Summary
Autoimmune haemolytic anaemias (AIHAs) are well-characterized disorders. They can be differentiated from one another and from other non-immune haemolytic anaemias by clinical, laboratory and serological testing. However, several misleading clinical presentations and/or serological findings may result in misinterpretation, delay and/or misdiagnosis. Such failures are avoidable by adequate clinical and serological experience of the responsible physicians and serologists or, at least, by an optimised bidirectional communication. As long as this has not been achieved, unpleasant failures are to be expected. A true diagnosis of AIHA can neither be verified by clinical nor serological findings alone. Thus, a collective clinical and serological picture remains obligatory for fulfilling the criteria of optimal diagnosis and therapy. Ultimately, the majority of pioneer scientific and practical work in this field stems from scientists who were simultaneously involved in both the clinic and serology.
Keywords: AIHA, Positive DAT, Negative DAT, C3d DAT, IgG DAT, Coombs-negative, Agglutination, Immune haemolysis, Autoimmune haemolytic anaemia
Introduction
With the exception of untreated paroxysmal nocturnal haemoglobinuria (PNH), the unifying characteristic of all immune haemolytic anaemias is a positive direct antiglobulin test (DAT). However, this finding is not always associated with autoimmune haemolytic anaemias (AIHAs), and immune haemolysis cannot always be excluded when the DAT remains negative. In general, results obtained by the DAT are of little or no value without relevant clinical information and detection of ‘true’ autoantibodies (aab) directed against autologous RBC antigens being capable of causing RBC destruction. In suspected cases, several aspects should be taken into consideration: medical history, signs, start, course, haemolytic character (extra- and/or intravascular haemolysis), current infections, co-morbidities and use of drugs. If haemolysis is recognisable, the DAT results are not only helpful in the verification of AIHA but also in its differentiation. In general, AIHAs are classified as warm, cold, paroxysmal cold (Donath-Landsteiner), mixed, and drug-induced. Each form is usually characterised by clinical and serological findings [1,2,3,4,5]. Confusion may occur for each type, but most commonly in AIHA of the warm type and in drug-induced forms.
In this article, the most relevant misleading findings will be addressed and critically discussed.
AIHA and Negative DAT
Several reports have repeatedly emphasised the occurrence of negative DAT in AIHA of the warm type, and the use of other methods for the detection of the causative autoantibodies (aab) in such cases [6,7,8,9,10,11,12,13,14]. The reasons behind this finding are attributed to either a low concentration or affinity of the aab, which may still play a role when the patients’ RBCs are washed prior to testing. In our experience, these aab can easily be detected when unwashed patients’ RBCs are tested, i.e. by using the gel or glass microcolum technique. However, such aab are at times rather difficult to characterise. These aab may be considered as significant only if haemolysis is recognisable and the course of haemolysis is typical for AIHA.
In rare cases, the causative aab are transiently detectable in the serum and eluate or only in the eluate, but not by DAT [2,3,9,12,15,16]. However, no eluate technique is currently available that invariably allows the detection of all types of aab [17,18,19]. In suspected cases, where the causative aab are not detectable by one method, at least one different technique should be used (fig. 1). An alternative method would be flow cytometry or other sensitive methods [10,11,13,20]. By use of gel cards and different elution techniques, we were able to detect the causative aab in all cases tested in our laboratory during the last two decades.
Fig. 1.
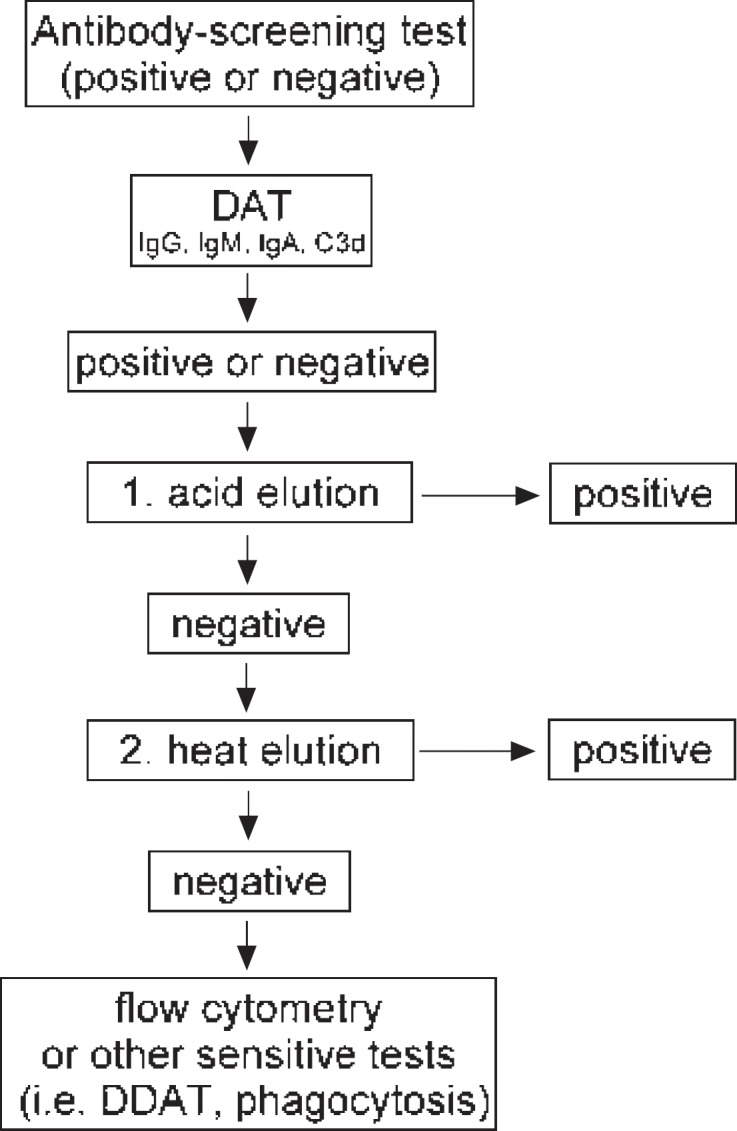
Testing procedure in all cases suspected to have any kind of immune haemolytic anaemia. Antibody screening test and elution should invariably be included. In isolated cases, the causative autoantibody could be detected in serum and/or eluate but not on patient's RBCs (negative DAT). DDAT = Dual direct antiglobulin test.
The reason why causative aab are not directly detectable by DAT in isolated cases but in the eluate is presumably the presence of diminished antigens on autologous RBCs as a response to the haemolytic attack. In fact, such affected patients do survive haemolysis even without treatment, as long as the expression of the involved antigen has not been restored. This phenomenon is most frequently observed in patients with aab to Gerbich antigens [15,21,22].
Additional reasons for the occurrence of a negative DAT in AIHA of the warm type are non-complement-activating IgM or IgA aab. The former aab could be detected by sensitive methods rather than by agglutination tests [23]. In comparison, IgA aab may escape detection when anti-IgA is not included in the applied test system [9,14,15]. To confirm the nature of true IgA aab, the eluate from the patients’ RBCs should also be tested by the indirect antiglobulin test using anti-IgA [15].
In conclusion, when sophisticated testing is employed, the causative aab in AIHAs, unlike those responsible for autoimmune thrombocytopenia, are detectable in the vast majority, if not all affected patients.
Positive DAT in the Absence of Haemolysis
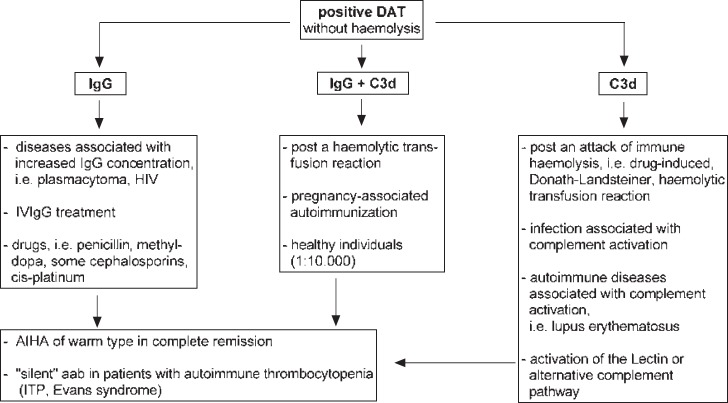
The observation of positive DAT in the absence of signs of immune haemolysis is relatively common. The significance of this phenomenon is evident in all laboratories dealing with RBC serology. Unfortunately, the vast majority of laboratories has only little or no information regarding the patients’ underlying disease(s) and/or treatment. In such cases, the interpretation of a positive DAT remains largely speculative at the serological side. If the affected patient does not have signs of haemolytic anaemia, the positivity observed may be related to a number of in vivo occurrences that may result in an IgG- and/or C3d-positive DAT (fig. 2).
Fig. 2.
Most relevant instances observed to be associated with positive DAT without haemolysis at time of serological testing.
IgG-Positive DAT without Haemolysis
Given the fact that the RBC membrane is negatively charged and IgG molecules are positively charged, these cells are susceptible for unspecific IgG attachment onto their surfaces, leading to an IgG-positive DAT [25]. This may occur in cases where the IgG concentration is significantly increased due to the underlying disease, i.e. plasmocytoma and HIV, or due to treatment with immunoglobulins [3,24]. In addition, these preparations may also contain antibodies to A and B RBC antigens. These antibodies may sometimes cause mild or, in rare cases, severe haemolysis [26]. Furthermore, some drugs may cause unspecific IgG adsorption onto RBCs, e.g. cephalosporins [3,24,27,28]. In conclusion, less than 1% of patients with an IgG-positive DAT may have immune haemolysis [3,24].
Drug-Induced IgG aab to RBCs
Some drugs may stimulate the production of IgG aab to RBCs, which do not always result in immune haemolysis. This phenomenon has most commonly been observed in patients who receive high doses of alpha-methyl dopa over a sustained period of time. A small number of these patients may develop haemolysis [3,27,28]. The list of drugs which have been implicated in causing a positive DAT is on the rise [27,29]. Interestingly, in almost all cases, drug-induced aab do not appear to cause complement activation [2,3,27,29]. The incidence of drug-induced aab is obscure, as these aab are either insignificant or result in immune haemolysis, which is usually indistinguishable from the ‘so-called’ idiopathic form [2,3,27,29].
Drug-Induced IgG-Positive DAT
Penicillin is the prototype of an IgG-positive DAT. These antibodies are penicillin-dependent, do not cause complement activation and, in rare cases, may result in haemolysis [3,27,29]. Similarly, older generations of cephalosporins have infrequently been reported to cause unspecific IgG adsorption and drug-dependent IgG antibodies similar to penicillin-dependent antibodies [3,27,29]. The true incidence of such antibodies is unknown, as they are insignificant or remain uncharacterised.
Pregnancy-Induced IgG aab to RBCs
Pregnancy-related alloimmunisation is well-known, whereas little is known about autoimmunisation. The importance of this phenomenon is reflected by two aspects: Firstly, pregnancy-induced aab to RBCs are, unlike those from patients with true AIHA, usually harmless for both the mother and child; and secondly, their detection may result in confusion and inadequate responses at the serological and clinical side. We have previously described this phenomenon in 2001 [30] and again in 2015 [31]. The question why these aab do not appear to cause significant haemolysis is obscure. Most importantly, alpha-methyl dopa is currently the standard drug for treatment of hypertension in pregnant women. Thus, in case of a positive DAT in pregnancy it has to be clarified whether or not this positivity might be related to the drug.
Transfusion-Induced aab to RBCs
RBC transfusion may stimulate not only the production of alloantibodies, but concomitantly aab or aab alone. Transfusion-induced aab may occur following serological transfusion reaction without significant haemolysis or following haemolytic transfusion reaction, and may persist for a long time in the circulation and/or on patients’ RBCs (positive DAT and/or eluate). Thus, a sophisticated medical history regarding previous blood transfusions is indicated in all cases. We first recognised this phenomenon in 1984 [32], and since then this finding has been supported by various groups [3,33,34]. This type of aab appears to occur for short or long periods of time in patients who develop haemolytic transfusion reactions, leading to confusion with true AIHA. A typical example is described in this issue [22]. This phenomenon had previously led to the assumption that patients with AIHA frequently develop alloantibodies, which should be considered prior to RBC transfusion in patients with AIHA [35].
Agglutinating aab
Agglutinating aab are usually of the cold and only rarely of the warm type [1,2,3]. The vast majority of these belong to the IgM class. However, IgG and IgA aab may also infrequently cause direct RBC agglutination. In all cases of agglutinating aab, the results obtained by DAT cannot be correctly interpreted. In addition, the classification and characterisation of such aab are associated with difficulties that may be overcome either by treatment with dithiothreitol (DDT) or inhibition of eluates with specific antibodies [36].
Positive C3d DAT in the Absence of aab to RBCs
One of the main prerequisites in understanding immunohaematology is an at least basic knowledge of the complement system, its functions and, most importantly, its interaction with RBCs. These cells express CD35 (CR1/C3b receptor), CD55 (decay accelerating factor; DAF), and CD59 (membrane inhibitor of reactive lysis) complement regulatory proteins. The former protein plays a central role in understanding C3d-positive DAT results. This component is the inactivated form of C3b which is involved in phagocytosis, formation of C3 convertase, and stimulation of complement activation [2,3,37,38]. Thus, C3d-coated RBCs represent cells which survive complement activation either by the classical pathway, i.e. due to drug-dependent antibodies or alloantibodies to RBCs, or due to activation of the alternative pathway in the presence of adjacent RBCs. While generation of C3b via the classical pathway is usually associated with haemolysis, the generation of C3b via the alternative pathway does not cause significant haemolysis. The phenomenon that adjacent RBCs are susceptible to free C3b in the circulation was demonstrated in 1984 [39] and later in 1992 [40]. This may give an explanation for the finding that DAT is positive with anti-C3d in 8% of hospital patients. In comparison, all patients with AIHA of the cold or the Donath-Landsteiner type have a positive C3d DAT. Patients with AIHA of the warm type show positive DAT with anti-IgG and C3d in 50-60%, and with anti-C3d in <13% [2,3]. However, the true incidence remains speculative due to many diagnostic pitfalls.
Evidence Suggesting C3-Independent C5 Activation
The observation that complement activation, via the classical and the alternative pathway, invariably results in coating of adjacent RBCs with C3d, but reactive haemolysis due to activation of the terminal complement components (C5b-9) does not result in positive C3d DAT can only be explained by direct activation of C5. This hypothesis is supported by a previous report on one patient who developed intravascular haemolysis, similar to that observed in PNH. In the aforementioned case, we demonstrated C5b-9 complexes on the RBC membrane, but did not observe any C3d on survived RBCs [41]. Recent findings have demonstrated C5 activation without C3 activation [42]. Presumably, the finding in the former publication and the debate related to negative DAT in patients with PNH have led to the detection of DAF and, subsequently, CD55 and CD59 deficiencies on RBCs of affected patients [43,44,45].
Confusion of Cold Agglutinins with Warm Agglutinins and vice versa
Agglutinating cold aab with high thermal amplitude and a relative low titre may sometimes lead to confusion with warm IgM aab, and vice versa, i.e. agglutinating warm aab may be confused with cold agglutinins [36,46]. In such cases, eluates from patients’ RBCs (fig. 1), and a comparison of haemolysis tests at room temperature and 37 °C are usually helpful. While cold haemolysins are active at 20 °C but not or only weakly (dependent on temperature amplitude) at 37 °C, warm aab are predominantly active at 37 °C. In addition, in vivo agglutinations due to warm aab, remain stable at core temperature, for example, skin discolouration [46]. Finally, eluates from pre-warmed and washed (at 37 °C) RBCs of patients with AIHA of the cold type remains negative.
Donath-Landsteiner aab with AIHA of the Warm Type
The Donath-Landsteiner aab are usually quite weak and rarely strong enough to cause not only a positive C3d DAT but also IgG DAT. In both cases, haemolysis is infrequently confused with AIHA of the warm type. However, Donath-Landsteiner haemolysis typically occurs in children and rarely in adults. In addition, the disease is usually acute and spontaneously reversible within several days [47].
Additive-Dependent Agglutination
Several chemicals that are used as preservatives in commercial serological reagents, such as albumin, EDTA, citrate and low-ionic strength saline, have been implicated in causing anomalous blood grouping [48,49,50,51,52,53,54,55]. This phenomenon is characterised by the discrepancy between reactions of serum samples with RBCs in the presence and in the absence of the causative chemical. For example, the antibody screening test is positive and the cross-match is negative, or the autocontrol is positive but both the DAT and eluate are negative. The positive reactions are usually strong and may lead to confusion with alloantibodies directed against a high-frequency antigen or with agglutinating aab.
Negative Eluates
As discussed above, none of the available or used eluate techniques are capable of capturing the causative aab in all cases. The use of more than one technique is indicated in suspected cases (fig. 1). In our experience, this is the case in most patients with AIHA related to warm IgM aab, and in roughly 3% of all affected patients [2,45].
Transplantation-Associated Immune Haemolysis
Many patients may develop mild or severe haemolysis following transplantation of haematopoietic stem cells, and less frequently, solid organs. In the former case, haemolysis is most often related to ABO blood group mismatches. Notably, these antigens are inherited independently of the HLA complex. In addition, some patients may develop aab and/or alloantibodies which may cause significant haemolysis [3]. The alloantibodies might be produced by the donor or recipient cells. Haemolysis related to the donors’ antibodies is known as passenger lymphocyte syndrome [3,57,58], which usually persists for a short amount of time. In contrast, transplantation-associated aab may cause severely persistent AIHA.
Non-Immune Haemolytic Anaemia
Frequently, patients with hereditary or acquired non-immune haemolytic anaemia might initially be suspected to have AIHA. However, the latter form can usually be excluded in the absence of aab and/or C3d on patients’ RBCs [1,2,3].
Disclosure Statement
The author declares no conflict of interest.
References
- 1.Dacie J. The Haemolytic Anaemias. Vol. 3. The Auto-Immune Haemolytic Anaemias. New York, Churchill Livingstone, 1992.
- 2.Salama A, Ahrens N, Kiesewetter H. Serological and clinical aspects of autoimmune hemolytic anemias. Infus Ther Med. 2002;29:206–217. [Google Scholar]
- 3.Petz LD, Garraty G. Immune Hemolytic Anemias, 2nd ed. Philadelphia, Churchill Livingstone, 2004.
- 4.Michel M. Classification and therapeutic approaches in autoimmune hemolytic anemia: an update. Expert Rev Hematol. 2011;4:607–618. doi: 10.1586/ehm.11.60. [DOI] [PubMed] [Google Scholar]
- 5.Bass GF, Tuscano ET, Tuscano JM. Diagnosis and classification of autoimmune hemolytic anemia. Autoimmun Rev. 2014;13:560–564. doi: 10.1016/j.autrev.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Gilliland BC. Coombs-negative immune hemolytic anemia. Semin Hematol. 1976;13:267–275. [PubMed] [Google Scholar]
- 7.Salama A, Mueller-Eckhardt C, Bhakdi S. A two-stage immunoradiometric assay with 125I-staphylococcal protein A for the detection of antibodies and complement on human blood cells. Vox Sang. 1985;48:239–245. doi: 10.1111/j.1423-0410.1985.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 8.Sokol RJ, Booker DJ, Stamps R, Jalihal S, Paul B. Direct Coombs test-negative autoimmune hemolytic anemia and low-affinity IgG class antibodies. Immunohematology. 1997;13:115–118. [PubMed] [Google Scholar]
- 9.Garratty G. Immune hemolytic anemia associated with negative routine serology. Semin Hematol. 2005;42:156–164. doi: 10.1053/j.seminhematol.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Barcellini W, Revelli N, Imperiali FG, Villa MA, Manera MC, Paccapelo C, Zaninoni A, Zanella A. Comparison of traditional methods and mitogen-stimulated direct antiglobulin test for detection of anti-red blood cell autoimmunity. Int J Hematol. 2010;91:762–769. doi: 10.1007/s12185-010-0578-9. [DOI] [PubMed] [Google Scholar]
- 11.Fayek MH, Saad AA, Eissa DG, Tawfik LM, Kamal G. Role of gel test and flow cytometry in diagnosis of Coombs’ negative autoimmune haemolytic anaemia. Int J Lab Hematol. 2012;34:311–319. doi: 10.1111/j.1751-553X.2011.01397.x. [DOI] [PubMed] [Google Scholar]
- 12.Segel GB, Lichtman MA. Direct antiglobulin (‘Coombs') test-negative autoimmune hemolytic anemia: a review. Blood Cells Mol Dis. 2014;52:152–160. doi: 10.1016/j.bcmd.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Bloch EM, Sakac D, Branch HA, Cserti-Gazdewich C, Pendergrast J, Pavenski K, Branch DR. Western immunoblotting as a new tool for investigating direct antiglobulin test-negative autoimmune hemolytic anemias. Transfusion. 2015;55:1529–1537. doi: 10.1111/trf.13082. [DOI] [PubMed] [Google Scholar]
- 14.Barcellini W. Pitfalls in the diagnosis of autoimmune haemolytic anaemia. Blood Transfus. 2015;13:3–5. doi: 10.2450/2014.0252-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Göttsche B, Salama A, Mueller-Eckhardt C. Autoimmune hemolytic anemia associated with an IgA autoanti-Gerbich. Vox Sang. 1990;58:211–214. doi: 10.1111/j.1423-0410.1990.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 16.Sachs UJ, Röder L, Santoso S, Bein G. Does a negative direct antiglobulin test exclude warm autoimmune haemolytic anaemia? A prospective study of 504 cases. Br J Haematol. 2006;132:655–656. doi: 10.1111/j.1365-2141.2005.05955.x. [DOI] [PubMed] [Google Scholar]
- 17.Panzer S, Salama A, Bödeker RH, Mueller-Eckhardt C. Quantitative evaluation of elution methods for red cell antibodies. Vox Sang. 1984;46:330–335. doi: 10.1111/j.1423-0410.1984.tb00094.x. [DOI] [PubMed] [Google Scholar]
- 18.Torloni AS, McCullough JS, Brecher ME, Tribble LJ, Hill MG. Microwave elution of red cell antibodies. Vox Sang. 1994;66:276–279. doi: 10.1111/j.1423-0410.1994.tb00329.x. [DOI] [PubMed] [Google Scholar]
- 19.Caruccio L, Byrne K, Procter J, Stroncek D. A novel method using formamide for the elution of antibodies from erythrocytes. Vox Sang. 2002;83:63–69. doi: 10.1046/j.1423-0410.2002.00181.x. [DOI] [PubMed] [Google Scholar]
- 20.Garratty G, Arndt P. Applications of flow cytofluorometry to transfusion science. Transfusion. 1995;35:157–178. doi: 10.1046/j.1537-2995.1995.35295125739.x. [DOI] [PubMed] [Google Scholar]
- 21.Zimring JC, Cadwell CM, Spitalnik SL. Antigen loss from antibody-coated red blood cells. Transfus Med Rev. 2009;23:189–204. doi: 10.1016/j.tmrv.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Sümnig A, Mayer B, Kiefel V, Greinacher A, Salama A. ‘Chameleonic’ serological findings leading to life-threatening haemolytic transfusion reactions. Transfus Med Hemother 2015; DOI: 10.1159/000437198. [DOI] [PMC free article] [PubMed]
- 23.Salama A, Mueller-Eckhardt C. Autoimmune haemolytic anaemia in childhood associated with non-complement binding IgM autoantibodies. Br J Haematol. 1987;65:67–71. doi: 10.1111/j.1365-2141.1987.tb06137.x. [DOI] [PubMed] [Google Scholar]
- 24.Garratty G. The significance of IgG on the red cell surface. Transfus Med Rev. 1987;1:47–57. doi: 10.1016/s0887-7963(87)70005-4. [DOI] [PubMed] [Google Scholar]
- 25.Bove JR, Holburn AM, Mollison PL. Non-specific binding of IgG to antibody-coated red cells. (the ‘Matuhasi-Ogata phenomenon') Immunology. 1973;25:793–801. [PMC free article] [PubMed] [Google Scholar]
- 26.Quinti I, Pulvirenti F, Milito C, Granata G, Giovannetti G, La Marra F, Pesce AM, Farrugia A, Coluzzi S, Girelli G. Hemolysis in patients with antibody deficiencies on immunoglobulin replacement treatment. Transfusion. 2015;55:1067–1074. doi: 10.1111/trf.12939. [DOI] [PubMed] [Google Scholar]
- 27.Salama A. Drug-induced immune hemolytic anemia. Expert Opin Drug Saf. 2009;8:73–79. doi: 10.1517/14740330802577351. [DOI] [PubMed] [Google Scholar]
- 28.Salama A, Mayer B. Diagnostic pitfalls of drug-induced immune hemolytic anemia. Immunohematology. 2014; 30(2):80-4. [PubMed]
- 29.Garratty G, Arndt P. Drugs that have been shown to cause drug-induced immune hemolytic anemia or positive direct antiglobulin tests: some interesting findings since 2007. Immunohematology. 2014;30:66–79. [PubMed] [Google Scholar]
- 30.Hoppe B, Stibbe W, Bielefeld A, Pruss A, Salama A. Increased RBC autoantibody production in pregnancy. Transfusion. 2001;41:1559–1561. doi: 10.1046/j.1537-2995.2001.41121559.x. [DOI] [PubMed] [Google Scholar]
- 31.Sürücü G, Yürek S, Mayer B, Salama A. Pregnancy-induced autoantibodies to red blood cells. Transfus Med Hemother 2015; DOI: 10.1159/000440672. [DOI] [PMC free article] [PubMed]
- 32.Salama A, Mueller-Eckhardt C. Delayed hemolytic transfusion reactions. Evidence for complement activation involving allogeneic and autologous red cells. Transfusion. 1984;24:188–193. doi: 10.1046/j.1537-2995.1984.24384225018.x. [DOI] [PubMed] [Google Scholar]
- 33.Ahrens N, Pruss A, Kähne A, Kiesewetter H, Salama A. Coexistence of autoantibodies and alloantibodies to red blood cells due to blood transfusion. Transfusion. 2007;47:813–816. doi: 10.1111/j.1537-2995.2007.01194.x. [DOI] [PubMed] [Google Scholar]
- 34.Zimring JC, Spitalnik SL, Roback JD, Hillyer CD. Transfusion-induced autoantibodies and differential immunogenicity of blood group antigens: a novel hypothesis. Transfusion. 2007;47:2189–2196. doi: 10.1111/j.1537-2995.2007.01468.x. [DOI] [PubMed] [Google Scholar]
- 35.Yürek S, Almahallawi M, Mayer B, Pruss A, Salama A. Precautions surrounding blood transfusion in autoimmune haemolytic anemias are overestimated. Blood Transfus 2015; doi: 10.2450/2015.0326-14. [DOI] [PMC free article] [PubMed]
- 36.Bartolmäs T, Mayer B, Yürek S, Genth R, Salama A. Paradoxical findings in direct antiglobulin test and classification of agglutinating autoantibodies using eluates and monospecific anti-human globulin sera. Vox Sang. 2015;108:58–63. doi: 10.1111/vox.12187. [DOI] [PubMed] [Google Scholar]
- 37.Freedman J. The significance of complement on the red cell surface. Transfus Med Rev. 1987;1:58–70. doi: 10.1016/s0887-7963(87)70006-6. [DOI] [PubMed] [Google Scholar]
- 38.Berentsen S, Sundic T. Red blood cell destruction in autoimmune hemolytic anemia: role of complement and potential new targets for therapy. Biomed Res Int. 2015;2015:363278. doi: 10.1155/2015/363278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salama A, Mueller-Eckhardt C. Binding of fluid phase C3b to nonsensitized bystander human red cells. A model for in vivo effects of complement activation on blood cells. Transfusion. 1985;25:528–534. doi: 10.1046/j.1537-2995.1985.25686071424.x. [DOI] [PubMed] [Google Scholar]
- 40.Salama A, Hugo F, Heinrich D, Höge R, Müller R, Kiefel V, Mueller-Eckhardt C, Bhakdi S. Deposition of terminal C5b-9 complement complexes on erythrocytes and leukocytes during cardiopulmonary bypass. N Engl J Med. 1988;318:408–414. doi: 10.1056/NEJM198802183180704. [DOI] [PubMed] [Google Scholar]
- 41.Salama A, Bhakdi S, Mueller-Eckhardt C. Evidence suggesting the occurrence of C3-independent intravascular immune hemolysis. Reactive hemolysis in vivo. Transfusion. 1987;27:49–53. doi: 10.1046/j.1537-2995.1987.27187121473.x. [DOI] [PubMed] [Google Scholar]
- 42.Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, Lambris JD, Warner RL, Flierl MA, Hoesel LM, Gebhard F, Younger JG, Drouin SM, Wetsel RA, Ward PA. Generation of C5a in the absence of C3:a new complement activation pathway. Nat Med. 2006;12:682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 43.Parker CJ. The pathophysiology of paroxysmal nocturnal hemoglobinuria. Exp Hematol. 2007;35:523–533. doi: 10.1016/j.exphem.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 44.Rachidi S, Musallam KM, Taher AT. A closer look at paroxysmal nocturnal hemoglobinuria. Eur J Intern Med. 2010;21:260–267. doi: 10.1016/j.ejim.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Salama A. Immunreaktionen gegen Erythrozyten; in Kiefel V (Hrsg): Transfusionsmedizin und Immunhämatologie, 4. Aufl. Heidelberg, Springer 2010, pp 79-89.
- 46.Salama A, Janvier D, Mayer B, Saison C, Moscatelli H, Aucouturier F, Yilmaz P, Arnaud L, Wild V, Knop S, Cartron JP. Lethal autoimmune hemagglutination due to an immunoglobulin A autoagglutinin with band 3 specificity. Transfusion. 2014;54:1988–1995. doi: 10.1111/trf.12638. [DOI] [PubMed] [Google Scholar]
- 47.Göttsche B, Salama A, Mueller-Eckhardt C. Donath-Landsteiner autoimmune hemolytic anemia in children. A study of 22 cases. Vox Sang. 1990;58:281–286. doi: 10.1111/j.1423-0410.1990.tb05000.x. [DOI] [PubMed] [Google Scholar]
- 48.Jones TE, Ayrton AS, Blajchman MA. Anomalous ABO grouping due to a new serum factor reactive with a reagent coloring material. Transfusion. 1973;13:150–152. doi: 10.1111/j.1537-2995.1973.tb05463.x. [DOI] [PubMed] [Google Scholar]
- 49.Hysell JK, Gray JM, Hysell JW, Beck ML. An anti-neomycin antibody interfering with ABO grouping and antibody screening. Transfusion. 1975;15:16–22. doi: 10.1046/j.1537-2995.1975.15175103504.x. [DOI] [PubMed] [Google Scholar]
- 50.Vengelen-Tyler V, Dearolf SJ, Haley S. Two examples of antibodies dependent upon the presence of inosine. Transfusion. 1981;21:315–319. doi: 10.1046/j.1537-2995.1981.21381201804.x. [DOI] [PubMed] [Google Scholar]
- 51.Howe SE, Sciotto CG, Berkner D. The role of carboxylic acids in EDTA-dependent panagglutination. Transfusion. 1982;22:111–114. doi: 10.1046/j.1537-2995.1982.22282177115.x. [DOI] [PubMed] [Google Scholar]
- 52.Shulman IA. Hasz LA, Simpson RB. Thimerosal dependent agglutination, a newly described blood bank problem. Transfusion. 1982;22:241–243. doi: 10.1046/j.1537-2995.1982.22382224950.x. [DOI] [PubMed] [Google Scholar]
- 53.Shulman IA, Simpson RB, Farmer CF, Lam H-T. Thimerosal-dependent agglutination complicating the serologic evaluation for unexpected antibodies. Transfusion. 1984;24:365–367. doi: 10.1046/j.1537-2995.1984.24484275584.x. [DOI] [PubMed] [Google Scholar]
- 54.Mason JM, Osborne PT, Hall AJ, Skolnik JS, Woods LL, Wood CL, Pierce SR, Beck ML. Example of a thimerosal-dependent antibody without apparent blood group specificity. Vox Sang. 1985;48:313–316. doi: 10.1111/j.1423-0410.1985.tb00188.x. [DOI] [PubMed] [Google Scholar]
- 55.Yasuda H, Ohto H, Motoki R, Uchikawa M. An EDTA-associated anti-B agglutinin: the role of ionized calcium. Transfusion. 1997;37:1131–1136. doi: 10.1046/j.1537-2995.1997.37111298088041.x. [DOI] [PubMed] [Google Scholar]
- 56.Joshi SR. Citrate-dependent auto-antibody causing error in blood grouping. Vox Sang. 1997;72:229–232. doi: 10.1046/j.1423-0410.1997.7240229.x. [DOI] [PubMed] [Google Scholar]
- 57.Seltsam A, Hell A, Heymann G, Salama A. Donor-derived alloantibodies and passenger lymphocyte syndrome in two of four patients who received different organs from the same donor. Transfusion. 2001;41:365–370. doi: 10.1046/j.1537-2995.2001.41030365.x. [DOI] [PubMed] [Google Scholar]
- 58.Petz, LD. Immune hemolysis associated with transplantation. Semin Hematol. 2005;42:145–155. doi: 10.1053/j.seminhematol.2005.05.017. [DOI] [PubMed] [Google Scholar]