Abstract
Objective:
To describe the clinical phenotype of idiopathic rapid eye movement (REM) sleep behavior disorder (IRBD) at presentation in a sleep center.
Methods:
Clinical history review of 203 consecutive patients with IRBD identified between 1990 and 2014. IRBD was diagnosed by clinical history plus video-polysomnographic demonstration of REM sleep with increased electromyographic activity linked to abnormal behaviors.
Results:
Patients were 80% men with median age at IRBD diagnosis of 68 y (range, 50–85 y). In addition to the already known clinical picture of IRBD, other important features were apparent: 44% of the patients were not aware of their dream-enactment behaviors and 70% reported good sleep quality. In most of these cases bed partners were essential to convince patients to seek medical help. In 11% IRBD was elicited only after specific questioning when patients consulted for other reasons. Seven percent did not recall unpleasant dreams. Leaving the bed occurred occasionally in 24% of subjects in whom dementia with Lewy bodies often developed eventually. For the correct diagnosis of IRBD, video-polysomnography had to be repeated in 16% because of insufficient REM sleep or electromyographic artifacts from coexistent apneas. Some subjects with comorbid obstructive sleep apnea reported partial improvement of RBD symptoms following continuous positive airway pressure therapy. Lack of therapy with clonazepam resulted in an increased risk of sleep related injuries. Synucleinopathy was frequently diagnosed, even in patients with mild severity or uncommon IRBD presentations (e.g., patients who reported sleeping well, onset triggered by a life event, nocturnal ambulation) indicating that the development of a neurodegenerative disease is independent of the clinical presentation of IRBD.
Conclusions:
We report the largest IRBD cohort observed in a single center to date and highlight frequent features that were not reported or not sufficiently emphasized in previous publications. Physicians should be aware of the full clinical expression of IRBD, a sleep disturbance that represents a neurodegenerative disease.
Commentary:
A commentary on this article appears in this issue on page 7.
Citation:
Fernández-Arcos A, Iranzo A, Serradell M, Gaig C, Santamaria J. The clinical phenotype of idiopathic rapid eye movement sleep behavior disorder at presentation: a study in 203 consecutive patients. SLEEP 2016;39(1):121–132.
Keywords: bed partner, dream-enacting behaviors, idiopathic REM sleep behavior disorder, medical consultation, nightmares, sleep quality
Significance.
IRBD is an early manifestation of a neurodegenerative disease. This study describes the clinical findings of a large IRBD cohort that was identified over a long observational period. Using rigorous electromyographic and audiovisual criteria on polysomnography for the diagnosis of IRBD, the detailed description of 203 consecutive patients confirmed previous well-known observations and highlighted important findings that were nor recognized or not sufficiently emphasized in the medical literature. General physicians, sleep specialists and neurologists should be aware of the full profile of IRBD described in this study to improve the early detection and correct identification of this condition. This is important for patient management and counseling and will be of great interest when neuroprotective strategies become available.
INTRODUCTION
Rapid eye movement (REM) sleep behavior disorder (RBD) is a condition characterized by nightmares and dream-enacting behaviors emerging in REM sleep.1 Patients with the idiopathic form of RBD (IRBD) have no cognitive and no motor complains.2–9 Population-based epidemiological data indicate that IRBD is not as rare as initially believed, mostly occurring in individuals older than 60 y.10 Longitudinal studies conducted in sleep centers showed that in most patients with an initial diagnosis of IRBD the synucleinopathies dementia with Lewy bodies (DLB), Parkinson disease (PD), and multiple system atrophy (MSA) eventually developed.11–15 Thus, it is accepted that IRBD represents the prodromal state of these neurodegenerative diseases.16,17
RBD occurs in 25% to 65% of patients with PD, antedating the onset of parkinsonism by several years in 20% to 30% of them.5,9,18–21 However, fewer than 1% of patients with PD complain about RBD symptoms to their primary care physician before receiving a diagnosis of PD.22 In individuals with IRBD, reasons for not seeking medical help include (1) belief that their dream-enacting behaviors represent a non-pathological phenomenon which is thought to be benign, (2) perception that symptoms are not severe or not frequent enough to consult a doctor, (3) belief that symptoms may resolve with time, (4) unawareness of the sleep behaviors in patients who sleep alone, and (5) embarrassment to report them.5,6,23 However, when patients finally decide to seek medical consultation they may receive no support or inappropriate advice because clinicians are not yet aware of RBD or it is misdiagnosed as another disorder such as sleepwalking or epilepsy.24 Thus, IRBD is often underdiagnosed because of lack of knowledge of the disorder. Education about the existence, clinical characteristics, and importance of IRBD is crucial to improve its early detection. Identification of individuals with IRBD is important for patient management and counseling, and to include them in future disease-modifying clinical trials.
Description of clinical manifestations of IRBD at presentation is limited to a few series.2,3,25,26 The majority of these series, however, combined subjects with primary (idiopathic) and secondary forms and in some studies RBD was not confirmed by polysomnography. We herein report a comprehensive and detailed characterization of a large IRBD cohort of 203 consecutive patients in whom a diagnosis was made during a 24-y period at the presentation to a sleep center, where we have identified several aspects that were not reported or not sufficiently emphasized in previous series.
METHODS
The cohort comprises all the 203 consecutive individuals in whom IRBD was diagnosed at the tertiary referral sleep center of the Hospital Clínic de Barcelona, Barcelona, Spain, between August 1990 and November 2014. The Hospital Clínic de Barcelona serves a population of about 500,000 from Barcelona city, one that is close in proximity to the hospital. The Hospital Clínic de Barcelona also admits referrals from residents living in other Barcelona neighborhoods, and other cities from Barcelona province, Catalonia, and Spain.
In the current study, the clinical histories of these 203 patients were reviewed. We first provide cross-sectional clinical and video-polysomnographic (V-PSG) data of patients at presentation, and then longitudinal data on the effect of drug therapy on RBD symptomatology and also on the development of neurodegenerative diseases with time. The study was approved by the Hospital Clinic of Barcelona ethics committee and available participants gave written informed consent.
Diagnosis of IRBD
All patients received a diagnosis of IRBD according to the following criteria: (1) history of dream-enacting behaviors; (2) V-PSG showing REM sleep with increased electromyographic (EMG) activity associated with abnormal behaviors; (3) absence of a neurodegenerative disease; (4) lack of motor and cognitive complaints, and (5) the clinical picture not better explained by another sleep disorder, medical disorder, medication, or substance abuse.1,5,27–30 Patients with a diagnosis of mild cognitive impairment (MCI)31 at the initial visit were excluded in this series because they were considered to have already started cognitive symptoms of a neurodegenerative disease. Patients with RBD who clearly identified the introduction of a medication (e.g., antidepressants, beta-blockers) with the onset of RBD symptomatology were excluded because they were considered to have secondary RBD, and not IRBD.
Clinical Assessment at the Initial Visit
At presentation, neurologists from the sleep center conducted a medical history that included demographic and clinical data, and current medications. As part of the routine clinical practice of the sleep center, a comprehensive semistructured sleep interview was conducted and focused on sleep habits and complaints, estimated age of RBD onset, dream recall and its content, self-awareness and characteristics of abnormal motor and vocal behaviors during sleep, resulting injuries from vigorous sleep behaviors, and overall subjective sleep quality. The bed partner or anyone who witnessed the patient's sleep was encouraged to substantiate and to complete the sleep history. Excessive daytime sleepiness was evaluated with the Epworth Sleepiness Scale32 and a score greater than 10 was considered indicative of excessive daytime sleepiness. Restless legs syndrome was diagnosed according to accepted criteria.33
V-PSG Confirmation of RBD
After the initial visit, all patients underwent an all-night V-PSG to confirm the diagnosis of RBD. V-PSG included electroencephalogram (C3, C4, O1, and O2, referred to the contralateral ear; F3 and F4 were added in 2007), right and left electro-oculograms, surface EMG of the mentalis, surface EMG of the right and left biceps brachii (from 1991 to 2007) or flexor digitorum superficialis (from 2008 to 2014) in the upper limbs, surface EMG of the right and left anterior tibialis in the lower limbs, electrocardiogram, nasal and oral airflow assessment, thoracic and abdominal movement assessment, and measurement of oxyhemoglobin saturation. Sleep stages were scored according to standard criteria with the allowance for REM sleep without atonia.30,34
For the diagnosis of RBD we did not establish a minimal amount of REM sleep time if clear dream enactment behaviors occurred during REM sleep. A repeat V-PSG was performed when the baseline study did not show clear behavioral and EMG RBD-features during the recording, no other causes of dream enactment behaviors were detected, and we were still convinced that clinical history was typical for RBD.
When patients were receiving clonazepam, melatonin, or antidepressants, the medication dose was gradually decreased and withdrawn at least 4 w before V-PSG, when possible. If patients had been using continuous positive airway pressure (CPAP) therapy for comorbid obstructive sleep apnea syndrome, V-PSG was done with the patients using the CPAP mask at the optimal prescribed pressure.
When V-PSG detected concomitant obstructive sleep apnea, patients were offered treatment with CPAP. Those who accepted this therapy underwent a second V-PSG study where CPAP was titrated to eliminate snoring, apneic events, arousals, and oxyhemoglobin desaturations in all body positions and sleep stages. We noted that in some patients with co-morbid obstructive sleep apnea the presence of EMG artifacts from apneic-related arousals did not allow to assess properly the EMG activity in REM sleep, making difficult to be sure whether the patient had RBD or not. This situation occurred in the initial V-PSG study and also persisted in some cases during the CPAP titration study. In these cases a third V-PSG study was performed with patients using their new prescribed CPAP mask in order to evaluate adequately the EMG activity in REM sleep without artifacts from respiratory events.
Treatment of RBD Symptomatology
When the diagnosis of IRBD was confirmed by V-PSG, patients were offered medical therapy for RBD symptomatology when clinically required (e.g., potential injurious dream-enacting behaviors, disturbing nightmares, and vigorous sleep behaviors resulting in patients or bed partners' restless sleep). Clonazepam was chosen as the first-line therapy1,25,35 and was started at 0.25 or 0.50 mg at bedtime. The dosage was increased during follow-up visits in 0.25- or 0.50-mg increments, taking into account the clinical response and side effects. Patients with comorbid obstructive sleep apnea were treated with clonazepam once apneas were eliminated with CPAP therapy. When clonazepam was ineffective or associated with side effects, it was either switched to melatonin or melatonin was added to clonazepam.35,36 Melatonin was initially prescribed at 3 mg at bedtime and it was increased in 3-mg increments until reaching optimal clinical response. During the follow-up visits, the effect of clonazepam and melatonin was clinically assessed by both the patient and the bed partner to reduce the frequency and intensity of the dream-enacting behaviors and nightmares. In each patient the effect of these drugs was classified as substantial success, partial success, and no response.3,5
Clinical Follow-Up
When clonazepam or melatonin was first prescribed, patients were followed at 1- to 3-mo intervals to evaluate the efficacy and potential side effects of these drugs. Patients were routinely followed every 3 to 12 mo at our sleep center after optimal therapy was reached.13,14 When we suspected the emergence of parkinsonism or cognitive decline during the follow-up visits, patients were formally assessed by neurologists of the Movement Disorders or Memory units through detailed clinical history, neurological examination, and neuropsychological tests, as previously described.13,14 PD,37 DLB,38 MSA,39 and MCI31 were diagnosed according to accepted criteria.
In the current study we reviewed whether in patients with IRBD a defined neurodegenerative syndrome developed with time and its types, with special interest to assess if patients with unnoticed clinical features at presentation were also prone to manifest a neurodegenerative disease with time.
Statistical Analysis
Descriptive data are reported as median, mean, standard deviation, number, and percentage. Duration of RBD was defined as the interval between the estimated reported onset of RBD symptoms and the time of the last follow-up visit or death. Follow-up duration was defined as the interval from diagnosis of IRBD at our sleep center with V-PSG to the time of the last evaluation or death. The onset of a defined neurodegenerative syndrome (PD, DLB, MSA, and MCI) was determined as the date when the diagnosis was made. Clinical comparisons of IRBD characteristics between men and women were done using the Student t-test, Mann-Whitney U test and chi-square test, as appropriate. P values less than 0.05 were considered to be significant. All analyses were done with SPSS version 18.0 (Armonk, IBM Corp, NY, USA).
RESULTS
Demographic Findings
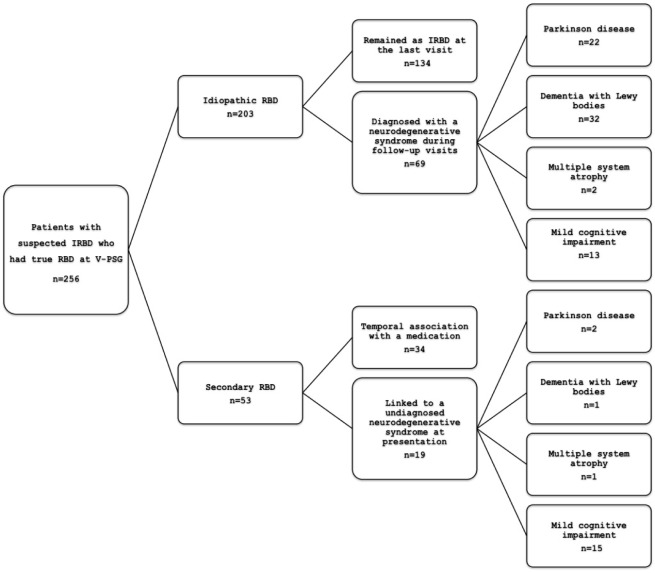
IRBD was diagnosed in 203 consecutive patients between 1990 and 2014 (Figure 1). During this same period of time, 53 additional patients were also referred to our sleep center because of suspected IRBD but when it was confirmed that they had RBD on V-PSG, we found that their RBD was secondary and not idiopathic. In these 53 cases we found that RBD was secondary to either a previously undiagnosed neurological condition (n = 19) or to the introduction of a medication (n = 34). The neurological disorders diagnosed at first assessment were PD (n = 2), DLB (n = 1), MSA (n = 1),40 and MCI (n = 15). Clear temporal association between the RBD onset and the introduction of a drug included two cases associated with beta-blockers (both with bisoprolol)41 and 32 with antidepressants (sertraline in eight, fluoxetine in five, venlafaxine in five, clomipramine in four, paroxetine in three, citalopram in three, escitalopram in two, duloxetine in one, and mirtazapine in one).
Figure 1.
Flow chart describing patients with idiopathic and secondary REM sleep behavior disorder.
The 203 patients with confirmed IRBD were 162 men (79.8%) and 41 women (20.2%) with a median age at estimated IRBD onset of 63 y (range, 40 to 81 y), median age at diagnosis of IRBD of 68 y (range, 50 to 85 y), and median interval between estimated IRBD onset and IRBD diagnosis of 4 y (range, 0.5 to 30 y). The median age at last visit was 74 y (range, 58 to 92 y) and the median follow-up from IRBD diagnosis to the last visit or to death was 5 y (range, 0.1 to 17 y).
Forty-eight patients, 43 men and 5 women (23.6%), were referred to our sleep center between August 1990 and December 2003, and 155 (76.4%) between January 2004 and November 2014 (Figure 2). All patients lived in Catalonia. Their origin was Barcelona city in 54.2% cases, Barcelona province in 37.4%, and other locations in Catalonia in 8.4%.
Figure 2.
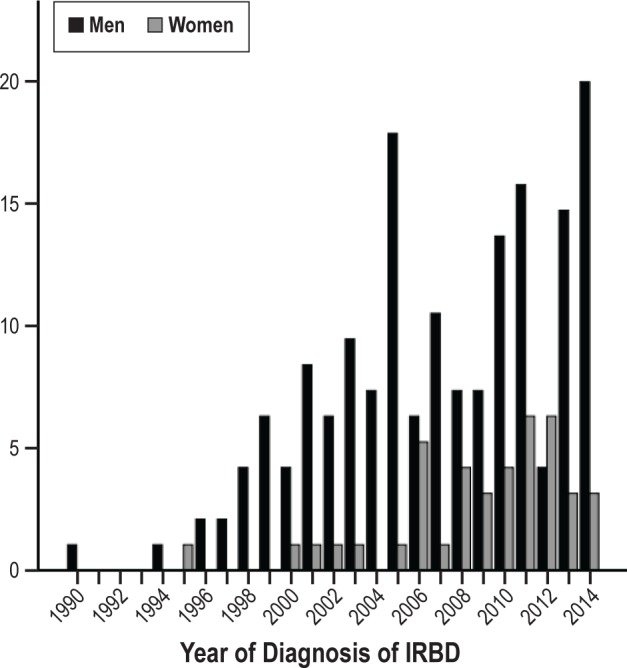
Graph depicting temporal distribution of men and women in whom idiopathic REM sleep behavior disorder was diagnosed between August 1990 and November 2014.
Five patients (2.5%) reported having a first-degree relative with dream-enacting behaviors during adulthood. None of these five relatives sought medical attention for these behaviors and thereby none had a formal diagnosis of RBD or other cause of dream-enacting behaviors. We had access to one of these five relatives, and evaluation through clinical history and V-PSG showed that he had obstructive sleep apnea and not RBD.
Medical History
Sixty patients (29.5%) had previously received a diagnosis of depression and in 32 of them (15.7%) depression antedated the onset of IRBD symptoms. Fifty-three of the 203 patients (26.1%) were taking antidepressants at presentation and none of them noticed a temporal relationship between the introduction of the antidepressant and the onset of RBD symptomatology.
At presentation, 25 patients (12.3%) had a previous diagnosis of obstructive sleep apnea syndrome and 21 of these 25 were under adequate CPAP therapy.
Seven patients (3.4%) had a medical history of sleepwalking. In six, sleepwalking onset was at childhood and disappeared at adolescence. A 69-y-old man had sleepwalking that started at childhood and persisted at the time of his first visit in our sleep center. He was self-referred because at the age of 67 y he started to experience violent sleep behaviors in bed (e.g., punching, kicking) and frightening nightmares (e.g., being attacked by unfamiliar people) that he had never experienced before. In these seven subjects with medical history of sleepwalking, V-PSG in our sleep center demonstrated RBD and no episodes of nonrapid eye movement (NREM) sleep parasomnia.
Three patients (1.5%) had a diagnosis of epilepsy because of seizures during wakefulness, and all three had been taking carbamazepine for more than 10 y. None of them had experienced seizures during sleep and they had been free of daytime seizures during the past 10 to 22 y. In these three patients, V-PSG in our sleep center confirmed RBD and ruled out the occurrence of epileptiform activity and seizures during both NREM sleep and REM sleep.
Reasons for Referral
One hundred eighty patients (88.7%) consulted their doctors because of dream-enacting behaviors and then were referred to our sleep center. Nine patients asked their primary physician to be referred after they learned in the media that our group had published in the medical literature that dream-enacting behaviors may herald a neurodegenerative disease. Two IRBD subjects (1%) first reported their own RBD symptoms while attending a medical interview to their spouses at our sleep center where we asked their spouses about dream-enacting behaviors. These two subjects were surprised to learn that these behaviors were not normal, when they always assumed that they were not medically important.
Twenty-three patients (11.3%) were not referred because of dream-enacting behaviors but because of possible obstructive sleep apnea (n = 15) or hypersomnia (n = 8) instead. However, specific questioning during the semistructured sleep interview at their first visit unmasked a concomitant chronic history typical of RBD. In these 15 patients with suspected sleep apnea, V-PSG showed RBD plus obstructive sleep apnea in nine cases, and RBD plus snoring without apneas in six. In the eight subjects referred because of hypersomnia V-PSG showed RBD; after clinical history and V-PSG, hypersomnia was attributed to concomitant obstructive sleep apnea in five and secondary to comorbid depression in three.
Sleep Data
Thirty-two patients (15.8%) slept alone and had no witness to complete the sleep history. The remaining 171 patients (84.2%) had a bed partner or a roommate who could describe the presence and characteristics of their abnormal sleep behaviors. Six patients (3.0%) were able to determine the date of onset of RBD because they associated it with a highly stressful situation (a robbery, a fraud, a cancer diagnosis) or a few days after a surgical procedure (a pacemaker implantation, and cardiac bypass surgery in two patients). Neuroimaging was unremarkable in all six subjects with IRBD onset associated with a life event. Most of the remaining patients and bed partners had difficulty stating the exact year when RBD symptoms started, the course of the symptoms, and the frequency of episodes per week or per month.
One hundred thirteen subjects (55.7%) were aware of their abnormal sleep behaviors. The remaining 90 (44.3%) had no recollection of their RBD episodes, and most of the relevant information had to be obtained from the bed partner.
One hundred forty-two patients (69.9%) reported good sleep quality and 66 of them (46.5%) were totally unaware of their abnormal sleep behaviors. The remaining 76 were aware of their abnormal sleep actions but considered them not disruptive enough to deserve medical consultation. The spouses of those patients who self-perceived sleeping well had to convince them to seek medical attention.
Eighteen patients (8.9%) had restless legs syndrome. Sixty-five patients (32.0%) complained of daytime somnolence. The mean Epworth Sleepiness Scale score was 8.3 ± 4.7 points, and 62 patients (32.5%) scored more than 10 points.
Dream-Enacting Behaviors (Tables 1 and 2)
Table 1.
Abnormal sleep behaviors.
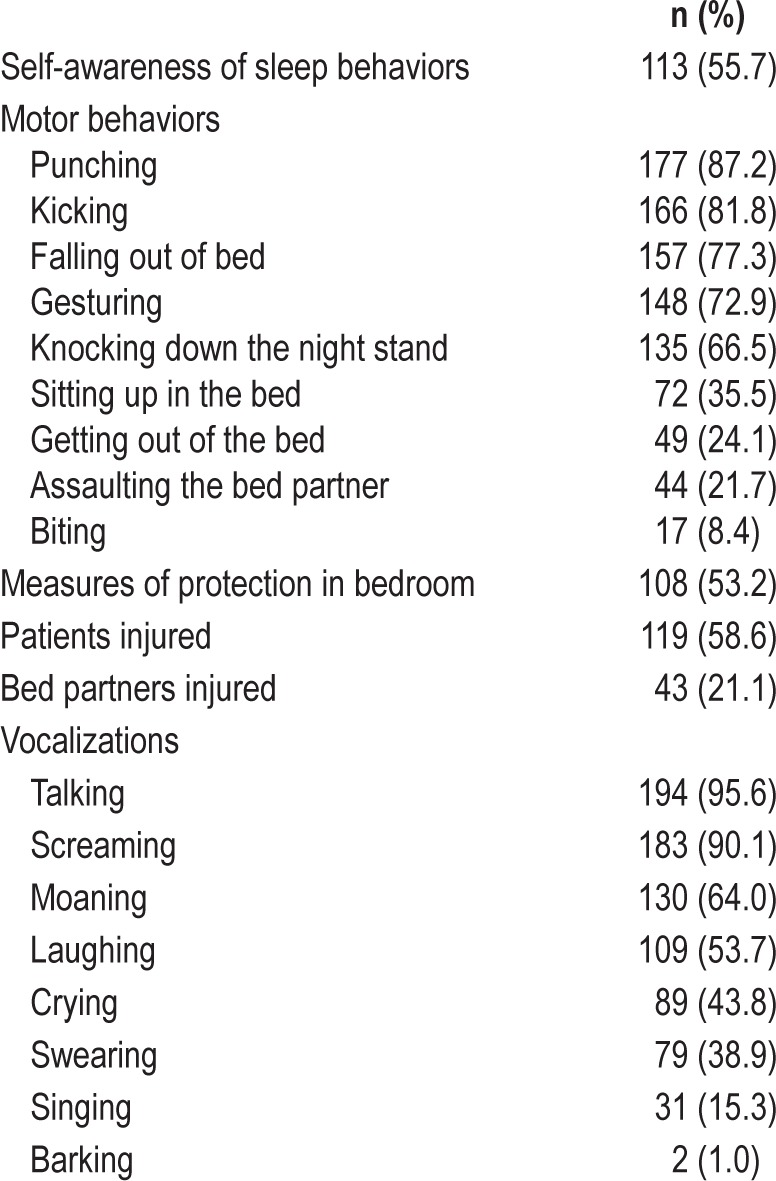
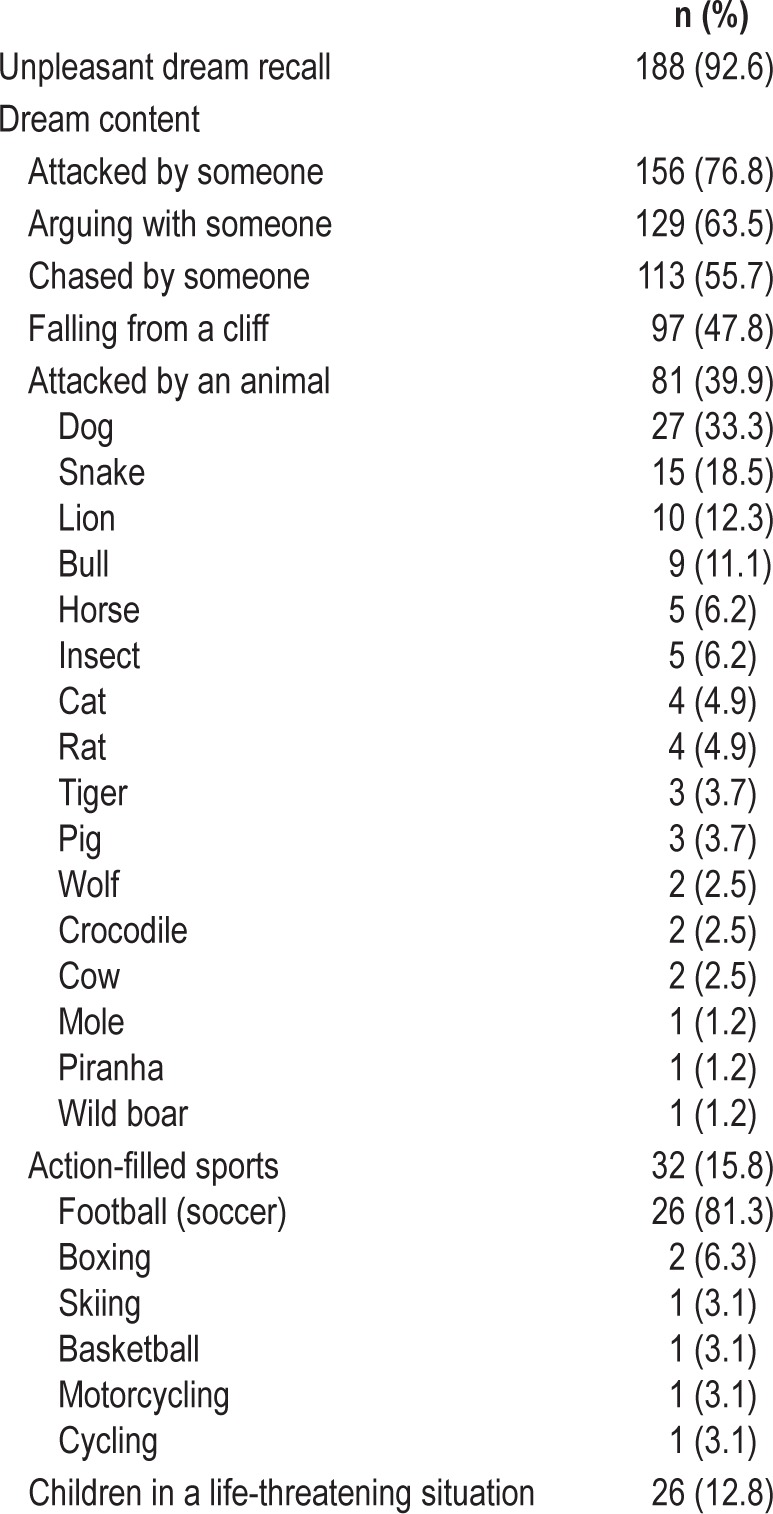
Table 2.
Unpleasant dream recall.
Most frequent motor behaviors were punching (87.2%) and kicking (81.8%) and most common vocalizations were talking (95.6%) and screaming (90.1%). Behaviors suggested to bed partners an emotional component involving violence (punching in 177 cases) or fear (screaming in 183 cases), but also joy (laughing in 109 cases, singing in 31 cases), suffering (crying in 89 cases, howling in one case), annoyance (swearing in 79 cases, barking in two cases, roaring in one case), and love (kissing in three cases). Occasionally, patients also displayed nonviolent elaborated activities such as giving a political speech (three cases), teaching a lesson (one case), and purposeful-looking gestures (shuffling, picking things, and riding). Patients who experienced these nonviolent behaviors also displayed aggressive behaviors during the same or different nights. In one patient, a behavior resembling sexual intercourse with an imaginary partner and accompanied by a disgusting comment occurred on a single night, as reported by his wife.
The majority of the behaviors were confined to the bed with the patient lying down or less frequently sitting up (35.5%). Forty-nine patients (24.1%) also left the bed; 21 (10.3%) got out of bed and awakened shortly after touching the floor with their feet, 18 (8.9%) walked round the bedroom, seven (3.4%) left the bedroom, and three (1.5%) left the house. Running was reported in two (1.0%) cases (one of them while dreaming of lions threatening him). In most of these 49 patients, however, leaving the bed occurred only 1 or 2 nights within a RBD history of several years. None of these 49 patients who got out of bed had a history of epilepsy and only one was a sleepwalker. V-PSG of all 49 subjects demonstrated RBD and did not show epileptiform activity, seizures, or a NREM sleep parasomnia.
One hundred fifty-seven patients (77.3%) fell out of bed (in most of the cases less than five times), 44 (21.7%) assaulted their spouses (pulling off their hair or grabbing them by the neck), and 17 bed partners (8.4%) were bitten. Sleep related injuries occurred in 119 patients (58.6%) and 43 spouses (21.1%). Injuries resulted from jumping out of bed, attempting strangulation, punching or biting the bed partner, and hitting the wall or the nightstand. Injuries included ecchymoses in 70 patients, lacerations in 43, bone fractures in 11 (ribs in four cases, toes in three, collar bone in two, carpus in one, and radius plus ulnar in one), and shoulder dislocation in 1. None of the violent sleep behaviors and injuries led to forensic or medical-legal consequences.
One hundred eight patients (53.2%) took measures to protect themselves and their spouses, such as sleeping in a separate bed or bedroom (56 cases), sleeping in a larger bed (one case), making changes in their bedroom (77 cases) such as removing or protecting the nightstand or other pieces of furniture with hard surfaces and sharp corners (23 cases), installing bed rails (15 cases), placing cushions, pillows, blankets, teddy bears, or a mattress on the floor adjacent to the bed (13 cases), using pillows, wooden screens, or chair barricades to separate the patient from the spouse, the wall, or the nightstand (12 cases), tying the hands into bed with ropes or straps (four cases), and wearing self-made padded gloves (one case).
Dream Recall (Tables 2 and 3)
Table 3.
Examples of dream content and related dream-enacting behaviors and vocalizations.
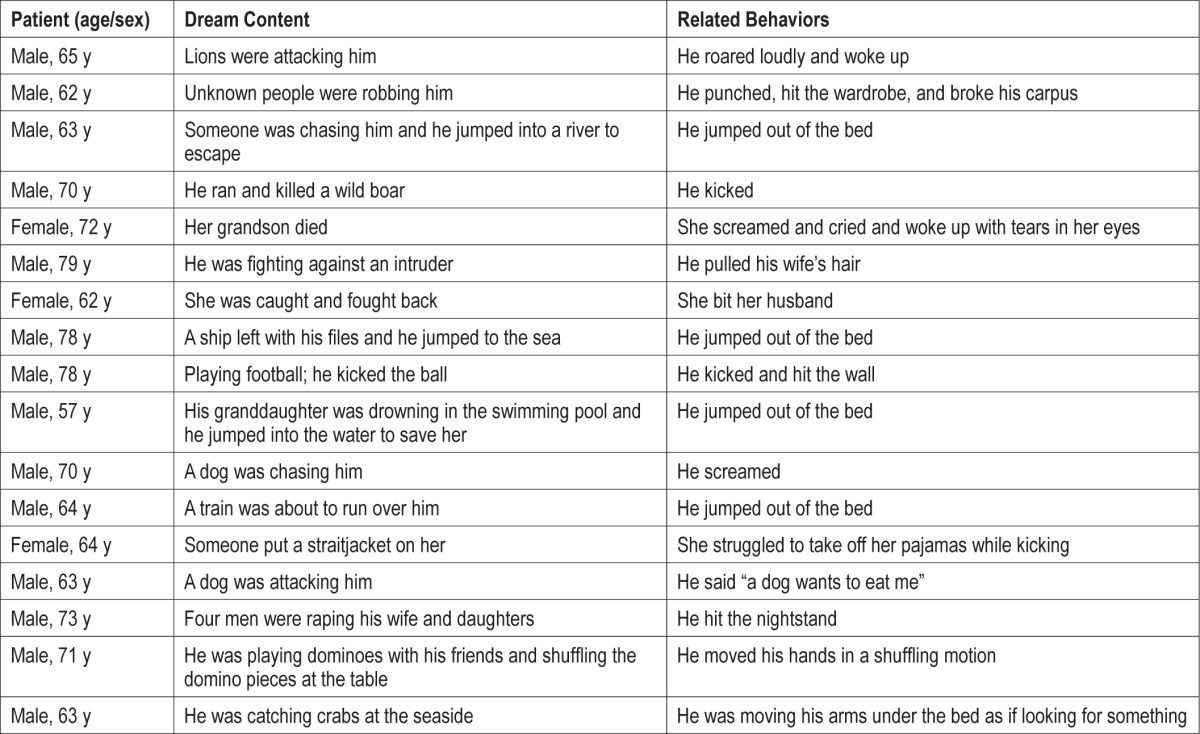
Fifteen patients (7.4%) did not recall unpleasant dreams. The remaining 188 patients (92.6%) reported nightmares, which were vivid, intense, and negatively toned. Most common nightmares included violence or stressful situations such as fighting (76.8%), arguing (63.5%), and being chased (55.7%) by an imaginary aggressor, usually unfamiliar people (e.g., strangers with a blurred face) or frightening animals. Patients were always involved in the nightmare, fighting back vigorously defending themselves or protecting their loved ones from a physical attack or, less frequently, from a humiliation. Nightmares often contained settings and activities related to the patients' past. Dreaming about playing sports included aggressive behaviors (e.g., kicking the opponent while playing football) or an exciting emotional component (e.g., “I was playing football and I scored a beautiful Chilena-goal”, “I was playing football and I desperately asked Messi -the famous Barcelona team football player- to pass me the ball”). Some patients, however, recalled enjoyable and funny dreams (e.g., “playing cards”, “friends telling jokes”) whereas the bed partner noted that the sleeper was laughing. Dreams did not include bizarre elements, situations, settings, or characters, and did not express concerns or worries about the patients' daily life, or references to friendliness, sexual interactions, or respiratory events (e.g., choking or drowning in the sea).
Twenty-eight subjects (13.8%) avoided watching thrillers and action films on television fearing that they may trigger nightmares that could lead to violent behaviors during their sleep. One hundred thirty subjects (69.1%), though, reported sleeping well despite recalling nightmares.
Sex Differences (Table 4)
Table 4.
Differences between men and women with IRBD.
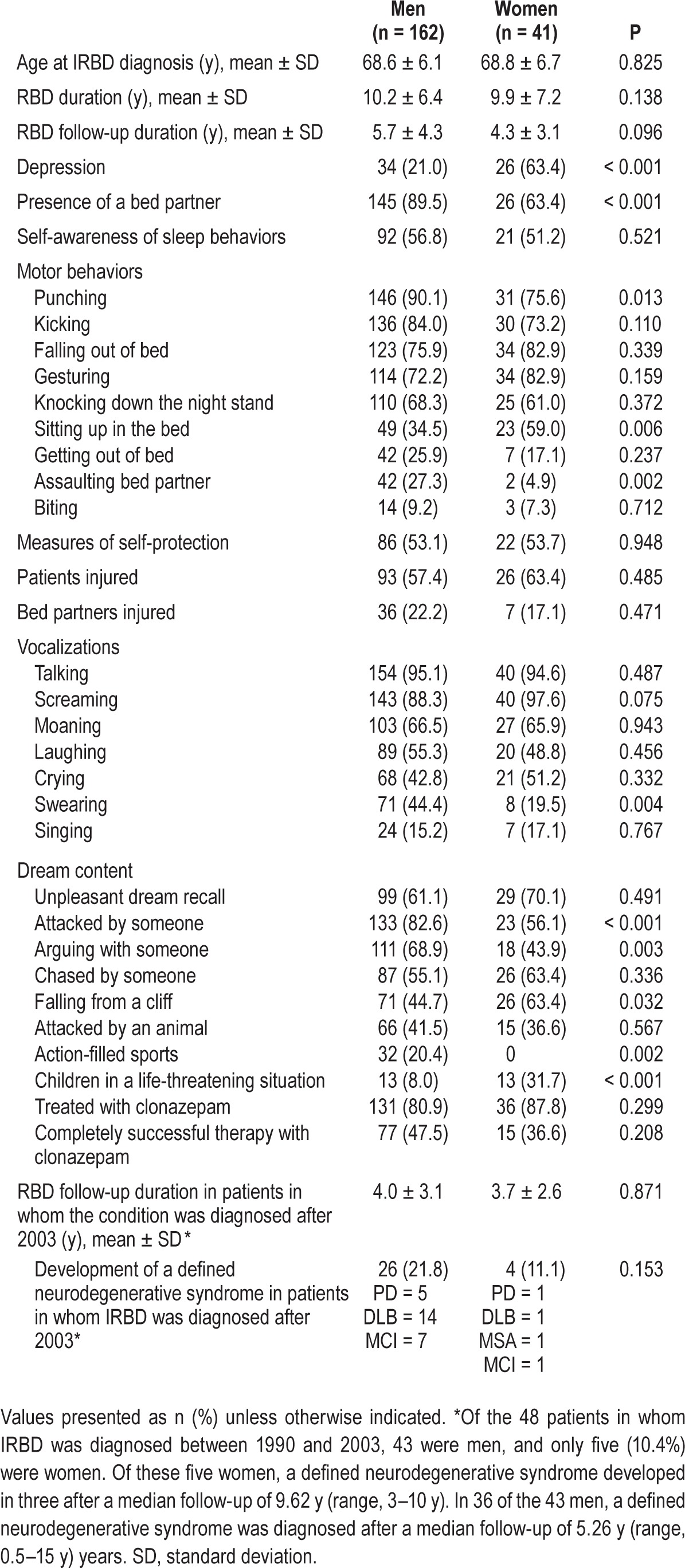
When compared to women, men displayed more frequently aggressive behaviors (e.g., punching, assaulting the bed partner) and vocalizations (e.g., swearing), recalled more violent and action-filled dreams (e.g., fights, arguments, sports) and were more likely to have a bed partner. When compared to men, women dreamed more commonly about children in life-threating situations and had depression more commonly. Of the 48 patients receiving a diagnosis between 1990 and 2003, only five (10.4%) were women. The proportion of women increased to 23.2% during the period 2004–2014 (Figure 2).
Polysomnographic Confirmation
At the time of baseline V-PSG study, 22 patients (10.8%) were receiving antidepressants that could not be withdrawn because of severe depression (n = 21) or severe bipolar disorder (n = 1). In these patients, as mentioned previously, development of RBD symptoms was not related to the introduction of antidepressants. Four patients taking clonazepam and one taking melatonin refused to withdraw these medications before baseline V-PSG. However, despite the use of clonazepam and melatonin the diagnosis of RBD could be made in these five individuals because V-PSG showed clear REM sleep with increased EMG activity associated with vigorous behaviors.
To confirm the diagnosis of IRBD a second V-PSG study had to be done in 32 (15.8%) patients. In 17 of these 32 patients (six of them were taking antidepressants) REM sleep was not recorded (n = 11) or we judged that there was not sufficient REM sleep time to assess if they had RBD (n = 6). In five additional patients, despite a compelling clinical history of IRBD and having sufficient REM sleep time (range, 15 to 119 min), increased EMG was not seen in REM sleep and typical RBD behaviors were not captured. In these five cases, a second V-PSG study confirmed the occurrence of IRBD showing excessive EMG activity linked to kicking and prominent body jerking in REM sleep. In seven additional patients we were not able to evaluate the EMG activity in REM sleep because of frequent EMG artifacts from apneic events. In the three remaining patients technical problems did not allow REM sleep to be evaluated properly.
Among these 32 subjects, a second V-PSG study confirmed IRBD in 26. The remaining six patients (3.0%) had to undergo a third V-PSG study because in the two previous studies there was not sufficient REM sleep time (n = 3, two of them were still taking antidepressants) or REM sleep was interfered by EMG artifacts from apneic events during both baseline and CPAP titration studies (n = 3). In all six patients, this third V-PSG confirmed IRBD.
At presentation, 21 patients were under CPAP therapy because of a previous diagnosis of obstructive sleep apnea. During follow-up visits we prescribed CPAP to 28 additional subjects (14.9%) because baseline V-PSG showed an increased apnea-hypopnea index.
Therapy for RBD Symptomatology
Before referral, physicians treated patients' dream-enacting behaviors with clonazepam (n = 26), other benzodiazepines (n = 14), antiepileptic drugs (n = 4, phenobarbital, lamotrigine, carbamazepine, and oxcarbamacepine), dopaminergic agents (n = 3, levodopa/carbidopa, ropinirol, and pramipexol), zolpidem (n = 2), melatonin (n = 1), haloperidol (n = 1), and imipramine (n = 1). Only patients taking clonazepam and melatonin reported a sustained therapeutic benefit with these compounds. With the exception of clonazepam and melatonin, we discontinued all these medications after we made the formal diagnosis of IRBD with V-PSG.
After the diagnosis of IRBD was made, we prescribed clonazepam to 167 patients (82.3%). The mean clonazepam effective dose was 1.0 ± 0.8 mg (range, 0.25–4), and treatment was considered completely successful in 92 (55.1%), partially successful in 52 (31.1%), and unsuccessful in 4 (2.4%). We have no information concerning the effect of clonazepam in 19 patients.
In 36 patients (17.7%), we initially considered it not necessary to start treatment because patients and bed partners judged the clinical RBD severity to be mild (according to the estimated frequency and intensity of the reported dream-enacting behaviors and recalled nightmares). Follow-up showed that lack of treatment in these 36 patients resulted in sleep related injuries in 11 patients and in 5 bed partners. During follow-up, the proportion of sleep related injuries was more frequent in those who did not received treatment than in those that were treated with clonazepam (36.1% versus 8.4%, P < 0.001).
Sixty-five patients (38.9%) experienced side effects related to clonazepam, including morning sedation (n = 35), dizziness (n = 9), sexual impotence (n = 7), urinary incontinence (n = 5), and others effects (e.g., fatigue, malaise, heartburn, dyspepsia, constipation). Clonazepam had to be withdrawn in 15 patients (9.0%) due to these adverse effects.
Thirty-two patients (15.7%) were treated with melatonin. Clonazepam was replaced by melatonin in eight subjects who experienced side effects. Melatonin was added to clonazepam in 24 patients in whom clonazepam dose had to be reduced due to side effects or in whom clonazepam was not successful. Of the eight patients taking only melatonin, the median dose was 6 mg (range, 1.9–9 mg), and it was considered partially successful in one patient and unsuccessful in four. In the three remaining patients we have no information regarding the effect on taking only melatonin. The only side effect reported related to melatonin was morning sedation in one subject.
In patients with concomitant obstructive sleep apnea syndrome, 23 of 49 subjects (46.9%) who received treatment with CPAP reported mild to moderate decrease (but not elimination) of the frequency and severity of unpleasant dreams and abnormal sleep behaviors.
Phenoconversion to a Neurodegenerative Disease
Sixty-nine patients (34.0%) received a diagnosis of defined neurodegenerative syndrome after a median follow-up of 5 y. Emerging disorders were DLB (n = 32), PD (n = 22), MSA (n = 2), and MCI (n = 13). Details and risk of conversion to a defined neurodegenerative syndrome for most patients were previously described in previous other studies.13,14
Among the subjects in whom IRBD was diagnosed during the period 2004–2014, the frequency of patients receiving a diagnosis of a defined neurodegenerative syndrome was similar between men and women (Table 4). During the period 1990– 2003 there were 43 men and five women. Of these five women, PD developed in three, and one died and one was lost both with the diagnosis of IRBD at their last visit. Among the 43 men, 36 (83.7%) received a diagnosis of defined neurodegenerative syndrome (DLB in 17, PD in 13, MSA in one and MCI in five), five remained idiopathic, and one died and one was lost both with the diagnosis of IRBD at their last visit.
A substantial proportion of patients presenting with uncommon IRBD features also converted to a defined neurodegenerative syndrome. Of the 23 subjects in whom dream-enacting behaviors were not the initial complaint at referral, nine (39.1%) had an eventual diagnosis of PD (n = 5), DLB (n = 2), MSA (n = 1), or MCI (n = 1). In two of the six patients (33.3%) who estimated the onset of RBD associated with a life event, a neurodegenerative disease developed (PD in one and DLB in one).
Of the 49 subjects who reported leaving the bed at night, 20 (40.8%) eventually received a diagnosis of defined neurodegenerative syndrome (DLB in 14, MCI in three, and PD in three). Three of the seven who reported leaving the bedroom received a diagnosis of MCI (n = 2) and DLB (n = 1). Of the three patients who reported leaving the house, DLB developed in two and one died with the diagnosis of IRBD at his last visit. Of the 69 patients in whom a defined neurodegenerative syndrome developed, DLB or MCI were more likely to develop than PD in those who left the bed (P = 0.043).
Among the 53 patients who were taking antidepressants at presentation, 12 (22.6%) later received a diagnosis of DLB (n = 5), PD (n = 4), MCI (n = 2), and MSA (n = 1), and the remaining 41 subjects remained idiopathic. In 22 of these 53 patients, antidepressants could not be withdrawn at the moment of the baseline V-PSG. Of these 22, nine (44.1%) reported that IRBD symptoms were initiated by a median of 5 y (range, 1–13 y) before the introduction of antidepressants. The other 13 patients (55.9%) reported that the start of antidepressant therapy antedated by a mean of 9 y (range, 1–37 y) the onset of IRBD. Of these 13, follow-up showed that in four patients RBD symptoms persisted when antidepressants were withdrawn after the resolution of depression (in two of these four subjects, a new V-PSG without taking antidepressants showed the persistence of RBD). Follow-up of the remaining nine patients showed that two died with the diagnosis of IRBD at their last visit, MCI developed in one, and six remained idiopathic after a median follow-up of two y (range, 0.5 to 6 y). These six patients were three men and three women, had a median age at IRBD diagnosis of 67.5 y (range, 58–85 y), had the diagnosis of major depression (n = 5) and bipolar disorder (n = 1), and were treated with citalopram (n = 3), venlafaxine (n = 1), clomipramine (n = 1), and mirtazapine (n = 1).
Of the five patients who had no abnormal sleep behaviors in baseline V-PSG (but were demonstrated in a second study confirming RBD), DLB developed in one, MCI developed in one, and three remained idiopathic.
DISCUSSION
To the best of our knowledge, this is the largest series evaluating the clinical features of patients with the idiopathic form of RBD. Previous publications describing the clinical characteristics of IRBD were either much smaller, included heterogeneous samples of patients with IRBD combined with secondary to neurological disorders, lacked confirmation of RBD with V-PSG, or were multicenter. Other publications focused in a single aspect of IRBD (e.g., age of symptom onset, dream content) and did not cover the full clinical phenotype, as we did.
The current work represents the characterization of 203 consecutive patients who received a diagnosis over a 24-y period. In addition to the already known profile, we have noticed the following findings that were not reported or were not sufficiently emphasized in previous publications: (1) an important proportion of IRBD patients report good sleep quality and is unaware of their nocturnal movements and vocalizations; (2) bed partners are essential to describe the behaviors and to convince their spouses to seek medical consultation; (3) the occurrence of IRBD can be elicited by specific questioning in some individuals referred for other problems; (4) leaving the bed and even walking may occur and appears to entail an increased risk for DLB; (5) some patients do not recall unpleasant dreams; (6) in some cases the correct diagnosis of IRBD cannot be made by a single V-PSG study; (7) even though the severity of IRBD symptoms may appear mild by clinical history, there is still an important risk of injuries if the parasomnia is left untreated; and (8) CPAP may improve (but not eliminate) IRBD symptomatology in subjects with comorbid obstructive sleep apnea.
Reasons for Referral
Only 23.6% of the patients were referred between 1990 and 2003, when IRBD was unknown among the majority of physicians. We noted that during this first period, neurologists and general practitioners treated IRBD symptoms before referral with either antiepileptic drugs (because dream-enacting behaviors were misinterpreted as being seizures) or with benzodiazepines (because doctors assumed that dream-enacting behaviors were simply indicative of poor sleep quality). The majority of the patients, though, presented between 2004 and 2014. This may be explained by several factors, including (1) an increased knowledge of the clinical importance of sleep disorders, (2) an increasing body of knowledge about IRBD and its clinical relevance among members of the medical profession, particularly neurologists and sleep specialists, (3) recognition by physicians from Barcelona city and province that our sleep center was capable and keen to make diagnoses, evaluate, and follow-up IRBD cases, and (4) coverage by the media of some of our publications. Overall, these factors could explain why in the period 2004–2014, referral physicians treated their patients with clonazepam (the first- line therapy for RBD) and more patients were identified. Despite these factors, underdiagnosis persists in most of the individuals in the general population with IRBD.10
The median interval between estimated onset of IRBD symptoms and medical consultation was 4 y. A large group of patients were aware of their sleep behaviors but they considered them nonpathological or not severe enough to require medical consultation. Failure to recognize IRBD manifestations as pathological can be attributed to mild severity of the symptoms in some patients who were not concerned by their injury potential, and the belief that their dream-enacting behaviors constitute a normal phenomenon. Many patients believed that dream-enacting behaviors were a benign phenomenon and decided to seek medical advice only when these behaviors became violent, resulted in injuries, lasted for several years, and did not resolve with time. In many instances, the insistence of the bed partner was crucial to convince their spouses (particularly to those patients who were completely unaware of their actions) to consult a doctor. This is important to recognize when using self-administered questionnaires for IRBD screening.
In some individuals, a history of dream-enacting behaviors was elicited only upon specific questioning when they presented because of other symptoms. This finding was also noted in another study where only one of 11 IRBD patients was referred because of suspected parasomnia.42
It is thought that in the general population there is a group of individuals with a mild form of IRBD who are not likely to seek medical help.4 Interestingly, eight of our 23 patients in whom a RBD history was not a spontaneous complaint were some years later diagnosed with PD, DLB or MCI. This is in agreement with several studies showing that the majority of the PD patients (in whom RBD preceded parkinsonism) did not seek medical attention because of dream-enacting behaviors prior to their diagnosis of PD.5,22,43 It appears that patients with milder forms who do not seek medical attention for IRBD are also at high risk for development of a neurodegenerative disease.
Gender Differences
Our work confirms previous reports showing that patients in whom IRBD is diagnosed in a sleep center are predominantly men.2,3,14,15,25,26 It is unclear why men predominate in IRBD. A plausible explanation is a referral bias. In our study the clinical expression in men was more violent than in women, with men displaying more vigorous sleep behaviors and recalling more aggressive and action-filled dreams. Because sex hormones are normal in men with IRBD,44 our data are in line with the hypothesis that IRBD manifests differently in men and women, making men more prone to seek medical attention.45–47 This difference may have a biological basis, because males show more physical aggressiveness than females in most mammalian species.48,49
Age at Onset
The minimum age at estimated IRBD onset was 40 y and the minimum age at diagnosis was 50 y. This is in concordance with studies showing that individuals younger than 50 y in whom RBD was diagnosed, they do not have an idiopathic condition. These subjects have secondary forms of RBD associated with narcolepsy, parasomnia overlap disorder or the introduction of antidepressants.4 In people younger than 40 y, the underlying conditions of severe dream-enacting behaviors are sleepwalking, sleep terrors, frontal lobe epilepsy, dissociative states, and malingering, but not idiopathic RBD.4
Dream Content and Enactment
Our data are in agreement with previous series showing that dream content ranges from frightening to funny and that dream-enacting behaviors may be nonviolent and elaborated or violent, leading to injuries and requiring measures of protection.50–52 However, we noted that despite this impressive clinical picture, IRBD seems not to disrupt sleep quality because an important number of patients are unaware of their actions and report good sleep quality. This might be explained by several factors including that sleep architecture is not importantly disrupted,30 abnormal behaviors are usually brief, and that IRBD is characterized by an important night-to-night variability where patients can spend nonviolent RBD nights.
IRBD activities were mainly confined to the bed. However, 49 patients left the bed, with some leaving the room and even the house. Although these behaviors were only displayed once or twice within several years of RBD history, our data indicate that ambulation does not exclude the occurrence of IRBD.53,54 We found that a neurodegenerative disease linked to cognitive impairment (DLB and MCI) with time was likely to develop in these patients. More common causes of nocturnal wandering were excluded in all 49 IRBD subjects. However, it is impossible to know if these episodes of ambulation in our IRBD subjects represented RBD episodes or confusional awakenings in subjects with predementia. In all our patients with comorbidities that may mimic RBD symptoms (e.g., sleepwalking, obstructive sleep apnea, epilepsy) V-PSG demonstrated frank RBD and did not show NREM sleep parasomnia episodes, seizures, or epileptiform activity.51,52,54,55
In our series, none of the violent sleep behaviors and injuries led to forensic or medical-legal consequences, although this has been reported in IRBD.56
Previously Unnoticed or Not Sufficiently Emphasized Clinical Features
They included onset of IRBD associated with a life-event episode,4,57 perceiving good sleep quality, lack of dream recall, nocturnal ambulation, and coexistence with previous diagnosis of sleepwalking and epilepsy.58 These features complement the full characterization of the IRBD clinical phenotype. Follow-up of the patients with these features showed that PD and DLB developed in an important number of them, indicating that the diagnosis of IRBD that we made was correct. This also suggests that the development of a neurodegenerative disease is independent of the clinical presentation of IRBD.
The Role of Antidepressants
Medications, especially antidepressants, are associated with RBD.59 It is still unclear if antidepressants can trigger the clinical manifestations of RBD in the setting of a latent neuro-degenerative process. When patients report a clear temporal association between the introduction of an antidepressant and the onset of RBD, the sleep disorder is considered secondary.27–29 Alternatively, when the antidepressant is not temporally associated with the onset of RBD, the sleep disorder is labeled as idiopathic.27–29 However, it is possible that in some patients taking antidepressants for years a hypothetically cumulative effect of the drug might be responsible for precipitating the onset of RBD. We identified a small group of six elderly patients with severe affective disorders who might belong to this category where V-PSG could not be performed without withdrawing antidepressants. These six individuals have remained idiopathic after a median follow-up of two (range 0.5 to 6) y. Longer follow-up, neuroimaging data, and postmortem neuro-pathology of these subjects are needed to elucidate this aspect.
PSG Confirmation
In all 203 cases, V-PSG revealed REM sleep with increased EMG activity linked to abnormal behaviors. In 15.7% of the patients, baseline V-PSG was not confirmatory of RBD mainly because sufficient REM sleep was not achieved (probably due to the use of antidepressants or due to the first-night effect), and because artifacts from untreated obstructive sleep apnea did not allow evaluation of EMG activity. When we stopped the use of antidepressants and provided therapy with CPAP, the diagnosis of IRBD could be performed with a second V-PSG study. In addition, in four IRBD patients baseline V-PSG was not definitive to confirm the occurrence of IRBD because it showed lack of frank behavioral motor and vocal manifestations. A second study, though, confirmed IRBD in these four cases. This suggests an important night-to-night variability in the clinical manifestations of IRBD that in some cases may require a second confirmatory study.60–62
Therapy for RBD Symptomatology
Clonazepam was used in the first article formally describing human IRBD in 19861 and is currently considered the first-line therapy for this parasomnia.35,63,64 We noted that clonazepam was very effective and relatively well tolerated, and therefore we still decide to use it as a first choice even though melatonin was first reported to be effective in 1997.65
Half of our subjects with comorbid obstructive sleep apnea reported that CPAP therapy improved (but not resolved) their IRBD symptomatology before the introduction of clonazepam or melatonin. This is in agreement with the description that patients with obstructive sleep apnea may experience the same dream-enacting behaviors and disturbing dreams that those with confirmed IRBD.55 Thus, it seems that the coexistence of obstructive sleep apnea may aggravate the typical RBD symptoms of subjects with confirmed IRBD. The reported partial improvement with CPAP might result from a decrease in dream recall and subsequently a reduction in dream-enacting behaviors.66
Strengths and Limitations
Strengths of the current study are the large number of individuals receiving a diagnosis over a long period of time and that inclusion criteria were restrictive, requiring both clinical history and V-PSG confirmation of abnormal REM sleep behaviors linked with increased EMG activity. Some limitations should be noted. First, because the study is retrospective complete information was not available in some instances. Second, dream content was assessed through a semistructured interview and not through systematic analyses.67 Finally, most cases presenting to our sleep center may be more likely to suffer the most severe form of IRBD. Thus, some of our findings may not be generalizable to all IRBD patients in the community.
CONCLUSIONS
In summary, we have identified several aspects in IRBD that should not be missed by the general physician, neurologist, and the sleep specialist when facing a subject presenting with dream-enacting behaviors. We believe that our study helps to better characterize and recognize the full clinical spectrum of IRBD, a condition that currently is considered a specific marker of PD and DLB. Education of the general population and physicians on the characteristics and importance of IRBD would improve early detection of this condition. This will be of great interest when neuroprotective strategies become available.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest. There was no off-label or investigational use.
REFERENCES
- 1.Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW. Chronical behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9:293–308. doi: 10.1093/sleep/9.2.293. [DOI] [PubMed] [Google Scholar]
- 2.Sforza W, Krieger J, Petiau C. REM sleep behavior disorder: clinical and physiopathological findings. Sleep Med Rev. 1997;1:57–69. doi: 10.1016/s1087-0792(97)90006-x. [DOI] [PubMed] [Google Scholar]
- 3.Olson EJ, Boeve BF, Silber MH. Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain. 2000;123:331–9. doi: 10.1093/brain/123.2.331. [DOI] [PubMed] [Google Scholar]
- 4.Schenck CH, Mahowald MW. REM sleep behavior disorder: clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep. 2002;25:120–38. doi: 10.1093/sleep/25.2.120. [DOI] [PubMed] [Google Scholar]
- 5.Iranzo A, Santamaria J, Rye DB, et al. Characteristics of idiopathic REM sleep behavior disorder and that associated with MSA and PD. Neurology. 2005;65:247–52. doi: 10.1212/01.wnl.0000168864.97813.e0. [DOI] [PubMed] [Google Scholar]
- 6.Gagnon JF, Postuma RB, Mazza S, Montplaisir J. Rapid-eye-movement sleep behaviour disorder and neurodegenerative diseases. Lancet Neurol. 2006;5:424–32. doi: 10.1016/S1474-4422(06)70441-0. [DOI] [PubMed] [Google Scholar]
- 7.Boeve B. REM sleep behaviour disorder. Updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann NY Acad Sci. 2010;1184:15–54. doi: 10.1111/j.1749-6632.2009.05115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnulf I. REM sleep behavior disorder: motor manifestations and pathophysiology. Mov Disord. 2012;27:677–89. doi: 10.1002/mds.24957. [DOI] [PubMed] [Google Scholar]
- 9.Cochen De Cock V. Recent data on rapid eye movement sleep behavior disorder in patients with Parkinson disease: analysis of behaviors, movements, and periodic limb movements. Sleep Med. 2013;14:749–75. doi: 10.1016/j.sleep.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Kang SH, Yoon- IY, Lee SD, Han JW, Kim TH, Kim KW. REM sleep behavior disorder in the Korean elderly population: prevalence and clinical characteristics. Sleep. 2013;36:1147–52. doi: 10.5665/sleep.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder. Neurology. 1996;46:388–92. doi: 10.1212/wnl.46.2.388. [DOI] [PubMed] [Google Scholar]
- 12.Schenck C, Boeve B, Mahowald M. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder: a 16-year update on a previously reported series. Sleep Med. 2013;14:744–8. doi: 10.1016/j.sleep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Iranzo A, Tolosa E, Gelpi E, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behavior disorder: and observational cohort study. Lancet Neurol. 2013;12:443–53. doi: 10.1016/S1474-4422(13)70056-5. [DOI] [PubMed] [Google Scholar]
- 14.Iranzo A, Fernández-Arcos A, Tolosa E, et al. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS ONE. 2014;9:e89741. doi: 10.1371/journal.pone.0089741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte-Marquez V, Montplaisir V. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology. 2009;72:1296–300. doi: 10.1212/01.wnl.0000340980.19702.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boeve BF. Idiopathic REM sleep behavior disorder in the development of Parkinson's disease. Lancet Neurol. 2013;12:469–82. doi: 10.1016/S1474-4422(13)70054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Postuma RB, Gagnon JF, Montplaisir J. Rapid eye movement sleep behavior disorder as a biomarker for neurodegeneration: the past 10 years. Sleep Med. 2013;14:763–7. doi: 10.1016/j.sleep.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Gagnon JF, Bédard MA, Fantini ML, et al. REM sleep behavior disorder and REM sleep without atonia in Parkinson's disease. Neurology. 2002;59:585–9. doi: 10.1212/wnl.59.4.585. [DOI] [PubMed] [Google Scholar]
- 19.Sixel-Doring F, Trautmann E, Mollenhauer B, Trenkwalder C. Associated factors for REM sleep behaviour disorder in Parkinson disease. Neurology. 2011;77:1048–54. doi: 10.1212/WNL.0b013e31822e560e. [DOI] [PubMed] [Google Scholar]
- 20.Plomhause L, Dujarin K, Duhamel A, et al. Rapid eye movement sleep behavior disorder in treatment-naïve Parkinson disease patients. Sleep Med. 2013;14:1035–7. doi: 10.1016/j.sleep.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 21.Gong Y, Hiong KP, Mao CJ, et al. Clinical manifestations of Parkinson disease and the onset of rapid eye movement sleep behaviour disorder. Sleep Med. 2014;15:647–53. doi: 10.1016/j.sleep.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 22.Schrag A, Hosrdfall L, Walters K, Noyce A, Petersen I. Prediagnostic presentations of Parkinson's disease in primary care: a case-control study. Lancet Neurol. 2014;14:57–64. doi: 10.1016/S1474-4422(14)70287-X. [DOI] [PubMed] [Google Scholar]
- 23.White C, Hill EA, Morrison I, Riha RL. Diagnostic delay in REM sleep behavior disorder (RBD) J Clin Sleep Med. 2012;8:133–6. doi: 10.5664/jcsm.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohayon MM, Schenck CH. Violent behavior during sleep: prevalence, comorbidity and consequences. Sleep Med. 2010;11:941–6. doi: 10.1016/j.sleep.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schenck CH, Hurwitz TD, Mahowald MW. REM sleep behavior disorder: an update on a series of 96 patients and a review of the world literature. J Sleep Res. 1993;2:224–31. doi: 10.1111/j.1365-2869.1993.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 26.Wing YK, Lam SP, Li SX, et al. REM sleep behavior disorder in Hong Kong Chinese: clinical outcome and gender comparison. J Neurol Neurosurg Psychiatry. 2008;79:1415–6. doi: 10.1136/jnnp.2008.155374. [DOI] [PubMed] [Google Scholar]
- 27.American Academy of Sleep Medicine. Chicago, IL: American Academy of Sleep Medicine; 2001. International classification of sleep disorders, revised: diagnostic and coding manual. [Google Scholar]
- 28.American Academy of Sleep Medicine. Westchester, IL: American Academy of Sleep Medicine; 2005. The international classification of sleep disorders. 2nd ed.: diagnostic and coding manual. [Google Scholar]
- 29.American Academy of Sleep Medicine. International classification of sleep disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 30.Lapierre O, Montplaisir J. Polysomnographic features of REM sleep behavior disorder: development of a scoring method. Neurology. 1992;42:1371–4. doi: 10.1212/wnl.42.7.1371. [DOI] [PubMed] [Google Scholar]
- 31.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 32.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 33.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. Sleep Med. 2003;4:101–9. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 34.Iber C, Ancoli-Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 35.Aurora RN, Zak RS, Magnati RK, et al. Best practice guide for the treatment of REM sleep behavior disorder (RBD) J Clin Sleep Med. 2010;6:85–95. [PMC free article] [PubMed] [Google Scholar]
- 36.Kunz D, Bes F. Melatonin as a therapy in REM sleep behavior disorder patients: an open-labeled pilot study on the possible influence of melatonin on REM-sleep regulation. Mov Disord. 1999;14:507–11. doi: 10.1002/1531-8257(199905)14:3<507::aid-mds1021>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 37.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinic-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies. Third report of the DLB consortium. Neurology. 2005;65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 39.Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–6. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaig C, Iranzo A, Tolosa E, Vilaseca I, Rey MJ, Santamaria J. Pathologically description of a non-motor variant of multiple system atrophy. J Neurol Neurosurg Psychiatry. 2008;79:1399–400. doi: 10.1136/jnnp.2008.145276. [DOI] [PubMed] [Google Scholar]
- 41.Iranzo A, Santamaría J. Bisoprolol-induced REM sleep behavior disorder. Am J Med. 1999;107:390–2. doi: 10.1016/s0002-9343(99)00245-4. [DOI] [PubMed] [Google Scholar]
- 42.Frauscher BF, Gschliesser V, Brandauer E, et al. REM sleep behavior disorder in 703 sleep-disorder patients: the importance of eliciting a comprehensive sleep history. Sleep Med. 2010;11:167–71. doi: 10.1016/j.sleep.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 43.Sixel-Döring F, Trautmann E, Mollenhauer B, Trenkwalder C. Rapid eye movement sleep behavioral events: a new marker for neurodegeneration in early Parkinson disease? Sleep. 2014;37:431–8. doi: 10.5665/sleep.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iranzo A, Santamaria J, Vilaseca I, Martinez de Osaba MJ. Absence of alterations in serum sex hormones levels in idiopathic REM sleep behavior disorder. Sleep. 2007;30:803–6. doi: 10.1093/sleep/30.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tatman JE, Sind JM. REM behavior disorder manifests differently in women and men. Sleep Res. 1996;25:380. [Google Scholar]
- 46.Bodkin CL, Schenck CH. Rapid eye movement sleep behavior disorder in women: relevance to general and specialty medical practice. J Womens Health. 2009;18:1955–63. doi: 10.1089/jwh.2008.1348. [DOI] [PubMed] [Google Scholar]
- 47.Bjonara KA, Dietrichs E, Toft M. REM sleep behavior disorder in Parkinson's disease-Is there a gender difference? Parkinsonism Related Disord. 2013;19:120–2. doi: 10.1016/j.parkreldis.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 48.Gregg TR, Siegel A. Brain structures and neurotransmitters regulating aggression in cats: implications for human aggression. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:91–140. doi: 10.1016/s0278-5846(00)00150-0. [DOI] [PubMed] [Google Scholar]
- 49.Kalin NH. Primate models to understand human aggression. J Clin Psychiatry. 1999;60:29–32. [PubMed] [Google Scholar]
- 50.Fantini ML, Corona A, Clerici S, Ferini-Strambi L. Aggressive dream content without daytime aggressiveness in REM sleep behavior disorder. Neurology. 2005;65:1010–5. doi: 10.1212/01.wnl.0000179346.39655.e0. [DOI] [PubMed] [Google Scholar]
- 51.Uguccioni G, Golmard JL, de Fontréaux AN, Leu-Semenescu S, Brion A, Arnulf I. Fight or flight? Dream content during sleepwalking/sleep terrors vs rapid eye movement sleep behavior disorder. Sleep Med. 2013;14:391–8. doi: 10.1016/j.sleep.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 52.Oudiette D, De Cock VC, Levault S, Leu S, Vidailhet M, Arnulf I. Nonviolent elaborate behaviors may also occur in REM sleep behavior disorder. Neurology. 2009;72:551–7. doi: 10.1212/01.wnl.0000341936.78678.3a. [DOI] [PubMed] [Google Scholar]
- 53.Ohayon MM, Mahowald MW, Dauvilliers Y, Krystal AD, Léger D. Prevalence and comorbidity of nocturnal wandering in the US adult general population. Neurology. 2012;78:1583–9. doi: 10.1212/WNL.0b013e3182563be5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin FC, Lai CL, Huang P, Liu CK, Hsu CY. The rapid-eye-movement sleep behavior disorder in Chinese-Taiwanese patients. Psychiatry Clin Neurosci. 2009;63:557–62. doi: 10.1111/j.1440-1819.2009.01998.x. [DOI] [PubMed] [Google Scholar]
- 55.Iranzo A, Santamaria J. Severe obstructive sleep apnea/hypopnea syndrome mimicking REM sleep behavior disorder. Sleep. 2005;28:203–6. doi: 10.1093/sleep/28.2.203. [DOI] [PubMed] [Google Scholar]
- 56.Schenck CH, Lee SA, Cramer Bornemann MA, Mahowald MW. Potentially lethal behaviors associated with rapid eye movement sleep behavior disorder (RBD): review of the literature and forensic implications. J Forensic Sci. 2009;54:1475–84. doi: 10.1111/j.1556-4029.2009.01163.x. [DOI] [PubMed] [Google Scholar]
- 57.Mysliwiec V, O'Reilly B, Plochinski J, Kwon HP, Germain A, Roth BJ. Trauma associated sleep disorder: a proposed parasomnia encompassing disruptive nocturnal behaviors, nightmares, and REM without atonia in trauma survivors. J Clin Sleep Med. 2014;10:1143–8. doi: 10.5664/jcsm.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manni R, Terzaghi M, Zambrelli E. REM sleep behaviour disorder in elderly subjects with epilepsy: frequency and clinical aspects of the comorbidity. Sleep. 2006;29:934–7. doi: 10.1016/j.eplepsyres.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 59.Gagnon JF, Postuma RB, Montplaisir J. Update on the pharmacology of REM sleep behavior disorder. Neurology. 2006;67:742–7. doi: 10.1212/01.wnl.0000233926.47469.73. [DOI] [PubMed] [Google Scholar]
- 60.Zhang J, Lam SP, Ho CKW, et al. Diagnosis of REM sleep behavior disorder by video-polysomnography study: is one night enough? Sleep. 2008;31:1179–85. [PMC free article] [PubMed] [Google Scholar]
- 61.Bliwise DL, Rye DB. Elevated PEM (phasic electromyographic metric) rates identify rapid eye movement behavior disorder patients on nights without behavioral abnormalities. Sleep. 2008;31:853–7. doi: 10.1093/sleep/31.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cygan F, Oudiette D, Leclair-Visonneau L, Leu-Semenescu S, Arnulf I. Night-to-night variability of muscle tone, movements, and vocalizations in patients with REM sleep behavior disorder. J Clin Sleep Med. 2010;6:551–5. [PMC free article] [PubMed] [Google Scholar]
- 63.Gugger JJ, Wagner ML. Rapid eye movement sleep behavior disorder. Ann Pharmacother. 2007;41:1833–41. doi: 10.1345/aph.1H587. [DOI] [PubMed] [Google Scholar]
- 64.McCarter SJ, Boswell C, St. Louis EK, et al. Treatment outcomes in REM sleep behavior disorder. Sleep Med. 2013;14:237–42. doi: 10.1016/j.sleep.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kunz D, Bes F. Melatonin effects in a patient with severe REM sleep behavior disorder: case report and theoretical considerations. Neuropsychobiology. 1997;4:211–4. doi: 10.1159/000119383. [DOI] [PubMed] [Google Scholar]
- 66.Carrasco E, Santamaria J, Iranzo A, et al. Changes in dreaming induced by CPAP in severe obstructive sleep apnea syndrome patients. J Sleep Res. 2006;15:430–6. doi: 10.1111/j.1365-2869.2006.00553.x. [DOI] [PubMed] [Google Scholar]
- 67.Hall CS, Van de Castle RL. The content analysis of dreams. New York: Appleton-Century-Crofts; 1966. [Google Scholar]