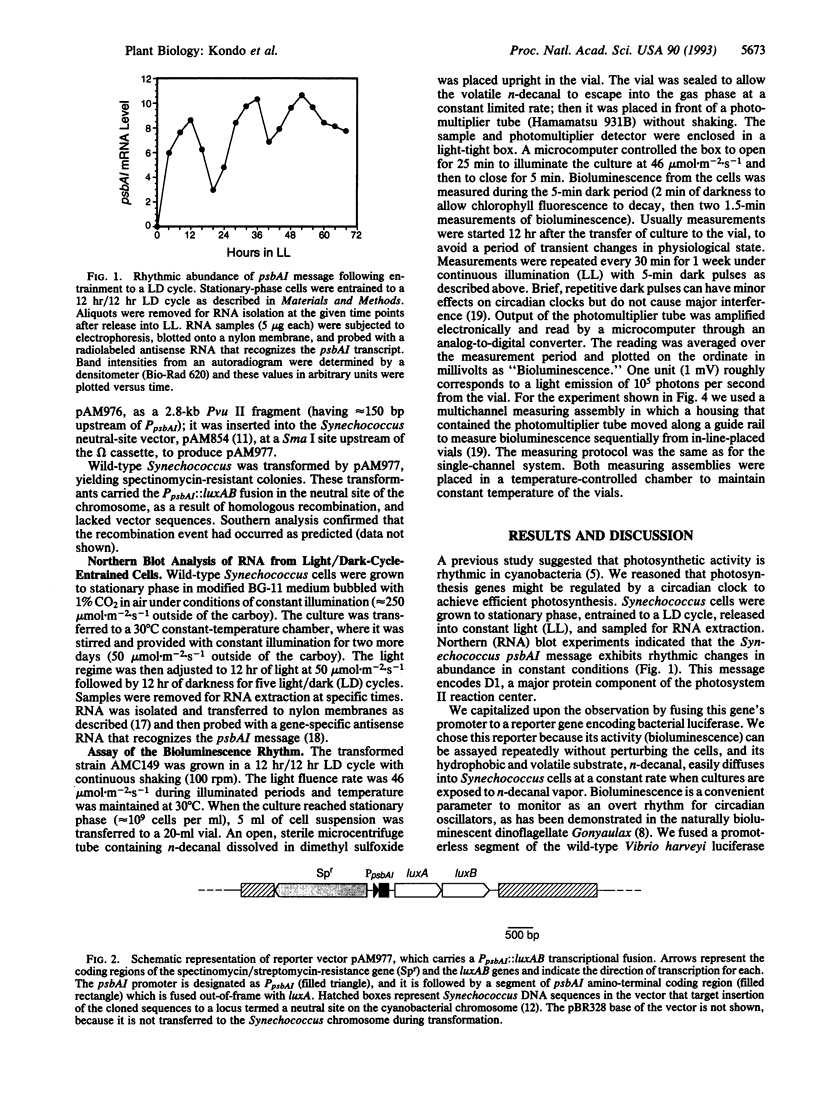
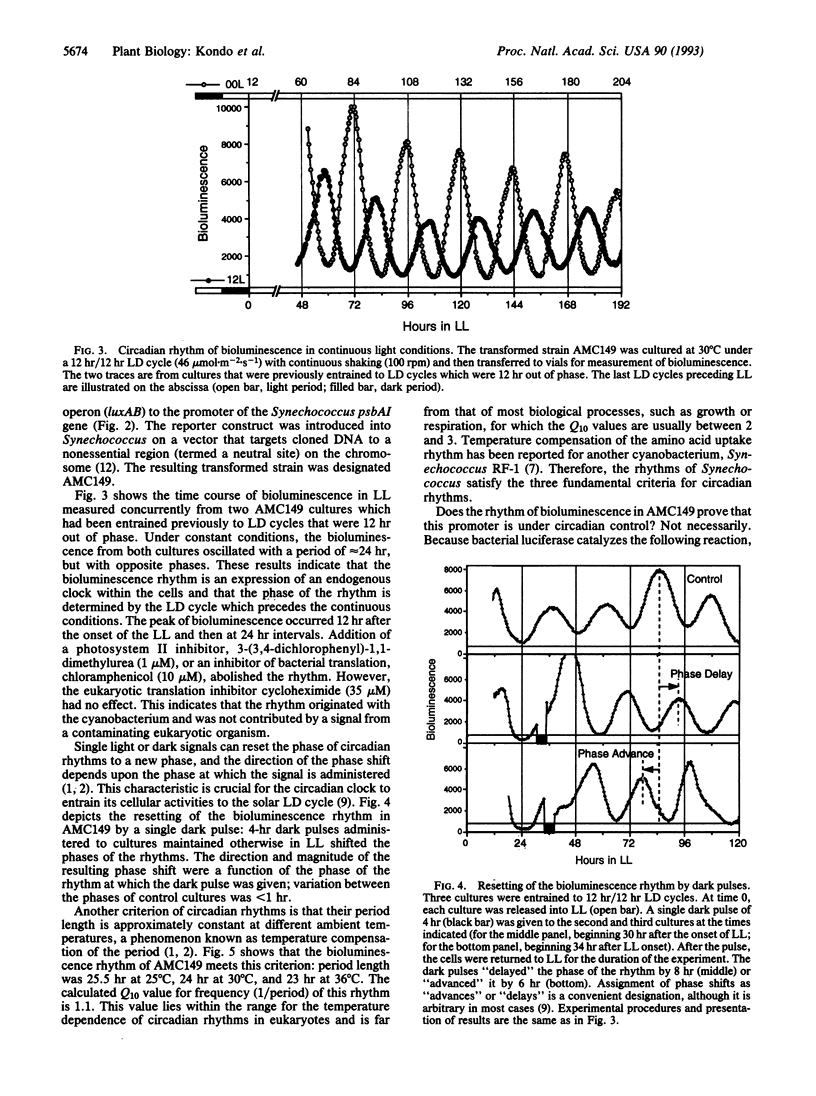
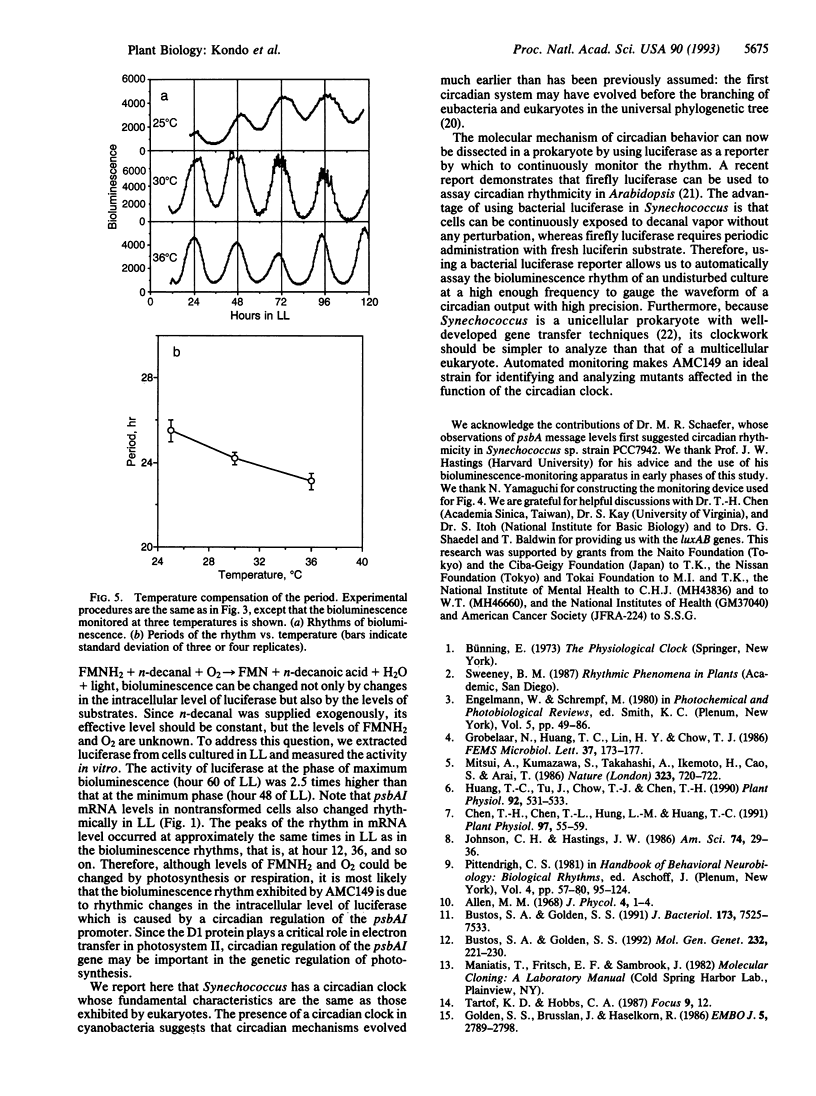
Abstract
We have used a luciferase reporter gene and continuous automated monitoring of bioluminescence to demonstrate unequivocally that cyanobacteria exhibit circadian behaviors that are fundamentally the same as circadian rhythms of eukaryotes. We also show that these rhythms can be studied by molecular methods in Synechococcus sp. PCC7942, a strain for which genetic transformation is well established. A promoterless segment of the Vibrio harveyi luciferase structural genes (luxAB) was introduced downstream of the promoter for the Synechococcus psbAI gene, which encodes a photosystem II protein. This reporter construction was recombined into the Synechococcus chromosome, and bioluminescence was monitored under conditions of constant illumination following entrainment to light and dark cycles. The reporter strain, AMC149, expressed a rhythm of bioluminescence which satisfies the criteria of circadian rhythms: persistence in constant conditions, phase resetting by light/dark signals, and temperature compensation of the period. Rhythmic changes in levels of the native psbAI message following light/dark entrainment supported the reporter data. The behavior of this prokaryote disproves the dogma that circadian mechanisms must be based on eukaryotic cellular organization. Moreover, the cyanobacterial strain described here provides an efficient experimental system for molecular analysis of the circadian clock.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin T. O., Berends T., Bunch T. A., Holzman T. F., Rausch S. K., Shamansky L., Treat M. L., Ziegler M. M. Cloning of the luciferase structural genes from Vibrio harveyi and expression of bioluminescence in Escherichia coli. Biochemistry. 1984 Jul 31;23(16):3663–3667. doi: 10.1021/bi00311a014. [DOI] [PubMed] [Google Scholar]
- Brusslan J., Haselkorn R. Resistance to the photosystem II herbicide diuron is dominant to sensitivity in the cyanobacterium Synechococcus sp. PCC7942. EMBO J. 1989 Apr;8(4):1237–1245. doi: 10.1002/j.1460-2075.1989.tb03497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos S. A., Golden S. S. Expression of the psbDII gene in Synechococcus sp. strain PCC 7942 requires sequences downstream of the transcription start site. J Bacteriol. 1991 Dec;173(23):7525–7533. doi: 10.1128/jb.173.23.7525-7533.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos S. A., Golden S. S. Light-regulated expression of the psbD gene family in Synechococcus sp. strain PCC 7942: evidence for the role of duplicated psbD genes in cyanobacteria. Mol Gen Genet. 1992 Mar;232(2):221–230. doi: 10.1007/BF00280000. [DOI] [PubMed] [Google Scholar]
- Chen T. H., Chen T. L., Hung L. M., Huang T. C. Circadian Rhythm in Amino Acid Uptake by Synechococcus RF-1. Plant Physiol. 1991 Sep;97(1):55–59. doi: 10.1104/pp.97.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Expression of a family of psbA genes encoding a photosystem II polypeptide in the cyanobacterium Anacystis nidulans R2. EMBO J. 1986 Nov;5(11):2789–2798. doi: 10.1002/j.1460-2075.1986.tb04569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Huang T. C., Tu J., Chow T. J., Chen T. H. Circadian Rhythm of the Prokaryote Synechococcus sp. RF-1. Plant Physiol. 1990 Feb;92(2):531–533. doi: 10.1104/pp.92.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni R. D., Schaefer M. R., Golden S. S. Transcriptional and posttranscriptional components of psbA response to high light intensity in Synechococcus sp. strain PCC 7942. J Bacteriol. 1992 Jun;174(11):3775–3781. doi: 10.1128/jb.174.11.3775-3781.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar A. J., Short S. R., Chua N. H., Kay S. A. A novel circadian phenotype based on firefly luciferase expression in transgenic plants. Plant Cell. 1992 Sep;4(9):1075–1087. doi: 10.1105/tpc.4.9.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Kandler O., Wheelis M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]




