Abstract
Study Objectives:
Recent studies have suggested that structural abnormalities in insomnia may be linked with alterations in the default-mode network (DMN). This study compared cortical thickness and structural connectivity linked to the DMN in patients with persistent insomnia (PI) and good sleepers (GS).
Methods:
The current study used a clinical subsample from the longitudinal community-based Korean Genome and Epidemiology Study (KoGES). Cortical thickness and structural connectivity linked to the DMN in patients with persistent insomnia symptoms (PIS; n = 57) were compared to good sleepers (GS; n = 40). All participants underwent MRI acquisition. Based on literature review, we selected cortical regions corresponding to the DMN. A seed-based structural covariance analysis measured cortical thickness correlation between each seed region of the DMN and other cortical areas. Association of cortical thickness and covariance with sleep quality and neuropsychological assessments were further assessed.
Results:
Compared to GS, cortical thinning was found in PIS in the anterior cingulate cortex, precentral cortex, and lateral prefrontal cortex. Decreased structural connectivity between anterior and posterior regions of the DMN was observed in the PIS group. Decreased structural covariance within the DMN was associated with higher PSQI scores. Cortical thinning in the lateral frontal lobe was related to poor performance in executive function in PIS.
Conclusion:
Disrupted structural covariance network in PIS might reflect malfunctioning of antero-posterior disconnection of the DMN during the wake to sleep transition that is commonly found during normal sleep. The observed structural network alteration may further implicate commonly observed sustained sleep difficulties and cognitive impairment in insomnia.
Citation:
Suh S, Kim H, Dang-Vu TT, Joo E, Shin C. Cortical thinning and altered cortico-cortical structural covariance of the default mode network in patients with persistent insomnia symptoms. SLEEP 2016;39(1):161–171.
Keywords: insomnia, neuroimaging, structural covariance, default mode network, MRI
Significance.
The current study found decreased structural connectivity between anterior and posterior regions of the default mode network (DMN) in patients with persistent insomnia symptoms compared to good sleepers. Decreased structural connectivity within the DMN was associated with higher levels of sleep disturbance and impaired working memory. Altered connectivity may further contribute to sustained sleep difficulties and cognitive impairment commonly reported by insomnia patients. The DMN, which is associated with diverse forms of self-relevant mentalizing and helps modulate consciousness, may be associated with insomnia. This may especially be clinically relevant for insomnia patients, who frequently complain about worrying about their own sleep during the day and report sleep misperception.
INTRODUCTION
Insomnia, characterized by difficulty initiating and maintaining sleep despite adequate opportunity, is one of the most common sleep complaints of the adult general population. The most consistent observation on the pathophysiology of insomnia is hyperarousal, which is pervasive across neurophysiological, cognitive, and autonomic domains.1 However the neural correlates of insomnia still remain poorly understood.
Recent structural magnetic resonance imaging (MRI) studies that provide a unique in vivo assessment of brain structural integrity suggest that insomnia is associated with cortical morphology deviated from the range of healthy sleepers.2,3 Different parameters (e.g., smoothing kernel size) and methods (e.g., use of optimized voxel-based morphometry, use of diffeomorphic nonlinear registration) chosen across studies and generally small sample sizes (n < 29) may have caused inconsistency of finding in structural abnormalities in insomnia.4 The previous findings have often included part of regions belonging to the default mode network (DMN), such as the me-dial prefrontal, anterior cingulate, and precuneus.2,3 Another study found no cortical structural abnormalities.5
Recently, there has been great interest in studies examining cortico-cortical structural covariation network that is derived from correlational analysis of morphometrics between various cerebral regions.6–11 Structural covariance is a between-subject analysis based on the assumption that brain areas that show similar variations in volumes across subjects are connected.6 Unlike “direct” structural connectivity estimated using diffusion tensor imaging-based tractography,12 such morphological covariance may indicate altered neural connectivity resulting from relatively long-term processes such as neurodevelopment13 or neurodegeneration.14 Analyses of cortical thickness indeed demonstrates structural covariance within regions of the DMN in healthy development and aging, and have been applied in meaningful areas of investigation.13,15
The DMN is a network of brain regions that display increased activity at wakeful rest in the absence of cognitively demanding tasks, and plays a central role in the modulation of consciousness.16,17 During sleep, functional connectivity within the DMN becomes reduced, particularly during slow wave sleep as compared to wakefulness: in good sleepers, the descent to slow wave sleep is characterized by a functional dissociation between frontal (anterior cingulate, medial pre-frontal) and posterior (precuneus, posterior cingulate) regions of the DMN.18,19 This decoupling realizes a breakdown of cortical connectivity in the descent to sleep, which is associated with the reduction of consciousness.
The current study first assessed structural abnormalities in a large sample of patients with persistent insomnia symptoms (PIS, n = 57) compared to good sleepers (GS, n = 40) using a cortical thickness metric that has been suggested to increase sensitivity of voxel-based morphormetry (VBM), which can be diluted by sulcogyral folding.20 We then investigated structural covariance in PIS and GS groups, looking at differences of volumetric cross-correlations between regions and comparing group differences, with the hypothesis that altered connectivity of the DMN would characterize PIS. Furthermore, we assess the relationship between structural covariance of the DMN in PIS and GS and sleep quality or neuropsychological scores.
METHODS AND MATERIALS
Study Design and Sample
From a database as part of a population-based cohort study, namely the Korean Genome and Epidemiology Study,21 we randomly selected 60 patients with PIS who completed questions about insomnia at 3 time points at every 2 years. Inclusion into the PIS group was assessed by the presence of the following four insomnia symptoms during the past month: (1) difficulty initiating sleep; (2) difficulty maintaining sleep; (3) experience of early morning awakenings; and (4) unrefreshed sleep using a 4-point Likert scale. Individuals who endorsed at least 1 of these 4 symptoms more than 3–4 times a week (score ≥ 3 on any item) were categorized as having insomnia symptoms. All participants were followed over 4 years with biennial exams, at 3 data points spaced 2 years apart. We defined the PIS group as the presence of insomnia symptoms at all 3 consecutive assessments. Forty-four GSs who reported no insomnia symptoms at all time points were also randomly chosen from the original database and were age and gender-matched with the PIS group.
All patients with PIS and GS underwent subsequently brain MRI scans. Among the PIS and GS, 7 participants were finally excluded due to moderate or severe obstructive sleep apnea determined by respiratory polygraphy, resulting in a total sample of 97 participants (57 PIS and 40 GS). Participants taking sleep or antidepressant medication at any time point were excluded.
Informed consent was obtained from each participant, and the study was approved by the institutional review board.
Measures
Beck Depression Inventory (BDI)
The BDI is a 21-item self-report inventory used to assess the severity of depressive symptoms.22 Participants were asked to indicate which statement best describes the way they had felt over the past 2 weeks. BDI scores were calculated without the sleep item to avoid overlap with other sleep measures.
Pittsburgh Sleep Quality Index (PSQI)
Participants completed the PSQI, a self-report questionnaire assessing sleep quality and disturbances over a one-month interval.23 The scale yields a total score that ranges from 0–21, with higher scores indicating more difficulties with sleep. The questionnaire has 7 subscales including subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction. Subjective sleep duration was derived from the sleep duration subscale of the PSQI.
Respiratory Polygraphy (RP)
Overnight RP was performed with a comprehensive portable device (Embletta X-100; Embla Systems, Broomfield, CO, USA) and conducted at home. Apneas were defined when airflow was reduced by ≥ 90% of the baseline values for ≥ 10 sec, and apneas were further classified as obstructive if respiratory efforts were noted on either the chest or abdominal inductance channel, or as central if no respiratory effort was noted. Additionally, hypopneas were defined by ≥ 30% reduction of airflow ≥ 10 sec accompanied by ≥ 4% drop in oxygen saturation (SpO2). The apnea-hypopnea index (AHI) was calculated by averaging the total number of obstructive apneas and hypopneas per hour of sleep.24
Data were scored by 2 well-trained technicians with ≥ 5 years of experience with RP monitoring and scoring based on standard AASM scoring guidelines.25 Internal consistency for detecting apneas and hypopneas was high (Cronbach α = 0.996 and 1.00 for each rater), and interrater reliability was also very strong (Cronbach α = 0.998). The scorers were also blinded to each other's scoring results.
Neuropsychological Assessments
All participants underwent a neuropsychological test battery (30 minutes) including 6 broad cognitive domains. Verbal Memory was assessed with the Story Recall Test26 from the logical memory test in the Wechsler Memory Scale-III.27 Visual memory was assessed using Visual Reproductions (WMS-III),27 and Language Processing was measured using the Controlled Oral Word Category Association Test.28 Additionally, we used the Trail Making Test29 and the Digit Symbol Test (Weschler Adult Intelligent Scale-III) for assessing Processing Speed and Mental Flexibility and Visual Information Processing, respectively. Executive Functioning was measured using the Stroop Test.29 All tests were administered according to standard protocol.
MRI Acquisition and Image Processing
All scans were performed on a GE Signal HDxt 1.5T MR imaging scanner (GE Medical Systems, Waukesha, WI) with an 8-channel head coil. T1-weighted MRI was performed using sagittal spoiled gradient recalled acquisition with the following parameters: TR = 10.9 ms; TE = 5.01 ms; NEX = 1; flip angle = 13°; matrix size = 256 × 256; FOV = 240 mm; slice thickness = 1.2 mm; 165 slices. Each image underwent automated correction for intensity non-uniformity and intensity standardization.30 To control for changes in relation to brain volume, MR images were linearly registered into the MNIICBM 152 template.31
Measurement of Cortical Thickness
Outer and inner cortical interfaces were extracted using the CIVET image processing pipeline developed at Montreal Neurological Institute.32 Briefly, images were first classified into gray matter (GM), white matter (WM), and cerebrospinal fluid using Statistical Parametric Mapping version 8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) with default priors. We then used a surface-fitting algorithm33 that iteratively warped a surface mesh to fit the GM-WM interface in the classified image. This surface was inflated along a Laplacian map to generate the outer pial surface. These 2 surfaces were nonlinearly aligned to a symmetric surface template using a spherical registration that guarantees point correspondence across all subjects.34 We measured cortical thickness across 80,492 of vertices spanning on the cortical surface. Thickness data were blurred using a surface-based diffusion-smoothing with a kernel size of 20 mm full width at half maximum that preserves cortical topologic features.
Statistical Analysis
All statistical tests included age and sex as covariates. As BDI scores in PIS was significantly lower than in GS (P < 0.001), effects of depression were also corrected in the analysis of cortical thickness and covariance network. Statistical analyses were performed using SurfStat toolbox (http://www.math.mcgill.ca/∼keith/surfstat/).35
Prior to analysis, 6 composite scores were computed from the neuropsychological tests by transforming all scores into Z-scores and then averaged to represent each domain: verbal memory, visual memory, language processing, processing speed and mental flexibility, visual information processing, and executive functioning.
To compare demographic, clinical, and neuropsychological parameters between groups, t-tests were used.
Group Comparison of Cortical Thickness
We assessed differences at each surface point between patient and GS groups using two-tailed t-tests. The main body of structural MRI studies (n = 3/4 = 75%, Table S1, supplemental material) in insomnia have used VBM. We thus performed VBM for the purpose of data reproducibility in a different morphometric analysis as well as of the sensitivity of each analysis (see supplemental material).
Mapping Cortico-Cortical Covariance Using Cortical Thickness Correlations
Most neuroimaging studies, whether or not examining neo-cortical areas implicated in the DMN in insomnia, (Table S1) have reported that regions involved in the DMN (8/8/12 = 67% in all neuroimaging studies) presented metabolic (5/8 = 63%) and structural changes (3/4 = 75%) in patients with insomnia compared to healthy controls. These studies consistently found changes in the medial frontal cortex (mFC) including medial prefrontal cortex (mpFC) and/or the anterior cingulate cortex (aCC). The precuneus (PC) was identified as the second most common locus of changes of the DMN regions (2/10 = 20%). The location of mFC clusters found in the literature were averaged at MNI coordinate = (left: −4,16,36; right: 4,16,36). The location of PC changes has not been specified in previous insomnia studies. Thus, we selected the representative voxels (left: −10,−57,50; right: 10,−57,50) from a review of studies of the PC.16 The mFC and PC voxels are shown in Figure S1 (supplemental material). We projected these voxels to the closest points on the cortical surface. Subsequently, we investigated structural covariance by correlating cortical thickness of each seed (mFC, PC) with the thickness at all other surface points of the entire cortex. These pairwise correlations constructed covariance maps in each GS and PIS group. This was achieved by applying the following equations following previous studies,11,36 and we used the linear model fitted for the thickness T at a surface point i (Equation 1):
At each point i, we assessed covariance alterations in PIS compared to GS by fitting a linear model containing independent variables as main effects of group (GS or PIS) and cortical thickness Tseed, and the interaction term group × Tseed, i.e.,
Assessing Relationship of Cortical Thickness and Covariance Strength to Sleep Quality and Neuropsychological Parameters
To investigate whether decreased sleep quality or impaired cognitive function was associated with cortical thinning, we first correlated either PSQI or each of neuropsychological scores presenting significant impairment relative to GS with cortical thickness while correcting for age, sex and depression levels. To assess the association between clinical parameters and the strength of mFC and PC covariance network, we fitted linear models that included PSQI or each of neuropsychological scores as a main effect Emain, seed thickness, and the interaction term between seed thickness and Emain. The model was accordingly defined as:
In each GS and PIS group, we assessed the significance of the interaction term in this model.
Correction for Multiple Comparisons
We corrected significance using the false discovery rate (FDR) procedure,37 with FDR < 0.05. In the analysis of demographic data, Bonferroni-adjustment was applied to control for family-wise error rate.
RESULTS
Demographics, Clinical Characteristics and Neuropsychological Scores (Table 1)
Table 1.
Demographic information of participants (n = 97).
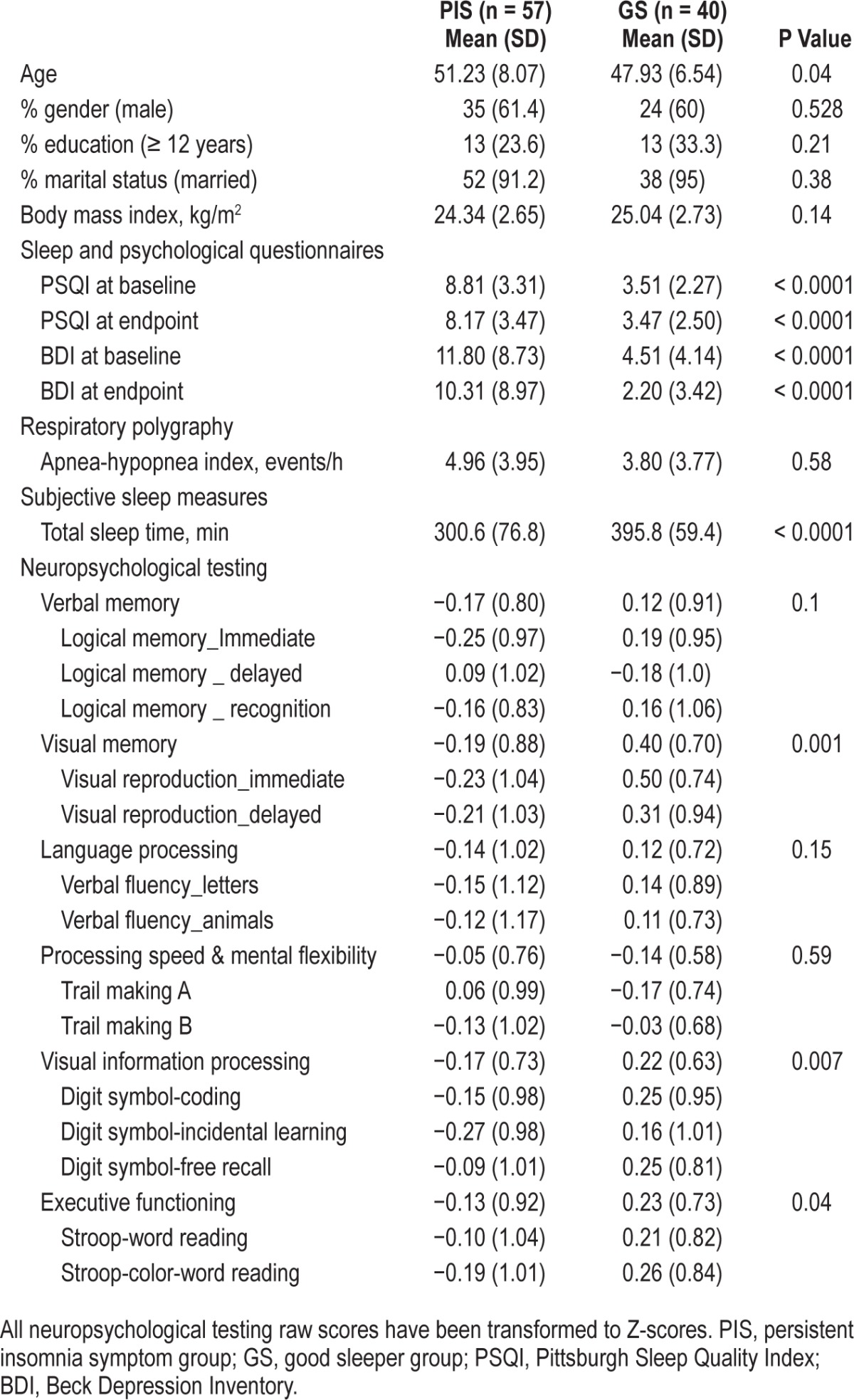
The PIS group was slightly older than the GS group (P = 0.04), and had significantly worse scores on the PSQI and BDI (P < 0.0001). There was one person in the GS group and 13 in the PIS group who reached the threshold for pathological BDI scores (< 16). The PIS group had significantly lower scores on visual memory (P = 0.001), visual information processing (P = 0.007), and executive functioning (P = 0.04) compared to the GS group.
Analysis of Cortical Thickness in PIS and GS
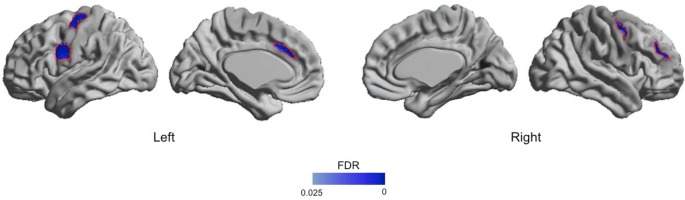
Analysis of mean cortical thickness over each hemisphere showed significant cortical thinning in both hemispheres of patients with PIS compared to GS (left: 2.61 ± 0.13 mm vs. 2.66 ± 0.11 mm, P < 0.05; right: 2.60 ± 0.14 vs. 2.67 ± 0.12, P < 0.01). Regional analyses (Figure 1) revealed cortical thinning in PIS, which was circumscribed to the left medial frontal cortex (more precisely anterior cingulate cortex) (t > 2.6, FDR < 0.05), bilateral precentral cortices (FDR < 0.05), and right lateral prefrontal cortex (right: FDR < 0.05). No cortical thickening was found in PIS compared to GS (FDR > 0.3).
Figure 1.
Patterns of cortical thinning in patients with persistent insomnia compared to good sleepers. Age, gender and the severity of depression were adjusted. Significant clusters, corrected for multiple comparisons (FDR < 0.05), are shown in dark blue and outlined in red, and are located in the bilateral precentral, left anterior cingulate and right middle frontal cortices. Additional clusters at an uncorrected threshold of P < 0.025 are shown in light blue. FDR, false discovery rate.
Our data were well-reproduced in different morphometry as VBM showed a similar pattern of GM density decreases (Figure S2, Table S2, supplemental material).
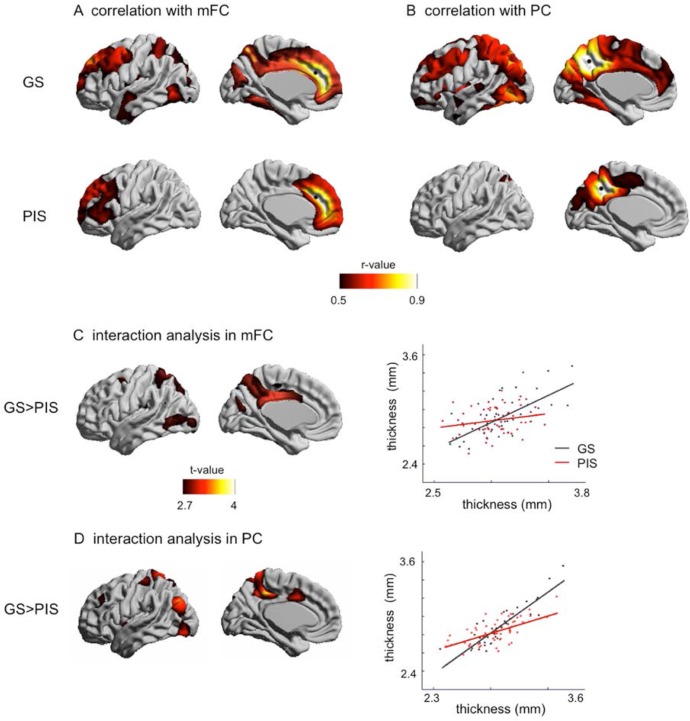
Analysis of Cortico-Cortical Morphological Covariance (Figure 2)
Figure 2.
Cortical-cortical covariance. (A) Significant correlations (r > 0.5, FDR < 0.05) of cortical thickness with medial frontal cortex (mFC) are mapped. (B) correlations with precuneus (PC). In GS, both mFC and PC covariance mappings reveal cortical areas involved in the default mode network. In PIS, the pattern of correlations is limited to areas neighboring the seed point (black circle), suggesting network disruption in the frontoparietal area. (C,D) the interaction between correlation and group indeed shows that the most marked decrease of mFC/PC structural covariance was found in medial parietal and posterior cingulate cortices.
We previously found hemispherically similar atrophy pattern in PIS. Vertex-wise one-sample t-tests on asymmetry (2 × [L − R] / [L + R]; L/R denotes the cortical thickness at a given vertex in the left hemisphere and R the cortical thickness at the mirror vertex in the right hemisphere) of cortical thickness between PIS and GS groups indeed confirmed no significant unilateral atrophy (FDR > 0.1). We thus averaged local thicknesses at the same location between the left and right sides to facilitate the subsequent covariance network analysis.
Our analysis of cortical thickness correlations (Equation 1) showed that in GS, regions presenting high covariance (r > 0.5; FDR < 0.001) with the mFC seed included prefrontal cortex, precuneus, lateral parietal cortex as well as scattered clusters in the temporal lobe. Noteworthy, these cortices are similar to areas of the DMN that were found in other imaging modalities.38 In contrast, the PIS group only displayed a significant correlation of the mFC with most of the frontal cortices, but not with the posterior regions appearing in GS (r > 0.5).
Mapping the covariance of cortical thickness between the PC and other regions in GS (Figure 2B) revealed a pattern similar to the mFC network (Figure 2A), yet the PC network showed a stronger correlation with posterior cortices and included a larger extent of parietal and occipital cortices. In PIS, however, the areas presenting high correlation with PC thickness were limited within the posterior brain (FDR < 0.001).
Therefore, compared to GS, there was a smaller extent of mFC and PC structural covariance in PIS, which was limited within areas neighboring the seed points.
The interaction analysis using Equation 2 confirmed that the covariance of mFC or PC with the posterior brain of GS indeed significantly decreased in PIS (FDR < 0.05). The most marked decrease in covariance occurred in the frontoparietal junctional area, including medial parietal and posterior cingulate cortices (Figure 2C, 2D).
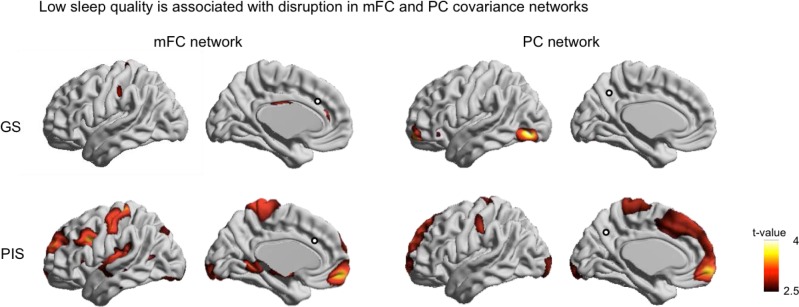
Association of Sleep Quality to Cortical Thickness and Its Covariance Network (Figure 3)
Figure 3.
Association between sleep quality and structural covariance of cortical thickness. Decreased correlations to the seed (i.e., mFC or PC indicated by circle) in relation to low subjective sleep quality are shown. In GS, only scattered focal areas present disrupted covariance networks. In PIS, decreased mFC covariance relating to low sleep quality was found in the lateral prefrontal and central cortices, where cortical thinning in PIS compared to GS was found (Figure 1), as well as in the medial prefrontal cortex, parahippocampal gyrus and focal areas of occipital cortex (FDR < 0.05). We found decreased PC covariance mainly in frontal cortical areas including anterior/posterior cingulate and prefrontal cortices in relation to lower sleep quality (FDR < 0.05). The decreased covariance was also mapped in focal areas of central cortex, and occipital cortex (FDR < 0.05).
Cortical thickness did not change in relation to variation of age, gender, education, depression (BDI) in either GS or PIS group (FDR > 0.3). We found no correlation between subjective sleep quality (PSQI) and cortical thickness changes neither in PIS nor GS (FDR > 0.2).
With respect to thickness covariance, a significant association between the decrease in the PC covariance and depressed mood was found in the orbitobasal frontal cortex and lateral occipital cortex in PIS (FDR < 0.05; Figure S3, supplemental material). No significant association was found for the mFC covariance (FDR > 0.1).
In GS, only focal and scattered areas of mFC and PC covariance maps altered in relation to PSQI variation (FDR < 0.05, Figure 3). In PIS, mFC covariance decreased relating to low sleep quality in diverse areas including the lateral and medial prefrontal, central, and occipital cortices, as well as parahippocampal gyrus (FDR < 0.05). Repetition of this analysis with respect to the PC in PIS showed decreased structural covariance with anterior/posterior cingulate, prefrontal, central and occipital cortices in relation to lower sleep quality (FDR < 0.05). In the areas of significant association in PIS, we computed the effect size (Cohen's D; D < 0.2: small, 0.2 < D < 0.5: moderate; D > 0.8: large) within each group. The analysis showed that the effect size of GS was much smaller than that of PIS (D = 0.18 vs. 0.86).
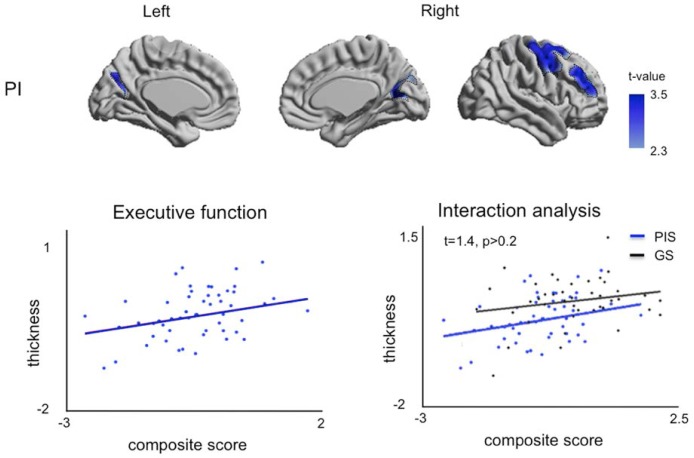
Association of Neuropsychological Parameters to Cortical Thickness and Its Covariance Network (Figure 4)
Figure 4.
Association of neuropsychological parameters to cortical thickness in the PIS group. The t-value indicates poor performance in executive function associated with cortical thinning and is thresholded at FDR < 0.05. In the left plot, thickness was averaged in the clusters presenting significant association. In this region, an interaction analysis showed no group difference in correlation even though the GS group showed no correlation in any cortical area due to its large variability (FDR > 0.1).
As we found that executive function, visual memory and visual information processing in PIS were significantly poorly performed compared to GS (Table 1), we analyzed the association with respect to only these functions.
In GS, changes in cortical thickness were not associated to variations in neuropsychological scores (t < 1.5; FDR > 0.2). In PIS, right lateral prefrontal area and bilateral areas of junction between precuneus and cuneus presented cortical thinning relating to lower performance in executive function (t = 3.4, FDR < 0.005, Figure 4). In these areas, the interaction analysis (group × executive function) however found no difference in the correlation between the groups, as the trend in the GS group was similar to that in the PIS (P > 0.2).
In GS and PIS, the mFC and PC covariance did not alter in association to changes in neuropsychological scores (t < 1.2; FDR > 0.3).
DISCUSSION
Our study investigated cortical thickness changes in patients with PIS and assessed their alterations in structural covariance by analyzing cortico-cortical correlations of thickness metrics. Compared to GS, our analysis showed morphological alterations in the PIS group were circumscribed to multiple cortices primarily including frontal and parietal cortices. Analysis of structural covariance revealed disruption in the link between these two cortices in the PIS group. Further analyses showed that decreased structural covariance related to low sleep quality extended beyond the areas presenting initial network disruption. Cortical thinning in areas of DMN in persistent insomnia symptoms was associated with cognitive impairment. This is the first study to investigate cortical thickness changes as well as structural covariance in individuals with persistent insomnia symptoms. Furthermore, we performed analyses on the largest sample ever published in the neuroimaging of insomnia patients. Cross-method validation using voxel-based morphometry indeed confirmed that changes in our large sample size are highly reproducible even though changes with a larger effect size are observed using the cortical thickness metric (supplemental material).
Cortical Thinning in Insomnia Patients
Bilateral patterns of cortical thinning in patients with PIS were found compared to GS, specifically mapped in the central cortex, anterior cingulate cortex, lateral prefrontal areas and occipital cortex. These areas well-overlap with regions presenting GM loss which have been found in a recent voxel-based morphometry study of patients with chronic insomnia.2,39 A study also found a similar pattern of changes, which did not reach a significant level after correction.3
Our finding is also consistent with evidence from various functional imaging studies in chronic insomnia disorder: lack of glucose metabolism decrease in the anterior cingulate cortex during NREM sleep relative to wakefulness,40 hypo-activation in the lateral prefrontal cortex during wakefulness compared to controls,40 and reduced modulation of the lateral prefrontal cortex with increasing task difficulty,41 abnormal excitability of the pre- and post-central area during transcranial magnetic stimulation42,43 and altered gammaaminobutyric acid level in the occipital lobe and anterior cingulate cortex44,45 during daytime tasks. Although our study investigated persistent insomnia symptoms and not insomnia disorder, the large overlap of spatial pattern between brain functional alterations and morphological changes in relation to chronic insomnia suggests that abnormal brain responses in specialized cortical areas are subtended by structural neural deficits.
We found no aging effects in PIS and GS groups. The aging effect on cortical thinning is generally limited in non-elderly group even though longitudinal studies of seniors found a relatively wide-range of effect.46,47 Our subjects, despite a large sample size, have a narrow age range and are not the elderly, which may result in an insufficient effect size for our cross-sectional study design.
Disruption in Structural Covariance between the DMN and Other Cortices in Insomnia
Previous functional connectivity studies indicate a disconnection within regions of the DMN, particularly between anterior and posterior regions during the transition from wake to slow wave sleep in good sleepers.19 In insomnia patients, multiple structural imaging studies2,39,48 have found volume changes in the DMN regions, including the medial prefrontal, anterior cingulate, and precuneus.
Consistent with previous studies, the assessment of cortical thickness covariance in our study showed that disruption in the DMN in insomnia was also structural, resulting mainly from reduced thickness covariance between the frontal and parietal cortices. Fronto-parietal functional dissociation is manifested during slow wave sleep18,19 as well as at a state of wakeful rest under prolonged sleep deprivation, as shown in resting-state functional MRI studies.49–51 This result has implications for the pathophysiology of insomnia. Since this functional connectivity decrease in the DMN was interpreted as reflecting the loss of consciousness during deep sleep and a marker of sleep intensity, one could expect that a decrease in structural connectivity in this network would also reflect a propensity to sleep. Our finding of a decreased structural connectivity in the DMN in individuals with persistent insomnia might thus appear contradictory. However, similarly to the lack of glucose metabolism decrease during NREM sleep relative to wakefulness,40 it is possible that it is a lack a connectivity decrease during NREM sleep relative to wakefulness that characterizes insomnia. In that regard, a decreased structural covariance between anterior and posterior nodes of the DMN, as we observed, might reflect an altered connectivity at baseline in insomniacs, which would impair any further connectivity decrease within the DMN during sleep attempts. We thus speculate that it is not the functional decoupling per se that characterizes the loss of consciousness during sleep, but rather the progressive decrease in coupling during the descent to deep sleep. This altered structural connectivity in insomnia may further contribute to sustained sleep difficulties and cognitive impairment frequently reported by insomniacs. Our results in patients with persistent insomnia symptoms indeed showed a significant association between altered DMN structural connectivity and low sleep quality. Furthermore regions involved in the DMN presented cortical thinning in relation to cognitive impairment. Future studies assessing functional connectivity changes in the DMN across the sleep wake-cycle in insomnia patients are warranted to further support this model.
We observed that the structural covariance between the DMN and the medial/lateral prefrontal cortices, central cortex, occipital cortex as well as parahippocampal gyrus was decreased in relation to lower sleep quality mainly in insomnia patients, which was not likely in controls due to a small effect size, This suggests that higher insomnia severity is associated with more extended levels of structural damage in the pathological network of insomnia. These regions have been also reported in past studies as primary brain foci that presented morphological alteration in relation to subjective/objective sleep severity.2,39,48
Relationship between Cognitive Impairment and Cortical Thinning of the DMN Regions in Insomnia
Our analysis showed that impaired executive function is related to cortical thinning in lateral prefrontal cortex and the junction between the precuneus and cuneus, which are part of or proximal to the DMN. Interaction analysis, however, suggested that it is unclear this relationship is only present in insomnia. A further study with a larger sample size may clarify this.
It has been frequently reported that cognitive impairment in insomnia is related to functional abnormality in prefrontal cortex,52–55 which is considered a hub to process such cognitive functions.56,57 Studies analyzing the effect of sleep deprivation to brain function have observed increased activation in the prefrontal cortex during a complex memory task with simultaneous decreased activation in DMN regions.58 This may reflect adaptive cognitive functioning in insomnia, in which the prefrontal cortex may become overused to compensate for the dysfunction of DMN areas given a “difficult-to-solve” task. Chronic overuse of the prefrontal cortex in insomnia may eventually lead to structural modifications in this structure as seen in our study.
Functional connectivity between the prefrontal cortex, parietal cortex, and sensory regions including the occipital cortices becomes stronger when executive function or visuospatial reasoning is performed.59–61 The found connectional modifications in the parietal and occipital lobes in relation to cognitive impairment in PIS may thus indicate secondary changes due to the propagation of the primary damages in the prefrontal cortex through its neuronal pathway to these regions.
Similarities and Differences between Structural Covariance and Other Imaging-Based Connectivity
A central question lies in what the observed corticocortical thickness covariance network implies compared to findings derived from MR diffusion-weighted imaging of white matter pathways or from functional MR imaging. Despite a large overlap between functional connectivity and diffusion MRI-based networks,38,62,63 recent studies suggest that functional connectivity more closely agrees with structural networks derived from morphological covariance than those from WM tractography. This is based on anti-correlation analyses of the DMN, in which the attentional network and DMN operate in opposition.6,64–67 Intriguingly, the anti-correlation observed in the functional analysis is also found in the thickness covariance networks.68,69 The pattern of structural network alteration in PIS found in our study is concordant with functional studies in insomnia. It should be noted that the different time scales of the structural covariance and functional networks might lead to a fundamentally different interpretation: i.e., structural covariance uniquely reflects brain organization along a relatively long-term time course.14,68,70 Moreover, the proposed approach does not assess a direct, causal connection. Thus, it remains unclear whether the network alteration found in our study precedes or results from insomnia.
Limitations
Despite our findings, a number of limitations should be considered when interpreting the results of this study. One limitation that should be noted is the insufficient information we had regarding long-term medication use in all participants. While no participants in this study were on current medication at the time of the experiment, we cannot exclude the possibility that long-term medication had an impact on the structural differences observed in our study. Previous structural MRI studies observed, for example, that long-term use of antidepressants for more than 3 years was associated with increased hippocampal volumes in patients with major depressive disorder.71 However such volume change was not observed after 8 weeks72 or 10 months of treatment,73 suggesting that effects on brain structure are mostly found after very prolonged and chronic medication exposure.
Additionally, we did not exclude any participants because of high depression scores on the Beck Depression Inventory. While this would have been ideal, past studies have reported that persistent insomnia is strongly associated with depression at baseline compared to normal sleep, and it is difficult to separate the two due to high correlation between insomnia and depression.74,75
CONCLUSION
This report constitutes the most comprehensive structural imaging study of insomnia, demonstrating anatomical alterations and disrupted structural connectivity, as well as their implications on cognitive function and sleep quality. Our results suggest that patients with persistent insomnia symptoms present altered structural connectivity mainly within regions of the DMN that reduce their capacity to perform the normal transition to sleep accompanied by a functional disconnection between the anterior and posterior regions of the DMN. This altered connectivity may further contribute to sustained sleep difficulties and cognitive impairment commonly reported by insomnia patients.
DISCLOSURE STATEMENT
This study was supported by grants from the Korean Center for Disease Control & Prevention and the Korean Ministry for Health and Welfare [Grant 2005-E71001-00, Grant 2006-E71005-00, Grant 2007-E71001-00, Grant 2008-E71001-00, Grant 2009-E71002-00, Grant 2010-E71001-00, Grant 2011-E71004-0] and by the National Research Foundation of Korea (NRF) grant funded by the Korean government (NRF-2012-0009830). Dr. Hosung Kim is supported in part by a National Institutes of Health (NIH) grant (R01HD072074). Dr. Dang-Vu is supported by the Canadian Institutes of Health Research, the Fonds du Recherche du Québec – Santé, the Natural Sciences and Engineering Research Council of Canada, and the Canada Foundation for Innovation. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Siheung Central hospital for providing support for MRI scans. We would like to thank Hyun Kim and Sunmi Lee for their support with organizing the neuropsychological data.
REFERENCES
- 1.Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Altena E, Vrenken H, Van Der Werf YD, van den Heuvel OA, Van Someren EJ. Reduced orbitofrontal and parietal gray matter in chronic insomnia: a voxel-based morphometric study. Biol Psychiatry. 2010;67:182–5. doi: 10.1016/j.biopsych.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Winkelman JW, Benson KL, Buxton OM, et al. Lack of hippocampal volume differences in primary insomnia and good sleeper controls: an MRI volumetric study at 3 Tesla. Sleep Med. 2010;11:576–82. doi: 10.1016/j.sleep.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Dang-Vu TT. Structural brain modifications in primary insomnia: myth or reality? Sleep. 2013;36:965–6. doi: 10.5665/sleep.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spiegelhalder K, Regen W, Baglioni C, et al. Insomnia does not appear to be associated with substantial structural brain changes. Sleep. 2013;36:731–7. doi: 10.5665/sleep.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans AC. Networks of anatomical covariance. NeuroImage. 2013;80:489–504. doi: 10.1016/j.neuroimage.2013.05.054. [DOI] [PubMed] [Google Scholar]
- 7.Rykhlevskaia E, Gratton G, Fabiani M. Combining structural and functional neuroimaging data for studying brain connectivity: a review. Psychophysiology. 2008;45:173–87. doi: 10.1111/j.1469-8986.2007.00621.x. [DOI] [PubMed] [Google Scholar]
- 8.Alexander-Bloch AF, Vertes PE, Stidd R, et al. The anatomical distance of functional connections predicts brain network topology in health and schizophrenia. Cereb Cortex. 2013;23:127–38. doi: 10.1093/cercor/bhr388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernhardt BC, Worsley KJ, Besson P, et al. Mapping limbic network organization in temporal lobe epilepsy using morphometric correlations: insights on the relation between mesiotemporal connectivity and cortical atrophy. NeuroImage. 2008;42:515–24. doi: 10.1016/j.neuroimage.2008.04.261. [DOI] [PubMed] [Google Scholar]
- 10.Bernhardt BC, Chen Z, He Y, Evans AC, Bernasconi N. Graph-theoretical analysis reveals disrupted small-world organization of cortical thickness correlation networks in temporal lobe epilepsy. Cereb Cortex. 2011;21:2147–57. doi: 10.1093/cercor/bhq291. [DOI] [PubMed] [Google Scholar]
- 11.Lerch JP, Worsley K, Shaw WP, et al. Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. Neuroimage. 2006;31:993–1003. doi: 10.1016/j.neuroimage.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 12.Spiegelhalder K, Regen W, Prem M, et al. Reduced anterior internal capsule white matter integrity in primary insomnia. Hum Brain Mapp. 2014;35:3431–8. doi: 10.1002/hbm.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raznahan A, Lerch JP, Lee N, et al. Patterns of coordinated anatomical change in human cortical development: a longitudinal neuroimaging study of maturational coupling. Neuron. 2011;72:873–84. doi: 10.1016/j.neuron.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen ZJ, He Y, Rosa-Neto P, Gong G, Evans AC. Age-related alterations in the modular organization of structural cortical network by using cortical thickness from MRI. Neuroimage. 2011;56:235–45. doi: 10.1016/j.neuroimage.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Spreng RN, Turner GR. Structural covariance of the default network in healthy and pathological aging. J Neurosci. 2013;33:15226–34. doi: 10.1523/JNEUROSCI.2261-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 17.Legrand D, Ruby P. What is self-specific? Theoretical investigation and critical review of neuroimaging results. Psychol Rev. 2009;116:252–82. doi: 10.1037/a0014172. [DOI] [PubMed] [Google Scholar]
- 18.Horovitz SG, Braun AR, Carr WS, et al. Decoupling of the brain's default mode network during deep sleep. Proc Natl Acad Sci U S A. 2009;106:11376–81. doi: 10.1073/pnas.0901435106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samann PG, Wehrle R, Hoehn D, et al. Development of the brain's default mode network from wakefulness to slow wave sleep. Cereb Cortex. 2011;21:2082–93. doi: 10.1093/cercor/bhq295. [DOI] [PubMed] [Google Scholar]
- 20.Hutton C, Draganski B, Ashburner J, Weiskopf N. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage. 2009;48:371–80. doi: 10.1016/j.neuroimage.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baik I, Shin C. Prospective study of alcohol consumption and metabolic syndrome. Am J Clin Nutr. 2008;87:1455–63. doi: 10.1093/ajcn/87.5.1455. [DOI] [PubMed] [Google Scholar]
- 22.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 23.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatr Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 24.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28:499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 26.Baek MJ, Kim HJ, Ryu HJ, et al. The usefulness of the story recall test in patients with mild cognitive impairment and Alzheimer's disease. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2011;18:214–29. doi: 10.1080/13825585.2010.530221. [DOI] [PubMed] [Google Scholar]
- 27.Hunsley J, Lee CM. Introduction to clinical psychology : an evidence-based approach. Second ed. Hoboken, NJ: John Wiley and Sons, Inc.; 2014. [Google Scholar]
- 28.Kang YW ND. Seoul neuropsychogical screening battery. Seoul: Human Brain Research & Consulting; 2003. [Google Scholar]
- 29.Lee JH, Lee KU, Lee DY, et al. Development of the Korean version of the Consortium to Establish a Registry for Alzheimer's Disease Assessment Packet (CERAD-K): clinical and neuropsychological assessment batteries. J Gerontol B Psychol Sci Soc Sci. 2002;57:P47–53. doi: 10.1093/geronb/57.1.p47. [DOI] [PubMed] [Google Scholar]
- 30.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 31.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- 32.Fahim C, Yoon U, Das S, et al. Somatosensory-motor bodily representation cortical thinning in Tourette: effects of tic severity, age and gender. Cortex. 2010;46:750–60. doi: 10.1016/j.cortex.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Kim JS, Singh V, Lee JK, et al. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage. 2005;27:210–21. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 34.Robbins S, Evans AC, Collins DL, Whitesides S. Tuning and comparing spatial normalization methods. Med Image Anal. 2004;8:311–23. doi: 10.1016/j.media.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 35.Chung MK, Worsley KJ, Nacewicz BM, Dalton KM, Davidson RJ. General multivariate linear modeling of surface shapes using SurfStat. Neuroimage. 2010;53:491–505. doi: 10.1016/j.neuroimage.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernhardt BC, Klimecki OM, Leiberg S, Singer T. Structural covariance networks of the dorsal anterior insula predict females' individual differences in empathic responding. Cereb Cortex. 2014;24:2189–98. doi: 10.1093/cercor/bht072. [DOI] [PubMed] [Google Scholar]
- 37.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc. 1995;57:289–300. [Google Scholar]
- 38.Honey CJ, Sporns O, Cammoun L, et al. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106:2035–40. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joo EY, Noh HJ, Kim JS, et al. Brain gray matter deficits in patients with chronic primary insomnia. Sleep. 2013;36:999–1007. doi: 10.5665/sleep.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–8. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 41.Drummond SP, Walker M, Almklov E, Campos M, Anderson DE, Straus LD. Neural correlates of working memory performance in primary insomnia. Sleep. 2013;36:1307–16. doi: 10.5665/sleep.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Werf YD, Altena E, van Dijk KD, et al. Is disturbed intracortical excitability a stable trait of chronic insomnia? A study using transcranial magnetic stimulation before and after multimodal sleep therapy. Biol Psychiatry. 2010;68:950–5. doi: 10.1016/j.biopsych.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 43.Huang Z, Zhan S, Li N, Ding Y, Wang Y. Abnormal recovery function of somatosensory evoked potentials in patients with primary insomnia. Psychiatry Res. 2012;198:463–7. doi: 10.1016/j.psychres.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 44.Morgan PT, Pace-Schott EF, Mason GF, et al. Cortical GABA levels in primary insomnia. Sleep. 2012;35:807–14. doi: 10.5665/sleep.1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plante DT, Jensen JE, Winkelman JW. The role of GABA in primary insomnia. Sleep. 2012;35:741–2. doi: 10.5665/sleep.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thambisetty M, Wan J, Carass A, An Y, Prince JL, Resnick SM. Longitudinal changes in cortical thickness associated with normal aging. NeuroImage. 2010;52:1215–23. doi: 10.1016/j.neuroimage.2010.04.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao Z, Hu B, Liang C, Zhao L, Jackson M. A longitudinal study of atrophy in amnestic mild cognitive impairment and normal aging revealed by cortical thickness. PloS One. 2012;7:e48973. doi: 10.1371/journal.pone.0048973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winkelman JW, Plante DT, Schoerning L, et al. Increased rostral anterior cingulate cortex volume in chronic primary insomnia. Sleep. 2013;36:991–8. doi: 10.5665/sleep.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bosch OG, Rihm JS, Scheidegger M, et al. Sleep deprivation increases dorsal nexus connectivity to the dorsolateral prefrontal cortex in humans. Proc Natl Acad Sci U S A. 2013;110:19597–602. doi: 10.1073/pnas.1317010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Havas JA, Parimal S, Soon CS, Chee MW. Sleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performance. NeuroImage. 2012;59:1745–51. doi: 10.1016/j.neuroimage.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 51.Samann PG, Tully C, Spoormaker VI, et al. Increased sleep pressure reduces resting state functional connectivity. Magma. 2010;23:375–89. doi: 10.1007/s10334-010-0213-z. [DOI] [PubMed] [Google Scholar]
- 52.Vignola A, Lamoureux C, Bastien CH, Morin CM. Effects of chronic insomnia and use of benzodiazepines on daytime performance in older adults. J Gerontol B Psychol Sci Soc Sci. 2000;55:P54–62. doi: 10.1093/geronb/55.1.p54. [DOI] [PubMed] [Google Scholar]
- 53.Bonnet MH, Arand DL. 24-Hour metabolic rate in insomniacs and matched normal sleepers. Sleep. 1995;18:581–8. doi: 10.1093/sleep/18.7.581. [DOI] [PubMed] [Google Scholar]
- 54.Varkevisser M, Kerkhof GA. Chronic insomnia and performance in a 24-h constant routine study. J Sleep Res. 2005;14:49–59. doi: 10.1111/j.1365-2869.2004.00414.x. [DOI] [PubMed] [Google Scholar]
- 55.Harrison Y, Horne JA. The impact of sleep deprivation on decision making: a review. J Exp Psychol Appl. 2000;6:236–49. doi: 10.1037//1076-898x.6.3.236. [DOI] [PubMed] [Google Scholar]
- 56.Monsell S. Task switching. Trends Cogn Sci. 2003;7:134–40. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- 57.Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- 58.Chee MW, Choo WC. Functional imaging of working memory after 24 hr of total sleep deprivation. J Neurosci. 2004;24:4560–7. doi: 10.1523/JNEUROSCI.0007-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bray S, Almas R, Arnold AE, Iaria G, Macqueen G. Intraparietal sulcus activity and functional connectivity supporting spatial working memory manipulation. Cereb Cortex. 2015;25:1252–64. doi: 10.1093/cercor/bht320. [DOI] [PubMed] [Google Scholar]
- 60.Gazzaley A, Rissman J, D'Esposito M. Functional connectivity during working memory maintenance. Cogn Affect Behav Neurosci. 2004;4:580–99. doi: 10.3758/cabn.4.4.580. [DOI] [PubMed] [Google Scholar]
- 61.Shokri-Kojori E, Motes MA, Rypma B, Krawczyk DC. The network architecture of cortical processing in visuo-spatial reasoning. Sci Rep. 2012;2:411. doi: 10.1038/srep00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Honey CJ, Thivierge JP, Sporns O. Can structure predict function in the human brain? Neuroimage. 2010;52:766–76. doi: 10.1016/j.neuroimage.2010.01.071. [DOI] [PubMed] [Google Scholar]
- 63.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Damoiseaux JS, Greicius MD. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct Func. 2009;213:525–33. doi: 10.1007/s00429-009-0208-6. [DOI] [PubMed] [Google Scholar]
- 65.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.He Y, Chen Z, Gong G, Evans A. Neuronal networks in Alzheimer's disease. Neuroscientist. 2009;15:333–50. doi: 10.1177/1073858409334423. [DOI] [PubMed] [Google Scholar]
- 67.He Y, Dagher A, Chen Z, et al. Impaired small-world efficiency in structural cortical networks in multiple sclerosis associated with white matter lesion load. Brain. 2009;132:3366–79. doi: 10.1093/brain/awp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gong G, He Y, Chen ZJ, Evans AC. Convergence and divergence of thickness correlations with diffusion connections across the human cerebral cortex. Neuroimage. 2012;59:1239–48. doi: 10.1016/j.neuroimage.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 69.Reid AT, Evans AC. Structural networks in Alzheimer's disease. Eur Neuropsychopharmacol. 2013;23:63–77. doi: 10.1016/j.euroneuro.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 70.Montembeault M, Joubert S, Doyon J, et al. The impact of aging on gray matter structural covariance networks. Neuroimage. 2012;63:754–9. doi: 10.1016/j.neuroimage.2012.06.052. [DOI] [PubMed] [Google Scholar]
- 71.Frodl T, Jager M, Smajstrlova I, et al. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. Journal Psychiatry Neurosci. 2008;33:423–30. [PMC free article] [PubMed] [Google Scholar]
- 72.Kong L, Wu F, Tang Y, et al. Frontal-subcortical volumetric deficits in single episode, medication-naive depressed patients and the effects of 8 weeks fluoxetine treatment: a VBM-DARTEL study. PloS One. 2014;9:e79055. doi: 10.1371/journal.pone.0079055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vythilingam M, Vermetten E, Anderson GM, et al. Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biol Psychiatry. 2004;56:101–12. doi: 10.1016/j.biopsych.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 74.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 75.Vgontzas AN, Fernandez-Mendoza J, Bixler EO, et al. Persistent insomnia: the role of objective short sleep duration and mental health. Sleep. 2012;35:61–8. doi: 10.5665/sleep.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.