through infant hearing screening programmes can improve the linguistic and educational outcomes for each child [5]. Children with hearing impairment would benefit by use of hearing aids, assistive listening devices, and cochlear implants. However, the production of hearing aid devices does not meet the need that has been generated. In the Indian scenario, the situation is further grim. Most developed countries have successfully finalised universal neonatal hearing screening programmes, however in India, such programmes are still nascent. Financial and social constraints have augmented its nationwide application. Hence, the current study was planned to understand the practicality of these protocols in a financially constrained country.
Objectives
To evaluate the incidence of hearing impairment among neonates in a rural setup.
To detect permanent hearing impairment of moderate to severe degree in the frequency range important for speech recognition in neonates, at earliest possible time.
To provide appropriate intervention (medical/surgical/rehabilitation) following the detection of a permanent hearing impairment.
Materials and Methodology
It was a prospective non randomized observational study conducted over a span of 3 years from February 2012 to January 2015.
Inclusion Criteria
All neonates admitted into Shree Krishna Hospital, Karamsad during the study period were screened for hearing loss prior to discharge from the hospital.
Exclusion Criteria
Cases of congenital meatal atresia were excluded from the study.
Parents/grandparents of the neonates were informed and counselled about the study and neonatal hearing screening programme. Each participant’s demographic details along with their perinatal and postnatal data were taken, and ear, nose, and throat examination was done. Details were filled in the participants’ proforma, in order to identify high risk neonates. All neonates born or admitted during the study period in the SK Hospital, Karamsad, (Anand) underwent hearing assessment using Distortion Product Oto-Ascoustic Emission(DPOAE) as the first level of hearing screening. Neonates who failed the first screening were subjected to a second level of hearing screening after 10 days by performing 2nd DPOAE test. This was done in the Department Of Otorhinolaryngology and Head and Neck Surgery at S.K Hospital, Karamsad (Anand) by a trained health worker using an Intelligent Hearing System Smart (IHS) OAE4.70SN:IHS5267(24:1056)DP-OAE, which is a completely automated analysis system that gives a “PASS” or “REFER” result. “PASS” suggests that the neonate has no hearing impairment in the specific frequency tested and “REFER” indicates that the neonate has hearing impairment. IHSDP-OAE passing criteria data were overall 70 % “PASS” in all frequencies and 80 % “PASS” in frequencies ranging from 1000 to 4000 Hz. Neonates who could not pass the 2nd DP-OAE test underwent confirmatory test auditory brain evoked responses (ABR) also known as brain evoked response audiometry (BERA) at the age of 3 months which was done by the audiologist. Irrespective of the results of the screening test, all the high risk (HR) neonates underwent ABR at 3 months of age. The criteria for high risk(HR) neonates were adopted from the American Joint Committee statement on Infant Hearing screening.(JCIH), 2000 updated in 2007 [6, 7].
Infants who were found positive for hearing impairment by BERA test showing abnormal wave morphology were advised for remedial measures of audiological rehabilitation, surgical intervention, or medical management.
Results
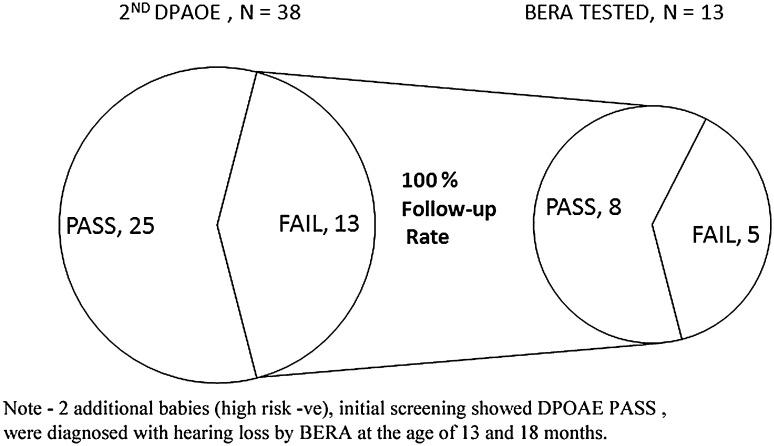
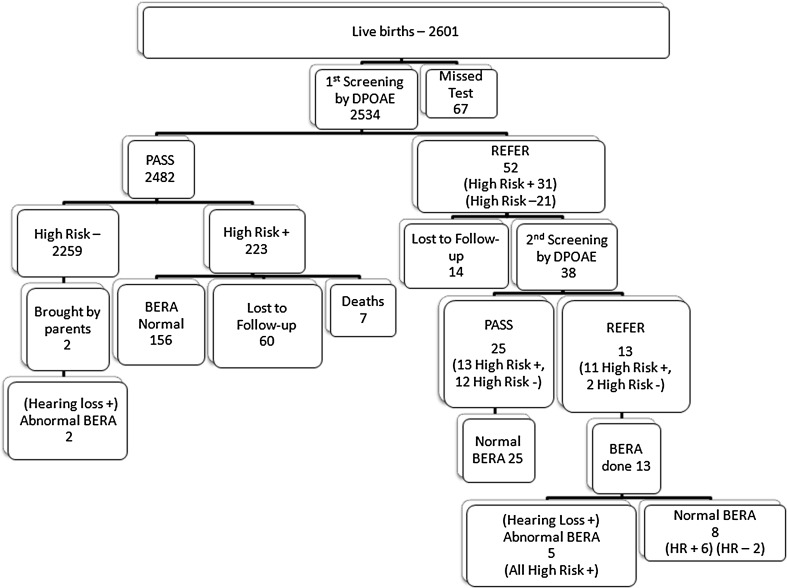
During the study period, total 2534 (97.42 %) neonates were screened out of 2601 live births. Out of total 2534 neonates, 254 (10 %) had one or more high risk factor. Among the 2534 neonates that were screened initially, 52 (2.05 %) babies failed (Refer) the 1st screening (Table 1). Out of these 52 failed neonates, 38 came for follow up (73.07 % follow up rate) for 2nd screening. Out of which, 13 neonates failed the 2nd screening (Fig. 1). Confirmatory test for hearing—BERA was done in 13 refer/failed neonates which showed abnormal wave morphology in 5 neonates (38.4 %) suggestive of bilateral profound sensorineural hearing impairment and 8 neonates showed normal BERA wave form patterned, thus suggestive of normal hearing. Through our protocol, total five cases were detected with hearing impairment which were confirmed by BERA, while two more cases came to us as their parents suspected impaired hearing at 13 and 18 months. On further testing of these two babies, BERA showed abnormal wave morphology. Therefore, total number of neonates who had bilateral profound sensorineural hearing impairment was seven at the end of the study. All of these seven hearing impaired neonates were sent to the audiologist for rehabilitation for hearing aid trial. One of these neonates underwent further workup for cochlear implant surgery but had a hypoplastic VIIIth nerve which was diagnosed on magnetic resonance imaging (MRI). Two neonates are undergoing a hearing aid trial. The remaining four have under gone hearing aid trials and one is currently undergoing a pre-operative check up for Cochlear Implant Surgery.
Table 1.
Results of 1st and 2nd DPOAE screening
| Total live birth-2601 Middle test-67 |
Total neonates | Test passed | Refer/test failed |
|---|---|---|---|
| Initial screening (1st DPOAE) | 2534 | 2482 (97.95 %) | 52 (2.05 % initial refer rate) |
| 2nd OAE rescreening (1st DPOAE refer cases) | 38 (78.07 % follow up rate 14—lost to follow-up) | 25 | 13 |
Confirmation with BERA showed moderate to severe SNHL—7 cases (5 à High risk +ve, 2 àHigh risk −ve). 2 HR −ve neonates brought by the parents at the age of 13 and 18 months respectively, confirmed by abnormal BERA waveform
Fig. 1.
showing results of 2nd DPOAE testing. Two additional babies, initial screening showed DPOAE PASS with HR −ve, were diagnosed with hearing loss by BERA at the age of 13 and 18 months
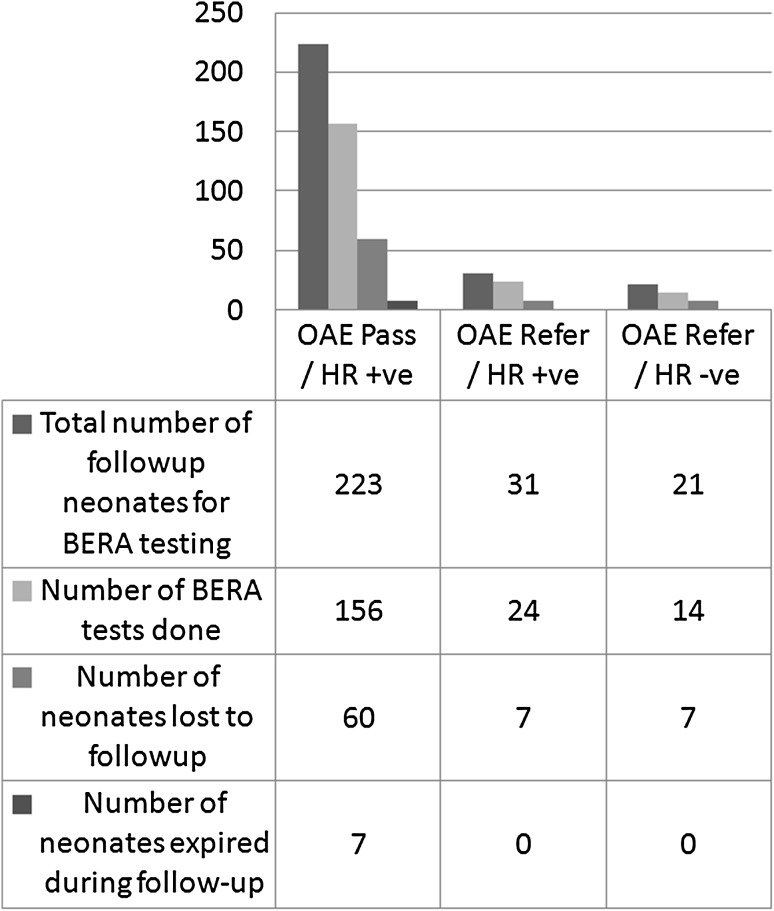
There were total 275 (10.57 %) neonates who were supposed to undergo confirmatory BERA testing, 74 (26.9 %) of these were lost to follow up and 7 neonates expired in this duration due to various causes (Fig. 2).
Fig. 2.
Distribution of BERA tested cases
Algorithm (Fig. 3) gives a gist of the entire programme.
Fig. 3.
Algorithm of the programme
Discussion
In our study, the possible burden of hearing impairment was evaluated in babies born in a rural based hospital in Gujarat.Total 2534 neonates were screened, out of them 52 failed and 2482 (97.94 %) neonates passed in the 1st DPOAE test with 2.05 % refer rate. Total 7 (2 per 1000) neonates were detected with hearing impairment 10 % neonates had one or other high risk factor. Out of high risk neonates, 1.8 % were diagnosed with hearing impairment in high risk group. Overall the follow-up rate was 72.7 %.
A number of limitations were noted. A noiseless nursery like surrounding was required and hence the infant had to be physically transported to the audiology room for testing. Occasionally the babies woke up during the test and started crying. The test could only be conducted when the babies were in deep sleep which was difficult to plan. Next, conducting a follow up from 1st DPOAE to 2nd Rescreening and then for BERA testing was probably the greatest obstacle in our setup. This was solved by coinciding the immunization visit with screening. The biggest hurdle was convincing the parents/family of the need for fixation of a hearing aid in ABR abnormal babies, probably attributable to the stigma attached to it.
Screening coverage was 97.42 %, which was better than the recommended bench mark of >95 % by JCIH 2007 suggesting, that screening is feasible [7]. Out of 2534 neonates only 52 (2.05 %) failed the initial or first screening with DPOAE test. The 2.05 % refer rate is within the recommended benchmark of <4 % [7, 8]. Of the total 2534 neonates screened by the two stage DPOAE hearing screening protocol and final confirmatory test with BERA at the age of three months, we could detect a total of seven neonates (2 per 1000) with hearing impairment. This was in agreement with the other studies which respectively stated that the average incidence of hearing impairment in neonates was 1.8–4.00 per 1000 [9–13]. However, one study from Cochin shows hearing loss in 10.3 per 1000 in high risk category [14]. In a study previously done in our institute, the prevalence of hearing loss in children <2 years was only 10.8 per 1000 [15].This could have been because we had included children <2 years who came with complaints of hearing impairment or speech difficulties.
In the last 10 years, three studies based on universal neonatal studies are running in the country, one each in the north, south and west [11–13]. The study protocols have significant overlapping to ours. The study from north and south had coverage of 80–84 % which was lower than ours. The referral rate after 1st DPOAE screening of the Vellore study matched with ours of 2 %. However, the study from Delhi showed a marginal 4.1 % and the study in Pune saw a refer rate of 9.5 %; both rates being higher than the recommended benchmark of <4 % [7]. The prevalence of hearing loss in all three was identical of 2–4 per 1000. Another study found the incidence rate of 5.6 per 1000 [16]. All these studies conclude that universal hearing screening is feasible, even though follow-up was their greatest obstacle. Recruitment and training of technical staff were the pillars to these studies. Follow-up rate is the most significant determinant of the efficacy of the programme. In our study, the follow-up rate after 1st DPOAE screening was 73.09 % which does not meet the benchmark recommended by JCIH 2007 [7]. However, the follow-up rate after 2nd DPOAE for confirmatory diagnostic BERA test was 100 %. On evaluation of lost to follow up we realized that convincing the parents of neonates to come for follow up was indeed the most challenging task at hand, attributed to their unwillingness to submit their child to additional testing. Some parents believed that their child responded to sound and hence ignored the audiologist’s advice. Other reasons for lost follow-up include, difficulty travelling from the patient’s house to the hospital, mainly due to the large distance. Additional factors were unreported change of patient address, financial constraints, and the parents’ inability to take time off from work.
During the initial hearing screening, we conducted counselling and awareness regarding hearing impairment due to which we were able to correctly diagnose two more neonates who were initially screened out and were not associated with any risk factors, but were brought back for further evaluation by the concerned parents, and were confirmed for hearing impairment.
Most countries have well established hearing screening programs for newborns, it is not mandatory but their policies are drafted towards early detection of congenital hearing loss. India is also quickly adopting early detection measures, where in congenital deafness is 1 of the 9 diseases included in the Rashtriya Bal Swasthya Karyakram (RBSK) that mandate screening protocols and early intervention services [17]. India has one of the largest newborn population in the world making universal screening an uphill task, as documented by John et al. [18]. However, the aforementioned study followed an inflexible inclusion criterion and relied majorly on the working hours whereby a greater bulk of the society is bound to get missed. Our study based in a rural area has shown its acceptance and feasibility in a rural setup. A survey to evaluate presence of such programs in India showed that dismal 38.09 % medical colleges had such a program [19]. This survey had a very poor response rate of 17 %, and even though it was sent out to only 165 medical colleges and 20 audio logical institutions, it is possible that the presence of such universal screening is very poor in India. Therefore, it is safe to say that the RBSK is bound to fail unless capacity is built up rapidly. A feedback study done to evaluate the parents’ perception to universal neonatal hearing screening gave positive results [20]. Universal hearing screening programs have lowered that age of identification of congenital deafness to 9.59 months, however it is still lower than the target of 3 months as per JCIH [7, 21]. According to an informal consultation held at the WHO headquarters in 2009, there is lack of epidemiological data regarding the incidence of neonatal hearing loss in most countries [22]. This study helps in summating the database regarding the incidence of neonatal hearing impairment in India. The main aim of the programme was to detect neonatal hearing loss at the earliest; but at the same time we educated every parent or guardian regarding the need. Whatever may be the result of the hearing screening, the parents were counselled to remain vigilant on their babies’ behavioural response to sound, because the test was not infallible and needed to be complemented by other methods also. The increased knowledge and awareness in the minds of the parents was responsible for the co-incidental identification of two false negatives.
Conclusion
This study was performed over a period of 3 years in a tertiary care hospital with minimal allocation of resources and according to a simple protocol. Despite its challenges this study generated data that can potentially be compared to nationwide statistics. A state or nation-wide study can be planned using the same principles with screening centres spread across the districts. Gujarat has an electronic capture system in which new-borns are tracked using eMamta for antenatal care, delivery status and immunization. Incorporating hearing screening program into this system is highly possible and would allow better follow up and tracking of new-borns with failed screens. There would also be a need for non-specialist staff in order to achieve a satisfactory referral rate with the two stage hearing screening protocol. Finally, the role of greater public awareness cannot be over emphasized in early screening for neonatal hearing loss.
Acknowledgement
We thank the Indian Council of Medical Research for providing financial support of this program. We acknowledge Mr. Ajay Phatak,MPH for his valued assistance and guidance from the beginning. We also acknowledge Ms. Ayushi Patel, Mr Jaydeep Chhastiya and Mr Hemant Patel (Audiologist) for their instrumental support.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
The project was funded by the Indian Council of Medical Research (ICMR) (Reference No 5/8/10-9(Oto)/CFP/11-NCD-1).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Research involving human participants and/or animals
This article does not contain any studies with animals performed by any of the authors.
References
- 1.Bachhman KR, Arvedson JV. Early identification and intervention for children who are hearing impaired. Paediatrics. 1998;19:155–165. doi: 10.1542/pir.19-5-155. [DOI] [PubMed] [Google Scholar]
- 2.Nagapoonirma P, Ramesh A, et al. Universal Hearing Screening. Indian J Paediatr. 2007;74(6):545–549. doi: 10.1007/s12098-007-0105-z. [DOI] [PubMed] [Google Scholar]
- 3.Rehabiltation Council of India; Status of Disability in India, 2000, New Delhi, 172–185
- 4.Jewel J. Newborn hearing screening—experience at a tertiary hospital in northwest India. Int J Otolaryngol Head Neck Surg. 2013;2:211–214. doi: 10.4236/ijohns.2013.25044. [DOI] [Google Scholar]
- 5.WHO 2015 Media Centre; Deafness and Hearing Loss; Last updated February 2014. http://www.who.int/mediacentre/factsheets/fs300/en/. Accessed 23 Feb 2015
- 6.Joint Committee on Infant Hearing Position Statement; Principles and guidelines for early hearing detection and intervention programmes. Paediatrics. 2000;106:798–817. doi: 10.1542/peds.106.4.798. [DOI] [PubMed] [Google Scholar]
- 7.Joint Committee on Infant Hearing Policy Statement (2007) JCIH Position statement updates. Paediatrics. 2007;120(4):898. doi: 10.1542/peds.2007-2333. [DOI] [Google Scholar]
- 8.Aishwaraya N, Heramba G, Jayshree S, Roopa N, Bin N Overcoming Challenges of Delivering a Newborn Hearing Screening Program in a Tertiary Care Hospital in India: 7th Australian Newborn Hearing Screening Conference, Auckland, New Zealand, 1–27
- 9.Sukumaran TU. Newborn hearing screening program. Indian Pediatr. 2011;48:351–353. doi: 10.1007/s13312-011-0079-9. [DOI] [PubMed] [Google Scholar]
- 10.Morton C, Nance W. Newborn hearing screening—a silent revolution. New Eng J Med. 2006;354:2151–2164. doi: 10.1056/NEJMra050700. [DOI] [PubMed] [Google Scholar]
- 11.Gupta S, Sah S, Som T, Saksena M, Yadav CP, Sankar MJ, Thakar A, Agarwal R, Deorari A, Paul VK. Challenges of implementing universal newborn hearing screening at a tertiary care centre from India. Indian J Pediatr. 2015 doi: 10.1007/s12098-015-1688-4. [DOI] [PubMed] [Google Scholar]
- 12.Augustine AM, Jana AK, Kuruvilla KA, Danda S, Lepcha A, Ebenezer J, Roshna RP, Tyagi A, Balraj A. Neonatal hearing screening—Experience from a tertiary care hospital in Southern India. Indian Pediatr. 2014;51:179–183. doi: 10.1007/s13312-014-0380-5. [DOI] [PubMed] [Google Scholar]
- 13.Vaid N, Shanbhag J, Nikam R, Biswas A. (2009); Neonatal hearing screening - the Indian experience. Cochlear Implants Int. 2009;10(Suppl 1):111–114. doi: 10.1179/cim.2009.10.Supplement-1.111. [DOI] [PubMed] [Google Scholar]
- 14.Paul A. Early identification of hearing loss and centralized newborn hearing screening facility-the Cochin experience. Indian Pediatr. 2011;48(5):355–359. doi: 10.1007/s13312-011-0067-0. [DOI] [PubMed] [Google Scholar]
- 15.Mishra G, Sharma Y, Mehta K, Patel G. Efficacy of distortion product Oto-Acoustic Emission (OAE)/Auditory Brainstem Evoked Response (ABR) Protocols in Universal Neonatal Hearing Screening and Detecting Hearing Loss in Children <2 years of Age. Indian J Otolaryngol Head Neck Surg. 2012;65(2):105–110. doi: 10.1007/s12070-012-0553-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagapoornima P, Ramesh A, Rao S, Patricia PL, Gore M, Dominic M. Universal hearing screening. Indian J Pediatr. 2007;74(6):545–549. doi: 10.1007/s12098-007-0105-z. [DOI] [PubMed] [Google Scholar]
- 17.National Health Mission, Ministry of Health & Family Welfare Government of India, Rashtriya Bal Swathya Karyakram, Last updated 20th November 2014. http://nrhm.gov.in/nrhm-components/rmnch-a/child-health-immunization/rashtriya-bal-swasthya-karyakram-rbsk/background.html. Accessed 6 Mar 2015
- 18.Nagapoornima P, Ramesh A, Rao S, Patricia PL, Gore M, Dominic M. Universal hearing screening. Indian J Otolaryngol Head Neck Surg. 2007;74(6):545–549. doi: 10.1007/s12098-007-0105-z. [DOI] [PubMed] [Google Scholar]
- 19.Kumar S, Mohapatra B. Status of newborn hearing screening program in India. Int J Pediatr Otorhinolaryngol. 2011;75(2011):20–26. doi: 10.1016/j.ijporl.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 20.Akilan R, Vidya R, Roopa N. Perception of ‘mothers of beneficiaries’ regarding a rural community based hearing screening service. Int J Pediatr Otorhinolaryngol. 2014;78(12):2083–2088. doi: 10.1016/j.ijporl.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Sirur GS, Rangasayee R. Age of identification of hearing impairment in Mumbai–a trend analysis. Int J Pediatr Otorhinolaryngol. 2011;75(12):1549–1552. doi: 10.1016/j.ijporl.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Newborn and infant hearing screening; Current issue and Duiding Principal for action. Outcome of a WHO Informal consultation held at WHO headquarters (2009) World Health Organization, Geneva, Switzerland