Abstract
Study Objectives:
Two commentaries recently published in SLEEP came to very different conclusions regarding how data from a mouse model of sleep-dependent neural plasticity (orientation-specific response potentiation; OSRP) fit with the synaptic homeostasis hypothesis (SHY). To assess whether SHY offers an explanatory mechanism for OSRP, we present new data on how cortical neuron firing rates are modulated as a function of novel sensory experience and subsequent sleep in this model system.
Methods:
We carried out longitudinal extracellular recordings of single-neuron activity in the primary visual cortex across a period of novel visual experience and subsequent sleep or sleep deprivation. Spontaneous neuronal firing rates and visual responses were recorded from the same population of visual cortex neurons before control (blank screen) or novel (oriented grating) stimulus presentation, immediately after stimulus presentation, and after a period of subsequent ad lib sleep or sleep deprivation.
Results:
Firing rate responses to visual stimuli were unchanged across waking experience, regardless of whether a blank screen or an oriented grating stimulus was presented. Firing rate responses to stimuli of the presented stimulus orientation were selectively enhanced across post-stimulus sleep, but these changes were blocked by sleep deprivation. Neuronal firing increased significantly across bouts of post-stimulus rapid eye movement (REM) sleep and slow wave sleep (SWS), but not across bouts of wake.
Conclusions:
The current data suggest that following novel visual experience, potentiation of a subset of V1 synapses occurs across periods of sleep. This finding cannot be explained parsimoniously by SHY.
Citation:
Durkin J, Aton SJ. Sleep-dependent potentiation in the visual system is at odds with the synaptic homeostasis hypothesis. SLEEP 2016;39(1):155– 159.
Keywords: synaptic plasticity, sensory processing, cerebral cortex, electrophysiology
Significance.
Understanding how sleep contributes to experience-dependent plasticity in the brain has important implications for human health and welfare. To promote our understanding of this process at the neuronal network and synaptic levels, we characterized the effects of sleep on a simple form of sensory-evoked plasticity in the adult mouse visual system. By quantifying the direction and magnitude of firing rate response changes in cortical neurons following novel visual experience and subsequent sleep, we have expanded upon our previous findings to provide additional evidence of synaptic potentiation during sleep. These data demonstrate that a predominant hypothesis in the field, the synaptic homeostasis hypothesis (SHY), cannot fully explain the beneficial effects of sleep on brain plasticity and cognitive function.
We are grateful to the authors of two commentaries recently published in SLEEP1,2 for helpful discussion of how our data on sleep-dependent visual system plasticity3 (and recent data from a number of other labs) could provide evidence for, or against, the synaptic homeostasis hypothesis (SHY). Here, we would like to present additional data to clarify why this form of plasticity cannot be explained parsimoniously by SHY.
Simply stated, the underlying assumption for SHY is that during waking experiences, synapses are strengthened, and during sleep, synapses are weakened. Aimed at explaining the cognitive benefits of sleep, SHY proposes that synapses throughout the brain undergo a global (if not necessarily uniform) decrease in strength as a function of sleep. Such a process could improve the function of neural circuits by reducing synaptic “noise” caused by strengthening of connections in wake. Proponents of the hypothesis have posited that “sleep is the price the brain pays for plasticity.”4 In other words, reduction in the neuronal signal-to-noise ratio through homeostatic synaptic downscaling is the sine qua non of why the brain has evolved to sleep. Such an incredibly far-reaching assertion requires a proportional amount of supportive evidence; Occam's razor must be applied, no matter how elegant the hypothesis seems. In support of SHY, converging electrophysiological, anatomical, and molecular data have shown subtle decreases in synaptic strength across the brain after a period of sleep when compared with a period of wake.5–8 Critically, however, such changes have been described primarily for rodents in their home cage in the absence of novel experience or learning.7,8 Thus, one fair criticism of SHY is that there is a paucity of data implicating downscaling as a mechanism for adaptive brain plasticity (e.g., during sleep-dependent memory consolidation). And while data simulations may indicate that SHY could improve neural circuit function9,10 (as described by Drs. Cirelli and Tononi in their commentary2), no experimental studies have conclusively demonstrated that sleep-dependent downscaling actually occurs in the context of sleep-dependent cognitive processes. Second, no studies have selectively interfered with downscaling during sleep (indeed, the cellular mechanism for sleep-dependent downscaling has not been clearly defined11)—so its function is unknown. Third, a strict interpretation of SHY is that sleep promotes cognitive functions exclusively through synaptic weakening—which is not supported by data from several labs indicative of sleep-dependent synaptic strengthening.3,12–18
Nonetheless, if further evidence was needed that SHY is highly influential in the field, one might cite the fact that discussion of our data has been centered on how it relates to SHY. As Dr. Heller correctly stated in his commentary,1 our previous findings do not disprove SHY. However, they do suggest that SHY does not account for some forms of sleep-dependent brain plasticity.3,17 The plasticity we recently described (orientation-specific response potentiation; OSRP) is initiated in primary visual cortex (V1) by presenting a novel visual stimulus (a flickering oriented grating). OSRP is expressed as a relative increase in V1 responses to the presented stimulus orientation over subsequent hours.3,19 This process relies on the same in vivo mechanisms as thalamocortical long-term potentiation (LTP),20 and critically, post-stimulus sleep deprivation interferes with OSRP.3 A parsimonious explanation is that in this case, synapses are potentiated during sleep. However, this simple interpretation runs counter to SHY, which does not allow for large-scale (i.e., circuit-wide) synaptic strengthening outside of wake.
Drs. Cirelli and Tononi commented that two factors suggest a mechanism consistent with SHY for sleep-dependent OSRP. First, they state that “visual responses were not recorded immediately after training,” conjecturing that enhancement of orientation-specific responses occurs across waking visual experience. This statement is simply not true; we showed that preference for the presented stimulus orientation is unchanged immediately after stimulus presentation, but only shifts in favor of the presented stimulus 6–12 hours later3—a finding consistent with what others have reported.19 Their second concern is that by comparing neuronal firing responses to stimuli of different orientations, rather than the absolute amplitude of visually evoked potentials (VEPs), we have obscured any absolute changes in V1 visual responses. The distinction between single neuron firing rate responses and VEPs is not germane; VEPs and V1 neuronal firing are correlated across stimulus conditions,21–23 and changes in VEP amplitudes are predicted by changes in V1 neuronal firing during visually induced response plasticity.24,25 The relative change in firing for various stimulus orientations was the salient feature of OSRP in our study, just as it was for prior studies using VEPs.19,20 Nonetheless, absolute changes in spontaneous26,27 or stimulus evoked28 neuronal firing rates have recently been found during homeostatic plasticity in the cortex (e.g., increases in firing rates following visual deprivation). Here, we present a meta-analysis of raw firing rate changes from a large number of in vivo stereotrode recordings (comprising the 23 mouse experiments previously reported3 and an additional 46 experiments subsequently carried out using identical methods), to address whether overall synaptic strength appears to go up or down in V1 with sleep. For all experiments, mice were implanted with 2 bundles of stereotrode wires (7 per bundle, spaced 1–2 mm apart in right-hemisphere V1) for single-neuron and local field potential recordings (reference and ground wires placed over left-hemisphere V1 and cerebellum, respectively) and nuchal EMGs as described previously.3 Spike trains from individual neurons were discriminated offline; only stably recorded and reliably discriminated V1 neurons (with single-unit spiking continuously recorded throughout the experiment) were included in subsequent analyses. All animal procedures were approved by the University of Michigan Committee on Use and Care of Animals.
To test whether synaptic downscaling is a likely mediator of OSRP, we first assessed whether V1 neuronal firing rates increased in non-anesthetized, head-fixed mice across waking visual experience (Figure 1A–1B). We found that firing rates for individual V1 neurons were virtually identical at the beginning and end of a 1-h waking visual stimulus period (3.4 ± 0.4 Hz and 3.4 ± 0.4 Hz for the first and last 5 min of stimulus presentation). Presentation of a blank screen over the same 1-h time window resulted in similar firing rate changes (mean changes of 0.01 ± 0.05 Hz and −0.11 ± 0.09 Hz across oriented grating stimulus presentation and blank screen presentation, respectively; Figure 1C). Immediately following stimulus presentation, firing rate responses to the presented stimulus orientation were only slightly (and not significantly) enhanced (N.S., RM ANOVA). Responses to stimuli of the orthogonal orientation, and spontaneous activity, showed a similar degree of change (increases of 0.15 ± 0.09 Hz and 0.17 ± 0.09 Hz, respectively, vs. 0.16 ± 0.08 Hz for presented stimulus responses; all N.S., RM ANOVA vs. baseline). Moreover, firing rate changes were similar in mice presented with a blank screen over the same time period (an increase of in spontaneous activity of 0.16 ± 0.17 Hz, also N.S., RM ANOVA; Figure 1D). Thus no significant spontaneous or stimulus evoked firing rate changes are present immediately following waking visual experience.
Figure 1.
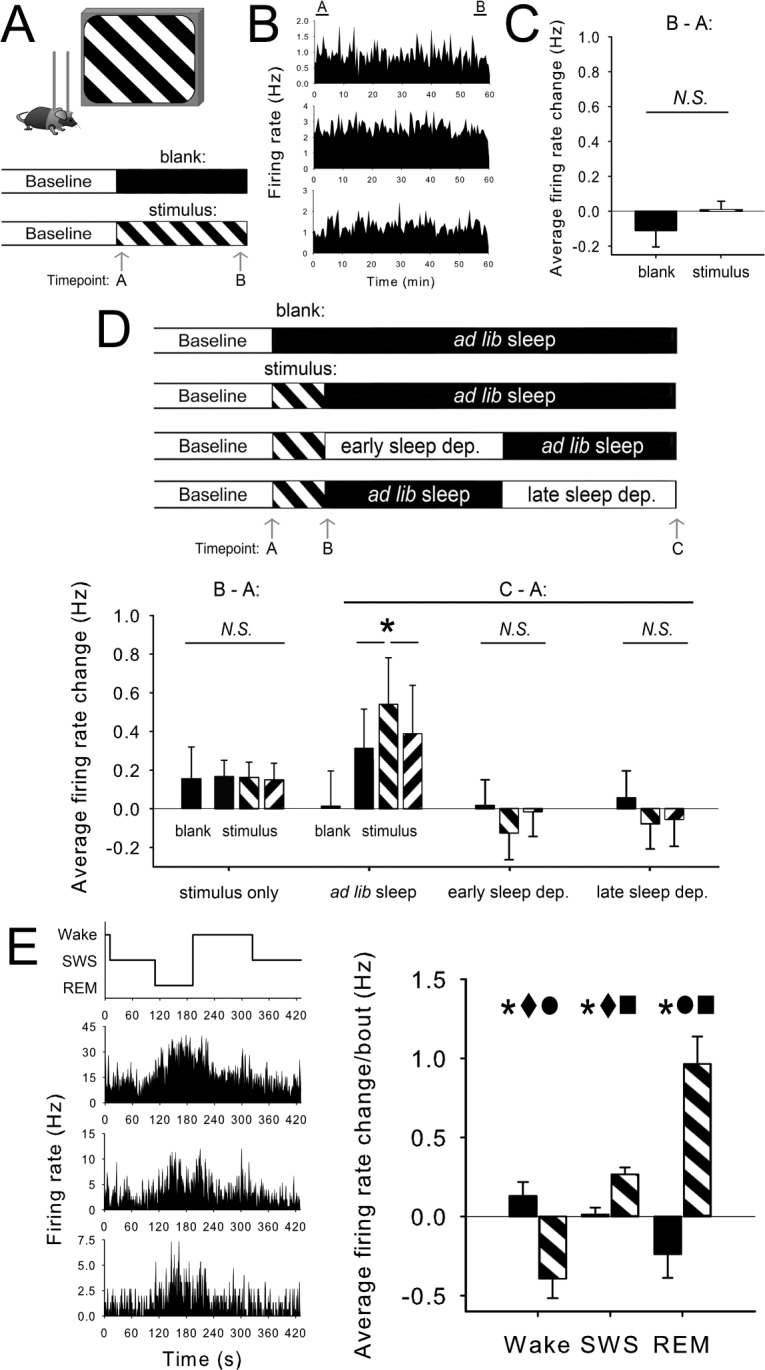
Cortical neurons' firing rates do not change across waking visual experience which induces OSRP, but do increase across subsequent sleep. (A) To assess firing rate changes over waking experience, firing rates were compared during the first (Timepoint A) and last (Timepoint B) 5-min windows of oriented grating (stimulus) presentation or blank screen (blank) presentation, beginning at lights-on. (B) Firing rate histograms for three representative V1 neurons during 1-h stimulus presentation. (C) Firing rate changes for individual neurons (in Hz) were not significantly changed across stimulus presentation, and were not different between stimulus (n = 20 experiments [8 from a previous study3], 268 neurons) and blank screen (n = 3 experiments, 44 neurons) conditions. (D) No differences in either spontaneous firing rate or orientation-specific responses (presented and orthogonal orientations are shown) were seen immediately after blank screen or stimulus presentation (Timepoint B). After subsequent ad lib sleep (Timepoint C; n = 11 experiments [4 from a previous study3], 137 neurons), firing rate responses were selectively enhanced for the presented stimulus. *P < 0.05 for presented vs. orthogonal, presented vs. blank, Holm-Sidak post hoc test, P < 0.05, RM ANOVA. Post-sleep firing rate changes following blank screen presentation (n = 8 experiments [4 from a previous study3], 105 neurons) were negligible. Subsets of mice underwent behavioral sleep deprivation in the first (early sleep dep., n = 14 experiments [4 from a previous study3], 176 neurons) or second (late sleep dep., n = 13 experiments [3 from a previous study3], 166 neurons) half of the post stimulus sleep period. In both sleep deprivation conditions, response rate changes across the day were negligible, and stimulus-specific potentiation of responses was lost. (E) To determine how neuronal firing changed during sleep and wake bouts, firing rates were averaged over the first and last 30 seconds of individual bouts of wake, SWS, or REM. Changes in firing were calculated for each bout ≥ 1 min over the first 4 h following presentation of oriented gratings (striped bars; n = 509, 1152, and 287 measurements from 4 mice for wake, SWS, and REM, respectively) or blank screen (black bars; n = 630, 1625, and 236 measurements from 4 mice). Bouts with zero firing were excluded from analysis. *P < 0.005 for stimulus vs. blank screen; ■, ●, and ♦ indicate P < 0.001 vs. wake, SWS, and REM, respectively in the stimulus condition, 2-way RM ANOVA with Holm-Sidak post hoc test.
We next asked whether evidence for sleep-dependent down-scaling was present in our recordings. We compared visually evoked firing rate responses (and spontaneous firing) 12 h after baseline visual response assessment, in freely-behaving animals which either were allowed ad lib sleep or were sleep deprived (Figure 1D). Neuronal firing was recorded continuously from mice across the post-stimulus interval to assess response changes associated with behavioral state, as previously described.3 Following uninterrupted post-stimulus sleep, neuronal firing rate responses to the presented stimulus orientation were selectively enhanced, increasing on average by 0.54 ± 0.24 Hz (P < 0.05, RM ANOVA vs. baseline), vs. 0.31 ± 0.20 Hz and 0.39 ± 0.25 Hz, respectively, for blank screen and the orthogonal stimulus orientation (P < 0.05 for both comparisons, RM ANOVA). These orientation-specific firing rate response increases were eliminated by post-stimulus sleep deprivation. When mice were deprived of sleep by gentle handling during either the first or last half of the day (early sleep dep. and late sleep dep., Figure 1D), V1 neurons' spontaneous activity and responses to stimuli were virtually unchanged from baseline.
Taken together, our data suggest that OSRP is dependent on selective, orientation-specific potentiation of V1 circuitry during post-stimulus sleep, resulting in enhanced firing rate responses to stimuli of the presented stimulus orientation. If these increases in firing rate are a function of sleep, one would predict that: (1) firing rate changes could be detected across individual bouts of either SWS or REM sleep, and (2) following stimulus presentation, firing rate increases would occur preferentially during sleep (vs. wake). To test these predictions, we quantified firing rates for individual neurons at the beginning and end of each bout of wake, SWS, and REM ≥ 1 min duration. Changes in firing rate across bouts were averaged for the 3 states over the first 4 h following presentation of oriented gratings or (for comparison) following presentation of blank screen (Figure 1E). Similar to what we found in our previous study,3 presentation of gratings led to significant increases in firing rate overall (main effect of stimulus presentation, F = 16.4, P < 0.001, two-way RM ANOVA). However, these increases were not uniform across states (stimulus × state interaction, F = 33.3, P < 0.001, two-way RM ANOVA). Relatively large increases in firing rate (0.96 ± 0.17 Hz) occurred across bouts of REM, with smaller increases (0.27 ± 0.04 Hz) occurring across bouts of SWS, and decreases in firing (−0.39 ± 0.12 Hz) occurring across bouts of wake (main effect of state, F = 12.0, P < 0.001; wake vs. REM, wake vs. SWS, and REM vs. SWS, P < 0.001, Holm-Sidak post hoc test). State-specific firing rate changes were in the opposite direction in the hours following blank screen presentation, with mean per-bout changes of −0.24 ± 0.15 Hz, 0.01 ± 0.04 Hz, and 0.13 ± 0.09 Hz, respectively, in REM, SWS, and wake.
Three of our current findings are inconsistent with SHY. First, firing rates do not increase significantly across a novel waking experience that induces OSRP. Second, after this experience, stimulus-specific visual responses increase in a sleep-dependent manner. Third, firing rates in V1 increase significantly across individual bouts of post-stimulus SWS, and increase even more across bouts of post-stimulus REM. One caveat is that in these studies, we directly measured neuronal activity, but not synaptic strength. In response to changing sensory input, in vivo firing rate change increases (as measured here) may result from Hebbian plasticity mechanisms,20,29 homeostatic mechanisms,26 or alterations in membrane excitability.30 Nonetheless, our data present a case where there is no evidence for homeostatic downscaling of synapses during sleep, and where downscaling is not a parsimonious mechanistic explanation for sleep-dependent plasticity. Rather, in light of what is already known about OSRP,20 the most parsimonious explanation of our current and past3,17,29 findings is that cortical synapses are strengthened during sleep.
Hypotheses are useful for advancing our understanding only when they can be amended or falsified. Because SHY has been so influential, two questions neuroscientists must ask are: (1) whether synaptic potentiation associated with novel learning can occur during sleep and, (2) whether synaptic potentiation, downscaling, or both are present in the context of naturally occurring sleep-dependent plasticity. The answer to the first question is “yes” - our lab and others3,12–17 have already provided substantial evidence that synaptic potentiation can occur during sleep instead of wake. The second question can only be answered with data from the brain in the context of experience-dependent plasticity, not with rigid adherence to one hypothesis about the function of sleep.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by a training grant to Ms. Durkin from the National Institutes of Health (T32 NS076401), a Graduate Research Fellowship to Ms. Durkin from the National Science Foundation, and research grants to SJA from the National Institutes of Health (DP2 MH104119, R00 EY021503), the Brain and Behavioral Research Foundation, and the Alfred P. Sloan Foundation. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Heller C. The ups and downs of synapses during sleep and learning. Sleep. 2014;37:1157–8. doi: 10.5665/sleep.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cirelli C, Tononi G. Sleep and synaptic homeostasis. Sleep. 2015;38:161–2. doi: 10.5665/sleep.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aton SJ, Suresh A, Broussard C, Frank MG. Sleep promotes cortical response potentiation following visual experience. Sleep. 2014;37:1163–70. doi: 10.5665/sleep.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81:12–34. doi: 10.1016/j.neuron.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cirelli C, Gutierrez CM, Tononi G. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron. 2004;41:35–43. doi: 10.1016/s0896-6273(03)00814-6. [DOI] [PubMed] [Google Scholar]
- 6.Maret S, Faraguna U, Nelson AB, Cirelli C, Tononi G. Sleep and waking modulate spine turnover in the adolescent mouse cortex. Nat Neurosci. 2011;14:1418–20. doi: 10.1038/nn.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–8. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- 8.Vyazovskiy VV, Olscese U, Lazimy YM, et al. Cortical firing and sleep homeostasis. Neuron. 2009;63:865–78. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nere A, Hashmi A, Cirelli C, Tononi G. Sleep-dependent synaptic down-selection (I): modeling the benefits of sleep on memory consolidation and integration. Front Neurol. 2013;4:143. doi: 10.3389/fneur.2013.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashmi A, Nere A, Tononi G. Sleep-dependent synaptic down-selection (II): single-neuron level benefits for matching, selectivity, and specificity. Front Neurol. 2013;4:148. doi: 10.3389/fneur.2013.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tononi G, Cirelli C. Time to be SHY? Some comments on sleep and synaptic homeostasis. Neural Plast. 2012;2012:415250. doi: 10.1155/2012/415250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang G, Lai CS, Cichon J, Ma L, Li W, Gan WB. Sleep promotes branch-specific formation of dendritic spines after learning. Science. 2014;344:1173–8. doi: 10.1126/science.1249098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vecsey CG, Baillie GS, Jaganath D, et al. Sleep deprivation impairs cAMP signalling in the hippocampus. Nature. 2009;461:1122–5. doi: 10.1038/nature08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heller H, Ruby N, Rolls A, Makam M, Colas D. Adaptive and pathological inhibition of neuroplasticity associated with circadian rhythms and sleep. Behav Neurosci. 2014;128:273–82. doi: 10.1037/a0036689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chauvette S, Seigneur J, Timofeev I. Sleep oscillations in the thalamocortical system induce long-term plasticity. Neuron. 2012;75:1105–13. doi: 10.1016/j.neuron.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mascetti L, Foret A, Schrouff J, et al. Concurrent synaptic and systems memory consolidation during sleep. J Neurosci. 2013;33:10182–90. doi: 10.1523/JNEUROSCI.0284-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aton SJ, Seibt J, Dumoulin M, et al. Mechanisms of sleep-dependent consolidation of cortical plasticity. Neuron. 2009;61:454–66. doi: 10.1016/j.neuron.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ulloor J, Datta S. Spatio-temporal activation of cyclic AMP response element-binding protein, activity-regulated cytoskeletal-associated protein and brain-derived nerve growth factor: a mechanism for pontine-wave generator activation-dependent two-way active-avoidance memory processing in the rat. J Neurochem. 2005;95:418–28. doi: 10.1111/j.1471-4159.2005.03378.x. [DOI] [PubMed] [Google Scholar]
- 19.Frenkel MY, Sawtell NB, Diogo AC, Yoon B, Neve RL, Bear MF. Instructive effect of visual experience in mouse visual cortex. Neuron. 2006;51:339–49. doi: 10.1016/j.neuron.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 20.Cooke SF, Bear MF. Visual experience induces long-term potentiation in the primary visual cortex. J Neurosci. 2010;30:16304–13. doi: 10.1523/JNEUROSCI.4333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Creutzfeldt O, Rosina A, Ito M, Probst W. Visually evoked response of single cells and of the EEG in primary visual area of the cat. J Neurophysiol. 1969;32:127–39. doi: 10.1152/jn.1969.32.2.127. [DOI] [PubMed] [Google Scholar]
- 22.Schroeder CE, Tenke CE, Givre SJ, Arezzo JC, Vaughan HCJ. Striate cortical contribution to the surface-recorded pattern-reversal VEP in the alert monkey. Vision Research. 1991;31:1143–57. doi: 10.1016/0042-6989(91)90040-c. [DOI] [PubMed] [Google Scholar]
- 23.Schroeder CE, Mehta AD, Givre SJ. A spatiotemporal profile of visual system activation revealed by current source density analysis in the awake macaque. Cereb Cortex. 1998;8:575–92. doi: 10.1093/cercor/8.7.575. [DOI] [PubMed] [Google Scholar]
- 24.Sawtell NB, Frenkel MY, Philpot BD, Nakazawa K, Tonegawa S, Bear MF. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron. 2003;38:977–85. doi: 10.1016/s0896-6273(03)00323-4. [DOI] [PubMed] [Google Scholar]
- 25.Fischer QS, Graves A, Evans S, Lickey ME, Pham TA. Monocular deprivation in adult mice alters visual acuity and single-unit activity. Learn Mem. 2007;14:277–86. doi: 10.1101/lm.392107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hengen K, Lambo M, SD VH, DB K, Turrigiano G. Firing rate homeostasis in visual cortex of freely behaving rodents. Neuron. 2013;80:335–42. doi: 10.1016/j.neuron.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keck T, Keller G, Jacobson R, Eysel U, Bonhoeffer T, Hubener M. Synaptic scaling and homeostatic plasticity in the mouse visual cortex in vivo. Neuron. 2013;80:327–34. doi: 10.1016/j.neuron.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 28.Mrsic-Flogel TD, Hofer SB, Ohki K, Reid RC, Bonhoeffer T, Hubener M. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron. 2007;54:961–72. doi: 10.1016/j.neuron.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 29.Aton SJ, Broussard C, Dumoulin M, et al. Visual experience and subsequent sleep induce sequential plastic changes in putative inhibitory and excitatory cortical neurons. Proc Natl Acad Sci U S A. 2013;110:3101–6. doi: 10.1073/pnas.1208093110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahon S, Charpier S. Bidirectional plasticity of intrinsic excitability controls sensory inputs efficiency in layer 5 barrel cortex neurons in vivo. J Neurosci. 2012;32:11377–89. doi: 10.1523/JNEUROSCI.0415-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


