Abstract
Study Objective:
To investigate the influence of sleep disordered breathing (SDB) on weight loss in overweight/obese veterans enrolled in MOVE!, a nationally implemented behavioral weight management program delivered by the National Veterans Health Administration health system.
Methods:
This observational study evaluated weight loss by SDB status in overweight/obese veterans enrolled in MOVE! from May 2008–February 2012 who had at least two MOVE! visits, baseline weight, and at least one follow-up weight (n = 84,770). SDB was defined by International Classification of Diseases, Ninth Revision, Clinical Modification codes. Primary outcome was weight change (lb) from MOVE! enrollment to 6- and 12-mo assessments. Weight change over time was modeled with repeated-measures analyses.
Results:
SDB was diagnosed in one-third of the cohort (n = 28,269). At baseline, veterans with SDB weighed 29 [48] lb more than those without SDB (P < 0.001). On average, veterans attended eight MOVE! visits. Weight loss patterns over time were statistically different between veterans with and without SDB (P < 0.001); veterans with SDB lost less weight (−2.5 [0.1] lb) compared to those without SDB (−3.3 [0.1] lb; P = 0.001) at 6 months. At 12 mo, veterans with SDB continued to lose weight whereas veterans without SDB started to re-gain weight.
Conclusions:
Veterans with sleep disordered breathing (SDB) had significantly less weight loss over time than veterans without SDB. SDB should be considered in the development and implementation of weight loss programs due to its high prevalence and negative effect on health.
Citation:
Janney CA, Kilbourne AM, Germain A, Lai Z, Hoerster KD, Goodrich DE, Klingaman EA, Verchinina L, Richardson CR. The influence of sleep disordered breathing on weight loss in a national weight management program. SLEEP 2016;39(1):59–65.
Keywords: MOVE!, obesity, population health, sleep apnea, veterans, weight loss
Significance.
Overall, the effect of SDB on weight loss appears to be smaller than the effect of weight loss on SDB severity. From a clinical perspective, targeting weight loss among individuals with SDB should continue to be a top priority to slow the progression of SDB as well as the severity of SDB symptoms. Our findings also highlight the need for more effective weight loss programs among overweight and obese veterans because weight loss of at least 10 kg or 10% of body weight is currently recommended to achieve significant improvements in SDB symptoms.
INTRODUCTION
Obesity is a known risk factor for sleep disordered breathing (SDB), a condition that includes obstructive and central sleep apnea (SA) and upper airway resistance syndrome.1,2 SDB is associated with impairments in daytime functioning, including sleepiness, motor vehicle accidents, psychosocial problems, decreased cognitive function, and reductions in quality of life.2,3 With SDB, medical comorbidities (e.g., diabetes, hypertension, cardiovascular disease, stroke, heart failure, and obesity) are common.1,3
The burden of obesity is substantial in the Veterans Health Administration (VHA) with an estimated 78% of veterans being overweight (37%) and obese (41%).4 In one study, nearly half of outpatient veterans self-reported risk factors for SA.5 Another study found that the odds of receiving a diagnosis of obstructive SA exceeded the odds of a diagnosis of diabetes, hypertension, hyperlipidemia, or psychiatric conditions in obese veterans treated in primary care.6 Similar to patterns seen in the general population, medical comorbidities have also been shown to be significantly greater in veterans with SA than veterans without SA.7,8 Behavioral weight loss is one treatment option for SDB that can reduce the apnea-hypopnea index and snoring while improving sleep efficiency, oxygenation,1,9,10 and cardiometabolic outcomes.10–12 However, few studies to date have assessed whether SDB13 or continuous positive airway pressure (CPAP) treatment14 influences weight loss among overweight and obese adults. The high rate of SDB among veterans7 provides an important opportunity to investigate the effects of SDB on weight loss within a nationally implemented behavioral weight management program (MOVE!). Hence, we conducted an evaluation of MOVE! to investigate the influence of SDB on weight loss.
Previously, observational and experimental studies have suggested that sleep disturbances may increase the risk of obesity and associated cardiometabolic diseases15–17 due to altered glucose metabolism, appetite dysregulation16 resulting in excess food intake,18 and decreased energy expenditure.17 Conversely, optimal levels of both sleep quality and quantity may increase the likelihood of weight loss success19 while minimizing the theorized pathways leading from sleep loss to cardiometabolic risks.16 Because adults with SDB would be expected to have more sleep disturbances and poorer sleep quality than adults without SDB, we hypothesized that over-weight and obese MOVE! participants with a SDB diagnosis would lose less weight over time than those without SDB.
METHODS
Setting and Study Population
As previously described, MOVE! is a behavioral weight management program available at all VHA facilities.20–22 This preventive medicine program aims to promote weight loss through lifestyle interventions of diet and physical activity and is delivered primarily through group modalities. Although national guidelines and standard curricula are available to each VHA facility, the implementation of MOVE! varies among facilities. Eligibility for MOVE! includes individuals seeking medical care within the VHA; age between 18 and 69 y; have a measured body mass index (BMI) > 30 kg/m2 or 25.0 to 29.9 kg/m2 with at least one obesity-related comorbidity (e.g., diabetes, hypertension, hypercholesterolemia/dyslipidemia, obstructive SA, degenerative joint disease, or metabolic syndrome) or elevated waist circumference (> 40 inches for men or > 35 inches for women); and have no contraindications to weight loss23 such as pregnancy or terminal/acute illness. Because a very small percentage of the MOVE! participants are not veterans (< 1%), the term ‘veteran’ is used in this report to refer to all individuals who receive care at the VHA. National patient care databases from the VHA were used to identify veterans enrolled in MOVE! between May 5, 2008 and February 29, 2012 (n = 285,784). This specific MOVE! evaluation was exempted from institutional review board review as part of quality improvement work requested by VHA policy leaders.
Outcomes
Body weight was measured as part of ongoing clinical care within the VHA. Baseline, 6- and 12-mo body weight measures were retrieved for each MOVE! veteran from the VHA patient care databases. Baseline weight was measured within 30 d of MOVE! enrollment. The closest weight within a 60-d window of the follow-up target date (180 d for 6 mo and 365 d for 12 mo) was selected/defined as weight for 6- and 12-mo follow-ups.23 Weight was coded missing if not available at baseline, 6 mo, and/or 12 mo. Outliers were defined as baseline weight less than 91 lb or greater than 600 lb; 6- or 12-mo weight less than 72 lb or greater than 650 lb; weight change from baseline greater than 100 lb; baseline BMI less than 18.5 kg/m2 or greater than 87.9 kg/m2; 6- or 12-mo BMI less than 15 kg/m2 or greater than 90.4 kg/m2; and absolute BMI change from baseline greater than 15 kg/m2.
The primary outcome was weight change at 6 mo and 12 mo from baseline weight. A secondary outcome was clinically significant weight loss defined as > 5% weight loss from baseline (1 = yes, 0 = no). Weight gain at 6 mo and 12 mo was defined as yes (weight at 12 mo > weight at baseline) or no (weight at 12 mo ≤ weight at baseline).
Independent Variables
SDB was defined by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic codes (327.2 [organic sleep apnea], 780.51 [insomnia with sleep apnea], 780.53 [hypersomnia with sleep apnea], 780.57 [unspecified sleep apnea], 786.03 [apnea]) using VHA administrative databases from 2002 through 2012. MOVE! participants were classified into either SDB or non-SDB groups depending on whether or not they had been diagnosed with SDB prior to MOVE! enrollment or within the first 3 mo of MOVE! enrollment. However, veterans who did not have a SDB diagnosis but who had other sleep disorder diagnoses were excluded (insomnia, sleep related movement disorder, and sleep deprivation [ICD-9-CM codes: 291.82, 292.85, 307.4, 327 excluding 327.2, 780.50, 780.52, 780.54, 780.55, 780.56, 780.58, 780.59, V694]) during the first 3 mo of MOVE! enrollment or the year prior to MOVE! enrollment from the analyses. This exclusion was done in order to isolate the effects of SDB on weight loss; including those with other sleep disorders might have biased results toward the null hypothesis, because of the effect of non-SDB sleep disturbances on weight.19,24
Demographic covariates included age, sex, race (White, Black, or Other/Missing), VHA facility (medical center or community-based outpatient clinics), marital status (married or single), and service connection disability (< 50% and ≥ 50%). Service connection disability refers to whether a veteran is disabled because of injuries or diseases incurred during military service and receives a monthly compensation for the disability.25,26 The scale for the service connection ranges from 0% to 100% (in increments of 10%) with greater disability and compensation for those with 100% service connection disability.26
Statistical Analyses
t-tests and chi-square tests were used to compare the baseline continuous and categorical characteristics, respectively, by SDB status. Repeated-measures analyses were used to model weight changes at 6 mo and 12 mo as the primary outcome; and SDB, time of weight assessment (months), and the interaction between SDB and months as predictors. The likelihood ratio test determined that the unstructured covariance fit the data better than compound symmetry or autoregressive covariance structures for the models. All the repeated-measures models were fitted with unstructured covariance. First, a parsimonious model (Model 1) was fitted adjusting for age, sex, baseline weight, race, and facility. Next, marital status, service connection, and number of MOVE! visits (time-varying covariate) were added (Model 2). Both age and baseline weights were centered around their means. Age was modeled as a quadratic term. Predicted weight changes over time were graphed using the average marginal effects.
Secondary analyses examined clinically significant weight loss at 6 mo and 12 mo (> 5% weight loss from baseline), separately. Statistical tests for clinically significant weight loss at 6 mo and 12 mo included chi-square tests with SDB status, and logistic regression models with the aforementioned independent variables for Model 1 and Model 2. Statistical analyses were performed using SAS (version 9.3, SAS Institute, Triangle Park, NC, USA).
RESULTS
Among the veterans enrolled in MOVE! (n = 285,784), the study sample for this report excluded veterans with fewer than two MOVE! visits (n = 129,715), age younger than 17 y or older than 69 y (n = 13,535), and baseline BMI < 25 kg/m2 for those with baseline BMI values (n = 1,247). Veterans were also excluded if they never received a diagnosis of SDB but other sleep disorders had been diagnosed (n = 7,464). After all of the aforementioned exclusions, a total of 133,823 veterans remained in the sample. Approximately one-third of the veterans from the study sample were excluded for missing baseline weight or baseline BMI (n = 45,650) or no weight at 6- or 12-mo assessments (n = 3,096). Finally, 307 veterans were excluded for outliers in weight, BMI, or change scores (weight > 100 lb (n = 225); BMI < 15 kg/m2 (n = 25). The final analytical sample consisted of 84,770 veterans.
The majority of the cohort consisted of older (60–69 y), white, obese males who received health care at VHA medical centers (Table 1). Overall, veterans averaged 2.7 medical comorbidities, with the most frequent diagnoses being hypertension (66%), dyslipidemia (61%), and diabetes (38%). In addition, 43% of the cohort had at least one mental health disorder.
Table 1.
Demographics and health characteristics of MOVE! cohort.
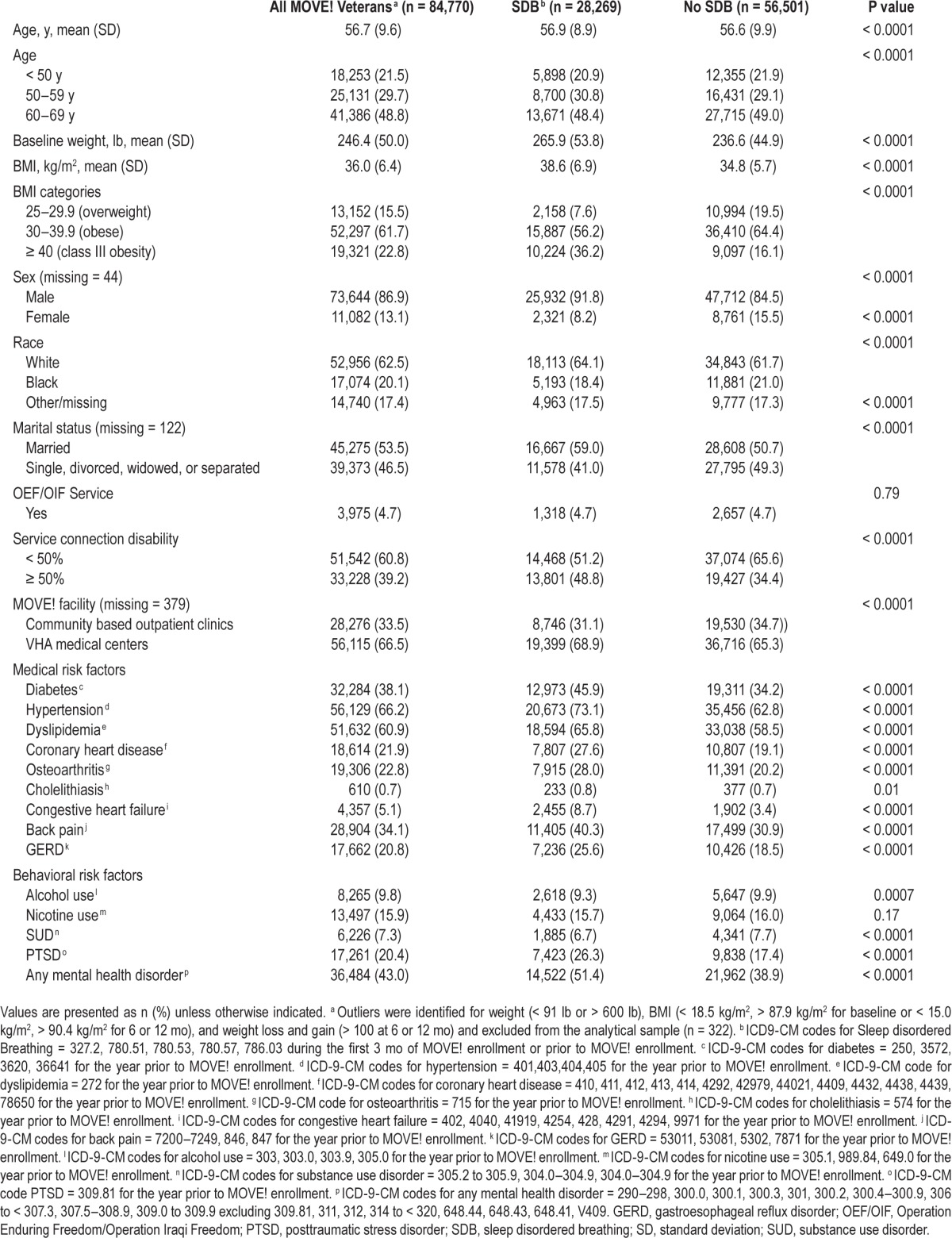
One-third of the cohort had a diagnosis of SDB. Clinically and statistically significant differences were observed among veterans with and without SDB for the majority of demographic and health characteristics (Table 1). Veterans with SDB were more likely to be male and to have a greater number of medical comorbidities and any mental health disorder (P < 0.001). Of those with a diagnosis of SDB, other sleep disorders in addition to the SDB diagnosis were diagnosed in 18%. At baseline, veterans with SDB weighed 29 (48) lb more than those without SDB. Class III obesity (BMI > 40 kg/m2) was observed in twice as many veterans with SDB (36%) versus those without SDB (16%). No clinically or statistically significant differences in SDB were observed for veterans who served or did not serve in the Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) campaigns. OEF/OIF veterans were younger (39.8 [10.2] y) and less obese (BMI = 34.5 [4.9]) than veterans who did not serve in OEF/OIF campaigns (57.5 [8.8] y, P < 0.0001; BMI = 36.1 [6.5], P < 0.0001).
On average, veterans attended eight MOVE! visits during the 12 mo including and following MOVE! enrollment (Table 2). Weight assessments were obtained for 66% (n = 55,960), 22% (n = 18,975), and 12% (n = 9,835) of the veterans at both 6 and 12 mo, 6 mo only, and 12-mo-only assessments, respectively. Weight loss patterns over time were statistically different between veterans with and without SDB (P < 0.001; Model 1, Figure 1). At 6 mo, predicted weight loss was significantly less for veterans with SDB (−2.5 [0.1] lb) compared to veterans without SDB (−3.3 [0.1] lb; P = 0.001). From 6 to 12 mo, predicted weight change was −0.1 (0.08) and 0.3 (0.06) lb for veterans with and without SDB, respectively. Overall, predicted 12-mo weight loss from baseline was −2.6 (0.1) lb for veterans with SDB and −3.0 (0.1) lb for veterans without SDB. Absolute difference in weight loss between veterans with and without SDB was 0.8 lb at 6 mo and 0.4 lb at 12 mo (predicted weight loss for veterans without SDB minus predicted weight loss for veterans with SDB). Relative difference in weight loss was 32% at 6 mo ([3.3 − 2.5] / 2.5) and 15% at 12 mo ([3.0 − 2.6] / 2.6) between veterans with and without SDB. Adjusting for additional confounders (Model 2) did not significantly change the magnitude (β estimates) or the strength (P values) of the association between SDB and weight change.
Table 2.
Unadjusted weight changes by sleep disordered breathing status among the MOVE! cohort.
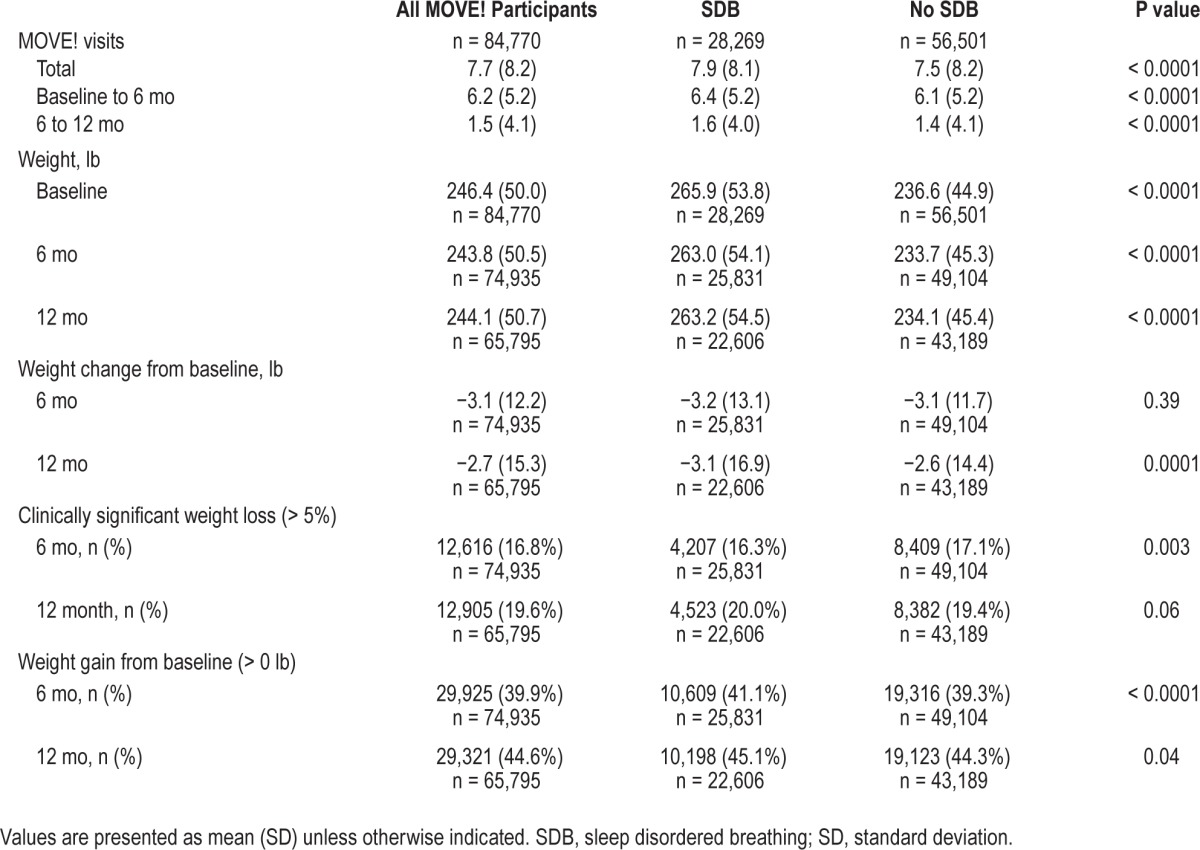
Figure 1.
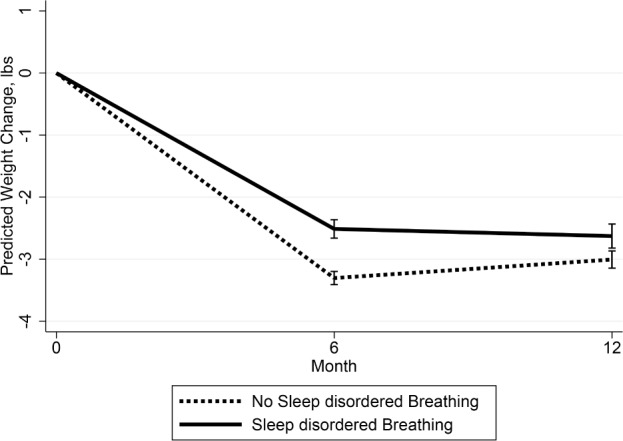
Predicted weight changes (lb) and 95% confidence interval using the average marginal effects from baseline among MOVE! study participants by sleep disordered breathing status (SDB). (Based on repeated measures analyses with weight changes at 6 and 12 mo as the outcome, SDB, time of weight assessment (6 or 12-months), and the interaction between SDB and months as predictors, and age, sex, baseline weight, race, and facility as potential confounders.)
Fewer veterans with SDB (16%) achieved clinically significant weight loss at 6 mo compared to those without SDB (17%) based on chi-square analyses (P = 0.004) and logistic regression models adjusting for confounders (P = 0.0003; data not shown). At 12 mo, an additional 4% and 2% achieved clinically significant weight loss among veterans with (20%) and without SDB (19%), respectively (P = 0.07). A greater percentage of veterans with SDB achieved clinically significant weight loss at 12 mo compared to veterans without SDB adjusting for potential confounders in the logistic regression models (P = 0.04; data not shown).
DISCUSSION
In this novel study, MOVE! participants with SDB lost statistically significantly less weight than those without SDB, although the difference was small (< 1 lb). For both groups, the absolute weight loss (2–3 lb) was modest. At the clinical level, this modest weight loss may be particularly problematic because veterans with a diagnosis of SDB weighed an average of 29 lb more and experienced greater medical comorbidity than veterans without SDB at MOVE! enrollment. Findings from several randomized controlled trials suggest that 10– 16% weight loss can reduce apnea-hypopnea index scores by 20–50%, resulting in a reduction of SDB symptoms and cardiovascular consequences.9,10,12 Hence, these findings suggest that enhancements to MOVE! programming may be needed to attain greater weight loss not only for the optimal treatment of SDB but also to alleviate SDB symptoms and to deter the progression of SDB.12
A majority of the MOVE! participants (55% with SDB and 56% without SDB [P = 0.04]) did not gain weight from baseline to 12 mo. MOVE! participation was not associated with progressive weight gain (1.1 to 2.2 lb/y among US adults27 or 4.4 lb for the year prior to MOVE! enrollment for veterans28) that is usually observed with aging and resulting in medical complications. Even the modest weight loss of 2–3 lb observed in this study may positively affect population health by slowing the progression of weight-related conditions including SDB and its associated medical comorbidities and impairments. Our findings also raise the possibility that concurrent treatment of SDB may optimize weight loss outcomes among affected participants.
Few veterans (20% with and 19% without SDB at 12 mo) achieved clinically meaningful weight loss in MOVE!. Continued quality improvements of the current MOVE! program are recommended to achieve and maintain the recommended weight loss of 10% or more to reduce SDB symptoms and deter the progression of SDB.12 Screening, diagnosis and treatment of SDB for all veterans at MOVE! enrollment may be an efficient and cost-effective method to enhance weight loss and slow the progression of SDB and subsequent cardiovascular and cardiometabolic disorders among overweight and obese veterans. Sleep improvement due to weight loss may motivate veterans to maintain their weight loss and prevent weight regain. Ongoing evaluation of weight loss and SDB symptoms among MOVE! participants may aid in the development of more effective behavioral interventions for SDB and obesity in this vulnerable population.
The evidence is mounting that SDB is on one or more pathways in the development of diabetes8 and cardiovascular disease.1 Unlike treatment of SDB with conventional methods such as CPAP machines, weight loss has the additional benefit of reducing the risk of diabetes and cardiovascular disease in this high-risk population. A recent study demonstrated greater improvements in cardiovascular risk factors (insulin resistance and serum triglycerides levels) among those randomly assigned to a weight loss intervention alone compared to those assigned to CPAP alone or CPAP combined with weight loss interventions.29 Weight loss may become the first line of defense and preferred treatment for SDB because it reduces cardiovascular risks and avoids the low adherence and high patient burden of CPAP use.30,31
Poor sleep quality may exacerbate mental health symptoms, accelerate the onset of cardiometabolic disturbances, and undermine compliance with medical treatments.32 In the current study, baseline characteristics of veterans with SDB revealed a medically complex patient population. Similar to studies from the general US population, veterans with SDB were more likely to be male and seriously obese. Medical comorbidities and service connection disability were consistently greater in veterans with SDB compared to those without SDB. Over half of the veterans with SDB had at least one comorbid mental health disorder, compared to 38% among the veterans without SDB.
Strengths and Limitations
Strengths of this observational study include the evaluation of a weight treatment program in a naturalistic and integrated health care setting; SDB diagnosis information gathered from ICD-9-CM codes in medical records (versus self-reports); and the evaluation of weight loss changes over a 1-y period in a nationally implemented weight loss program. The primary limitation of this study is the statistically but not necessarily clinically significant weight loss difference (0.4 lb) between SDB and non-SDB groups. This small effect size may be due to various methodological issues resulting in measurement error that should be addressed in future studies. Weight was measured in a clinical rather than research setting. Greater measurement error would be expected for weight assessments in a clinical setting6 compared to a research setting with standardized protocols (e.g., the removal of shoes prior to weight assessments and weighing on a calibrated scale). Due to the administrative nature of the data, we were not able to screen for SDB, confirm SDB diagnoses with objective sleep tests, or explore the influence of various SDB parameters (type of SA [central or obstructive], severity, treatment adherence [compliance with nightly CPAP use of 7 h or more33], sleep quality, or other barriers) on weight loss. We suspect that a significant percentage of veterans in the non-SDB comparison group of the analytic sample had undiagnosed SDB. Hence, our findings are probably biased toward the null hypnosis and provide a conservative estimate of the weight loss differences between the SDB and non-SDB groups. Although speculative, we expect that the observed differences in weight loss would be greater over time if the presence or absence of SDB was clinically confirmed rather than administratively determined. We also hypothesize that weight loss among individuals with a diagnosis of SDB may follow the dose-response relationship observed for hours of CPAP use and normal daytime functioning.33 Finally, future studies examining the influence of SDB on weight loss should examine potential confounders because the small weight loss difference may be explained by such factors.
Future investigations may want to include a SDB screen in their design as well as quantify treatment adherence for SDB. In addition, other factors should be investigated to determine their influence not only on the considerably greater baseline weight but also slower weight loss pattern among veterans with SDB compared to veterans without SDB. These include health (mobility/disability unrelated to service, tolerance of physical exercise, and chronic pain), lifestyle (occupational and/or household-related activity, time, support from family or friends, history of exercise habits,34 behaviors intended to compensate for poor sleep such as daytime napping, and other leisure activities),34 behavioral (propensity to overeat or binge eating, negative body image, motivation and self-efficacy to change health behaviors,35 and strong personal incentives35), and environmental (access to safe, convenient, welcoming, and affordable facilities35) factors. Despite these limitations and potential biases, our findings suggest that SDB may impede weight loss.
CONCLUSIONS
Nationally, participation in the MOVE! program was associated with a lack of weight gain in overweight and obese veterans with SDB. This finding is clinically relevant because SDB has been shown to have a dose-response relationship with body weight, with the severity of symptoms increasing with weight. Hence, the prevention of weight gain, over time, is clinically significant and may slow the progression of SDB. Our findings also highlight the need for more effective weight loss programs among overweight and obese veterans because weight loss of at least 10 kg or 10% of body weight is currently recommended to achieve significant improvements in SDB symptoms. Possible enhancements for accelerated weight loss that could be tested in future trials include (1) increasing the intensity of the intervention by more frequent contacts and/ or lengthening the intervention; (2) providing more directive diet modifications and plans; (3) using efficacious protocols such as the Diabetes Prevention Program for clinically meaningful weight loss; (4) assessing and treating SDB at MOVE! enrollment; (5) providing weight loss motivation targeted for obese veterans with SDB; (6) emphasizing physical activity for weight maintenance and sleep improvement; and (7) emphasizing sleep hygiene behavioral interventions, such as abstaining from alcohol and caffeine, that may also aid in weight loss. Finally, the influence of SDB on weight loss needs to be confirmed in overweight and obese civilians and other high-risk populations.
In this study, veterans with SDB had significantly less weight loss over time than veterans without SDB. Even though the absolute difference in weight loss was small (< 1 lb), the relative difference in weight loss was large (32% at 6 mo) for veterans with and without SDB. However, the effect of SDB on weight loss appears to be smaller than the positive effect of weight loss on SDB severity.31 Thus, from a clinical perspective, targeting weight loss among individuals with SDB should continue to be a top priority. SDB should be considered in the development and implementation of weight loss programs due to its high prevalence and negative effect on health.
DISCLOSURE STATEMENT
This was not an industry supported study. This report was conducted as a joint quality improvement evaluation involving VA Health Services Research and Development Quality Enhancement Research Initiative (QUERI) programs, VHA National Center for Health Promotion & Disease Prevention (NCP), and VA Serious Mental Illness Resource and Evaluation Center (SMITREC). It is part of a larger quality improvement evaluation of MOVE! conducted by the SMITREC at the request of NCP. Funding was obtained by Dr. Janney and Dr. Goodrich from the QUERI programs for Diabetes and Mental Health research as a locally initiated project (QLP 55-017). Dr. Janney was supported by the VA Center for Clinical Management Research, Health Services Research and Development as a postdoctoral fellow at the VA Ann Arbor Healthcare System. Dr. Klingaman was supported by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment. The funding bodies had no role in the manuscript other than financial support. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. Dr. Kilbourne is a co-author of the workbook “Overcoming Bipolar Disorder: A Comprehensive Workbook for Managing Your Symptoms & Achieving Your Life Goals” (New Harbinger Publications, Inc., 2008) and receives publication royalties. The other authors have no conflicts of interest to disclose. MOVE! was developed internally by VA behavioral change experts to meet the needs of Veterans and is not a commercial product.
ACKNOWLEDGMENTS
The authors are grateful to Wyndy Wiitala, PhD,and David Ratz, MS for their statistical expertise and guidance, and Kenneth R. Jones, PhD and Linda S. Kinsinger, MD, MPH for their constructive feedback and comments regarding the manuscript and the research.
CAJ initiated the study and was responsible for the study design. CAJ had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. CRR, KDH, EAK, DEG, AG, and AMK contributed to the study design. ZL and LV performed the data extraction and statistical analyses. CAJ wrote the manuscript. All authors provided critical review of the manuscript.
Partially presented at the Society of Behavioral Medicine Conference, 35th Annual Meeting and Scientific Sessions, Philadelphia, PA. April 23–26, 2014; and Albert J. Silverman Research Conference, University of Michigan Department of Psychiatry, Ann Arbor, MI, April 16, 2014.
REFERENCES
- 1.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118:1080–111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 2.Leinum CJ, Dopp JM, Morgan BJ. Sleep-disordered breathing and obesity: pathophysiology, complications, and treatment. Nutr Clin Pract. 2009;24:675–87. doi: 10.1177/0884533609351532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291:2013–6. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]
- 4.Washington DC: 2014. Veterans Health Administration, Patient Care Services, and Health Promotion and Disease Prevention, Fiscal Year 2013 Evaluation Report: MOVE! Weight Management Program for Veterans, U.S. Department of Veterans Affairs, Editor. [Google Scholar]
- 5.Mustafa M, Erokwu N, Ebose I, Strohl K. Sleep problems and the risk for sleep disorders in an outpatient veteran population. Sleep Breath. 2005;9:57–63. doi: 10.1007/s11325-005-0016-z. [DOI] [PubMed] [Google Scholar]
- 6.Noel PH, Copeland LA, Pugh MJ, et al. Obesity diagnosis and care practices in the Veterans Health Administration. J Gen Intern Med. 2010;25:510–6. doi: 10.1007/s11606-010-1279-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharafkhaneh A, Richardson P, Hirshkowitz M. Sleep apnea in a high risk population: a study of Veterans Health Administration beneficiaries. Sleep Med. 2004;5:345–50. doi: 10.1016/j.sleep.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Boyko EJ, Seelig AD, Jacobson IG, et al. Sleep characteristics, mental health, and diabetes risk: a prospective study of U.S. military service members in the Millennium Cohort Study. Diabetes Care. 2013;36:3154–61. doi: 10.2337/DC13-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anandam A, Akinnusi M, Kufel T, Porhomayon J, El-Solh AA. Effects of dietary weight loss on obstructive sleep apnea: a meta-analysis. Sleep Breath. 2013;17:227–34. doi: 10.1007/s11325-012-0677-3. [DOI] [PubMed] [Google Scholar]
- 10.Tuomilehto HP, Seppa JM, Partinen MM, et al. Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2009;179:320–7. doi: 10.1164/rccm.200805-669OC. [DOI] [PubMed] [Google Scholar]
- 11.Tuomilehto H, Seppa J, Uusitupa M, et al. The impact of weight reduction in the prevention of the progression of obstructive sleep apnea: an explanatory analysis of a 5-year observational follow-up trial. Sleep Med. 2014;15:329–35. doi: 10.1016/j.sleep.2013.11.786. [DOI] [PubMed] [Google Scholar]
- 12.Araghi MH, Chen YF, Jagielski A, et al. Effectiveness of lifestyle interventions on obstructive sleep apnea (OSA): systematic review and meta-analysis. Sleep. 2013;36:1553–62. doi: 10.5665/sleep.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borel AL, Leblanc X, Almeras N, et al. Sleep apnoea attenuates the effects of a lifestyle intervention programme in men with visceral obesity. Thorax. 2012;67:735–41. doi: 10.1136/thoraxjnl-2011-201001. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Niu X, Xiao Y, Dong J, Lu M, Kong W. Effect of continuous positive airway pressure on leptin levels in patients with obstructive sleep apnea: a meta-analysis. Otolaryngol Head Neck Surg. 2015;152:610–8. doi: 10.1177/0194599814562719. [DOI] [PubMed] [Google Scholar]
- 15.Grandner MA. Addressing sleep disturbances: an opportunity to prevent cardiometabolic disease? Int Rev Psychiatry. 2014;26:155–76. doi: 10.3109/09540261.2014.911148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knutson KL. Does inadequate sleep play a role in vulnerability to obesity? Am J Hum Biol. 2012;24:361–71. doi: 10.1002/ajhb.22219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanlon EC, Van Cauter E. Quantification of sleep behavior and of its impact on the cross-talk between the brain and peripheral metabolism. Proc Natl Acad Sci U S A. 2011;108:15609–16. doi: 10.1073/pnas.1101338108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomson CA, Morrow KL, Flatt SW, et al. Relationship between sleep quality and quantity and weight loss in women participating in a weight-loss intervention trial. Obesity (Silver Spring) 2012;20:1419–25. doi: 10.1038/oby.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahwati LC, Lance TX, Jones KR, Kinsinger LS. RE-AIM evaluation of the Veterans Health Administration's MOVE! Weight Management Program. Transl Behav Med. 2011;1:551–60. doi: 10.1007/s13142-011-0077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinsinger LS, Jones KR, Kahwati L, et al. Design and dissemination of the MOVE! Weight-Management Program for Veterans. Prev Chronic Dis. 2009;6:A98. [PMC free article] [PubMed] [Google Scholar]
- 22.VHA National Center for Health Promotion & Disease Prevention (NCP) MOVE! Weight Management Program. [Accessed July 15, 2014]. http://www.move.va.gov.
- 23.Kahwati LC, Lewis MA, Kane H, et al. Best practices in the Veterans Health Administration's MOVE! Weight management program. Am J Prev Med. 2011;41:457–64. doi: 10.1016/j.amepre.2011.06.047. [DOI] [PubMed] [Google Scholar]
- 24.Nedeltcheva AV, Kilkus JM, Imperial J, Schoeller DA, Penev PD. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435–41. doi: 10.1059/0003-4819-153-7-201010050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCarthy JF, Blow FC, Valenstein M, et al. Veterans Affairs Health System and mental health treatment retention among patients with serious mental illness: evaluating accessibility and availability barriers. Health Serv Res. 2007;42:1042–60. doi: 10.1111/j.1475-6773.2006.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.U.S. Department of Veterans Affairs. Disability Compensation. [Accessed March 16, 2015]. http://www.benefits.va.gov/compensation/types-disability.asp.
- 27.Hutfless S, Gudzune KA, Maruthur N, et al. Strategies to prevent weight gain in adults: a systematic review. Am J Prev Med. 2013;45:e41–51. doi: 10.1016/j.amepre.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Dahn JR, Fitzpatrick SL, Llabre MM, et al. Weight management for veterans: examining change in weight before and after MOVE! Obesity (Silver Spring) 2011;19:977–81. doi: 10.1038/oby.2010.273. [DOI] [PubMed] [Google Scholar]
- 29.Chirinos JA, Gurubhagavatula I, Teff K, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med. 2014;370:2265–75. doi: 10.1056/NEJMoa1306187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnes M, Goldsworthy UR, Cary BA, Hill CJ. A diet and exercise program to improve clinical outcomes in patients with obstructive sleep apnea--a feasibility study. J Clin Sleep Med. 2009;5:409–15. [PMC free article] [PubMed] [Google Scholar]
- 31.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–21. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 32.Lunsford-Avery JM. Sleep dysfunction prior to the onset of schizophrenia: a review and neurodevelopmental diathesis-stress conceptualization. Clin Psychol Sci Pract. 2013;20:291–320. [Google Scholar]
- 33.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711–9. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith SS, Doyle G, Pascoe T, Douglas JA, Jorgensen G. Intention to exercise in patients with obstructive sleep apnea. J Clin Sleep Med. 2007;3:689–94. [PMC free article] [PubMed] [Google Scholar]
- 35.Igelstrom H, Martin C, Emtner M, Lindberg E, Asenlof P. Physical activity in sleep apnea and obesity-personal incentives, challenges, and facilitators for success. Behav Sleep Med. 2012;10:122–37. doi: 10.1080/15402002.2011.574763. [DOI] [PubMed] [Google Scholar]


