Abstract
Study Objectives:
We used quantitative genetic models to assess whether area-level deprivation as indicated by the Singh Index predicts shorter sleep duration and modifies its underlying genetic and environmental contributions.
Methods:
Participants were 4,218 adult twin pairs (2,377 monozygotic and 1,841 dizygotic) from the University of Washington Twin Registry. Participants self-reported habitual sleep duration. The Singh Index was determined by linking geocoding addresses to 17 indicators at the census-tract level using data from Census of Washington State and Census Tract Cartographic Boundary Files from 2000 and 2010. Data were analyzed using univariate and bivariate genetic decomposition and quantitative genetic interaction models that assessed A (additive genetics), C (common environment), and E (unique environment) main effects of the Singh Index on sleep duration and allowed the magnitude of residual ACE variance components in sleep duration to vary with the Index.
Results:
The sample had a mean age of 38.2 y (standard deviation [SD] = 18), and was predominantly female (62%) and Caucasian (91%). Mean sleep duration was 7.38 h (SD = 1.20) and the mean Singh Index score was 0.00 (SD = 0.89). The heritability of sleep duration was 39% and the Singh Index was 12%. The uncontrolled phenotypic regression of sleep duration on the Singh Index showed a significant negative relationship between area-level deprivation and sleep length (b = −0.080, P < 0.001). Every 1 SD in Singh Index was associated with a ∼4.5 min change in sleep duration. For the quasi-causal bivariate model, there was a significant main effect of E (b0E = −0.063; standard error [SE] = 0.30; P < 0.05). Residual variance components unique to sleep duration were significant for both A (b0Au = 0.734; SE = 0.020; P < 0.001) and E (b0Eu = 0.934; SE = 0.013; P < 0.001).
Conclusions:
Area-level deprivation has a quasi-causal association with sleep duration, with greater deprivation being related to shorter sleep. As area-level deprivation increases, unique genetic and nonshared environmental residual variance in sleep duration increases.
Citation:
Watson NF, Horn E, Duncan GE, Buchwald D, Vitiello MV, Turkheimer E. Sleep duration and area-level deprivation in twins. SLEEP 2016;39(1):67– 77.
Keywords: area-level deprivation, dizygotic, monozygotic, sleep duration, twins
Significance.
More deprived areas were associated with reduced sleep duration, even after accounting for genetic and shared environmental confounding. This suggests that area-level deprivation may decrease habitual sleep duration. Future studies are needed to define the specific elements endemic to poor living environments that contribute most to sleep curtailment.
INTRODUCTION
Sleep has been found in nearly every animal in which it has been sought,1 is necessary for life,2 and plays a crucial role in memory consolidation,3 hormonal regulation,4 and clearance of byproducts of neurotransmission from the brain.5 Adequate, regular sleep is critical for optimal functioning of human metabolism,6–12 immunity,13–15 cardiovascular systems,16–21 cognition,22,23 psychology,24 and functional ability.25–29 Short sleep is associated with increased mortality risk.30 Despite its clear importance, substantial intraindividual and interindividual variation exists in nightly sleep duration.
Nearly a third of individual differences in sleep duration are associated with genetic variations,6 but during an adult's work and caregiving years environmental factors play an ever increasing role in determining sleep length.31 The environmental effect on sleep duration is a complex construct influenced by numerous individual, social, and societal level decisions and policies. On a daily basis people decide bed and wake times and nap schedules that influence sleep duration and timing. Consumption of sleep affecting substances such as caffeine, alcohol, prescription or nonprescription medications, and illegal drugs also plays a role. The use of disrupting technology (e.g., televisions, tablet computers, smart phones) in bedrooms is yet another individual choice with sleep implications. Social factors include economic status, employment situation, neighborhood location, and family size, including an individual's roles and responsibilities. Societal factors include shift work regulations, timing of television programming, shopping mall and grocery store hours, public and private transportation schedules, and access to sleep affecting substances. All three environmental determinants of sleep length—individual, social, and societal—reflect and influence the cultural perception of the importance of sleep in our society.
Recent research suggests social factors strongly influence sleep. Socioeconomic disadvantage and related infrastructure dilapidation, crime, pollution, and noise all have negative effects on physical health.32–34 Living in these areas triggers release of stress hormones such as cortisol and epinephrine that promote mental and physiological arousal to the detriment of sleep. In the United States, one in five individuals adjust their sleeping habits to avoid environmental noise, which can result in psychosocial distress.35 Fear of neighborhood crime is associated with difficulty falling and staying asleep.36 Disadvantaged neighborhoods are associated with low sleep quality and increase the odds of inadequate sleep duration (defined as ≤ 6 h per night) by 43% in their residents.32,37 Beyond these direct relationships, sleep quality has been shown to partially mediate the relationship between disadvantaged neighborhoods and subjective physical health suggesting that poor sleep may be a pathway to poor health in troubled neighborhoods.38
We sought to further examine social level determinants of sleep duration by focusing on an objective measure of area-level deprivation (Singh Index) using a genetically informed twin design. Twins are identical in age, and if reared together are naturally well matched for shared family background and numerous childhood and adolescent exposures. As such, twin comparisons can be used to account for uncontrolled third-variable confounders that typically differ among unrelated individuals. This approach is particularly helpful when investigating the relationship between area-level deprivation and sleep duration because many aspects of these phenotypes are genetically influenced, and random assignment to area-level conditions is obviously not possible.
Using twin research methods, we wished to learn if the unique environmental influences of area-level deprivation predict sleep duration after accounting for familial factors (e.g., genetics and shared environment)—a “quasi-causal” association.39 We also sought to examine area-level deprivation as an environmental moderator of the heritability of sleep duration—a gene × environment interaction effect. Therefore, the goals of this study were to: (1) determine the magnitude of genetic and environmental influences on area-level deprivation and sleep duration; (2) determine if a quasi-causal relationship exists between area-level deprivation and sleep duration; and (3) determine if area-level deprivation moderates genetic influences on sleep duration.
METHODS
Participants
The University of Washington Twin Registry is a community-based sample of adult twins reared together; construction methods are described in detail elsewhere.40,41 On enrollment, all twins completed a survey with items on sociodemographics, general physical and mental health, and lifestyle behaviors. Standard questions about childhood similarity that determine zygosity with greater than 90% accuracy when compared with DNA-based methods were used to classify twins as identical (monozygotic; MZ) or fraternal (dizygotic; DZ).42–44 We used data from surveys completed from 2008–2013; residential street addresses (used to obtain census tract data) were not available prior to 2008. Sleep duration and residence data were collected concurrently, and questionnaires were completed by twins independently. Written informed consent was provided as approved by the university's institutional review board. The final sample included 2,377 MZ and 1,841 DZ pairs.
Measures
Singh Index
Socioeconomic status (SES) was measured using a census-based area-level deprivation index. The Singh Index45 is a composite measure of SES based on 17 different area-level indicators at the census tract level drawn from the 2000 Census: (1) percentage of population aged ≥ 25 y with < 9 y of education; (2) percentage of population aged ≥ 25 y with at least a high school diploma; (3) percentage of employed persons aged ≥ 16 y in white-collar occupations; (4) median family income; (5) income disparity (the Log of 100 times the ratio of number of households with < $10,000 income to number of households with ≥ $50,000 income); (6) median home value; (7) median gross rent; (8) median monthly mortgage; (9) percentage of owner-occupied housing units (home ownership rate); (10) percentage of civilian labor force population aged > 16 y unemployed (unemployment rate); (11) percentage of families below poverty level; (12) percentage of population below 150% of the poverty threshold; (13) percentage of single-parent households with children aged < 18 y; (14) percentage of households without a motor vehicle; (15) percentage of households without a telephone; (16) percentage of occupied housing units without complete plumbing; and (17) percentage of households with more than one person per room (crowding). The Index is a normally distributed latent variable derived through factor analysis and is interpreted as an overall index of area-level deprivation, with higher scores indicating greater deprivation (i.e., lower SES). It is important to note that the Singh Index is based on census tracts, which are much larger administrative units than neighborhoods. Therefore, we use the terms “area-level” and “area” rather than “neighborhood” throughout this manuscript to reference circumscribed geographical regions. The Singh Index at the census tract level was calculated by standardizing the aforementioned 17 indicators by their corresponding means and standard deviations for Washington State, and then multiplying these standardized values by the 17 coefficients. Thus, the Singh Index values for our twin sample shown in Table 1 are standardized to facilitate comparison between different areas within Washington State. Table 2 presents descriptive statistics on the 17 census tract variables that comprise the Singh Index; again, these values have been standardized to facilitate comparison between different areas within Washington State. Rates of change per one unit change of the Singh Index are based on these Washington State standardized values.
Table 1.
Descriptive statistics, twin correlations, and standardized ACE components for sleep duration and area-level-level socioeconomic deprivation.
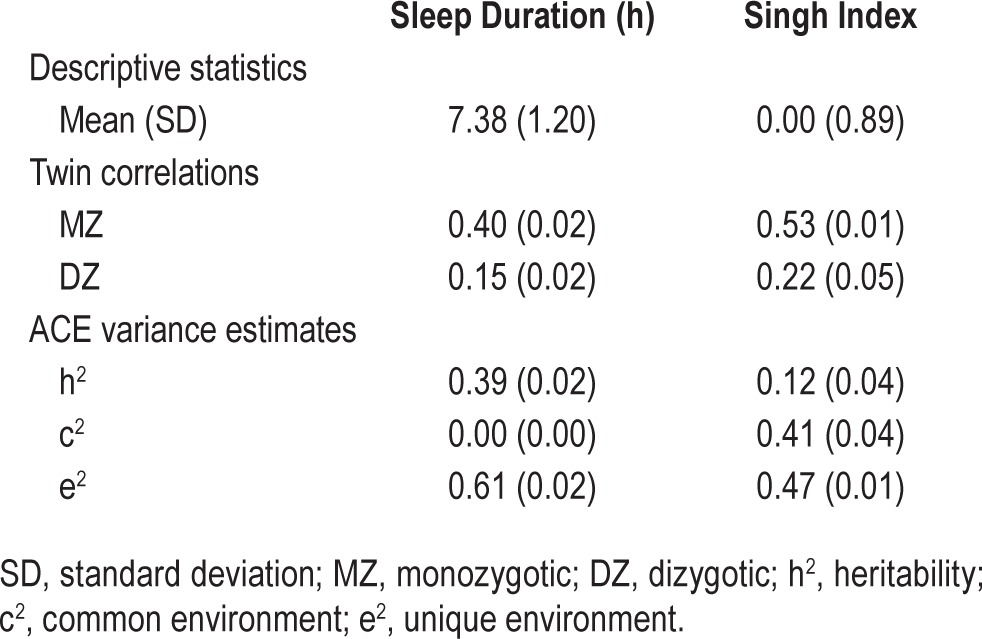
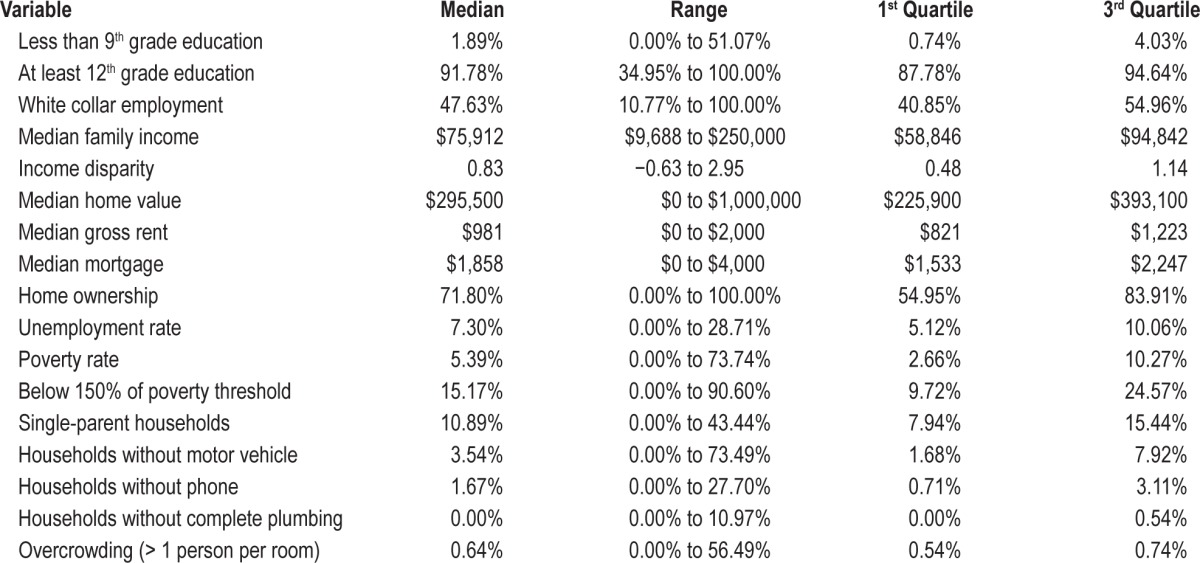
Table 2.
Distribution of the 17 census tract variables comprising the Singh Index.
To apply the Index to the University of Washington Twin Registry, the participants' home addresses were geocoded with a minimum match score of 100%. Addresses that failed the automatic geocoding process were matched manually by individually reviewing each address and correcting data entry errors. The Singh Index was replicated with the Census of Washington State and the Census Tract Cartographic Boundary Files from 2000 and 2010 by using ArcGIS 9.3.1, MS Access, and R software. Because the values shown in Table 1 are state based, in order to facilitate interpretation of the results to a broader representation of census tracts across the United States, our twin data were restandardized based on Singh's original publication45 for comparison to the US population, as shown in Table 3.46 Note that the values in Table 3 are shown merely to facilitate comparison of area-level deprivation of our twin sample to the broader US, whereas interpretation of associations between our sleep outcomes and area-level deprivation are based solely on the values specifically standardized for Washington State (i.e., the distribution shown in Table 1).
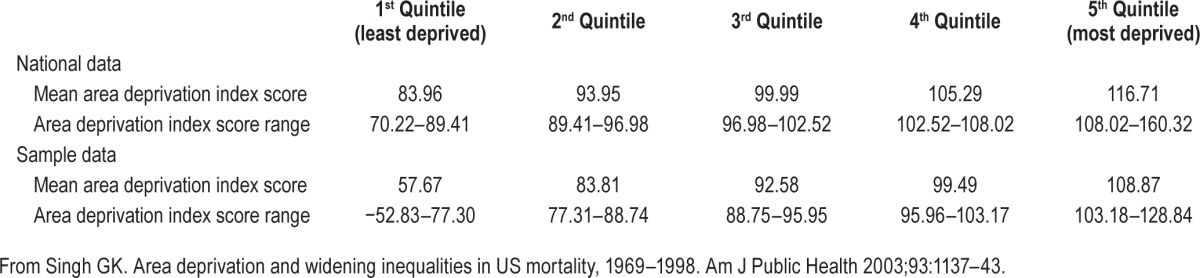
Table 3.
Comparison of twin sample area deprivation scores with nationally representative data.
Sleep Duration
Twins were asked, “On average, how long do you sleep per night?” Participants entered values for hours and minutes, and a total sleep duration variable was created, with hours being the unit of measure (Table 1).
Statistical Analyses
All analyses were conducted using latent variable path analysis using the computer program Mplus (Muthén and Muthén 2012 v. 7.0, Los Angeles, CA) and maximum likelihood estimation. Analyses controlled for linear effects of age, sex, ethnicity, household income, and educational attainment. We used likelihood ratio tests to compare nested models.
Univariate Biometric Decomposition
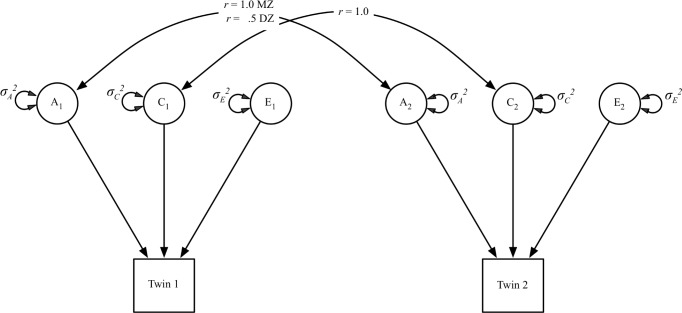
Although it was not the primary goal of our analysis, we began by using the classic twin model to decompose the variance of sleep duration and the Singh Index into three components: additive genetic (A) variance, shared environmental (C) variance, and nonshared environmental (E) variance. The classic twin model is illustrated in Figure 1. The (A) variance components, which represent the additive effect of an individual's genes, correlate r = 1.0 between MZ twins (who share 100% of their genetic sequence) and r = 0.5 between DZ twins (who share on average 50% of their segregating genes). The (C) variance components correlate at 1.0 regardless of degree of genetic relatedness, because it represents environmental experiences that make members of the same family more alike. The (E) variance components, which represent environmental experiences unique to the individual, do not correlate between twins. It should be noted that (E) variance is confounded with measurement error in the absence of a measurement model.
Figure 1.
Path diagram of the classical twin model. The phenotype is influenced by additive genetic (A), shared environmental (C), and nonshared environmental (E) factors. Genetic variance components correlate at r = 1.0 for monozygotic twins and r = 0.5 for dizygotic twins. Shared environmental variance components correlate at r = 1.0 regardless of pair type.
Causal Pathways Versus Gene-Environment Correlation
Examination of the association between the Singh Index and sleep duration within pairs of MZ and DZ twins raised in the same family provides the closest approximation of the causal effect of area-level deprivation on sleep duration short of random area assignment. Assessing this relationship within twin pairs allows us to control for the effects of many measured and unmeasured confounds that vary between families, such as underlying genetic or environmental backgrounds that area-level deprivation and sleep duration may share.
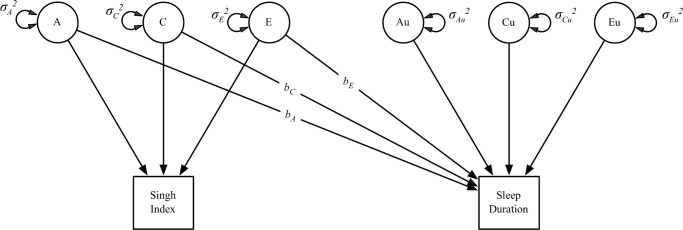
The bivariate twin model is essentially a regression model in which the outcome (sleep duration) is regressed on the (A), (C), and (E) terms of the predictor (Figure 2). The outcome also comprises residual variation not explained by the predictor that can also be partitioned into (A), (C), and (E) components. The logic of this design is as follows: if area-level deprivation has a causal relationship with sleep duration, then the association should be observable both between twin pairs (pairs who live in deprived areas on average sleep less) and within pairs (the member of a pair in the more deprived area should sleep less than their co-twin in the less deprived area). The within-pair association, which is the most valid measure of the causal effect, is represented by the bE path in Figure 2.39
Figure 2.
Path diagram of a bivariate twin model (only one twin shown for clarity). The main effect of the Singh Index on sleep duration is divided into a genetic regression (bA), a shared environmental regression (bC), and a nonshared environmental regression (bE). The regression of sleep duration on the (A) and (C) components of the Singh Index represents the between-twin pair or population-level effect; the nonshared environmental regression represents the within-twin pair or causal effect of Singh Index on sleep duration.
If, however, we observe that sleep duration varies as a function of the Singh Index between twins pairs but not within twin pairs, we can conclude that the observed effect of area-level deprivation is induced by noncausal processes involving shared genetic background (bA path in Figure 2) for area-level stress and sleep (referred to as gene-environment correlation [rGE]). Shared environmental factors (e.g., area-level deprivation during childhood) may also be inducing this correlation. We chose to use rGE in our example, however, because sleep duration shows little to no influence from the shared environment.
The twin design, of course, cannot control for all possible confounds of a causal relationship, but only for those which are shared by pairs of MZ twins who were raised together. The DZ twins allow us to infer whether any confounds that do exist are attributable to genetic or family environmental variance. The twin design therefore allows us to establish a quasi-causal effect of area-level deprivation on sleep duration.39
Gene × Environment Interaction
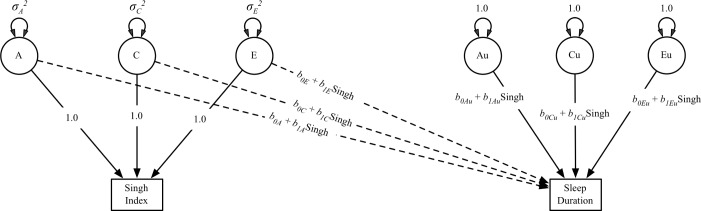
The twin model also allows us to test for moderation of the variance components of sleep duration by area-level deprivation, a form of gene × environment interaction. The aforementioned bivariate model can be extended to allow moderation of the ACE components.47 The three regression parameters relating the Singh Index to sleep duration (bA, bC, and bE) and the three residual variances of sleep duration all can be modified by the Singh Index, as illustrated in Figure 3. For each of the modified paths, the Singh Index is the moderating variable; the b0 terms are the values of the ACE variances where the Singh Index = 0; and the b1 terms represent the rate of increase or decrease in a given variance component as a function of the Singh Index.
Figure 3.
Path diagram of the fully saturated model fit to the data (Model 3; only one twin shown for clarity). Successive models were fit by fixing parameters to zero and conducting likelihood ratio tests whether adding parameters resulted in a significant improvement in model fit. The (A), (C), and (E) latent variables (represented with circles) are the additive genetic, shared environmental, and non-shared environmental variance components of the Singh Index. The Au and Eu latent variables represent residual additive genetic and nonshared environmental variance in sleep duration. In this model, the main effect of the Singh Index on sleep duration (captured in the dotted single-headed paths from the [A] and [E] components of the Singh Index to sleep duration) is permitted to vary with level of the Singh Index. Similarly, the variance in sleep duration that remains after controlling for the main effect of the Singh Index (double-headed paths from Au, Cu, and Eu to sleep duration) also varies as a function of Singh Index.
For moderating variables such as the Singh Index that can differ between twins from the same family, rGE that is nonstatic with respect to the moderator must be accounted for when testing for gene × environment effects to reduce the inflated false-positive rate that results from failure to do so.48 To account for rGE that depends on the level of the moderator, the regression of sleep duration on the ACE components of the Singh Index are also allowed to vary as a function of the Singh Index (i.e., the effect that the Singh Index has on sleep duration can depend on level of the Singh Index). We present a path diagram of the fully saturated model fit to the data in Figure 3.
RESULTS
Descriptive Statistics and Univariate Biometric Analyses
The study sample included 4,218 twin pairs (2,377 MZ and 1,841 DZ) who provided complete data on both outcomes and exposures. The sample was predominantly female (62%), and Caucasian (91%), with a mean age of 38.2 y (standard deviation [SD] = 18). In general, the twins were well educated and economically secure, with 34% having a bachelor's degree or higher and 35% having an annual income in excess of $80,000/y. The mean sleep duration was 7.38 h (SD = 1.20) per night and the Singh Index mean was 0.00 (SD = 0.89).
We computed descriptive statistics for sleep duration and the Singh Index and conducted univariate biometric analyses of their variation (Table 1). Sleep duration was moderately heritable (39%), showed no evidence of shared environmental variance, and had large nonshared environmental variance (61%). The Singh Index showed modest variance attributable to additive genetic factors (12%), moderate shared environmental variance (41%), and moderate nonshared environmental variability (47%), indicating nearly half of all variance in Singh Index is not shared between twins. Men and women differed slightly but significantly in the Singh Index, with men living in less deprived area levels on average (Cohen d = −0.09, 95% confidence interval [CI] = −0.13 to −0.05). Sex also influenced sleep duration, with women getting slightly less sleep on average than men (Cohen d = −0.05, 95% CI = −0.09 to −0.01). DZ twins lived in slightly more deprived areas on average (Cohen d = −0.06, 95% CI = −0.11 to −0.02). It should be noted that d = 0.20 is a widely accepted threshold for a “small” effect size; the Cohen d effect sizes we report are considered negligible and represent > 96% overlap between distributions.
Causal Pathways Versus rGE
The uncontrolled, unstandardized phenotypic (observed) regression of sleep duration on the Singh Index showed a significant negative relationship between area-level deprivation and sleep length (b = −0.080, P < 0.001), meaning that, on average, each unit increase in the Singh Index is associated with a 5-min decrease in total daily sleep duration. Alternatively, because the standard deviation of the Singh Index is 0.89, the amount of change in sleep duration associated with a 1 SD change in the Singh Index is 89% of 5 min, or ∼4.5 min. The correlation between the Singh Index and sleep duration is strongest for shorter sleep durations with r = −0.11 (95% CI: −0.14 to −0.07) for < 7 h sleep versus r = −0.05 (95% CI: −0.07 to −0.03) for the whole group. Thus we are likely presenting a conservative estimate of this relationship.
Distribution of the 17 census tract variables comprising the Singh Index is presented in Table 2. Variability in range and difference between the first and third quartiles was observed for all variables with the exception of plumbing and overcrowding where differences in range exceeded interquartile differences. For the Singh Index as a whole, the range is −2.34 to 4.91 and the first to third interquartile range is −0.58 to 0.49. Table 3 presents Singh Index scores for our twin sample in comparison to standardized, nationally representative Singh Index scores.45 Our twin sample was less deprived overall, but had a wide range of scores up to and including households in the most deprived fifth quintile. Indeed, mean values in the fourth and fifth quintile were similar between our twin sample (99.49 and 108.87, respectively) and the national sample (105.29 and 116.71, respectively). Our twin sample showed a greater overall range than the nationally representative Singh Index scores.
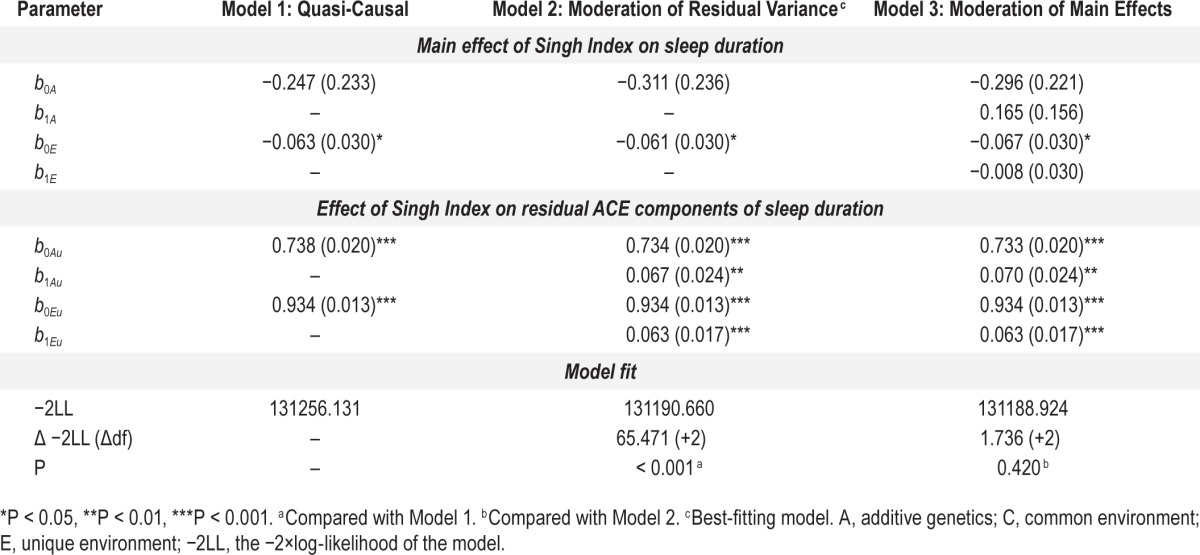
As noted previously, both sleep duration and Singh Index showed influences from genetic factors (39% and 12%, respectively), leaving open the possibility that the phenotypic association between area-level socioeconomic deprivation and sleep duration is present because of a genetically induced correlation between the two phenotypes (rGE), rather than to differential exposure to area-level socioeconomic factors. To test whether this result was consistent with a causal hypothesis as opposed to rGE, we fit the bivariate quasi-causal model in Figure 2, which yields an estimate of the phenotypic effect that is unbiased by possible genetically induced correlation existing between the Singh Index and sleep duration. The results are given in the first column of Table 4, labeled Model 1: Quasi-causal. The quasi-causal pathway was smaller than it was in the uncontrolled model, but remained statistically significant (b0E = −0.063, P < 0.05). The estimate of the common genetic background to area-level deprivation and sleep duration was not statistically significant (b0A = −0.247, P > 0.05). These results suggest that the relation between the Singh Index and sleep duration cannot be attributable to underlying genetic factors common to both phenotypes, and is instead a result of differential exposure to area-level socioeconomic factors. The residual variance in sleep duration was a combination of genetic (b0Au = 0.738, P < 0.001; equivalent to 44% of the total variance) and nonshared environmental (b0Eu = 0.934, P < 0.001; equivalent to 56% of the total variance) variance.
Table 4.
Parameter estimates and model fit statistics for quasi-causal and gene × environment models.
This main effect is illustrated in the bar plots in Figure 4. The outermost bars represent the mean sleep duration for MZ twins concordant for lower area-level SES (lower 50%; far left bar) and higher area-level SES (upper 50%; far right bar); their comparison can be considered the phenotypic (nongenetically controlled) effect of the Singh Index on sleep duration. The within-twin pair effect (genetically controlled) is illustrated in the comparison of MZ and DZ twins discordant for area-level SES (middle four bars). As the model predicted, the difference between higher (upper 50%) and lower (bottom 50%) area-level socioeconomic deprivation is attenuated (but not absent) within discordant MZ twins, evidence for genetic confounding in this association. Differences between DZ twins discordant for higher versus lower area-level SES are larger than those for MZ twins, again consistent with genetic confounding—the within-twin pair effect should be larger in DZ twins because they share fewer genes than MZ twins.
Figure 4.
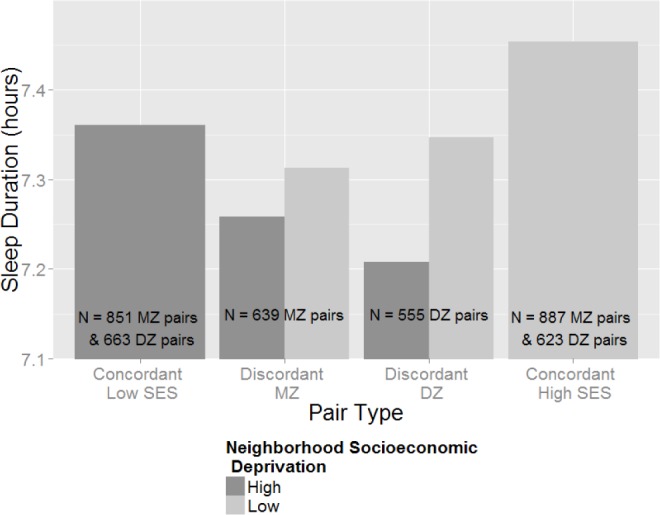
Sleep duration as a function of the Singh Index in various pair types. This bar plot demonstrates the phenotypic (nongenetically controlled) and within-twin pair (genetically controlled) effect of area-level deprivation on sleep duration in twins. The phenotypic effect is evident comparing the concordant low-SES mean to the concordant high-SES mean (two outermost bars) whereas the attenuation observed within-twin pairs is evident comparing MZ twins discordant for higher vs. lower area-level SES (middle-left two bars). The effect of the Singh Index on sleep duration is less attenuated in discordant DZ twins (middle-right two bars) who share on average only 50% of their genes. DZ, dizygotic; MZ, monozygotic; SES, socioeconomic status.
Gene × Environment Interaction
We next fit a model that allowed for heteroscedasticity of the residual (A) and (E) variance components of sleep duration (paths from the latent variables Au and Eu to sleep duration in Figure 3), which resulted in a significant improvement in model fit (P < 0.001; Model 2 in Table 4), suggesting that residual variance in sleep duration after controlling for the main effects of Singh Index is not constant across all levels of the Singh Index. Allowing the main effects of the Singh Index on sleep duration (the dotted paths from the [A] and [E] components of the Singh Index to sleep duration in Figure 3) to be modified by different levels of the Singh Index, which yields unbiased estimates of moderation of residual (A) and (E) variance in sleep duration, did not improve model fit (P = 0.420; Model 3 in Table 4). Therefore, Model 2 was considered the best-fitting, most parsimonious model.
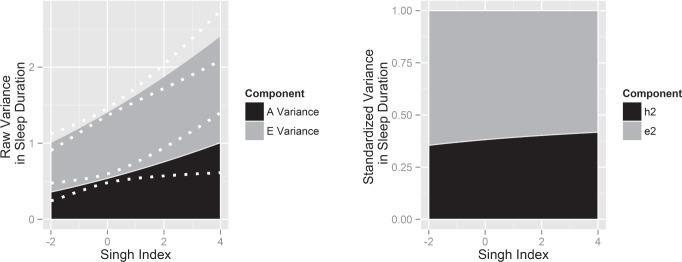
The parameter estimates in Model 2 suggest that, because both (A) and (E) variances increase as a function of the Singh Index, that the primary effect of the Singh Index was on the total variance of sleep duration. At mean levels of area-level deprivation, residual genetic variance equaled 0.734 (P < 0.001), and increased by approximately 0.067 SDs for each additional unit of the Singh Index (P < 0.001). Residual nonshared environmental variance equaled 0.934 (P < 0.001) at mean area-level deprivation and increased at a rate of 0.063 SDs per unit of the Singh Index (P < 0.001). These results, in both raw and standardized form, are illustrated in Figure 5. Evident from this figure is that residual genetic variance in sleep duration increases as a function of area-level deprivation, as does the heritability of sleep duration.
Figure 5.
Raw residual variance (left) and standardized residual variance (right) in sleep duration as a function of area-level deprivation. The stacked variance plot on the left illustrates how the (A), (E), and total residual variance in sleep duration increases with units of the Singh Index. The dotted white lines represent the 95% confidence intervals around the change in residual variance. The stacked variance plot on the right shows the proportion of total residual variance in sleep duration attributable to genetic factors (h2) and nonshared environmental factors (e2) vary as a function of the Singh Index.
As previously noted, the structural equation models predicted that the total phenotypic variance in sleep duration was greater in more socioeconomically deprived areas. This heteroscedasticity is illustrated in Figure 6, which shows box plots overlaid with violin plots (which show the probability density of the data) of sleep duration by quintile of the Singh Index. Evident in this figure is that the distribution of sleep duration becomes more platykurtic at higher levels of area-level deprivation. Also evident is the phenotypic main effect that area-level deprivation has on sleep duration; the proportion of the individuals falling in the normal sleep range (7–9 h, demarcated by the green dotted lines in Figure 6)6,24,49,50 decreases with increasing quintile of the Singh Index, such that at the greatest levels of area-level deprivation, more than 30% of the sample gets less than the minimum recommended amount of sleep. The increased variance as a function of Singh Index also appears to be driven by the lower end of the sleep duration spectrum. The proportion of the sample getting short sleep (< 5 h; demarcated by the red dotted line in Figure 6) steadily increases across levels of the Singh Index, doubling from the first quintile to the fifth. However, no such systematic change is evident at the higher end of the sleep duration spectrum (> 9 h [The last quintile of Singh Index shows a higher percentage of individuals falling above 9 hours of sleep. It should be noted, however, that outliers are unstable and there does not appear to be a trend in the 9-hour percentages.]).
Figure 6.
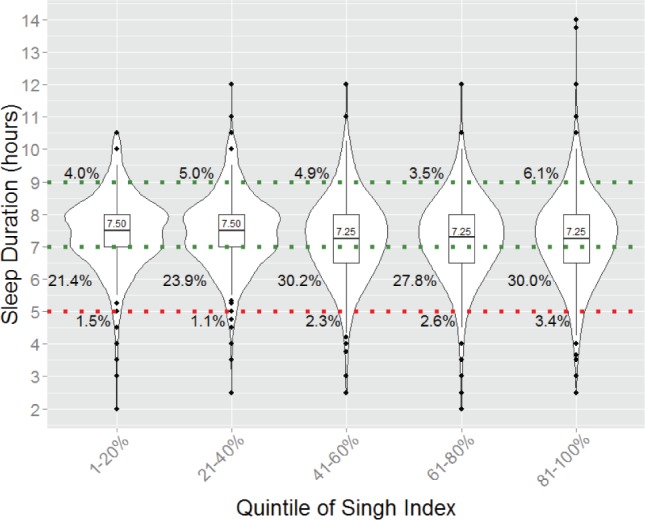
Box plots overlaid with violin plots, sleep duration as a function of quintile of Singh Index, with median sleep duration of each quintile labeled. Normal sleep (7–9 h) falls between the two dotted green lines. The dotted red line denotes short sleep (5 h). The sample was divided according to quintiles of the Singh Index. The boxplots grow wider as a function of increasing Singh Index, demonstrating that the 25th and 75th percentiles cover a wider range of sleep duration at greater levels of area-level deprivation. Similarly, the violin plots (which provide information about the probability density of the subsamples) grow flatter and wider with increasing area-level deprivation. The percentage next to each violin plot is the proportion of the subsample sleeping for greater than 9 h each night (topmost percentage), fewer than 7 h (middle row of percentages), and fewer than 5 h (bottommost percentages). Evident in this figure is that the percentage of individuals receiving less sleep increases as level of Singh Index increases, but no systematic differences are observed for longer sleep durations.
DISCUSSION
This study demonstrates a quasi-causal effect of area-level deprivation on sleep duration. Overall, as area-level deprivation increases sleep duration decreases, a finding that remains significant following complete adjustment for familial factors (e.g., shared environment and genetics) with the twin study design. A dose-response effect was present, with twins living in the most deprived areas exhibiting the greatest propensity for short sleep.
Socioeconomic status is inversely associated with many health outcomes, yielding a socioeconomic health gradient in many domains. Sleep is no exception, with recent evidence showing SES has substantive and far-reaching implications for sleep quality and duration. Individuals of lower SES report shorter sleep duration, poorer sleep quality, longer sleep latency, more daytime sleepiness, and weekend oversleep.51 Research using data from the National Health and Nutrition Examination Survey showed that lower SES—as defined by reduced income and education, use of public insurance, and low food security—was associated with subjective endorsement of habitual very short sleep duration (< 5 h/night).52 This relationship exists whether SES is determined objectively or subjectively and cuts across ages and life stages. In adults, after adjusting for objective measures of SES, low subjective SES was linked with nonoptimal sleep duration, poor sleep quality, daytime sleepiness, and irregular sleep habits.51,53 Pregnant women with low SES, as defined by self-reported household income < $50,000/y, showed reduced sleep quality and fragmented sleep.54 In children and adolescents, objective indices of parental SES such as household income and parental education are linked with shorter and poorer sleep, increased nocturnal awakenings, and daytime sleepiness.55,56 Our findings add to this literature by introducing the notion of causality through use of twin research methods. We show a significant nonshared environmental pathway from the Singh Index to sleep duration. By accounting for genetic and shared environmental factors within twin pairs, we increase the likelihood this finding is driven by the environmental contribution of area-level deprivation. This is consistent with the social-ecological model of sleep,57 where the relationship between sleep and health is influenced by individual-level factors, as well as those at the social-environmental level that exist outside the individual in the neighborhood in which they live.
Which elements endemic to poor living environments contribute most to reduced and poor sleep? Our comprehensive composite measure does not specifically address this question but this is an area worthy of further research. Factors such as ambient noise levels (particularly at night),58,59 threat of crime and violence,60 and resource scarcity and the resultant psychological stress response,61 air pollution,62 and crowded and inadequate sleeping environments63 are all potential contributors. Health and social factors that apply downward pressure on SES, such as chronic pain64 and psychological illness,65 may also interfere with attempts to obtain adequate sleep. Detailed research that collects individual level, real-time data on noise exposure, activity levels, stress hormones, and sleep length and quality are needed to better define the opportunities for intervention to improve sleep health in those living in deprived areas.
In addition to the quasi-causal finding, we revealed a gene × environment interaction effect of area-level deprivation on sleep duration. This effect involved the residual sleep duration variance present after accounting for the main effect of the Singh Index on sleep duration. That is, it modified genetic factors unique to sleep duration and not those shared between sleep duration and the Singh Index. This interaction showed that more deprived areas were associated with increased total phenotypic variance in sleep duration. This included both residual genetic and nonshared environmental components, which both increased with increases in area-level deprivation.
Numerous experimental and epidemiological studies show the relationship between sleep duration and health follows a U-shaped trend,6,24,30,66,67 with untoward associations observed at sleep durations both shorter and, for some studies, longer than the 7–9 h of sleep per night generally considered necessary for human physiological homeostasis. We found that sleep duration variance increased as a function of increasing area-level deprivation. This increased variability can only represent individuals sleeping outside this “normal” range, with potential attendant consequences for health and well-being, particularly for those sleeping shorter durations.
As previously mentioned, residual genetic and nonshared environmental variance in sleep duration increased with increasing Singh Index and the gene × environment interaction did not involve shared genetic factors between the two. Taken together, these findings do not support the notion of deprived areas activating genetic pathways that control sleep duration. A more likely interpretation is that deprived areas facilitate existing genetic and nonshared environmental determinants of sleep duration. This phenomenon occurs in a dose-response manner, but accelerates in the highest quintile of the Singh Index, suggesting efforts to extend sleep durations would be maximally influential in the most deprived areas.
This study has a number of strengths. The Singh Index is robust and validated, incorporating 17 different area-level indicators, whereas other studies in this domain explore many fewer indicators of SES, which risks misspecification bias of the independent variable. Our sample size is large, and the twin method provides powerful opportunities to explore relationships between variables not available with unrelated subjects. This study also has several limitations. Our subjective measure of sleep duration was not validated. Also, the small change in sleep duration for every one unit change in SD of the Singh Index may not be an important clinical difference on an individual basis. Our sample was less deprived than a nationally representative sample. However, our sample had similar mean deprivation values in the higher quintiles and a broader range than the nationally representative sample. We adjusted for multiple covariates, but the possibility of residual confounding exists. Our twins were predominantly younger adult Caucasian women and the unique SES of our twin sample does not mirror that of other populations, and therefore our results should be applied to the general population with caution. In particular, future research should examine the relation between area-level deprivation and sleep duration in racial/ethnic minorities. It should be noted that our sample was derived from the community and not from a clinical population seeking health care, which increases the generalizability of the results. Self-reported sleep duration, which is commonly used in observational studies, approximates objective measures of sleep length,68,69 although recent studies suggest it may be biased by overestimation.70 Future studies assessing quasi-causal associations and gene × environment interactions between area-level deprivation and sleep duration would benefit from direct objective measurement of the sleep variable with wrist actigraphy.
In conclusion, we found a quasi-causal relationship and gene × environment interaction between area-level deprivation as defined by the Singh Index and subjective sleep duration. More deprived areas were associated with reduced sleep duration, a robust finding present after accounting for genetic and shared environmental confounding, which asserts that area-level deprivation may decrease habitual sleep duration. We found that increased area-level deprivation was also associated with increased variance in sleep duration from both a genetic and nonshared environmental standpoint. Because health is likely optimized within a defined sleep duration, increased variance likely implies movement to extremes with attendant untoward consequences for health, particularly for short sleepers. Future studies are needed to reveal specific aspects of deprived areas that contribute to this phenomenon as a prelude to interventional studies aimed at improving sleep and overall health for denizens of these communities.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by NIH grants R01AG042176, K23HL083350, P30NR011400, OD006547, and a University of Washington General Clinical Research Center Pilot Grant. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Siegel JM. Do all animals sleep? Trends Neurosci. 2008;31:208–13. doi: 10.1016/j.tins.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat: an update of the 1989 paper. Sleep. 2002;25:18–24. doi: 10.1093/sleep/25.1.18. [DOI] [PubMed] [Google Scholar]
- 3.Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81:12–34. doi: 10.1016/j.neuron.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomez-Gonzalez B, Dominguez-Salazar E, Hurtado-Alvarado G, et al. Role of sleep in the regulation of the immune system and the pituitary hormones. Ann N Y Acad Sci. 2012;1261:97–106. doi: 10.1111/j.1749-6632.2012.06616.x. [DOI] [PubMed] [Google Scholar]
- 5.Mendelsohn AR, Larrick JW. Sleep facilitates clearance of metabolites from the brain: glymphatic function in aging and neurodegenerative diseases. Rejuvenation Res. 2013;16:518–23. doi: 10.1089/rej.2013.1530. [DOI] [PubMed] [Google Scholar]
- 6.Watson NF, Harden KP, Buchwald D, et al. Sleep duration and body mass index in twins: a gene-environment interaction. Sleep. 2012;35:597–603. doi: 10.5665/sleep.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Padilha HG, Crispim CA, Zimberg IZ, et al. A link between sleep loss, glucose metabolism and adipokines. Braz J Med Biol Res. 2011;44:992–9. doi: 10.1590/s0100-879x2011007500113. [DOI] [PubMed] [Google Scholar]
- 8.Ayas NT, White DP, Al-Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–4. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 9.Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165:863–7. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 10.Aldabal L, Bahammam AS. Metabolic, endocrine, and immune consequences of sleep deprivation. Open Respir Med J. 2011;5:31–43. doi: 10.2174/1874306401105010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klingenberg L, Sjodin A, Holmback U, Astrup A, Chaput JP. Short sleep duration and its association with energy metabolism. Obes Rev. 2012;13:565–77. doi: 10.1111/j.1467-789X.2012.00991.x. [DOI] [PubMed] [Google Scholar]
- 12.Penev PD. Update on energy homeostasis and insufficient sleep. J Clin Endocrinol Metab. 2012;97:1792–801. doi: 10.1210/jc.2012-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faraut B, Boudjeltia KZ, Vanhamme L, Kerkhofs M. Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep Med Rev. 2012;16:137–49. doi: 10.1016/j.smrv.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Miller MA. Association of inflammatory markers with cardiovascular risk and sleepiness. J Clin Sleep Med. 2011;7:S31–3. doi: 10.5664/JCSM.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–62. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 16.Fang J, Wheaton AG, Keenan NL, Greenlund KJ, Perry GS, Croft JB. Association of sleep duration and hypertension among US adults varies by age and sex. Am J Hypertens. 2012;25:335–41. doi: 10.1038/ajh.2011.201. [DOI] [PubMed] [Google Scholar]
- 17.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 18.Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–92. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 19.Eguchi K, Hoshide S, Ishikawa S, Shimada K, Kario K. Short sleep duration is an independent predictor of stroke events in elderly hypertensive patients. J Am Soc Hypertens. 2010;4:255–62. doi: 10.1016/j.jash.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Eguchi K, Pickering TG, Schwartz JE, et al. Short sleep duration as an independent predictor of cardiovascular events in Japanese patients with hypertension. Arch Intern Med. 2008;168:2225–31. doi: 10.1001/archinte.168.20.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29:1009–14. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 22.Loerbroks A, Debling D, Amelang M, Sturmer T. Nocturnal sleep duration and cognitive impairment in a population-based study of older adults. Int J Geriatr Psychiatry. 2010;25:100–9. doi: 10.1002/gps.2305. [DOI] [PubMed] [Google Scholar]
- 23.Anderson B, Storfer-Isser A, Taylor HG, Rosen CL, Redline S. Associations of executive function with sleepiness and sleep duration in adolescents. Pediatrics. 2009;123:e701–7. doi: 10.1542/peds.2008-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watson NF, Harden KP, Buchwald D, et al. Sleep duration and depressive symptoms: a gene-environment interaction. Sleep. 2014;37:351–8. doi: 10.5665/sleep.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Transportation Safety Board. Collision of Two Canadian National/Illinois Central Railway Trains near Clarkston, Michigan, November 15, 2001 11/192002. Report No.: RAR-02-04
- 26.National Transpotation Safety Board. Aircraft Accident Report: Crash During Attempted Go-Around After Landing East Coast Jets Flight 81 Hawker Beechcraft Corporation 125-800A, N818MV Owatonna, Minnesota July 31, 2008 3/15/2011. Report No.: PB2011-910401 [Google Scholar]
- 27.National Transportation Safety Board. Highway Accident Report: Truck-Tractor Semitrailer Rollover and Motorcoach Collision With Overturned Truck Interstate Highway 94 Near Osseo, Wisconsin October 16, 2005, 9/16/2008. Report No.: HAR-08-02 [Google Scholar]
- 28.Sallinen M, Harma M, Akila R, et al. The effects of sleep debt and monotonous work on sleepiness and performance during a 12-h dayshift. J Sleep Res. 2004;13:285–94. doi: 10.1111/j.1365-2869.2004.00425.x. [DOI] [PubMed] [Google Scholar]
- 29.Tomasi D, Wang RL, Telang F, et al. Impairment of attentional networks after 1 night of sleep deprivation. Cereb Cortex. 2009;19:233–40. doi: 10.1093/cercor/bhn073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. 2009;18:148–58. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 31.Grandner MA. Sleep duration across the lifespan: implications for health. Sleep Med Rev. 2012;16:199–201. doi: 10.1016/j.smrv.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill TD, Burdette AM, Hale L. Neighborhood disorder, sleep quality, and psychological distress: testing a model of structural amplification. Health Place. 2009;15:1006–13. doi: 10.1016/j.healthplace.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Hill TD, Ross CE, Angel RJ. Neighborhood disorder, psychophysiological distress, and health. J Health Soc Behav. 2005;46:170–86. doi: 10.1177/002214650504600204. [DOI] [PubMed] [Google Scholar]
- 34.Ross CE, Mirowsky J. Neighborhood disadvantage, disorder, and health. J Health Soc Behav. 2001;42:258–76. [PubMed] [Google Scholar]
- 35.Aparicio-Ramon DV, Morales Suarez-Varela MM, Garcia Garcia A, et al. Subjective annoyance caused by environmental noise. J Environ Pathol Toxicol Oncol. 1993;12:237–43. [PubMed] [Google Scholar]
- 36.Steptoe A, O'Donnell K, Marmot M, Wardle J. Positive affect, psychological well-being, and good sleep. J Psychosom Res. 2008;64:409–15. doi: 10.1016/j.jpsychores.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Hale L, Do DP. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30:1096–103. doi: 10.1093/sleep/30.9.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hale L, Hill TD, Burdette AM. Does sleep quality mediate the association between neighborhood disorder and self-rated physical health? Prev Med. 2010;51:275–8. doi: 10.1016/j.ypmed.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 39.Turkheimer E, Harden KP. Behavior genetic research methods: testing quasi-causal hypotheses using multivariate twin data. In: Reis HT, Judd CM, editors. Handbook of research methods in personality and social psychology. 2nd ed. New York, NY: Cambridge University Press; 2014. [Google Scholar]
- 40.Afari N, Noonan C, Goldberg J, et al. University of Washington Twin Registry: construction and characteristics of a community-based twin registry. Twin Res Hum Genet. 2006;9:1023–9. doi: 10.1375/183242706779462543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strachan E, Hunt C, Afari N, et al. University of Washington Twin Registry: poised for the next generation of twin research. Twin Res Hum Genet. 2013;16:455–62. doi: 10.1017/thg.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eisen S, Neuman R, Goldberg J, Rice J, True W. Determining zygosity in the Vietnam Era Twin Registry: an approach using questionnaires. Clin Genet. 1989;35:423–32. doi: 10.1111/j.1399-0004.1989.tb02967.x. [DOI] [PubMed] [Google Scholar]
- 43.Spitz E, Moutier R, Reed T, et al. Comparative diagnoses of twin zygosity by SSLP variant analysis, questionnaire, and dermatoglyphic analysis. Behav Genet. 1996;26:55–63. doi: 10.1007/BF02361159. [DOI] [PubMed] [Google Scholar]
- 44.Torgersen S. The determination of twin zygosity by means of a mailed questionnaire. Acta Genet Med Gemellol (Roma) 1979;28:225–36. doi: 10.1017/s0001566000009077. [DOI] [PubMed] [Google Scholar]
- 45.Singh GK. Area deprivation and widening inequalities in US mortality, 1969-1998. Am J Public Health. 2003;93:1137–43. doi: 10.2105/ajph.93.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.HIP xchange. Area Deprivation Index. [cited 2015 April 27]. Available from: http://www.hipxchange.org/ADI.
- 47.Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Res. 2002;5:554–71. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- 48.van der Sluis S, Posthuma D, Dolan CV. A note on false positives and power in G x E modelling of twin data. Behav Genet. 2012;42:170–86. doi: 10.1007/s10519-011-9480-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dijk DJ, Duffy JF, Czeisler CA. Age-related increase in awakenings: impaired consolidation of nonREM sleep at all circadian phases. Sleep. 2001;24:565–77. doi: 10.1093/sleep/24.5.565. [DOI] [PubMed] [Google Scholar]
- 50.Weitzman ED, Czeisler CA, Zimmerman JC, Ronda JM. Timing of REM and stages 3 + 4 sleep during temporal isolation in man. Sleep. 1980;2:391–407. [PubMed] [Google Scholar]
- 51.Jarrin DC, McGrath JJ, Silverstein JE, Drake C. Objective and subjective socioeconomic gradients exist for sleep quality, sleep latency, sleep duration, weekend oversleep, and daytime sleepiness in adults. Behav Sleep Med. 2013;11:144–58. doi: 10.1080/15402002.2011.636112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whinnery J, Jackson N, Rattanaumpawan P, Grandner MA. Short and long sleep duration associated with race/ethnicity, sociodemographics, and socioeconomic position. Sleep. 2014;37:601–11. doi: 10.5665/sleep.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goodin BR, McGuire L, Smith MT. Ethnicity moderates the influence of perceived social status on subjective sleep quality. Behav Sleep Med. 2010;8:194–206. doi: 10.1080/15402002.2010.509193. [DOI] [PubMed] [Google Scholar]
- 54.Okun ML, Tolge M, Hall M. Low socioeconomic status negatively affects sleep in pregnant women. J Obstet Gynecol Neonatal Nurs. 2014;43:160–7. doi: 10.1111/1552-6909.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore M, Kirchner HL, Drotar D, Johnson N, Rosen C, Redline S. Correlates of adolescent sleep time and variability in sleep time: the role of individual and health related characteristics. Sleep Med. 2011;12:239–45. doi: 10.1016/j.sleep.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kelly RJ, El-Sheikh M. Marital conflict and children's sleep: reciprocal relations and socioeconomic effects. J Fam Psychol. 2011;25:412–22. doi: 10.1037/a0023789. [DOI] [PubMed] [Google Scholar]
- 57.Grandner MA, Hale L, Moore M, Patel NP. Mortality associated with short sleep duration: the evidence, the possible mechanisms, and the future. Sleep Med Rev. 2010;14:191–203. doi: 10.1016/j.smrv.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim SJ, Chai SK, Lee KW, et al. Exposure-response relationship between aircraft noise and sleep quality: a community-based cross-sectional study. Osong Public Health Res Perspect. 2014;5:108–14. doi: 10.1016/j.phrp.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frei P, Mohler E, Roosli M. Effect of nocturnal road traffic noise exposure and annoyance on objective and subjective sleep quality. Int J Hyg Environ Health. 2014;217:188–95. doi: 10.1016/j.ijheh.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 60.Lepore SJ, Kliewer W. Violence exposure, sleep disturbance, and poor academic performance in middle school. J Abnorm Child Psychol. 2013;41:1179–89. doi: 10.1007/s10802-013-9709-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Charles LE, Slaven JE, Mnatsakanova A, et al. Association of perceived stress with sleep duration and sleep quality in police officers. Int J Emerg Ment Health. 2011;13:229–41. [PMC free article] [PubMed] [Google Scholar]
- 62.Fang SC, Schwartz J, Yang M, Yaggi HK, Bliwise DL, Araujo AB. Traffic-related air pollution and sleep in the Boston Area Community Health Survey. J Expo Sci Environ Epidemiol. 2015;25:451–6. doi: 10.1038/jes.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu X, Liu L, Wang R. Bed sharing, sleep habits, and sleep problems among Chinese school-aged children. Sleep. 2003;26:839–44. doi: 10.1093/sleep/26.7.839. [DOI] [PubMed] [Google Scholar]
- 64.Campbell CM, Bounds SC, Kuwabara H, et al. Individual variation in sleep quality and duration is related to cerebral mu opioid receptor binding potential during tonic laboratory pain in healthy subjects. Pain Med. 2013;14:1882–92. doi: 10.1111/pme.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wiebe ST, Cassoff J, Gruber R. Sleep patterns and the risk for unipolar depression: a review. Nat Sci Sleep. 2012;4:63–71. doi: 10.2147/NSS.S23490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2007;30:1667–73. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hauri PJ, Wisbey J. Wrist actigraphy in insomnia. Sleep. 1992;15:293–301. doi: 10.1093/sleep/15.4.293. [DOI] [PubMed] [Google Scholar]
- 69.Lockley SW, Skene DJ, Arendt J. Comparison between subjective and actigraphic measurement of sleep and sleep rhythms. J Sleep Res. 1999;8:175–83. doi: 10.1046/j.1365-2869.1999.00155.x. [DOI] [PubMed] [Google Scholar]
- 70.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19:838–45. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]