Abstract
Study Objectives:
To further evaluate adolescent brain maturation by determining the longitudinal trajectories of nonrapid eye movement (NREM) sigma (11–15 Hz) power across childhood-adolescence.
Methods:
The maturational trend for sigma (11–15 Hz) power was evaluated in an accelerated longitudinal study of three overlapping age cohorts (n = 92) covering ages 6 to 18 y. Semiannually, sleep electroencephalography (EEG) was recorded from participants sleeping at home in their normal sleep environment while keeping their current school night schedules.
Results:
Sigma frequencies became faster with age. The frequency of the 11–15 Hz spectral peak increased linearly. Sigma frequency power (SFP) declined with age, but its trajectory was complex (cubic). Power in a group of low sigma subfrequencies declined with age. Power in a group of high sigma frequencies increased with age. Power in subfrequencies within 11–15 Hz also showed different trends across the night, with lower frequencies increasing across NREM periods and higher frequencies decreasing across NREM periods. The upper and lower boundaries for the sigma frequencies that changed across NREMPs shifted upward with age.
Conclusions:
We hypothesize that these maturational brain changes result from synaptic elimination which decreases sleep depth and streamlines circuits. SFP displays a maturational trajectory different from both delta and theta power. Theories on the function of sigma must be reconciled with its maturational trajectory. These findings further demonstrate the value of sleep EEG for studying noninvasively the complex developmental brain changes of adolescence.
Citation:
Campbell IG, Feinberg I. Maturational patterns of sigma frequency power across childhood and adolescence: a longitudinal study. SLEEP 2016;39(1):193–201.
Keywords: adolescence, brain maturation, EEG, NREM, sleep spindle, trajectory
Significance.
These longitudinal data demonstrate the maturational trend for a hallmark of non-rapid eye movement sleep, sigma frequency EEG. Low and high sigma frequency power (SFP) have different age trajectories with low SFP showing a trajectory similar to that of delta EEG power. These changes in SFP, along with the linear increase in the frequency of peak sigma power, likely reflect maturation of thalamocortical circuits. SFP provides an index of sleep spindle activity, currently a focus of research on sleep and cognition and psychiatric illness. Theories on the functional significance of spindles must ultimately integrate the maturational changes described here. These maturational changes further our understanding of adolescent brain development which we hypothesize is driven largely by synaptic elimination.
INTRODUCTION
The human brain undergoes extensive postnatal development that includes rapid synaptic growth in the first years of life and a steep decline in synaptic density across adolescence.1 Maturational changes are particularly prominent in the sleep electroencephalogram (EEG), where they are much more substantial in nonrapid eye movement (NREM) sleep than in rapid eye movement (REM) sleep. To investigate these developmental changes, we carried out a longitudinal study of normative sleep EEG across ages 6–18 y. Our previous reports described the age trajectories of fast Fourier transform (FFT) power in two major NREM frequency bands, delta (1–4 Hz) and theta (4–8 Hz)2,3 as well as changes in sleep duration,4 dynamics,5 and daytime sleepiness.6 Power in both delta and theta NREM EEG declines massively across adolescence, but their trajectories differ between ages 6 and 12 y. Here we report the longitudinal trajectories of FFT power in a third major group of NREM frequencies, those in the sigma band, typically defined as 12–15 Hz.
After the discovery of the inverse relation of sigma frequency power (SFP) and delta power,7 it became clear that SFP could provide an index of the amount of organized spindle burst activity without prior pattern recognition detection of spindles. The high amplitude of the sinusoidal waves that distinguish organized spindles dominates the much lower amplitude of background activity in 12–15 Hz NREM EEG. Several investigators are now using SFP to index the overall amount of organized spindle activity. However, it is widely recognized that SFP does not measure the density, mean frequency, or average duration of spindles. These biologically important features should eventually be assessed because they change with aging8 and probably with maturation as well.
Organized spindles produce a clear peak in the sigma frequency range of the EEG power spectrum of NREM. Shinomiya et al.9 were the first to report that the frequency of this peak increases across childhood and adolescence. A later longitudinal study by Tarokh and Carskadon10 also found an increase in peak spindle frequency. The slower frequency of spindle activity in children suggests that the lower boundary of the sigma band should be set at 11 Hz for studies in children and adolescents. Spindle density and duration also show developmental changes.11 NREM sigma and sleep spindles continue to change in adulthood and change further in the elderly years.8,12,13
Research into SFP maturation must take into account the possibility that power within sigma will not behave uniformly. Thus, the lower and higher frequencies within sigma respond differently to sleep deprivation, circadian changes, and can also exhibit different trends across the night.14–16 The neuronal mechanisms underlying spindle frequency oscillations have been the subject of basic investigations that focused on the interplay of cortical and thalamic structures in producing EEG oscillations in the sigma frequencies.17–19
Here, we analyzed our longitudinal recordings across childhood and adolescence (ages 6–18 y) to determine: (1) the maturational trajectory of the peak in SFP; (2) the maturational trend for SFP in 11–15 Hz; (3) whether the maturational trends within 11–15 Hz SFP differ among higher and lower subfrequencies; and (4) whether higher and lower subfrequencies show significant differences in across-night trends and how these trends change with age.
METHODS
Subjects
Our longitudinal data set is composed of three overlapping age cohorts. Cohort C6 (n = 25, 11 girls) entered the study at approximately age 6 y and was studied for 4 y. Cohort C9 (n = 30, 15 girls) entered the study at approximately age 9 y and was studied for 7 y. Cohort C12 (n = 37, 19 girls) entered the study at approximately age 12 y and was studied for 6 y. Thus, the three cohorts span ages 6 to 18 y with C6 and C9 overlapping for 1 y (ages 9–10 y) and C9 and C12 overlapping for 4 y (ages 12–16 y).
At the time of enrollment in the study, an interview with the parents screened subjects for the following exclusion criteria: psychiatric or behavioral disorders, epilepsy, head injury that resulted in loss of consciousness, and use of medication that affects the central nervous system. Parents provided informed consent and subjects older than 12 y provided assent. Subjects were paid for participating in the study. The UC Davis Institutional Review Board approved all procedures.
Experiment Design
All-night EEG was recorded semiannually in the subjects' homes in their usual sleep environment. Subjects in the C6 cohort were recorded for 2 consecutive weeknights while maintaining their current school year weeknight sleep schedules. Subjects in the C9 and C12 cohorts were recorded for 4 consecutive nights. On nights 1 and 2, subjects maintained their current habitual weeknight bedtime and rise time. On nights 3 and 4, subjects went to sleep at their current habitual weekday bedtime but slept as long as possible, up to 12 h.
It is well established that bedtimes shift to a later hour as children mature through adolescence. We did not require that our subjects between the ages 6 and 18 y maintain the same bedtimes and rise times. Instead we recorded subjects sleeping on their ongoing current schedules. However, we required that they maintain this schedule throughout the recording period and also for the 5 nights that preceded recording. We used actigraphy watches to confirm fidelity to these agreed-upon schedules. When subjects deviated from their scheduled bed or rise times, or took inadvertent naps, we rescheduled the EEG recordings.
EEG Recording and Analysis
EEG was recorded with electrodes at Fz, Cz, C3, C4, O1, and either O2 or Pz with A1 and A2 mastoid electrodes. Electrooculogram (EOG) was recorded with electrodes applied at left and right outer canthi referred to a forehead electrode. Chin EMG was not recorded. All recordings were made versus a reference, and electrode pairs such as C3-A2 were obtained by subtraction. For the first nine semiannual recordings in the C9 and C12 cohorts, signals were amplified and recorded on Grass H2O ambulatory recorders (200 Hz digitization rate, Grass Technologies, Natus Neurology, Warwick, RI). During the course of this longitudinal study, Grass Instruments discontinued support for the H2O amplifier. For the 10th recording and later, signals were amplified and recorded on Grass Aura ambulatory recorders (400 Hz digitization rate, Grass Technologies, Natus Neurology, Warwick, RI). Aura recorders were used for all eight recordings in the C6 cohort. The H2O and Aura recorders have the same low frequency hardware filter, but their high-frequency filters differ. The Aura has a three-pole, high-frequency filter with a −3 dB point at 100 Hz and an 18 dB/octave slope, leaving the sigma frequency waves unfiltered. The H2O high frequency filters have a lower −3 dB point (approximately 55 Hz as determined by our frequency response curves) which slightly reduces the amplitude of sigma frequency waves. We adjusted for this effect with a correction factor determined from the frequency-response curves for H2O and Aura amplifiers. We created the frequency response curves by recording various frequency sine waves of known amplitudes on both recorders. We multiplied the EEG power recorded on the H20 amplifiers by a correction factor whose values ranged from 1.021 for the 11.0–11.2 Hz band to 1.039 for the 14.9 to 15.1 Hz band.
Using a computer display of EEG and EOG recordings, trained raters scored each 20-sec epoch of EEG recording as wake, NREM, REM, or movement. Raters followed Rechtschaffen and Kales20 criteria, modified by collapsing stages 2, 3, and 4 into a single NREM sleep stage. Raters marked the presence of artifacts in addition to vigilance state score. Each record was scored by one rater and checked by another. Disagreements were reconciled by a senior laboratory scientist.
Selecting the channel with fewer artifacts, we analyzed either C3-A2 or C4-A1 with the spectral analysis algorithms of PassPlus (Delta Software, St. Louis, MO, USA). Fast Fourier Transform (FFT) parameters were as follows: 5.12-sec Welch tapered windows with 2.62-sec overlap for eight windows per 20-sec epoch. Data in the 11 to 15 Hz range were analyzed in 0.195-Hz bands, the maximum resolution for 5.12-sec windows. Previously we defined the sigma band as 12 to 15 Hz, but children have slower spindles than adults.13 We therefore analyzed 11–15 Hz to include the slower spindle-like activity. Because sleep durations on habitual school schedules declined across adolescence,4 we limited analyses to sleep durations common to 18-y-old subjects. For NREM sigma, SFP represents the average power in all artifact-free epochs in the first 5 h of NREM sleep, and for REM sigma, the power reported is the average power in all artifact-free epochs in REM periods 2 and 3. We analyzed REM periods 2 and 3 because older subjects sometimes failed to complete a fourth REM period and younger subjects often “skipped” their first REM period.
To perform trend analysis we analyzed the first four NREM periods (NREMPs) even if these periods extended beyond the first 5 h of NREM sleep. For each subject-night, average power in each NREM period was calculated for 0.195 Hz frequency bands between 11 and 15 Hz.
For each subject-night of data, we also determined the sigma peak frequency, i.e., the 0.195- Hz band between 11 and 15 Hz that showed a distinct peak in power. For the occasional subject-night that showed two distinct peaks, we chose the higher frequency peak which, in all cases, had greater power. One subject in the C9 cohort had infrequent, low-amplitude spindles. In this subject, power declined steadily across the sigma range without a clear peak. His data were not included in analyses of age effects on sigma peak frequency but were included in analyses of age effects on SFP.
Statistical Analysis
Prior to statistical analysis, multiple nights at each semiannual recording were averaged for each subject. For the C6 cohort, this average was based on 2 nights of recording. For the C9 and C12 cohorts, this average included not only the 2 nights on the habitual sleep schedule, but also a third consecutive night, on which subjects slept as long as possible. On this night, the first 5 h of NREM as well as REM periods 2 and 3 occurred prior to sleep extension and, therefore, could be included with the night 1 and 2 data. Data from the fourth night were excluded because of the sleep extension on night 3.
Age effects on sigma power were evaluated with mixed-effect analysis with age as both a fixed and random variable. Mixed-effect analysis is particularly suited for longitudinal data because it accounts for the correlation between data points recorded from the same subject over time.21,22 To make intercept data meaningful, age was centered by subtracting 6 from all subject ages. For 11–15 Hz power, linear, quadratic, and cubic age trends were evaluated. To determine if age effects were uniform across 11–15 Hz, linear age effects were examined in each 0.195-Hz band within 11–15 Hz. Mixed-effect analysis was also used to evaluate age-related changes in sigma peak frequency. Sex was included as a class variable to test for sex differences in the age related changes in sigma power and in sigma peak frequency.
Initially, we examined age-related changes in across-NREMP trends using a mixed-effect analysis with cycle and age factors and a cycle by age interaction. The cycle by age interaction was significant for nearly every frequency band between 11 and 15 Hz. To examine more closely how the trends across NREMPs changed with age, a mixed-effect analysis of the change in power across NREM periods was performed for each 0.195 Hz band for each recording period of each cohort.
In presenting results, data were averaged across subjects for each semiannual recording for each cohort. However, the statistical analysis used age as a continuous variable, calculated by subtracting date of birth from the date of the first night of EEG recording.
RESULTS
Frequency of Peak Sigma Power Increases Linearly with Age
We first examined the FFT spectra of our cohorts to establish that, similar to adults, these young subjects showed an augmentation or “peak” in 11–15 Hz power in NREM but not in REM sleep. Figure 1 shows that at age 12 y (the midpoint of the age range of this study) a clear sigma peak is present in the NREM spectrum but not the REM spectrum. This peak was present in all cohorts at each measurement period.
Figure 1.
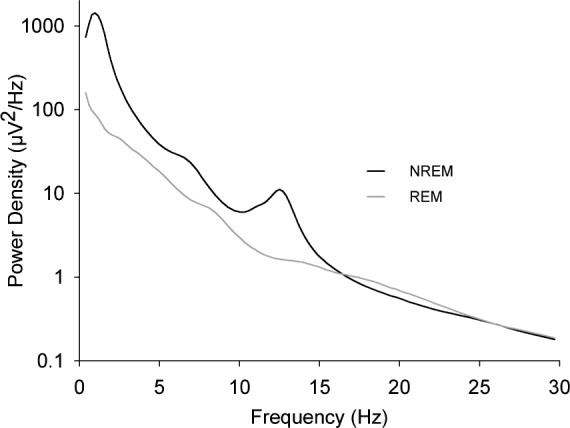
Power spectra at age 12 y for electroencephalogram recorded during the first 5 h of nonrapid eye movement (NREM) sleep (black line) or during the second and third rapid eye movement (REM) periods (gray line). Mean (n = 65) power density is plotted against the midpoint of the frequency band. A peak occurs in the sigma frequency band (11–15 Hz) in NREM but not in REM sleep in each age group.
The SFP peak occurred at steadily higher frequencies with increasing age. The frequency progression was strikingly linear (Figure 2). Linear mixed-effect analysis across ages 6–18 y revealed an intercept of 11.83 (± 0.05) Hz at age 6 y and a hugely significant increase of 0.102 (± 0.009) Hz/y (F1,865 = 128, P < 0.0001). There were no significant sex differences in linear intercept (F1,89 = 1.4, P = 0.24) or slope (F1,864 = 0.16, P = 0.69). There was good agreement between cohorts (Figure 2); in the 4 y of overlap between C9 and C12, the trends did not differ significantly (F1,413 = 0.05, P = 0.82).
Figure 2.
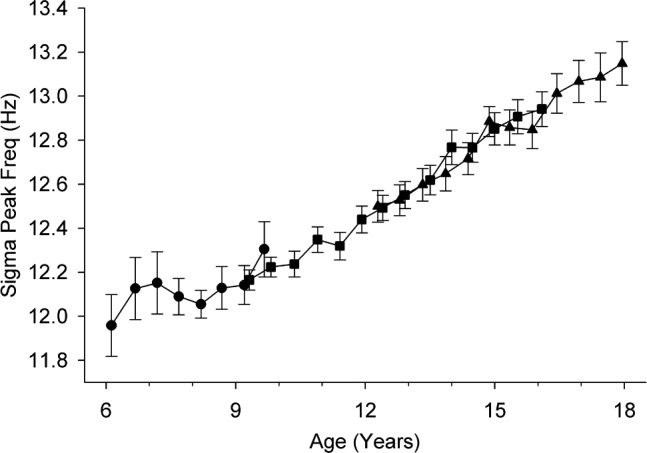
Maturational trajectory of the frequency of peak sigma power. The average (± standard error) peak frequency at each semiannual recording is plotted against age for each of the three cohorts, C6 (circles), C9 (squares), and C12 (triangles). The linear increase is highly significant (F1,865 = 128, P < 0.0001).
Maturational Pattern of NREM Sigma Power
NREM SFP showed a complex trajectory with age. Power in 11–15 Hz increased significantly across ages 6 to 12 y and then significantly declined between ages 12 and 16 y (Figure 3). A small increase in power (0.3 μV2/y) across ages 16 to 18 y was not significant (F1,101 = 0.51, P = 0.48). Overall, SFP showed a significant linear decline from age 6 to 18 y (F1,872 = 46.6, P < 0.0001). However, its trajectory was better fit by a cubic equation, 0.033*(age-6)3-0.74*(age-6)2+3.7*(age-6)+22, with all coefficients significantly different from 0 at P < 0.0001. The Bayesian information criterion (lower is better) for goodness of fit dropped from 5814 for the linear model to 5749 for the cubic model. Linear evaluation of the age change in SFP did not reveal significant sex differences in either intercept (F1,90 = 0.63, P = 0.43) or slope (F1,872 = 1.32, P = 0.25). Similarly, there were no significant sex differences in any of the coefficients of the cubic equation (P > 0.34 for all).
Figure 3.
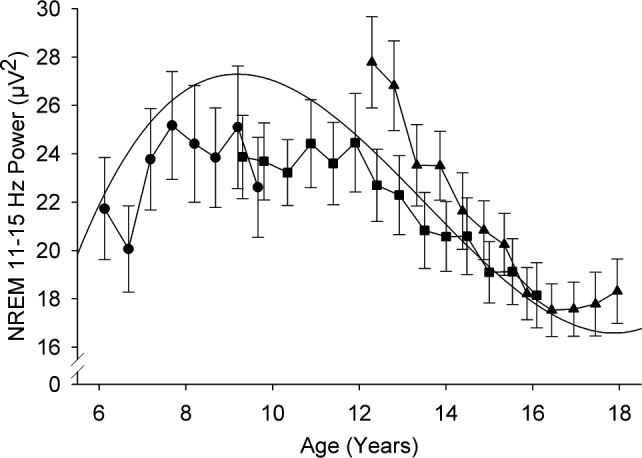
Maturational trajectory of sigma power shows a power increase at younger ages followed by a steep decrease across adolescence. Average (± standard error) power at each semiannual recording is plotted against age for the three cohorts. The trend line is a cubic function fit with mixed-effect analysis.
Unlike the close correspondence we found for the age curves of delta and theta power in C9 and C12 in their 4 y of overlap,3 SFP in these cohorts differed in the overlap years (Figure 3). A mixed-effect analysis of SFP across the ages of overlap revealed that the age 12 y intercept for C9 was 11.7 μV2 lower (F1,66 = 4.97, P = 0.029) than the 28.2 μV2 intercept for C12, and that the rate of decline for C9 was 1.2μV2/y less steep (F1,412 = 5.49, P = 0.020) than the 2.6 μV2/y rate of decline for C12. Despite these cohort differences, separate analysis of each cohort supported the aforementioned age trends. Thus, in the C6 cohort, sigma power increased (F1,142 = 7.68, P = 0.0067) by 1.1 μV2/y between ages 6 and 10 y. In the C9 cohort, sigma power was unchanged (F1,132 = 0.13, P = 0.72) between ages 9 and 12 y before declining (F1,185 = 29.2, P < 0.0001) by 1.50 μV2/y between ages 12 and 16 y. Analyzing the C12 cohort data alone showed a 2.59 μV2/y decline in SFP between ages 12 and 16 y (F1,244 = 48.6, P < 0.0001).
Maturational Changes in 0.195 Hz Subbands within 11–15 Hz
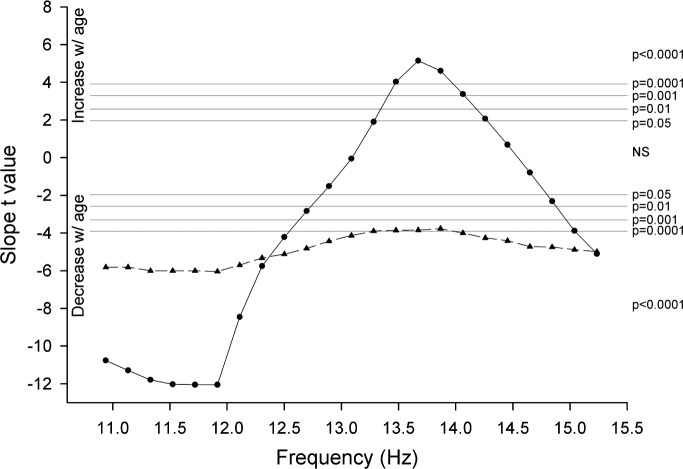
Previous investigators have shown that different subfrequencies within sigma can respond differently to experimental challenges. We examined 0.195 Hz subbands within the 11–15 Hz range to determine whether different subfrequencies manifest different maturational trajectories. We found that power in some low sigma frequencies declined with age but that power increased in some higher frequencies. To select these subfrequencies objectively, we computed t-values for the slopes of the age-related change in power for each 0.195 Hz band between 11 and 15.3 Hz. These results are shown in Figure 4. In all subfrequencies below 12.8 Hz, power declined significantly with age. Power in subfrequencies between 12.8 and 13.4 Hz did not change significantly with age. Power in subfrequencies between 13.4 and 14.4 Hz increased with age. Between 14.4 Hz and 14.9 Hz, power did not change significantly with age. Power in frequencies above 14.9 Hz declined significantly with age. These complex age patterns within NREM 11–15 Hz were entirely absent in the 11–15 Hz EEG of REM sleep. In REM, power in all subfrequencies between 11 and 15.3 Hz declined significantly (P < 0.0002) with age.
Figure 4.
Statistical analysis demonstrates significantly different maturational trajectories for the subfrequencies within sigma. For each 0.195 Hz subfrequency band between 10.8 and 15.3 Hz, the t-value for the slope of the age-related change in power is plotted against frequency band midpoint. Results are for the first 5 h of nonrapid eye movement (NREM) sleep (solid line, circles) and the second and third rapid eye movement (REM) periods (dashed line, triangles). Horizontal gray lines indicate significance level. Power in frequencies below 12.8 Hz and above 14.9 Hz declined significantly with age, and power in frequencies between 13.4 and 14.4 Hz increased significantly with age. In REM sleep, power in all sigma subfrequencies declined with age.
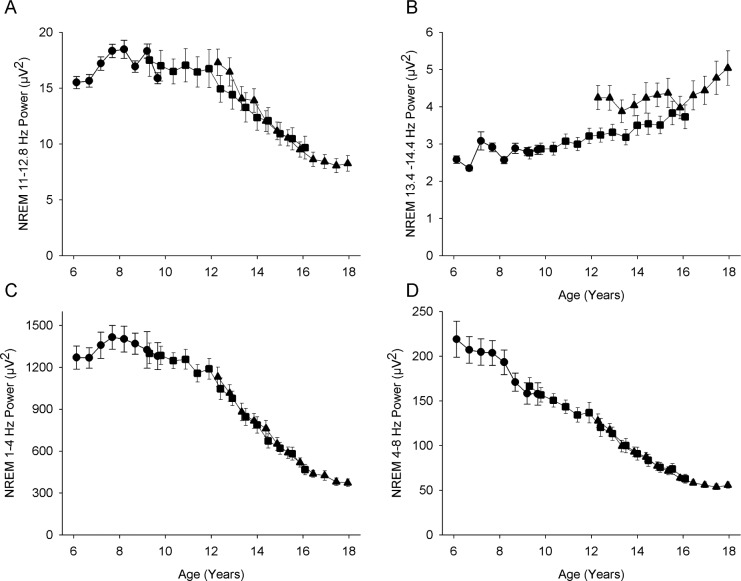
For NREM EEG, we summed power over 11.0 to 12.8 Hz (significant decline with age) and designated it low frequency SFP (SFP-LF). We summed power over 13.4 to 14.4 Hz (significant increase with age) and designated it high frequency SFP (SFP-HF). The maturational trends for power in the LF and HF sigma frequencies are shown in Figure 5, which also presents the trends in delta and theta we previously reported in these subjects.3 Power in SFP-LF showed an overall decline with age despite an initial increase in power between 6 and 8 y (Figure 5A). Older than age 8 y, power in the lower sigma frequencies declined slowly until age 12 y, when the rate of decline accelerated before leveling off at age 16 y. SFP-HF (Figure 5B) increased slowly but significantly between ages 8 and 15 y and then increased steeply between ages 15 and 18 y.
Figure 5.
Maturational trajectory of power in (A) 11.0–12.8 Hz, the frequency range that significantly decreases in power with age and (B) 13.4–14.4 Hz, the frequency range that significantly increases in power with age. Also shown for comparison are the maturational trends for (C) delta (1–4 Hz) and (D) theta (4–8 Hz) in these subjects. The trajectory for low frequency sigma power (A) strongly resembles that for delta power (C). Format as in Figure 3.
It is interesting to contrast the maturational trajectories of high and low SFP with the trajectories for delta (1–4 Hz) and theta (4–8 Hz) power. Delta and theta age curves differ in that delta power increases between ages 6 and 8 y before declining slowly to age 12 y, when its decline sharply accelerates, leveling off at about age 16.5 y. In contrast to delta, theta power declines steadily from our earliest measurements at age 6 y; nevertheless, its decline also accelerates between ages 12 and 16.5 y. The maturational trajectory for SFP-LF is similar in shape to that of delta, but the magnitude of the decline in sigma is somewhat smaller. The age trend for SFP-HF differs markedly from the trajectories of delta, theta, and SFP-LF.
Across-NREMP Trends in SFP
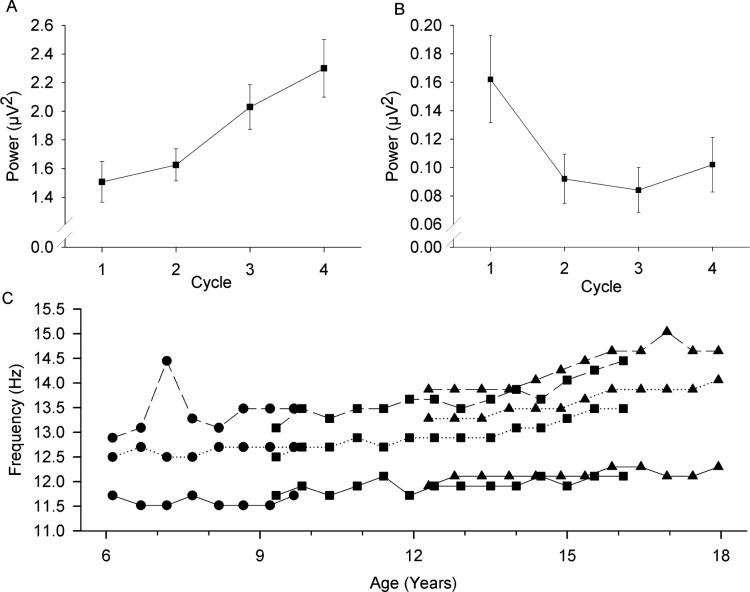
As mentioned in Methods, the across-NREMP trends in power showed a significant age interaction for nearly all subfrequencies between 11 and 15 Hz. Therefore, the cycle trends were analyzed separately for each semiannual recording in each cohort. Below 11 Hz, power decreased across NREMPs. Between 11 and 12.5 Hz, this trend reversed, and power increased across NREMPs (Figure 6A). The frequency at which this reversal occurs advanced with age (Figure 6C). At age 7 y, 11.5 Hz was the lowest frequency band for which power increased significantly (P < 0.05) across the night, whereas at age 18 y, 12.3 Hz was the lowest frequency for which power increased across the night. The highest within-sigma frequency that increased across the night also advanced with age. At age 7 y, the highest band for which power increased significantly across the night was 12.3 Hz; at age 18 y, it had increased to 13.7 Hz. With increasing frequency within 11–15 Hz, the across-cycle trend reversed again and power decreased across the night (Figure 6B). At age 7 y, decreasing power was evident by 13 Hz; at age 18 y the decrease in power across the night did not become significant until 14.6 Hz. We note here that the (changing) low and high frequencies that show opposite trends across NREMPs are not the same as the low and high frequencies that show opposite age trajectories. It is also important to note that the decrease in high frequency sigma power across NREMPs was entirely due to a sharp decrease from NREMP1 to NREMP2; NREMPs2–4 showed little change or even a slight increase across the night (Figure 6B).
Figure 6.
The trend in power across the night differs by frequency band. Mean power (± standard error) for nonrapid eye movement (NREM) periods 1–4 for the seventh recording of the C9 cohort (mean age = 12.4 y) is plotted against NREM period for (A) a subfrequency, 12.6–12.8 Hz, which increases in power across the night and for (B) a subfrequency, 14.6–14.7 Hz, which decreases in power across the night. The trends across the night change as children mature. Thus, Figure 6C shows for each recording period for each cohort, the lowest 0.195 Hz frequency band for which power increases significantly across the night (solid line), the highest 0.195 Hz frequency band for which power increases significantly across the night (dotted line), and the lowest 0.195 Hz frequency band at which the trend reverses and power decreases significantly across the night (dashed line).
DISCUSSION
As Purves23 and others have emphasized, postnatal brain maturation involves constructive as well as regressive changes. Thus, while supernumerary synapses are eliminated, surviving synapses become structurally more complex and more effective. We have interpreted the massive declines in both delta and theta power across adolescence as direct and indirect effects of synaptic elimination.2,3 The sigma trajectories are more complex and likely reflect constructive as well as regressive brain development. Overall, NREM sigma power shifts to higher frequencies across childhood/adolescence, suggesting that synaptic elimination has produced faster and presumably more effective circuity.
Peaks in NREM and REM Spectra
An a priori question for interpreting our data is whether SFP represents the intensity of the EEG in organized spindles. Basic support for this possibility is the long-standing evidence that the power spectrum of NREM sleep shows a peak at the sigma frequencies, whereas the REM spectrum does not. NREM but not REM sleep contains organized spindles; as previously mentioned, the amplitude of organized spindles is grossly higher than that of the background 11–15 Hz EEG, producing the sigma frequency peak. Most of the evidence showing NREM sigma peaks is from adults although several investigators9,24 have reported the same finding in children and adolescents. Our data here show strong sigma peaks in all cohorts. This finding supports the interpretation that the longitudinal changes in 11–15 Hz power found here are produced by developmental changes in organized sleep spindles.
The Linear Increase in Frequency of Peak Sigma Power
The increase in spindle peak frequency is remarkably linear and highly significant. This linear age-related change was first reported by Shinomiya et al.9 in cross-sectional data for frontal and centroparietal SFP. Our results in the central EEG versus contralateral mastoid confirm and extend their centroparietal findings in a longitudinal sample with larger numbers. We observed no inflection in the linear progression; therefore, it is likely that peak spindle frequency continues to increase beyond its value of 13.15 Hz at age 18 y. A physiological basis for this developmental increase in sigma frequency is proposed below under the heading “Maturational Increase in SFP-HF.”
Maturational Trajectory of SFP (11–15 Hz)
The overall age curve for SFP is biphasic, with a significant increase from age 6 to 12 y followed by a significant decrease across 12–18 y. This complex curve is better fit by a cubic than linear function, although an overall linear decline is itself significant. The complexity of the age curve for total SFP results, in part, from the opposing age trends of power in the low and high frequencies within 11–15 Hz. It is, therefore, more meaningful to discuss separately the trajectories of low and high frequency sigma power.
Maturational Decline in SFP-LF
Low frequencies within 11–15 Hz exhibit an age trajectory that parallels the curve for NREM delta power in these subjects. Both delta and low frequency spindle power increase from age 6 to 8 y, then decline slowly to age 12 y when their declines accelerate before slowing markedly at age 16–16.5 y. The sharp declines between 12 and 16 y reduce delta power by 61% and low SFP by 50% (see Figure 5). We previously reported significant sex differences in the delta decline, a difference we did not observe for the sigma decline. The absence of a significant sex difference may be biologically meaningful, or the larger interindividual differences in sigma power may have obscured existing sex differences. These interindividual differences created a C9-C12 cohort difference in sigma that we did not observe in other frequency bands. The change from the H2O to Aura amplifier cannot account for the cohort difference, not only because of the correction factor we applied, but also because the difference is greatest between ages 12 and 13 y when both cohorts were recorded on the H2O recorders.
We have previously interpreted the decline in NREM slow wave power across childhood/adolescence as a result of synaptic pruning. In 198225 we noted the similarity of the ontogenetic curve for NREM delta amplitude to the age curve for synaptic density reported by Huttenlocher.1 Synaptic pruning could directly decrease delta power by reducing the size of the neuronal pools capable of producing synchronous oscillations. Studies that concurrently measured gray matter thickness and EEG activity support the interpretation that these maturational changes are correlated with reduced slow wave EEG activity.26,27 Because current theory holds that slow wave (delta) and sleep spindle oscillations are controlled by the same thalamocortical networks,19 this relation might account for the similar maturational trajectories for SFP-LF and delta power.
However, the magnitude of the delta age decline is greater than that of sigma. We propose that although both are affected by the diminishing numbers of interconnected neurons, delta power is, in addition, reduced by the decreasing need for the recuperative processes of sleep. According to the restorative model of slow wave sleep first outlined in 1974,28 neuronal-metabolic changes produced by plastic brain activities during waking are reversed in NREM rather than REM sleep. The rate of reversal is proportional to the level (i.e., power) of high- amplitude NREM slow waves. In brief, the 1982 model hypothesized the following causal relationships: decreasing synaptic density across adolescence decreases the intensity (and hence metabolic rate) of waking neuronal activity.25 The decreased intensity of waking neuronal activity decreases the demand (i.e., “substrate”) for NREM recovery processes. This declining recuperative need augments the delta power decline directly produced by the effects of synaptic pruning on the size of neuronal pools.
Maturational Increase in SFP-HF
A group of higher sigma frequencies increases in power across adolescence. This increase appears unique to the upper frequency of the sigma range (13.4 to 14.4 Hz in our data). Power in all the other NREM EEG frequencies we have examined (0.3–30 Hz) either decreases significantly with age or does not change. We hypothesize that the linear increase in sigma peak frequencies is related to the age-dependent increase in 13.4 to 14.4 Hz power as follows. Prior to the increase in peak sigma frequencies, these fast EEG waves did not possess the higher amplitude sinusoidal patterns of organized spindle EEG. Instead, they behaved biologically as did the low-amplitude beta waves, which decrease in power with age. As the brain matures across childhood/adolescence, organized spindle EEG becomes faster, incorporating 13.4 – 14.4 Hz waves and increasing power in this band. This possibility can be investigated with the reanalyses of our digitized data with spindle detection and time-domain measurements.29
A possible physiological mechanism for the increase in spindle frequency is that it is a consequence of the reduction in deep sleep caused by synaptic pruning. Spindle wave frequency is inversely related to the duration and degree of thalamocortical hyperpolarization.30 Adrillon et al.31 recently proposed that the increased spindle frequency later in the sleep period when sleep is lighter reflects a decreased level of thalamocortical hyperpolarization. A similar mechanism might produce higher spindle frequencies across adolescence. As described previously, the intense waking brain activity (i.e., metabolic rate) produced by high synaptic density in childhood and early adolescence creates a high need for neuronal recovery. As synaptic pruning proceeds through adolescence, the intensity of waking neuronal activity falls, as evidenced by decreasing cerebral metabolic rate. This decline in waking brain metabolism reduces recuperative sleep need. Therefore, sleep becomes less deep, the degree of membrane hyperpolarization diminishes, and spindle frequency (and the frequency of peak sigma power) increases. Support for this interpretation is the finding that an experimental increase in sleep recuperative need produced by sleep deprivation decreases spindle frequency in adults.15 In this interpretation, the deeper sleep after sleep deprivation produces greater membrane hyperpolarization and slower spindle frequencies.
Alternatively (or, in addition), spindle peak frequencies might increase across adolescence because synaptic pruning streamlines circuits, making them more efficient and increasing the speed of central processing. Although some contradictory evidence exists, it has been proposed that sleep spindles are associated with both cognitive ability and memory consolidation.32,33 Bodizs et al.29 found an increase in fast spindle activity with age across adolescence and found that in girls, intelligence quotient (IQ) was correlated with density of fast spindle and that in boys IQ was correlated with fast spindle frequency. In a study of sleep and motor skills, spindle frequency was positively related to motor performance.34 The authors concluded that slower spindles indicate immature neural networks. If fast spindling EEG reflects the speed of neural transmission/processing, completion of the bulk of synaptic pruning at about age 16.5 y could account for the sharp increase in fast sigma power after that age. We have suggested elsewhere that synaptic pruning during adolescence contributes to the increase in cognitive power over the second decade of life.25
SFP-LF and SFP-HF Have Opposite Trends Across NREMPs
Power in several low sigma frequencies increases significantly across NREMPs, and power in several high sigma frequencies shows the reverse trend, decreasing significantly across the night. Werth et al.16 described a somewhat similar pattern in young adults, although the specific frequencies differ. In addition, our analyses show that the decline in power in high spindle subfrequencies is entirely produced by a decline from NREMP1 to NREMP2. This observation provides another example of the special biological significance of NREMP1, which is also demonstrated in human ontogenetic and nap studies.28,35,36
Adolescent brain maturation also affects the frequencies within sigma that change across NREMPs, raising the lower and upper boundaries of the sigma frequencies that increase in power, and also raising the lower boundary of frequencies that decrease in power. Whatever its mechanistic basis, the reliable across-night increase in EEG power in the low sigma frequencies is interesting because low frequency sigma power shows an age decline similar to that of delta, but, in contrast to delta, it increases across the night.
There are many other questions raised by spindle burst phenomena. The major issue is that of functional significance. We briefly discussed the proposal that spindles play a role in memory consolidation. It has also been proposed that spindles serve to maintain sleep by inhibiting sensory input.37 Theories on spindle function must be able to reconcile the substantial changes in sigma frequency and spindle activity across childhood and adolescence.
Spindles, along with NREM delta and theta, exemplify the extraordinary sensitivity of the NREM EEG to postnatal brain development. We have long argued that this close relationship holds powerful clues to such fundamental questions in human neurobiology as the function of sleep and the processes underlying late brain maturation and brain aging.
DISCLOSURE STATEMENT
This was not an industry supported study. United States Public Health Service grants R01MH062521 and R01HL116490 supported this research. The authors have indicated no financial conflicts of interest. All work performed at the University of California, Davis, Davis, CA.
ACKNOWLEDGMENTS
We greatly appreciate the work of the research associates and many undergraduate research assistants who helped collect and analyze data over the course of this longitudinal study. We also thank the study participants and their families.
REFERENCES
- 1.Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 2.Campbell IG, Feinberg I. Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proc Natl Acad Sci U S A. 2009;106:5177–80. doi: 10.1073/pnas.0812947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feinberg I, Campbell IG. Longitudinal sleep EEG trajectories indicate complex patterns of adolescent brain maturation. Am J Physiol Regul Integr Comp Physiol. 2013;304:R296–303. doi: 10.1152/ajpregu.00422.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feinberg I, Davis NM, de Bie E, Grimm KJ, Campbell IG. The maturational trajectories of NREM and REM sleep durations differ across adolescence on both school-night and extended sleep. Am J Physiol Regul Integr Comp Physiol. 2012;302:R533–40. doi: 10.1152/ajpregu.00532.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell IG, Darchia N, Higgins LM, et al. Adolescent changes in homeostatic regulation of EEG activity in the delta and theta frequency bands during non-rapid eye movement sleep. Sleep. 2011;34:83–91. doi: 10.1093/sleep/34.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell IG, Higgins LM, Trinidad JM, Richardson P, Feinberg I. The increase in longitudinally measured sleepiness across adolescence is related to the maturational decline in low-frequency EEG power. Sleep. 2007;30:1677–87. doi: 10.1093/sleep/30.12.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uchida S, Maloney T, March JD, Azari R, Feinberg I. Sigma (12-15 Hz) and delta (.3-3 Hz) EEG oscillate reciprocally within NREM sleep. Brain Res Bull. 1991;27:93–6. doi: 10.1016/0361-9230(91)90286-s. [DOI] [PubMed] [Google Scholar]
- 8.Guazzelli M, Feinberg I, Aminoff M, Fein G, Floyd TC, Maggini C. Sleep spindles in normal elderly: comparison with young adult patterns and relation to nocturnal awakening, cognitive function and brain atrophy. Electroencephalogr Clin Neurophysiol. 1986;63:526–39. doi: 10.1016/0013-4694(86)90140-9. [DOI] [PubMed] [Google Scholar]
- 9.Shinomiya S, Nagata K, Takahashi K, Masumura T. Development of sleep spindles in young children and adolescents. Clin Electroencephalogr. 1999;30:39–43. doi: 10.1177/155005949903000203. [DOI] [PubMed] [Google Scholar]
- 10.Tarokh L, Carskadon MA. Developmental changes in the human sleep EEG during early adolescence. Sleep. 2010;33:801–9. doi: 10.1093/sleep/33.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scholle S, Zwacka G, Scholle HC. Sleep spindle evolution from infancy to adolescence. Clin Neurophysiol. 2007;118:1525–31. doi: 10.1016/j.clinph.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Carrier J, Land S, Buysse DJ, Kupfer DJ, Monk TH. The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20-60 years old) Psychophysiology. 2001;38:232–42. [PubMed] [Google Scholar]
- 13.Nicolas A, Petit D, Rompre S, Montplaisir J. Sleep spindle characteristics in healthy subjects of different age groups. Clin Neurophysiol. 2001;112:521–7. doi: 10.1016/s1388-2457(00)00556-3. [DOI] [PubMed] [Google Scholar]
- 14.Dijk D-J, Shanahan TL, Duffy JF, Ronda JM, Czeisler CA. Variation of electroencephalographic activity during non-rapid eye movement and rapid eye movement sleep with phase of circadian melatonin rhythm in humans. J Physiol. 1997;505:851–8. doi: 10.1111/j.1469-7793.1997.851ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knoblauch V, Martens WL, Wirz-Justice A, Cajochen C. Human sleep spindle characteristics after sleep deprivation. Clin Neurophysiol. 2003;114:2258–67. doi: 10.1016/s1388-2457(03)00238-4. [DOI] [PubMed] [Google Scholar]
- 16.Werth E, Achermann P, Dijk D-J, Borbély AA. Spindle frequency activity in the sleep EEG: individual differences and topographic distribution. Electroencephalogr Clin Neurophysiol. 1997;103:535–42. doi: 10.1016/s0013-4694(97)00070-9. [DOI] [PubMed] [Google Scholar]
- 17.Buzsáki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci. 1988;8:4007–26. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nunez A, Curro Dossi R, Contreras D, Steriade M. Intracellular evidence for incompatibility between spindle and delta oscillations in thalamocortical neurons of cat. Neuroscience. 1992;48:75–85. doi: 10.1016/0306-4522(92)90339-4. [DOI] [PubMed] [Google Scholar]
- 19.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–85. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 20.Rechtschaffen A, Kales A. Washington, DC: Public Health Services, US Government Printing Office; 1968. A manual of standardized terminology, techniques and scoring systems for sleep stages of human subjects. [Google Scholar]
- 21.Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Stat. 1998;23:323–55. [Google Scholar]
- 22.Twisk JWR. Applied longitudinal data analysis for epidemiology. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- 23.Purves D. Body and brain: a trophic theory of neural connections. Cambridge: Harvard University Press; 1988. [DOI] [PubMed] [Google Scholar]
- 24.Jenni OG, Carskadon MA. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep. 2004;27:774–83. [PubMed] [Google Scholar]
- 25.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982/1983;17:319–34. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 26.Buchmann A, Ringli M, Kurth S, et al. EEG sleep slow-wave activity as a mirror of cortical maturation. Cereb Cortex. 2011;21:607–15. doi: 10.1093/cercor/bhq129. [DOI] [PubMed] [Google Scholar]
- 27.Whitford TJ, Rennie CJ, Grieve SM, Clark CR, Gordon E, Williams LM. Brain maturation in adolescence: concurrent changes in neuroanatomy and neurophysiology. Hum Brain Mapp. 2007;28:228–37. doi: 10.1002/hbm.20273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feinberg I. Changes in sleep cycle patterns with age. J Psychiatr Res. 1974;10:283–306. doi: 10.1016/0022-3956(74)90011-9. [DOI] [PubMed] [Google Scholar]
- 29.Bodizs R, Gombos F, Ujma PP, Kovacs I. Sleep spindling and fluid intelligence across adolescent development: sex matters. Front Hum Neurosci. 2014;8:952. doi: 10.3389/fnhum.2014.00952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steriade M, Llinas RR. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988;68:649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- 31.Andrillon T, Nir Y, Staba RJ, et al. Sleep spindles in humans: insights from intracranial EEG and unit recordings. J Neurosci. 2011;31:17821–34. doi: 10.1523/JNEUROSCI.2604-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Astori S, Wimmer RD, Luthi A. Manipulating sleep spindles--expanding views on sleep, memory, and disease. Trends Neurosci. 2013;36:738–48. doi: 10.1016/j.tins.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Fogel SM, Smith CT. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev. 2011;35:1154–65. doi: 10.1016/j.neubiorev.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Astill RG, Piantoni G, Raymann RJ, et al. Sleep spindle and slow wave frequency reflect motor skill performance in primary school-age children. Front Hum Neurosci. 2014;8:910. doi: 10.3389/fnhum.2014.00910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feinberg I, Maloney T, March JD. Precise conservation of NREM Period 1 (NREMP1) delta across naps and nocturnal sleep: implications for REM latency and NREM/REM alternation. Sleep. 1992;15:400–3. doi: 10.1093/sleep/15.5.400. [DOI] [PubMed] [Google Scholar]
- 36.Feinberg I, March JD, Floyd TC, Jimison R, Bossom-Demitrack L, Katz PH. Homeostatic changes during post-nap sleep maintain baseline levels of delta EEG. Electroencephalogr Clin Neurophysiol. 1985;61:134–7. doi: 10.1016/0013-4694(85)91051-x. [DOI] [PubMed] [Google Scholar]
- 37.De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003;7:423–40. doi: 10.1053/smrv.2002.0252. [DOI] [PubMed] [Google Scholar]