Abstract
Study Objectives:
One of the key processes in language development is generalization—the selection and extension of relevant features and information to similar objects and concepts. Little is known about how sleep influences generalization, and studies on the topic are inconclusive. Our aim was to investigate how a nap affects generalization in 16-mo-olds. We hypothesized that a nap is necessary for successful generalization of word meanings.
Methods:
Twenty-eight 16-mo-old, typically developing toddlers were randomly assigned to nap and wake groups. We trained toddlers with two novel object-word pairs and tested their initial ability to generalize. Toddlers took part in an intermodal preferential looking task, in which they were shown different colored versions of the original objects and heard one of the trained labels. If toddlers understand the label, they are expected to increase their looking time to the target. Looking behavior was measured with an automated eye tracker. Afterward, the nap group went to sleep, while the wake group stayed awake for approximately 2 h. We then repeated the test of their performance on the generalization task.
Results:
A significant interaction of group and session was found in preferential looking. The performance of the nap group increased after the nap, whereas that of the wake group did not change.
Conclusions:
Our results suggest that napping improves generalization in toddlers.
Citation:
Horváth K, Liu S, Plunkett K. A daytime nap facilitates generalization of word meanings in young toddlers. SLEEP 2016;39(1):203–207.
Keywords: abstraction, generalization, infancy, memory, nap
Significance.
Our research showed that sleep facilitates generalization of word meanings in 16-month-old toddlers. After learning an object-label association, infants who had a nap after learning were able to generalize the label to objects with a different color. We used an eye-tracking methodology which relies less on the toddlers' active participation than methods used previously. The observed association suggests that sleep does not merely promote passive consolidation of memories, but also promotes an active process of forgetting irrelevant information and retention of the key features of the category. This finding highlights the importance of napping in this age group. One implication of practical relevance is that nurseries and parents should be advised not to sacrifice their toddlers' daytime sleep.
INTRODUCTION
Generalization of word meanings to similar objects is a key process in acquiring knowledge about the environment, because it saves the cognitive effort of learning the names of objects individually. The underlying mechanisms involve consolidation processes, which can take place either online (i.e., same time with encoding) or offline (after encoding). A great body of literature suggests that in adults, sleep facilitates generalization offline1–6; however, the picture is less clear regarding toddlers who are in the most intensive stage of learning word meanings.
Generalization makes it possible to adapt previously gained knowledge to new situations on the basis of identified similarities. To be able to recognize the crucial commonalities an abstract representation is required.7 Sleep may play a role in selecting the relevant features that should be represented, and in promoting the forgetting of irrelevant information, thus, reorganizing memory.8 Several studies showed that after sleep, adults are more efficient in tasks that require generalization, e.g., synthetic speech recognition,3 finding a hidden rule in a mathematical problem,4 or a relational memory task.5,6
Recently, similar claims for the role of sleep in the generalization of word meaning in 9- to 16-mo-olds have been reported.8 Friedrich and colleagues,8 using event-related potentials, showed that during an initial training period infants were capable of learning specific word meanings but were not able to learn names for categories consisting of similar pictures. However, after a 1.5-h nap, infants (without further training) could generalize the category names for novel exemplars and remembered the specific word meanings as well. In contrast, the wake group seemed to forget the specific word meanings and did not show generalization. Moreover, the generalization effect was associated to a specific sleep electroencephalographic (EEG) oscillation, i.e. sleep spindles.
The opposite effect has been reported by Werchan and Gomez,9 who found that a period of wake, not sleep, facilitates generalization of word meanings. Toddlers age 2.5 y learned a label for a category consisting of three exemplar objects. The toddlers were also familiarized with a distracter (without labelling). Four hours later they were presented with four objects: a novel exemplar of the learned category, the distracter, and two novel objects, and were asked using the label of the category to point to the target image. The performance of children who had a nap between the two sessions was significantly worse than those who stayed awake.
To clarify the role of sleep in generalization of word meanings in infancy, we adapted a methodology that we have already used with 16-mo-old toddlers.10 In a recent study, we demonstrated that a nap facilitates the consolidation of novel object-word associations. The intermodal preferential looking (IPL) paradigm with automatic eye tracking makes it possible to obtain data on the behavioral level while relying less on the toddler's active participation. In the current study, we hypothesized that 16-mo-old toddlers would only be able to generalize novel object-word associations to object exemplars beyond the original training set after they had a nap.
METHODS
Participants
Data of 28 toddlers were analyzed (nap group: 14, wake group: 14, male: 16, female: 12). Nine additional toddlers were tested but excluded from further analysis due to noncompletion of the task (due to crying, refusing to participate) or insufficient eye-tracking data (five and four toddlers, respectively). Of the nine infants, excluded four were in the wake group. All toddlers were from families in which English was the primary language used and they were all Caucasian. On average, mothers spent 17.07 y in education. Parents were asked to complete the Oxford Communicative Development Inventory (OCDI)11 to obtain vocabulary measures prior to arrival, as well as the Sleep and Naps Oxford Research Inventory (SNORI) to collect data on the toddlers' sleeping patterns during the preceding week of the appointment. If the parents did not fill in the SNORI, they were asked whether their toddler had regular naps and whether there had been anything unusual in their child's sleeping pattern in the previous 3 d. Due to substantial missing data, we do not provide information on toddler's sleep assessed with the SNORI. Written informed consent was collected from the caregiver. The study was approved by the University of Oxford Central University Research Ethics Committee (MSD/IDREC/ C2/2012/11).
Stimuli
During the play phase, four novel objects (two objects are shown in Figure 1, the other two had the same shape but different colors) were used. Two objects (original objects) were named by the experimenter with the labels (tesh/tɛ∫ and ginn/g⌶n). The other two objects served as generalization objects and differed in color but not in shape or texture from the original objects. In the on-screen sessions, toddlers saw colored photographs of the trained original objects plus eight extra pictures (dog, cow, cat, duck, ball, book, cup, shoe) as familiar stimuli. The pictures were 1024 × 768 pixels colored photographs presented on a 50% gray background on a 1920 × 1080 pixel, 23-inch thin film transistor monitor.
Figure 1.
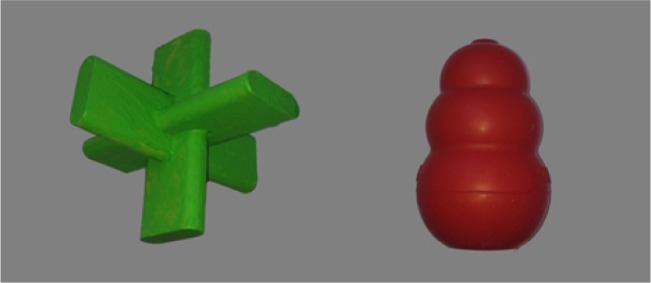
Two novel objects used in the experiment. The other two (generalization) objects were the same in shape but different in color.
The audio stimuli consisted of the aforementioned pseudowords (tesh/tɛ∫ and ginn/g⌶n, duration: 702 msec and 652 msec), eight familiar nouns (dog, cow, cat, duck, ball, book, cup, shoe) and two attention phrases (Ooh look!, Hey wow!) produced by a female native speaker of British English in a Southern accent. Audio stimuli were recorded in a sound-attenuating room with a solid state recorder sampling at 44.1 Hz in 16-bit stereo and were delivered by two speakers centrally located above the screen.
The stimuli were presented using custom-built routines in MATLAB (version 7.10.0.499, R2010a, The MathWorks, Inc., Natick, MA, USA) using Psychtoolbox version 3.12–14
Eye Tracking
Eye movements were tracked using a Tobii TX300 Eye Tracker (Tobii Technology AB, Sweden) with 120 Hz sampling rate with custom built routines (integrated in the stimuli presentation software above) in MATLAB using the Talk2Tobii Toolbox.15 The accuracy of the measurement is about 0.4° for binocular eye movements.
Procedure
The procedure was an adaptation of that used by Horvath and colleagues.10 Toddlers were randomly assigned to wake and nap groups during recruitment. To avoid causing sleep deprivation in the wake group, the testing of the wake group was scheduled at a time when the toddlers did not usually nap. To maximize the chance for a daytime sleep, the nap group was brought into the laboratory just before their usual nap time.
First, an interactive playing phase was conducted while toddlers sat on the floor, sofa, or the caregiver's lap. The experimenter introduced the two new original objects, one at a time. Toddlers played with and observed the toys, while the experimenter labelled each one six times using carrier sentences such as: Show mummy/daddy the X! Where is the X? The order of the presentation of the objects and the object-label pairing were counterbalanced across participants and groups. Afterward, toddlers were familiarized with the two generalization objects, which had the same shape as the novel objects but differed in color. The same carrier sentences were used but the label was replaced by a pronoun (it/this).
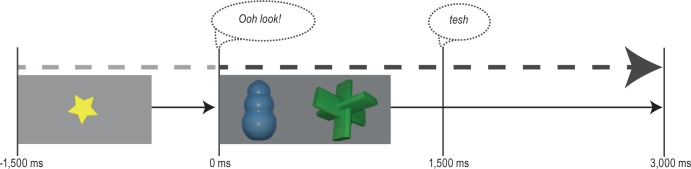
Then, an on-screen training and testing phase took place. Toddlers sat on the caregiver's lap approximately 80 cm from the screen and the eye tracker. Before the presentation of the experimental stimuli, a nine-point calibration was conducted with individual calibration points repeated until four good calibration points were obtained so that the eye tracker could identify the location of visual fixations. Each trial was 3,000 msec long, and was preceded by a 1,500- msec-long animation appearing in the middle of the screen, to direct toddlers' gaze toward the center. The onset of the audio label occurred 1,500 msec after the onset of the test objects. The time line of an example generalization testing trial is shown in Figure 2.
Figure 2.
The time line of the trials. Here, we show a testing trial for the generalization objects.
First, a block of four familiar testing trials was presented where pictures of two familiar objects were shown side by side (e.g., cat and duck) and one of them was labeled. We included these trials to provide toddlers with some time to understand the task. Then, a block of six novel object training trials were shown and named with its associated auditory label, one at a time either on the left or the right side of the screen (counterbalanced within toddlers). This was followed by a block of four novel object testing trials with the pictures of the two novel objects presented simultaneously, side by side. Then, to familiarize toddlers with the generalization objects on screen, we presented four generalization object trials where a picture of one of the generalization objects was shown, accompanied with an attention-getter sentence (without a label). Each generalization object was presented twice, once on each side of the screen. Finally, both generalization objects were presented side by side four times and one of the labels of the original objects was spoken. The side of the labeled generalization object was counterbalanced within toddlers. The order of the trials within a block was randomized.
The second testing session was approximately 1.5 h after the first. During this period, toddlers in the nap group had a daytime nap in the laboratory, whereas toddlers in the wake group played with their parents in the play area or they went for a walk in town. Toddlers wore an actiwatch (Mini Actiwatch, CamNtech Ltd, Cambridge, UK) around their ankle in order to ensure the nap group slept, and the wake group was awake. Toddlers could nap as long as they wanted (mean = 59 min, standard deviation = 29.69). The second testing session consisted of a block of four familiar object testing trials, a block of four generalization testing trials, and a block of four novel object testing trials in random order within block.
Data Processing and Statistical Analysis
Processing of data was performed in MATLAB (version 8.2.0.701, R2013b). The raw gaze data were smoothed with a three-point median filter. Fixations were identified automatically using custom routines on the basis of the spatial (within a circle with 35 pixel radius) and temporal characteristics (within 66.7 msec) of the smoothed gaze data. Statistical analysis and visualization of data were performed using IBM SPSS Statistics 22 (Armonk, NY, USA) and R (version 3.1.2).16
Using the fixation data obtained from the automatic eye tracker, we calculated the total amount of looking time at the target (T) and distracter (D) for both prenaming and post-naming phases of each trial as described by Horvath et al.10 We define a preference for the target as a difference measure (T − D). To control for object preferences independent of labeling, we calculated a difference measure for the prenaming phase of the trial (Tpre − Dpre). We also calculated a similar measure for the postnaming phase of the trial (Tpost − Dpost). Each phase of the trial was defined to begin 200 msec after stimulus onset (picture then label), because any saccades launched before this time could not be in response to the stimulus.17 To determine how labeling changes looking behavior, we define a naming effect as the difference between these difference measures, i.e., (Tpost − Dpost) − (Tpre − Dpre).
RESULTS
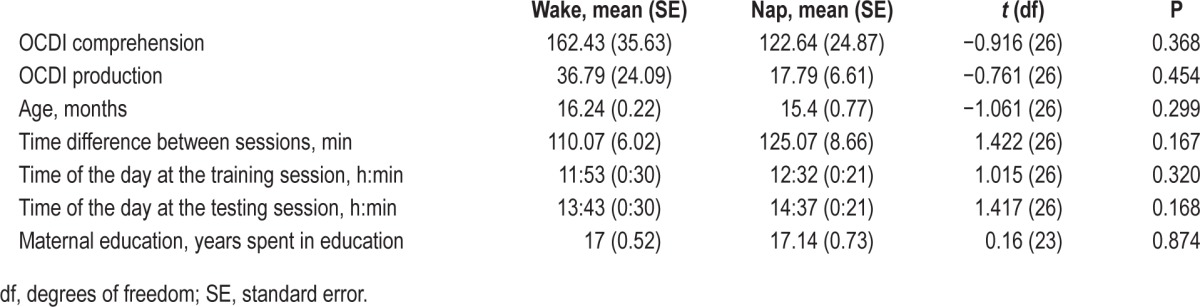
The data of 28 toddlers were analyzed. All toddlers had regular naps according to the caregiver and confirmed by the SNORI or by interview. There was no significant difference between the groups in OCDI score, age, time difference between the sessions, time of day of the training, and testing and maternal education. Descriptive and t-test statistics are presented in Table 1.
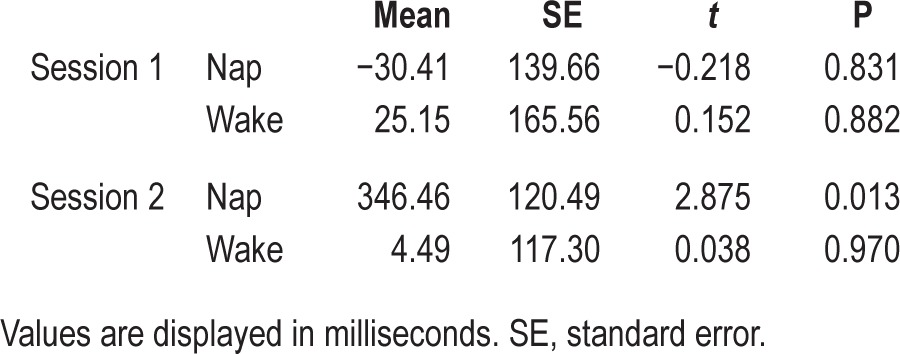
Table 1.
Descriptive and t-test statistics.
To determine whether the two groups changed in generalization performance over the 1.5-h period, including sleeping for the nap group, we used a repeated-measures analysis of covariance. Age, CDI comprehension score, and the time difference between the two sessions were included as covariates because they may influence test performance. There was no significant main effect of session (F(1,23) = 0.02, P = 0.882), but a significant interaction emerged between session and group (F(1,23) = 1.81, P = 0.049, η2 = 0.19). The interaction is shown in Figure 3. Follow-up comparisons revealed that the naming effect in the nap group increased (t(13) = −2.93, P = 0.012, Cohen d = - 0.78), whereas there was no change in the wake group (t(13) = 0.097, P = 0.924). One-sample t-tests indicated that only the nap group showed learning (i.e., naming effect was significantly different from zero) and exclusively in session two. Descriptive and t-test statistics are shown in Table 2. There was no correlation between sleep time and change in performance in the nap group.
Figure 3.
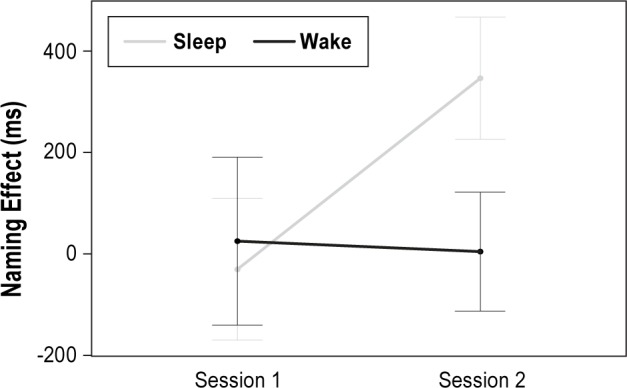
Change in naming effect between sessions by group. A significant interaction between session and group emerged. Means and standard errors are presented.
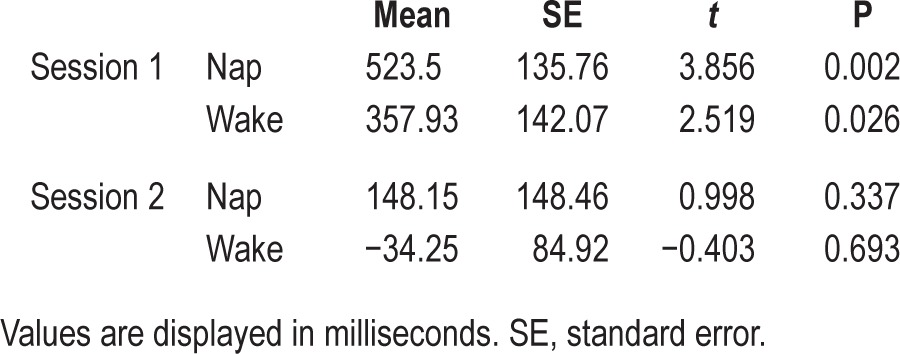
Table 2.
The naming effect in the generalization trials by session and group.
To rule out the possibility that toddlers' levels of attention were different in the two groups, we compared the mean total looking time of the trials in the nap and the wake group with independent sample t-tests. No significant difference emerged in either session 1 (t(26) = −0.784, P = 0.44, mean difference: −73.06 [−264.71 118.59], standard error [SE]: 93.24), or session 2 (t(26) = −0.257, P = 0.799, mean difference: −25.72 [−231.52 180.08], SE: 100.12).
Furthermore, we also analyzed the familiar object trials to confirm that both groups were on task and that they did not differ systematically. We conducted a repeated-measures analysis of covariance with the same covariates as for the generalization objects. There was neither a significant main effect of session (F(1,23) = 0.35, P = 0.56), nor an interaction between session and group (F(1,23) = 0.005, P = 0.945). One-sample t-tests were also performed to see whether both groups participated in the task. Means and t-statistics can be found in Table 3. Both groups showed a significant naming effect but only in the first session. An independent sample t-test confirmed that the two groups did not differ (session 1: t(26) = 0.843, P = 0.407, mean difference: 165.57 [−238.36 569.5], session 2: t(26) = 1.066, P = 0.296, mean difference: 182.4 [−169.16 533.97]).
Table 3.
The naming effect in the familiar trials by session and group.
DISCUSSION
This study provides additional evidence that sleep facilitates generalization of word meanings in toddlers and thereby confirms the results of Friedrich and colleagues8 with a method different from electrophysiology. In this study, we used automatic eye tracking in the IPL paradigm to investigate whether toddlers were able to generalize novel words to objects of similar kind. Toddlers were trained with two object-word pairs and tested with objects that had different colors but the same shape. Toddlers were not able to generalize the name of the object immediately after training. However, the sleep group generalized successfully after having a nap in the laboratory. Although Friedrich and colleagues8 trained with eight objects per category, in this study toddlers were shown only one exemplar of a category, which was enough for generalizing the word meaning.
The facilitating effect of sleep on generalization in infancy was first shown by Gomez and colleagues.18 Toddlers were presented with word strings with an underlying rule where the first word predicted the third. After a nap, toddlers were able to generalize this rule to stimuli which were previously not heard. This effect was observable after 24 h, but only in the nap group.19 The studies of Friedrich and colleagues and the current study extend this grammar-related observation to the acquisition of word meanings.
However, it has been observed elsewhere that wake, and not sleep, promotes the generalization of word meanings.9 It is important to note that in their study, Werchan and Gomez9 tested an older age group (2.5-y-olds), suggesting that the role of sleep in generalization might change during development.20 However, there are many studies in adults that show sleep dependent generalization.1–5 Thus, it is possible that other factors contributed to the contrasting pattern of results. Friedrich and colleagues8 suggest that contextual changes such as the change in background color and texture may account for different findings. Furthermore, Werchan and Gomez's9 task required pointing, which means that other factors influencing toddlers' levels of cooperation might affect the results. In addition, circadian effects cannot be ruled out because there was an almost a 2-h difference approaching significance in the time of day at testing and presumably learning.
Circadian effects might have an effect on our results as well. Although there was no time difference in the time of the day at training or testing, toddlers in the nap group were brought into the laboratory before their usual nap time, and toddlers in the wake group arrived after their usual nap. This might mean that they were in a different phase in their circadian cycle. However, we found no difference between the groups in total looking time, indicating similar levels of attention to the task.
Another limitation of our study is that neither of the groups seemed to participate in the familiar trials in the second session. This might have resulted from repeating the same familiar trials as those in the first session. Furthermore, as these trials were at the beginning of the testing session, sleep toddlers may have still been drowsy because they had just woken up, and wake toddlers would have required longer time to focus on the task as they were tired. However, the lack of any systematic differences between groups in the familiar trials suggests that the differences in performance in the generalization trials were not a consequence of different levels of participation in the task.
Experimenters were not blind to group membership in our study, which might have affected our results. In a previous study, experimenters who had experience with children could judge the group membership of the toddlers on the basis of their behavior.10 We attempted to minimize any confounding effects by using a predefined script during the interactive training phase. Moreover, during the on-screen training and testing phase the experimenter was in the control room. In addition, parents were blind to the hypothesis of the study; thus, it was unlikely they influenced the results.
To conclude, our study implies that during sleep not just a passive consolidation but an active ‘memory evolution’1 happens in the brain of a toddler. During this process, the key aspect of the category, which is a constant shape in the current study, is retained in association with the label while irrelevant information is forgotten.
DISCLOSURE STATEMENT
This was not an industry supported study. The actiwatches were provided by Russell Foster who is the co-supervisor of Dr. Horváth's PhD. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Russell Foster for providing the actiwatches for the study.
ABBREVIATIONS
- df
degrees of freedom
- Dpost
distracter looking time in the postnaming phase
- Dpre
distracter looking time in the prenaming phase
- IPL
Intermodal Preferential Looking
- OCDI
Oxford Communicative Development Inventory
- SE
standard error
- SNORI
Sleep and Naps Oxford Research Inventory
- Tpost
target looking time in the postnaming phase
- Tpre
target looking time in the prenaming phase
REFERENCES
- 1.Stickgold R, Walker MP. Sleep-dependent memory triage: evolving generalization through selective processing. Nat Neurosci. 2013;16:139–45. doi: 10.1038/nn.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaskell MG, Warker J, Lindsay S, et al. Sleep underpins the plasticity of language production. Psychol Sci. 2014;25:1457–65. doi: 10.1177/0956797614535937. [DOI] [PubMed] [Google Scholar]
- 3.Fenn KM, Nusbaum HC, Margoliash D. Consolidation during sleep of perceptual learning of spoken language. Nature. 2003;425:614–6. doi: 10.1038/nature01951. [DOI] [PubMed] [Google Scholar]
- 4.Wagner U, Gais S, Haider H, Verleger R, Born J. Sleep inspires insight. Nature. 2004;427:352–5. doi: 10.1038/nature02223. [DOI] [PubMed] [Google Scholar]
- 5.Ellenbogen JM, Hu PT, Payne JD, Titone D, Walker MP. Human relational memory requires time and sleep. Proc Natl Acad Sci U S A. 2007;104:7723–8. doi: 10.1073/pnas.0700094104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lau H, Alger SE, Fishbein W. Relational memory: a daytime nap facilitates the abstraction of general concepts. PloS One. 2011;6:e27139. doi: 10.1371/journal.pone.0027139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Son JY, Smith LB, Goldstone RL. Simplicity and generalization: Short-cutting abstraction in children's object categorizations. Cognition. 2008;108:626–38. doi: 10.1016/j.cognition.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedrich M, Wilhelm I, Born J, Friederici AD. Generalization of word meanings during infant sleep. Nat Commun. 2015;6:6004. doi: 10.1038/ncomms7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Werchan DM, Gomez RL. Wakefulness (not sleep) promotes generalization of word learning in 2.5-year-old children. Child Dev. 2014;85:429–36. doi: 10.1111/cdev.12149. [DOI] [PubMed] [Google Scholar]
- 10.Horvath K, Myers K, Foster R, Plunkett K. Napping facilitates word learning in early lexical development. J Sleep Res. 2015;24:503–9. doi: 10.1111/jsr.12306. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton A, Plunkett K, Schafer G. Infant vocabulary development assessed with a British communicative development inventory. J Child Lang. 2000;27:689–705. doi: 10.1017/s0305000900004414. [DOI] [PubMed] [Google Scholar]
- 12.Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–6. [PubMed] [Google Scholar]
- 13.Kleiner M, Brainard D, Pelli D. 2007, “What's new in Psychtoolbox-3?” Perception 36 ECVP Abstract Supplement, 2007.
- 14.Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10:437–42. [PubMed] [Google Scholar]
- 15.Deligianni F, Senju A, Gergely G, Csibra G. Automated gaze-contingent objects elicit orientation following in 8-month-old infants. Dev Psychol. 2011;47:1499–503. doi: 10.1037/a0025659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.R Core Development Team. Vienna, Austria: R Foundation for Statistical Computing; 2008. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 17.Haith MM, Hazan C, Goodman GS. Expectation and anticipation of dynamic visual events by 3.5-month-old babies. Child Dev. 1988;59:467–79. [PubMed] [Google Scholar]
- 18.Gomez RL, Bootzin RR, Nadel L. Naps promote abstraction in language-learning infants. Psychol Sci. 2006;17:670–4. doi: 10.1111/j.1467-9280.2006.01764.x. [DOI] [PubMed] [Google Scholar]
- 19.Hupbach A, Gomez RL, Bootzin RR, Nadel L. Nap-dependent learning in infants. Dev Sci. 2009;12:1007–12. doi: 10.1111/j.1467-7687.2009.00837.x. [DOI] [PubMed] [Google Scholar]
- 20.Gomez RL, Edgin JO. Sleep as a window into early neural development: shifts in sleep-dependent learning effects across early childhood. Child Dev Perspect. 2015;9:183–9. doi: 10.1111/cdep.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]