Abstract
Studies that estimate indoor aeroallergen exposure typically measure a pre-selected limited range of allergens. In this study, inhalable aeroallergen particles were quantified using the halogen immunoassay (HIA) to determine the contribution of fungal and non-fungal aeroallergens to total allergen exposure. Bioaerosols from 39 homes of fungal-allergic subjects were sampled using inhalable fraction samplers and immunostained by HIA using resident subject's immunoglobulin E (IgE) to detect allergen-laden particles. Fungal aerosols as well as particles carrying mite, cat, and cockroach allergens were identified and enumerated by HIA. Reservoir dust-mite (Der p 1), cat (Fel d 1), and cockroach (Bla g 1) allergen concentrations were quantified by ELISA. Fungal particles that bound subject's IgE in the HIA were 1.7 (bedroom)- and 1.4 (living room)-fold more concentrated than Der p 1, Fel d 1, and Bla g 1 allergen particles combined. Predominant fungal conidia that bound IgE were derived from common environmental genera including Cladosporium and other fungi that produce amerospores. Airborne mite, cat, and cockroach allergen particle counts were not associated with reservoir concentrations determined by ELISA. This study demonstrates that inhalable fungal aerosols are the predominant aeroallergen sources in Sydney homes and should be considered in future exposure assessments.
Keywords: Aeroallergens, Bioaerosols, Allergen exposure, Airborne fungi, Fungal allergens
Introduction
Personal exposure to aeroallergens is the major causal factor in the development of allergic sensitization (Chew et al., 2008; Platts-Mills et al., 2006) and respiratory symptoms (Lemanske and Busse, 2006). The domestic aeroallergens most commonly associated with allergic rhinitis and allergic asthma originate from a variety of sources including house dust-mites, cats, cockroaches, and fungi (Cho et al., 2006; Gruchalla et al., 2005), and these are the allergens that are most commonly measured in exposure studies. Multiple aeroallergens typically co-exist in a dynamic mixture of bioaerosols (Rabito et al., 2007), influenced by location, climate, time of year, time of day, and by the extent of dust disturbance (Custovic et al., 1999). Other variables associated with aeroallergen exposure may also influence clinical outcomes; these include the size distribution of aeroallergen particles (and hence their site of respiratory deposition), the quantity of allergen per particle, and other concurrent exposures, such as to endotoxin, which may modulate the effects of allergens (Platts-Mills and Woodfolk, 2011).
The contribution of a number of other characterized as well as uncharacterized allergens may be completely overlooked due to the limitations associated with traditional methods of allergen exposure assessment. The halogen immunoassay (HIA) has provided a new approach to characterize the diversity of aeroallergens through the immunostaining of individual bioaerosol particles that function as sources of aeroallergens. Allergen sources are identified by labeling allergens associated with the source particles with specific immunoglobulin E (sIgE) obtained from the serum of sensitized individuals. In some situations, bioaerosols (fungal spores and pollen) associated with IgE immunostaining can be identified morphologically (Green et al., 2005; Razmovski et al., 2000); however, many indoor bioaerosols derived from perennial sources are amorphous and their identification can only be made on the basis of immunostaining with allergen-specific monoclonal antibodies (mAbs).
In this study, the relative occurrence of indoor fungal aeroallergens was evaluated using the HIA and compared to the prevalence of other aeroallergen sources including the strictly defined mite, cat, and cockroach allergens that commonly constitute domestic aeroallergen exposure in most observational studies. Due to the lack of available fungal-specific antibodies and to maximize the detection of different sources of fungal aeroallergens by the HIA, we recruited from a cohort of subjects who are atypically allergic to fungi, but not to other allergen sources. This study provides the most comprehensive description to date of the diversity of aeroallergen exposure in homes.
Methods
Subject selection
Thirty-nine subjects (mean age: 20.8 years; range: 6 to 63 years, 20 males) were recruited through respiratory medicine clinics located in Sydney, Australia. Patients diagnosed with cystic fibrosis (CF) or severe asthma were preferentially recruited in order to obtain subjects with high serum concentrations of fungal-specific IgE (Henry et al., 2000; O'Driscoll et al., 2005; Skov et al., 2005). Twenty-five subjects were clinically diagnosed with CF, 21 of whom also had asthma, 12 had asthma without CF, 4 had CF without asthma, and 2 had rhinitis but not asthma or CF. All subjects resided in Sydney, Australia, a location with a warm temperate climate. The study was approved by the University of Sydney Human Research Ethics Committee, ACTR# 012605000155695. Written informed consent was obtained from all participants. For minors, a parent provided informed consent.
Skin prick tests
Skin prick testing (SPT) to allergen extracts from the fungal sources Alternaria alternata, Aspergillus mix, Cladosporium mix, Epicoccum purpurascens, Helminthosporium halodes, and Penicillium mix, and other allergen sources including cat fur, Dermatophagoides pteronyssinus (house dust-mite), and Lolium perenne (perennial rye grass) pollen (Stallergenes SA, Antony, France) was performed. Droplets of extract (50 μL) were applied to the volar surface of the forearm, and then, the skin was pricked under each extract with a sterile lancet. Positive (histamine 10 mg/mL) and negative (50% glycerol/normal saline) controls (Hollister-Steir Laboratories LLC, Spokane, WA) were included. Sensitization was defined as a wheal reaction ≥ 3 mm mean diameter measured after 15 min.
Determination of specific IgE concentration
Serum from peripheral blood was collected from all subjects at the time of SPT. Serum concentrations of sIgE to 3 fungal species, A. alternata, Aspergillus fumigatus, and Penicillium notatum (syn. Penicillium chrysogenum), were measured in all subjects using the Immulite 2000 immunoassay system (Seimens Healthcare, Erlangen, Germany). By convention, a specific IgE concentration of ≥0.35 kUA/L is considered to be indicative of sensitization.
House dust-mite, cat, and cockroach allergen content of reservoir dust
At the conclusion of air sampling, separate dust samples were collected from each subject's bedding, bedroom floor, and living room floor using a modified hand-held vacuum cleaner (Model 4071D, Makita Corp., Anjo, Japan) fitted with a 1-mm metal mesh pre-filter followed by a 20-μm pore size conical nylon bag filter that collected fine dust. For carpeted floors, an area of 1 m2 was vacuumed for 2 min. For hard flooring, an area of 2 m2 was vacuumed for 2 min. Bedding was vacuumed for 2 min, where 1 m2 of the blankets or quilt and the entire pillow was sampled. The weight of fine dust collected in the nylon bag filter was measured, and 50 mg of this fine dust was extracted in 1 mL of 1% bovine serum albumin in phosphate-buffered saline, 0.05% Tween 20 (BSA–PBS–Tween 20). Extracts were then assayed for the house dust-mite allergen Der p 1, the cat allergen Fel d 1, and the cockroach allergen Bla g 1 using 3 separate monoclonal antibody ELISAs, with reference to commercially produced individual-specific allergen standards (Indoor Biotechnologies Inc., Charlottesville, VA, USA). Allergen content was calculated as a mass concentration (μg of Der p 1 and Fel d 1, and units of Bla g 1, per gram of fine dust).
Collection of home bioaerosol samples
Indoor bioaerosol samples were collected simultaneously using two static air samplers. One sampler was placed in the subject's bedroom and the other in the living room. Each air sampler drew air through an Institute of Occupational Medicine (IOM) inhalable fraction sampling head (SKC Ltd, Dorset, UK) at a flow rate of 2.0 L/min. The IOM sampling head was fitted with a 0.8-μm pore size mixed cellulose ester protein-binding membrane (MCE) (Millipore Corp., Bedford, MA) for use in the HIA. The air sampling heads were positioned 1 meter above floor level. Constant air sampling for 24 h was not possible as this would lead to overloading of the MCE membranes, making them unsuitable for use in the HIA. A timer was used to turn the samplers on for six sampling periods, each lasting 1 h and spaced over a single 24-h period in order to collect samples that were likely to represent bioaerosol concentrations that coincided with various levels of human activity (06:00–07:00, 08:00–09:00, 11:00–12:00, 15:00–16:00, 19:00–20:00, and 22:00–23:00 h) including sleeping, eating meals, dressing, watching television/reading, and caring for children. Residents were given no instructions to change their regular home ventilation practices in any way. At the conclusion of sampling, it was reported by residents (and confirmed by observation) that no homes had been ventilated by open windows during the 24-h sampling period. Samples were not collected if rainfall occurred in the 24 h prior to sampling or during the sampling period, because rainfall can reduce concentrations of bioaerosols both outdoors and indoors.
HIA of fungal bioaerosols
The MCE membrane was removed from the IOM sampling head following sample collection and divided into 2 equal sections. The section for fungal bioaerosol analysis was placed in a humid chamber overnight to allow fungal spore germination as previously described (Green et al., 2003). Membrane sections were immunostained with the resident subject's serum IgE using the HIA adapted for fungal samples as previously described (Green et al., 2005). Immunostained samples were examined at ×200 magnification using bright field microscopy. Positively immunostained particles were characterized by purple immunostaining only if the subject's serum contained sIgE to antigens associated with those particles. The number of non-immunostained and immunostained fungal spores and hyphae was counted and, where possible, identified to genus level using morphological features (Smith, 1990). Pollen grains immunostained with IgE were also quantified, but not identified. All data were converted to counts per m3. Immunostaining controls included pooled negative control human sera (<0.05 kU/L total serum IgE) as an isotype control and fetal bovine serum as a species control.
HIA of Der p 1, Fel d 1, and Bla g 1 bioaerosols
The remaining half of the MCE was divided into 3 equal sections. Each section was immunostained using the HIA and mAbs specific for Der p 1, Fel d 1, or Bla g 1 allergen. These specific allergens are conventionally recognized as the most appropriate markers for exposure to allergens from house dust-mite, cats, and cockroaches, respectively. The HIA method used in this series of analyses was identical to that described for the HIA of fungal bioaerosols, with the exceptions that there was no overnight incubation in the humid chamber and commercially available mAbs directed against Der p 1, Fel d 1, or Bla g 1 allergen (Indoor Biotechnologies Inc., Charlottesville, VA.) diluted 1:1000 in BSA–PBS–Tween 20 were used in place of the subject's serum IgE as the primary antibody, and sheep anti-mouse IgG alkaline phosphatase conjugate (Sigma Chemical Co., St Louis, MO, USA) diluted 1:500 in BSA–PBS–Tween 20 was used as the detection antibody. Fetal bovine serum was used as an immunostaining species control. Particles that displayed positive immunostaining for the defined allergens Der p 1, Fel d 1, or Bla g 1, as well as non-immunostained particles, were enumerated using bright field microscopy at ×200 magnification. All counts were converted to counts per m3.
Statistical analyses
All statistical analyses were performed using Analyse-it for Microsoft Excel (version 2.20, Analyse-it Software Ltd., Leeds, UK). Spearman's correlation analyses were used to explore the associations between non-normally distributed datasets, with Pearson correlation being used for normally distributed datasets.
Results
Sensitization
Thirty-seven of the 39 subjects (94.9%) were SPT positive to at least one of the tested aeroallergen extracts. Thirty-four subjects (87.2%) were SPT positive to at least one fungal extract and 18 (46.2%) were polysensitized to fungi. Specifically, of the 39 subjects, 16 (41%) were SPT positive to A. alternata, 24 (61%) to Aspergillus mix, 5 (12%) to Cladosporium mix, 4 (10%) to E. purpurascens, 9 (23%) to H. halodes, 10 (25%) to Penicillium mix, 9 (23%) to cat fur, 20 (51%) to D. pteronyssinus (house dust-mite), and 12 (30%) to L. perenne (perennial ryegrass).
Serum concentration of specific IgE
Twenty-eight (71.8%) of the subjects tested positive for serum sIgE (≥ 0.35 kUa/L) to one or more of the 3 fungi tested. Geometric mean serum concentrations of sIgE to A. alternata, A. fumigatus, and P. notatum were 0.43, 2.9, and 0.53 kU/L, respectively. Although sIgE was elevated in subjects who had CF, concentrations of sIgE for most CF subjects were within the same range as those observed in the non-CF subjects.
HIA of fungal bioaerosols
The HIA made possible the visual identification of fungal conidia, spores, and hyphae in samples of inhalable airborne particulates that were collected from all homes. In total, 5042 fungal spores were counted from samples collected in 39 homes. Of these, 4637 could be identified to genus level; however, 405 could not be identified due to intense surface HIA immunostaining or the spores being morphologically indiscernible. This group was classified as ‘unidentified fungal spores’.
Counts derived from the genera Aspergillus, Penicillium, and other amerospore (unicellular spore)-producing taxa were expressed in HIA samples as a combined count of Aspergillus/Penicillium type. This method has been used by previous investigators (Pyrri and Kapsanaki-Gotsi, 2007).
A total of 563 hyphal fragments were collected. Hyphal fragments are morphologically indiscernible particles predominantly derived from septate Ascomycete or Basidiomycete groups. Due to a lack of distinguishable morphological phenotypes, these fungal-derived particles could not be identified to order, genus, or species.
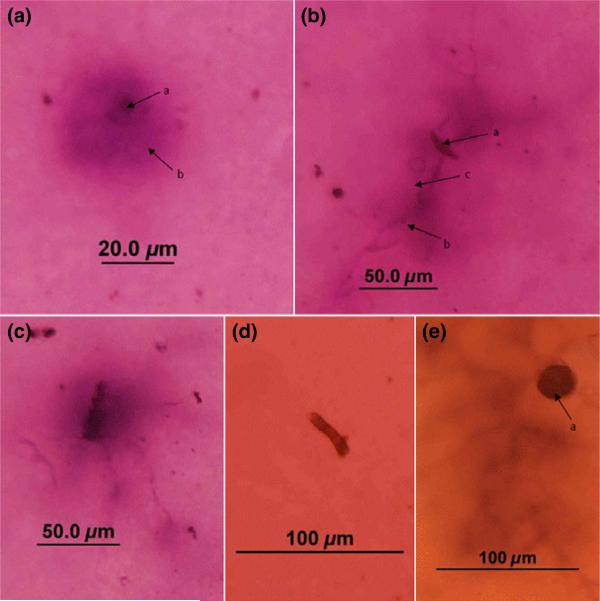
Compared to SPT and serum sIgE concentration as indicators of sensitization, greater numbers of subjects (37 of 39 subjects) had observable IgE immunostaining to airborne fungal spores and hyphae collected within their homes. No HIA immunostaining was observed in negative control samples stained with either pooled negative control human sera or the species control fetal bovine serum. Figure 1 shows examples of HIA immunostaining of bioaerosols sampled from subjects’ environments.
Fig. 1.
a: An immunostained unicellular amerospore (a) from a subject's bedroom, with immunostaining indicating allergen present in a zone surrounding the particle (b). b: An immunostained Curvularia spore (a) from a subject's bedroom. Immunostaining (b) around emerging hyphae (c) identifies this as the zone of allergen release. c: An immunostained morphologically indiscernible fragment and hyphae collected from a subject's bedroom and stained with the serum of the subject who was SPT positive to Aspergillus and Penicillium. d: A non-immunostained hyphal fragment from a subject's living room. e: An immunostained Epicoccum spore (a) collected from a subject's living room, stained with serum of a subject SPT positive to Alternaria, Helminthosporium, Aspergillus, and Penicillium, but SPT negative to Epicoccum
Prevalence and airborne concentrations of fungal bioaerosols calculated from HIA samples
The most common fungal genera identified in the HIA analysis were derived from the genera Aspergillus/Penicillium, Cladosporium, Alternaria, and Epicoccum. Morphologically indiscernible spores and hyphae were also prevalent (Tables 1 and 2) as were amorphous particulate allergen sources.
Table 1.
Prevalence and concentration of immunostained and non-immunostained bioaerosols from bedrooms identified microscopically from HIA samples.*
| Genera of fungalspores/conidia or type of other aerosol | Prevalence of bioaerosol(% of bedrooms in which observed) | Percentage of bedrooms with this bioaerosol whose resident had positive HIA immunostaining | Bedroom total particle concentrations as observed by HIA (stained + non-stained particles per m3) |
Bedroom HIA immunostain-positive concentrations (stained particles per m3) |
||||
|---|---|---|---|---|---|---|---|---|
| Median | Min | Max | Median | Min | Max | |||
| Cladosporium | 100 | 71.8 | 44 | 6 | 133 | 11 | 0 | 67 |
| Aspergillus/Penicillium | 97.4 | 78.9 | 47 | 0 | 333 | 17 | 0 | 167 |
| Epicoccum | 69.2 | 51.9 | 6 | 0 | 72 | 0 | 0 | 39 |
| Bipolaris | 66.7 | 65.4 | 6 | 0 | 44 | 0 | 0 | 39 |
| Curvularia | 56.4 | 45.5 | 3 | 0 | 44 | 0 | 0 | 33 |
| Alternaria | 53.8 | 71.4 | 3 | 0 | 50 | 0 | 0 | 50 |
| Pithomyces | 28.2 | 63.6 | 0 | 0 | 22 | 0 | 0 | 6 |
| Fusillaria | 20.5 | 87.5 | 0 | 0 | 22 | 0 | 0 | 14 |
| Leptosphaeria | 20.5 | 62.5 | 0 | 0 | 22 | 0 | 0 | 22 |
| Spegazzinia | 15.4 | 50.0 | 0 | 0 | 11 | 0 | 0 | 11 |
| Corprinus | 2.6 | 100 | 0 | 0 | 3 | 0 | 0 | 3 |
| Torula | 2.6 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| Pleospora | 2.6 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| Basidiospores | 2.6 | 0 | 0 | 0 | 6 | 0 | 0 | 0 |
| Unidentifiable fungi | 56.4 | 90.9 | 6 | 0 | 317 | 3 | 0 | 317 |
| Hyphal fragments | 84.6 | 57.6 | 14 | 0 | 67 | 0 | 0 | 22 |
| Pollen | 38.5 | 60.0 | 0 | 0 | 11 | 0 | 0 | 11 |
| Non-fungal non-pollen aerosols | 100 | 48.7 | 19400 | 4690 | 203000 | 0 | 0 | 533 |
The percentage of bedrooms where resident subjects displayed HIA IgE immunostaining to each category of bioaerosolis also shown.
Table 2.
Prevalence and concentration of immunostained and non-immunostained bioaerosols from living rooms identified microscopically from HIA samples*
| Genera of fungal spores/conidia or type of other aerosol | Prevalence of bioaerosol (% of living rooms in which observed) | Percentage of living rooms with this bioaerosol whose resident had positive HIA immunostaining | Living room total particle concentrations as observed by HIA (stained + non-stained particles per m3) |
Living room HIA immunostain-positive concentrations (stained particles per m3) |
||||
|---|---|---|---|---|---|---|---|---|
| Median | Min | Max | Median | Min | Max | |||
| Cladosporium | 100 | 59.0 | 50 | 6 | 306 | 8 | 0 | 139 |
| Aspergillus/Penicillium | 100 | 64.1 | 61 | 14 | 217 | 17 | 0 | 206 |
| Epicoccum | 69.2 | 44.4 | 6 | 0 | 44 | 0 | 0 | 19 |
| Bipolaris | 56.4 | 63.6 | 6 | 0 | 22 | 0 | 0 | 17 |
| Curvularia | 56.4 | 63.6 | 3 | 0 | 39 | 0 | 0 | 22 |
| Alternaria | 71.8 | 60.7 | 6 | 0 | 33 | 0 | 0 | 33 |
| Pithomyces | 33.3 | 61.5 | 0 | 0 | 11 | 0 | 0 | 11 |
| Fusillaria | 28.2 | 45.5 | 0 | 0 | 11 | 0 | 0 | 11 |
| Leptosphaeria | 10.3 | 25.0 | 0 | 0 | 11 | 0 | 0 | 6 |
| Spegazzinia | 15.4 | 33.3 | 0 | 0 | 11 | 0 | 0 | 6 |
| Corprinus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Torula | 2.6 | 0 | 0 | 0 | 14 | 0 | 0 | 0 |
| Pleospora | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Basidiospores | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Unidentifiable fungi | 46.2 | 94.4 | 0 | 0 | 67 | 0 | 0 | 67 |
| Hyphal fragments | 89.7 | 60.0 | 17 | 0 | 78 | 6 | 0 | 28 |
| Pollen | 33.3 | 76.9 | 0 | 0 | 17 | 0 | 0 | 11 |
| Non-fungal non-pollen aerosols | 100 | 59.0 | 16000 | 3750 | 227000 | 6 | 0 | 1970 |
The percentage of living rooms where resident subjects displayed HIA IgE immunostaining to each category of bioaerosolis also shown.
Airborne concentrations of all fungal spores measured by HIA (including all spores, both IgE-immunostained and non-immunostained) from bedrooms were significantly correlated (rs = 0.47, P < 0.01) with the respective fungal spore concentrations from living rooms of the same homes.
HIA of airborne Der p 1, Fel d 1, and Bla g 1 bioaerosols probed with mAbs
Most homes contained airborne particles that carried Der p 1, Fel d 1, and Bla g 1 allergens (Table 3). There were significantly greater numbers of airborne particles that contained Fel d 1 in the homes of cat owners compared to non-cat owners (geometric mean 47 particles/m3 (n = 10) vs 7.8 particles/m3 (n = 25), independent-samples t-test P < 0.05).
Table 3.
Incidence, geometric mean (95% confidence interval) of concentrations of airborne non-fungal, non-pollen particles from bedrooms and living rooms, showing positive immunostaining with antibodies specific for Der p 1, Fel d 1, and Bla g 1
| Bedroom |
Living Room |
|||
|---|---|---|---|---|
| Incidence (n = 39 homes) | Geometric Mean (95% CI) (/m3) | Incidence (n = 39 homes) | Geometric Mean (95% CI) (/m3) | |
| Der p 1 immunostained | 36 | 19.5 (12.5–30.6) | 36 | 31.6 (19.1–52.3) |
| Fel d 1 immunostained | 31 | 17.1 (8.75–33.5) | 30 | 22.9 (10.6–50.0) |
| Bla g 1 immunostained | 32 | 15.6 (9.20–26.6) | 32 | 19.2 (10.7–34.2) |
Der p 1, Fel d 1, and Bla g 1 allergen concentration in reservoir dust
Der p 1, Fel d 1, and Bla g 1 allergen concentrations in reservoir dust collected from bedding, bedroom floors, and living room floors are shown in Table 4. Highest concentrations of allergen in bedding and bedroom floor reservoir dust samples were predominantly derived from Der p 1, whereas Fel d 1 was highest in living room floor samples. In contrast, lower concentrations of Bla g 1 were quantified in each sampling location.
Table 4.
Geometric mean (95% confidence interval) of allergen concentrations in reservoir dust*
| Der p 1 (μg/g) | Fel d 1 (μg/g) | Bla g 1 (Units/g) | |
|---|---|---|---|
| Bedding | 6.54 (4.89–8.19) | 5.42 (2.70–8.15) | 0.75 (0.00–2.00) |
| Bedroom floor | 7.71 (6.27–9.16) | 4.69 (2.51–6.86) | 0.32 (0.00–1.69) |
| Living room floor | 5.31 (3.71–6.91) | 6.54 (4.52–8.56) | 0.41 (0.00–1.90) |
In the current study, Indoor Biotechnologies individual allergen standards were used and not the Universal Allergen Standard. A correction factor may be required when comparing allergen concentrations with studies that have chosen to adopt the Universal Allergen Standard (Filep et al., 2012).
No significant correlations were identified between airborne concentrations of Der p 1, Fel d 1, or Bla g 1 HIA immunostained particles and the concentration of corresponding allergens in reservoir dust derived from beds (Der p 1 rs = 0.02 P = 0.90, Fel d 1 rs = −0.09 P = 0.57, Bla g 1 rs = 0.05 P = 0.77), bedroom floors (Der p 1 rs = 0.24 P = 0.13, Fel d 1 rs = 0.09 P = 0.60, Bla g 1 rs = 0.03 P = 85), or living room floors (Der p 1 rs = −0.25 P = 0.12, Fel d 1 rs = 0.13 P = 0.44, Bla g 1 rs = −0.09 P = 0.57).
Ratio of IgE-binding fungal particles to IgE-binding non-fungal particles
Nineteen of 39 bedrooms and 23 of 39 living rooms contained amorphous particles that were positively immunostained with residents’ serum IgE (Tables 1 and 2). These particles had no clear fungal or pollen-like morphologies. In bedrooms, there were on average 2.9 times more positively immunostained airborne fungal spores and hyphae than positively immunostained non-fungal particles. Similarly, in living rooms, there were 3.1 times as many positively immunostained fungal particles than positively immunostained non-fungal particles. Non-fungal IgE-binding particles were not identifiable by their morphology but may represent particles that carry allergen derived from sources such as house dust-mite, cat, cockroach, or other bioaerosols.
Ratio of IgE-binding fungal particles to particles carrying Der p 1, Fel d 1, or Bla g 1 identified by mAb HIA
In bedrooms, there were on average 1.7 times more (95% CI: 0.71–2.7) IgE-immunostained airborne fungal spores and hyphae than airborne particles with Der p 1, Fel d 1, and Bla g 1 immunostaining. In living rooms, there were 1.4 times as many fungal particles (95% CI: 0.43–2.4) that were immunostained. Although there were wide variations in these ratios between homes, the ratios within homes (i.e., the ratio in bedrooms vs the ratio in living rooms) were weakly but significantly correlated (r2 = 0.11, P < 0.05).
Discussion
Characterizing the complete diversity of aeroallergen exposure in domestic environments has not been possible utilizing existing methods of assessment. In this study, the use of HIA has enabled, for the first time, an approximation of the complete spectrum of domestic exposure to inhalable aeroallergen-carrying particles. In the HIA, a combination of fungal-allergic serum and particle morphology was used to identify particles carrying fungal allergens, while allergen-specific monoclonal antibodies were used in the HIA to identify mite, cat, and cockroach allergen-carrying particles. These results were compared to traditional immunoassay measures of allergen exposure.
This study demonstrates that fungal aeroallergens are among the most prevalent domestic aeroallergens in Sydney indoor environments. HIA immunostaining also showed that fungal sensitized subjects’ IgE reactivity was to a broader diversity of fungal bioaerosols than indicated by traditional diagnostic methods. Overall, the HIA data reported in this study suggest that exposure to fungal allergens is both underestimated and may be very common in Australian indoor environments.
Several limitations associated with HIA immunostaining may confound the interpretation of this study. The capacity to immunostain IgE-binding fungal particles as well as amorphous (non-fungal, non-pollen) particles will differ between sera according to their content of IgE specific to different allergens collected in the homes. An alternative design for this study could have been to use a serum pool, but this approach was not pursued as the aim of the study was to maximize the relationship between individual exposure and sensitization. While this may maximize the capacity to identify fungi in houses which are possibly clinically relevant to the individual, it also precludes the analysis comparing the sum of staining the particles with a group of allergen-specific monoclonal antibodies with measures of either amorphous or identifiable IgE-binding particles. It is feasible that the human sera used were identifying other uncharacterized non-fungal domestic allergens, for example it has been shown that allergic sensitization to detritus from non-mite, non-cockroach domestic arthropods (flies, silver-fish, moths, etc.) is surprisingly common (Baldo and Panzani, 1988). It is also feasible that other cat, mite, and cockroach particles do not carry the single, specific allergens quantified in the ELISAs and these may have been detected by IgE to other minor allergens that may be present on the particle.
In addition, subjects were predominantly fungal sensitized CF subjects and this may have biased the results toward the detection of unicellular amerospore genera such as Aspergillus, Penicillium, and Paecilomyces that are capable of colonizing CF subjects and mediating allergic bronchopulmonary aspergillosis (Skov et al., 2005). Furthermore, HIA enables the semi-quantification of the number of collected particles that function as aeroallergen sources but does not provide quantification of the amount of allergen associated with the particle. The initiation of spore germination improves the detectable thresholds of the HIA but it may not be reflective of the allergen burden within the sampled environment and result in the overestimation of fungal allergen burden. Recent modifications to the HIA have included the use of fluorescent probes to immunostain allergen, which improves the ability to detect antigens from amerospore-producing fungal genera. Utilizing these improved approaches in future studies may provide improved quantification of the actual fungal allergen burden within indoor environments.
Although this study demonstrated the ubiquity of fungal aeroallergens compared to other indoor allergen sources in an Australian domestic setting, the study does not evaluate the clinical relevance of exposure to fungi. Previous studies have provided no clear consensus on the health impacts of low-level exposure to fungal allergens (Mendell et al., 2011). While a generalized elevated exposure to fungi has been associated with increased incidence of adverse health effects in some epidemiological studies (Gent et al., 2002; Stark et al., 2005; Turyk et al., 2006; Yazicioglu et al., 2004), other studies that have investigated direct measures such as total fungal propagules, specific types of fungi, or concentration of fungal metabolites have not found associations with symptoms (Dharmage et al., 2002; Garrett et al., 1998; Jovanovic et al., 2004; Katz et al., 1999). The relationship between exposure, allergic sensitization, and symptoms at times appears paradoxical and requires further study utilizing innovative immunoassays such as HIA that can provide detailed insight into personal exposure within the subjects own environment.
Compared to many other locations around the world, Sydney typically features high concentrations of house dust-mite allergen in reservoir dust. Cat ownership is also relatively common in Sydney, so although Sydney features a warm temperate climate and homes are often well ventilated and dry, our finding that fungi were the dominant airborne allergen sources in the homes studied here is likely to also be relevant to other similar localities.
To date, most indoor allergen exposure assessment studies have used reservoir allergen concentrations as proxy measures of longitudinal human exposure, due mainly to the ease with which samples may be collected and analyzed. In contrast, air sampling takes an integrated measurement of short-term personal exposure that provides important information related to inhaled exposure. This is especially the case when air sampling has been conducted using inhalable fraction air samplers over sampling intervals representative of domestic exposure, such as those used in the current experiments. Only poor (Karlsson et al., 2002; Paufler et al., 2001; Swanson et al., 1989) or variable (Gore et al., 2006) correlations have been identified between house dust-mite and cat allergen measurements obtained from air samples and reservoir dust samples. This lack of correlation is probably due to variations associated with the settled reservoir and airborne dust populations as well as factors including differential re-aerosolization. Differential re-aerosolization of different-sized particulates will additionally lead to short-term changes in human inhaled exposure, especially during anthropogenic activities such as vigorous cleaning; however, when a large number of samples are collected as an average over time, as was done in these experiments, a better representation of average human exposure is likely. Gore et al., (2006) identified correlations between inhaled allergen and reservoir dust allergen concentrations in 3 of 6 combinations of allergens and reservoir dust sampling location. Similarly, in the present study using differing methods to assay airborne and reservoir samples, no correlations were identified between the numbers of airborne particles with Der p 1, Fel d 1, or Bla g 1 HIA immunostaining and the concentrations of these same allergens in reservoir dust collected from the same locations.
In conclusion, this study demonstrated that residents of homes in Sydney, Australia, are exposed to a wide diversity of fungal aeroallergens as well as traditionally measured allergen sources in the indoor environment. Methods such as HIA provide new insight into the complexity of domestic aeroallergen exposure as well as the diversity of aeroallergen sources in the environment.
Practical Implications.
Indoor allergen exposure assessment studies have primarily focused on a limited range of allergen sources in samples derived from reservoir dust samples. Using an innovative immunodiagnostic approach, this study demonstrates that fungal bioaerosols are the dominant source of aeroallergen exposure in the domestic environment, providing unique insight into domestic aeroallergen exposure.
Acknowledgements
The authors thank the study participants, Samantha Forbes RN, Merilyn McArthur RN, Margherita Pitman, and other staff of The Children's Hospital at Westmead CF Clinic, The Royal Prince Alfred Hospital CF Clinic, and The Royal Prince Alfred Hospital Clinical Immunology Laboratory. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
References
- Baldo BA, Panzani RC. Detection of IgE antibodies to a wide range of insect species in subjects with suspected inhalant allergies to insects. Int. Arch. Allergy Appl. Immunol. 1988;85:278–287. doi: 10.1159/000234518. [DOI] [PubMed] [Google Scholar]
- Chew GL, Perzanowski MS, Canfield SM, Goldstein IF, Mellins RB, Hoepner LA, Ashby-Thompson M, Jacobson JS. Cockroach allergen levels and associations with cockroach-specific IgE. J. Allergy Clin. Immun. 2008;121:240–245. doi: 10.1016/j.jaci.2007.08.024. [DOI] [PubMed] [Google Scholar]
- Cho SH, Reponen T, Bernstein DI, Olds R, Levin L, Liu X, Wilson K, LeMasters G. The effect of home characteristics on dust antigen concentrations and loads in homes. Sci. Total Environ. 2006;371:31–43. doi: 10.1016/j.scitotenv.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custovic A, Simpson B, Simpson A, Hallam C, Craven M, Woodcock A. Relationship between mite, cat, and dog allergens in reservoir dust and ambient air. Allergy. 1999;54:612–616. doi: 10.1034/j.1398-9995.1999.00062.x. [DOI] [PubMed] [Google Scholar]
- Dharmage S, Bailey M, Raven J, Abeyawickrama K, Cao D, Guest D, Rolland J, Forbes A, Thien F, Abramson M, Walters EH. Mouldy houses influence symptoms of asthma among atopic individuals. Clin. Exp. Allergy. 2002;32:714–720. doi: 10.1046/j.1365-2222.2002.01371.x. [DOI] [PubMed] [Google Scholar]
- Filep S, Tsay A, Vailes L, Gadermaier G, Ferreira F, Matsui E, King EM, Chapman MD. A multi-allergen standard for the calibration of immunoassays: CREATE principles applied to eight purified allergens. Allergy. 2012;67:235–241. doi: 10.1111/j.1398-9995.2011.02750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett MH, Rayment PR, Hooper MA, Abramson MJ, Hooper BM. Indoor airborne fungal spores, house dampness and associations with environmental factors and respiratory health in children. Clin. Exp. Allergy. 1998;28:459–467. doi: 10.1046/j.1365-2222.1998.00255.x. [DOI] [PubMed] [Google Scholar]
- Gent JF, Ren P, Belanger K, Triche E, Bracken MB, Holford TR, Leaderer BP. Levels of household mold associated with respiratory symptoms in the first year of life in a cohort at risk for asthma. Environ. Health Perspect. 2002;110:A781–A786. doi: 10.1289/ehp.021100781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore RB, Curbishley L, Truman N, Hadley E, Woodcock A, Langley SJ, Custovic A. Intranasal air sampling in homes: relationships among reservoir allergen concentrations and asthma severity. J. Allergy Clin. Immun. 2006;117:649–655. doi: 10.1016/j.jaci.2005.12.1351. [DOI] [PubMed] [Google Scholar]
- Green BJ, Mitakakis TZ, Tovey ER. Allergen detection from 11 fungal species before and after germination. J. Allergy Clin. Immun. 2003;111:285–289. doi: 10.1067/mai.2003.57. [DOI] [PubMed] [Google Scholar]
- Green BJ, Sercombe JK, Tovey ER. Fungal fragments and undocumented conidia function as new aeroallergen sources. J. Allergy Clin. Immun. 2005;115:1043–1048. doi: 10.1016/j.jaci.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Gruchalla RS, Pongracic J, Plaut M, Evans R, Visness CM, Walter M, Crain EF, Kattan M, Morgan WJ, Steinbach S, Stout J, Malindzak G, Smartt E, Mitchell H. Inner city asthma study: relationships among sensitivity, allergen exposure, and asthma morbidity. J. Allergy Clin. Immun. 2005;115:478–485. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Henry M, Bennett DM, Kiely J, Kelleher N, Bredin CP. Fungal atopy in adult cystic fibrosis. Resp. Med. 2000;94:1092–1096. doi: 10.1053/rmed.2000.0918. [DOI] [PubMed] [Google Scholar]
- Jovanovic S, Felder-Kennel A, Gabrio T, Kouros B, Link B, Maisner V, Piechotowski I, Schick KH, Schrimpf M, Weidner U, Zollner I, Schwenk M. Indoor fungi levels in homes of children with and without allergy history. Int. J. Hyg. Environ. Health. 2004;207:369–378. doi: 10.1078/1438-4639-00302. [DOI] [PubMed] [Google Scholar]
- Karlsson A-S, Renström A, Hedrén M, Larsson K. Comparison of four allergen-sampling methods in conventional and allergy prevention classrooms. Clin. Exp. Allergy. 2002;32:1776–1781. doi: 10.1046/j.1365-2222.2002.01553.x. [DOI] [PubMed] [Google Scholar]
- Katz Y, Verleger H, Barr J, Rachmiel M, Kiviti S, Kuttin ES. Indoor survey of moulds and prevalence of mould atopy in Israel. Clin. Exp. Allergy. 1999;29:186–192. doi: 10.1046/j.1365-2222.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- Lemanske RF, Busse WW. Asthma: factors underlying inception, exacerbation, and disease progression. J. Allergy Clin. Immun. 2006;117:S456–S461. doi: 10.1016/j.jaci.2005.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell MJ, Mirer AG, Cheung K, Tong M, Douwes J. Respiratory and allergic health effects of dampness, mold, and dampness-related agents: a review of the epidemiologic evidence. Environ. Health Perspect. 2011;119:748–756. doi: 10.1289/ehp.1002410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Driscoll BR, Hopkinson LC, Denning DW. Mold sensitization is common amongst patients with severe asthma requiring multiple hospital admissions. BMC Pulmonary Med. 2005;5:4. doi: 10.1186/1471-2466-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paufler P, Gebel T, Dunkelberg H. Quantification of house dust mite allergens in ambient air. Rev. Environ. Health. 2001;16:65–80. doi: 10.1515/reveh.2001.16.1.65. [DOI] [PubMed] [Google Scholar]
- Platts-Mills TA, Woodfolk JA. Allergens and their role in the allergic immune response. Immunol. Rev. 2011;242:51–68. doi: 10.1111/j.1600-065X.2011.01021.x. [DOI] [PubMed] [Google Scholar]
- Platts-Mills TA, Erwin EA, Woodfolk JA, Heymann PW. Environmental factors influencing allergy and asthma. Chem. Immunol. Allergy. 2006;91:3–15. doi: 10.1159/000090225. [DOI] [PubMed] [Google Scholar]
- Pyrri I, Kapsanaki-Gotsi E. A comparative study on the airborne fungi in Athens, Greece, by viable and non-viable sampling methods. Aerobiologia. 2007;23:3–15. [Google Scholar]
- Rabito FA, Iqbal S, Holt E, Grimsley LF, Islam TM, Scott SK. Prevalence of indoor allergen exposures among New Orleans children with asthma. J. Urban Health. 2007;84:782–792. doi: 10.1007/s11524-007-9216-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razmovski V, O'Meara TJ, Taylor DJ, Tovey ER. A new method for simultaneous immunodetection and morphologic identification of individual sources of pollen allergens. J. Allergy Clin. Immun. 2000;105:725–731. doi: 10.1067/mai.2000.105222. [DOI] [PubMed] [Google Scholar]
- Skov M, McKay K, Koch C, Cooper PJ. Prevalence of allergic bronchopulmonary aspergillosis in cystic fibrosis in an area with a high frequency of atopy. Resp. Med. 2005;99:887–893. doi: 10.1016/j.rmed.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Smith G. Sampling and Identifying Allergenic Pollens and Moulds. Blewstone Press; San Antonio: 1990. [Google Scholar]
- Stark PC, Celedon JC, Chew GL, Ryan LM, Burge HA, Muilenberg ML, Gold DR. Fungal levels in the home and allergic rhinitis by 5 years of age. Environ. Health Perspect. 2005;113:1405–1409. doi: 10.1289/ehp.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson MC, Campbell AR, Klauck MJ, Reed CE. Correlations between levels of mite and cat allergens in settled and airborne dust. J. Allergy Clin. Immun. 1989;83:776–783. doi: 10.1016/0091-6749(89)90014-6. [DOI] [PubMed] [Google Scholar]
- Turyk M, Curtis L, Scheff P, Contraras A, Coover L, Hernandez E, Freels S, Persky V. Environmental allergens and asthma morbidity in low-income children. J. Asthma. 2006;43:453–457. doi: 10.1080/02770900600758333. [DOI] [PubMed] [Google Scholar]
- Yazicioglu M, Asan A, Ones U, Vatansever U, Sen B, Ture M, Bostancioglu M, Pala O. Indoor airborne fungal spores and home characteristics in asthmatic children from Edirne region of Turkey. Allergol. Immunopathol. (Madr) 2004;32:197–203. doi: 10.1016/s0301-0546(04)79239-3. [DOI] [PubMed] [Google Scholar]