Abstract
Study Objectives:
Obstructive sleep apnea (OSA) is associated with an increased prevalence of metabolic syndrome (MetS), even in patients with morbid obesity. Our goal was to address whether continuous positive airway pressure (CPAP) treatment improved glucose metabolism in this population.
Methods:
A prospective randomized controlled trial was performed in severe OSA patients with morbid obesity without diabetes in two university referral hospitals. Patients received conservative (CT) versus CPAP treatment for 12 weeks. MetS components, homeostasis model assessment of insulin resistance (HOMA-IR) and oral glucose tolerance were assessed at baseline and after treatment.
Results:
A total of 80 patients completed the study (42 CPAP and 38 CT patients). After 12 w of CPAP treatment, weight loss was similar in both groups and physical activity, prevalence of MetS, and HOMA-IR did not change in either group. In the CPAP group impaired glucose tolerance (IGT) reversed in nine patients and IGT developed in none, whereas IGT reversed in five patients and IGT developed in five patients in the CT group (P = 0.039 in the Fisher test). Changes in 2-h plasma glucose after glucose load were greater in the CPAP group than in the CT group (CPAP: −0.5 ± 1.5 versus CT: 0.33 ± 1.9, P = 0.007).
Conclusions:
The improvement of glucose tolerance in morbidly obese patients with severe obstructive sleep apnea, without changes in homeostasis model assessment of insulin resistance, supports an improvement in peripheral insulin resistance after continuous positive airway pressure treatment.
Clinical Trials Registration:
Citation:
Salord N, Fortuna AM, Monasterio C, Gasa M, Pérez A, Bonsignore MR, Vilarrasa N, Montserrat JM, Mayos M. A randomized controlled trial of continuous positive airway pressure on glucose tolerance in obese patients with obstructive sleep apnea. SLEEP 2016;39(1):35–41.
Keywords: continuous positive airway pressure, glucose tolerance, insulin resistance, obstructive sleep apnea
Significance.
Observational studies have suggested that obstructive sleep apnea (OSA) is associated with impaired glucose metabolism and metabolic syndrome. To date, it remains unclear whether sleep apnea treatment with CPAP is beneficial for glucose metabolism. This is the first study to investigate the effects of CPAP on glucose metabolism in a specific population of morbidly obese patients. These patients represent an extreme model for studying the relation between OSA and metabolic dysfunction because of an increased risk for both disorders. We demonstrated that an effective treatment of OSA with CPAP improves glucose tolerance in morbidly obese patients with severe OSA, without changes in HOMA-IR or metabolic syndrome. These results support an improvement in peripheral insulin resistance after CPAP treatment.
INTRODUCTION
Obesity, and particularly central adiposity, has been recognized as a significant risk factor in the pathophysiology of obstructive sleep apnea (OSA) in adults.1 Furthermore, obesity is not only a major risk factor for sleep apnea but also for cardio-metabolic diseases. Metabolic syndrome (MetS) is a cluster of risk factors that include central obesity, dysglycemia, raised blood pressure, elevated triglyceride levels, and low high-density lipoprotein (HDL) cholesterol levels,2 and is associated with an increased risk for diabetes, cardiovascular events, and mortality in the general population.2–4 Central obesity and insulin resistance are key features of MetS.2
Data from both epidemiological and clinical studies suggest an independent association of OSA with the different components of MetS, particularly insulin resistance, hypertension, and abnormal lipid metabolism.5 The common association between OSA and obesity makes it difficult to separate the role each one plays in their metabolic consequences.6,7 We recently reported that even in morbidly obese patients, OSA was associated with MetS independently of central obesity. Furthermore, the metabolic profile progressively worsened with increasing OSA severity,8 suggesting that OSA can play a significant role in the development of insulin resistance and metabolic disturbances beyond obesity.
Continuous positive airway pressure (CPAP) is the treatment of choice for moderate-to-severe OSA in adults. CPAP prevents upper airway collapse during sleep and can ameliorate intermittent hypoxia and sympathetic overactivation, both of which are pathophysiological mechanisms involved in the metabolic and cardiovascular consequences of OSA.9
Many randomized controlled trials have shown that CPAP treatment clearly leads to an improvement in OSA patient health status,10 but few of them have analyzed its effect on the metabolic profile, and those that have reported contradictory results.11–15 It has been suggested that CPAP could positively affect metabolic variables only in patients with severe OSA without morbid obesity.16 However, the data supporting this hypothesis are scarce and further studies are needed to better identify what type of patients with OSA stand to benefit from CPAP treatment.
We performed a randomized controlled trial (RCT) comparing 12 w of CPAP to conservative treatment (CT) in a population of morbidly obese patients with severe OSA without clinically overt diabetes. We hypothesized that, compared with CT 12 w of CPAP treatment would primarily improve insulin resistance and secondarily lead to a greater improvement in glucose tolerance, MetS, and metabolic profile.
METHODS
Trial Design
A parallel RCT comparing 12 w of CPAP treatment to CT was designed.
Participants and Study Settings
Patients included in the obesity surgery program were studied prospectively in two referral sleep clinics in Barcelona from January 2009 through July 2011. Inclusion criteria for the obesity surgery program were: age between 18 and 65 y; a body mass index (BMI) ≥ 40 kg/m2 or BMI ≥ 35 kg/m2 with comorbidity related to obesity (hypertension, heart disease, degenerative osteoarthritis, and respiratory complications). Eligible patients had an apnea-hypopnea index (AHI) > 30 after a full overnight polysomnography (PSG). The exclusion criteria were: current or previous CPAP treatment, previously known diabetes mellitus or diabetic treatment, unstable cardiovascular conditions, severe cognitive or psychiatric disorders, chronic obstructive pulmonary disease, pregnancy, past or current history of alcohol abuse, refusal to participate, disabling daytime sleepiness, professional drivers or professionals performing potentially dangerous activities. The study protocol was approved by both Ethical Committees (PR052/08, 07/064/797). All participants gave their informed written consent. The trial registration number was NCT 01029561.
Interventions and Protocol
Included patients were randomized to receive individualized lifestyle counseling therapy plus CPAP (CPAP group) or conservative treatment (CT group) consisting only of individualized lifestyle counseling therapy.
At baseline and after 12 w, each participant completed a detailed questionnaire on medical history, cardiovascular risk factors, and current medication. Exercise level and sleep duration were recorded in a self-administered International Physical Activity Questionnaire17 (IPAQ) and a sleep diary for 15 consecutive days. Excessive daytime sleepiness was quantified by the Epworth Sleepiness Scale. Anthropometric characteristics included BMI, neck circumference, waist circumference, waist/hip ratio, and percentage of body fat mass measured by electrical bioimpedance (BIA 101, Akern Bioresearch, Florence, Italy). Clinical blood pressure (BP) was measured according to Spanish guidelines.18
Ethical Issues
After the indication for bariatric surgery, patients generally spend more than 1 y on the waiting list for surgery. During this time, patients receive medical care from an endocrinologist and respiratory and sleep studies are performed in order to reduce the perioperative complications associated with untreated sleep apnea. For ethical reasons, patients were included in our study after the first evaluation by the endocrinologist and were prioritized for overnight PSG, in order to avoid a delay in the beginning of treatment. At the end of the 12-w study period, CPAP treatment was initiated in all CT patients.
Polysomnography
OSA was determined by a full overnight PSG. The PSG (Siesta, Compumedics, Melbourne, Australia) included recording of oronasal flow (termistor and cannula), thoracoabdominal movements, electrocardiogram, submental and pretibial electromyography, electrooculogram, electroencephalogram, pulse oximetry, and body position sensor. Rechtschaffen and Kales' criteria were used for the visual scoring of sleep stages. Apnea was defined as a cessation of flow for at least 10 sec, and hypopnea as any flow reduction of at least 10 sec, accompanied by a fall of ≥ 3% in SpO2 or microarousal. The AHI and the arousal index were defined as the number of apneas/hypopneas and arousals, respectively, per hour of sleep.
CPAP Titration and Compliance
CPAP titration was performed by an overnight PSG with manual CPAP titration or by autotitration devices.19 Objective treatment compliance was determined by dividing the number of hours recorded by the CPAP device's built-in hour meter by all the nights of the study period. Patients with an average use time < 4 h per night were considered non-compliant.
Glucose Metabolism, Lipoproteins, and MetS Definitions
At baseline and after 12 w of CPAP or CT, a venous blood sample was obtained from all patients in fasting conditions; glycosylated hemoglobin (HbA1c), fasting plasma glucose (FPG), total cholesterol, triglycerides, and HDL cholesterol were determined with standard laboratory methods. In patients with FPG < 6.7 mmol/L, plasma glucose measurements were obtained 5 min before and 2 h after administration of 75-g oral glucose (2-h PG). Based on the results of the oral glucose tolerance test (OGTT), normal glucose tolerance was defined as 2-h PG < 7.8 mmol/L, impaired glucose tolerance (IGT) as 2-h PG from 7.8 to 11.0 mmol/L, and diabetes as 2-h PG ≥ 11.1 mmol/L.20 Insulin levels were determined by the automated chemiluminescence method and insulin resistance was estimated using the homeostasis model assessment (HOMA-IR).21 MetS was defined in accordance with the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) modified criteria,2 and metabolic index (MI) as the number of individual MetS components for each patient.
Outcomes
The primary outcome was the change in insulin resistance measured by HOMA-IR. Other secondary outcomes were change in glucose tolerance measured by the oral glucose tolerance test, percentage of metabolic syndrome according to NCEP-ATP III modified criteria, and metabolic profile.
Sample Size, Randomization, and Statistical Analysis
Sample size calculations showed that 42 subjects were needed in each group to detect a difference greater or equal to 1 unit of HOMA-IR, assuming a standard deviation of 1.5 based on previous results in the clinical database of morbid obesity in one of our centers, with a minimum power of 80%. The dropout rate for the study was estimated at 15%.
Simple randomization was performed by a statistician not involved in the study with a computer-generated sequence of random numbers for balanced allocation. Math.random() function was used to generate the random allocation sequence prior to study activation. We did not use any restriction or blocking method. Randomization was assigned using the central database, where the previously reported number generation algorithm was stored. According to eligibility screening by the research coordinator, the system generated a unique number that could not be modified or erased.
The treatment arm was not blinded to the participants, care providers, nor to the person assessing the statistical analysis. Continuous variables were shown as mean ± standard deviation for normally distributed data or median (and interquartile range) for non-normally distributed data, and categorical variables as proportions. The intention-to-treat principle was applied for the analysis of the differences between treatment groups, but missing data were not imputed in order to avoid the dilution effect. Changes from baseline of treatment with CPAP and conservative treatment were calculated by subtracting the value after the 12-w intervention period from the value before the period, the t-test or the Mann-Whitney U test were used (continuous variables). We aggregated patients with IGT and those who screened positive for diabetes in the IGT category, as only three patients proved to be diabetic in the OGTT. Changes after 12 w in IGT and MetS were categorized as improved, worsened, or unchanged. The chi-square test or the Fisher exact test was used. Two-way analysis of variance (ANOVA) was used to assess changes in 2-h PG over time (repeated measures) and the interaction between it and the treatment group. This analysis was adjusted for basal BMI using analysis of covariance (ANCOVA). Two-sided P < 0.05 were considered to indicate statistical significance. Statistical analysis was performed with the statistical software package SPSS V-19 (IBM Corporation, Armonk, NY, USA).
RESULTS
Two hundred forty-three patients were initially assessed for the study. Figure 1 shows exclusion causes and follow-up of participants. Ninety-eight patients were recruited, 44 of whom were assigned to CT and 54 to CPAP. Twelve patients in the CPAP group and six in the CT group discontinued follow-up; therefore, 80 patients completed the study and were analyzed. Patients who discontinued the study had lower BP and FBG compared to patients who completed the study (Table S1, supplemental material).
Figure 1.

Study flow chart.
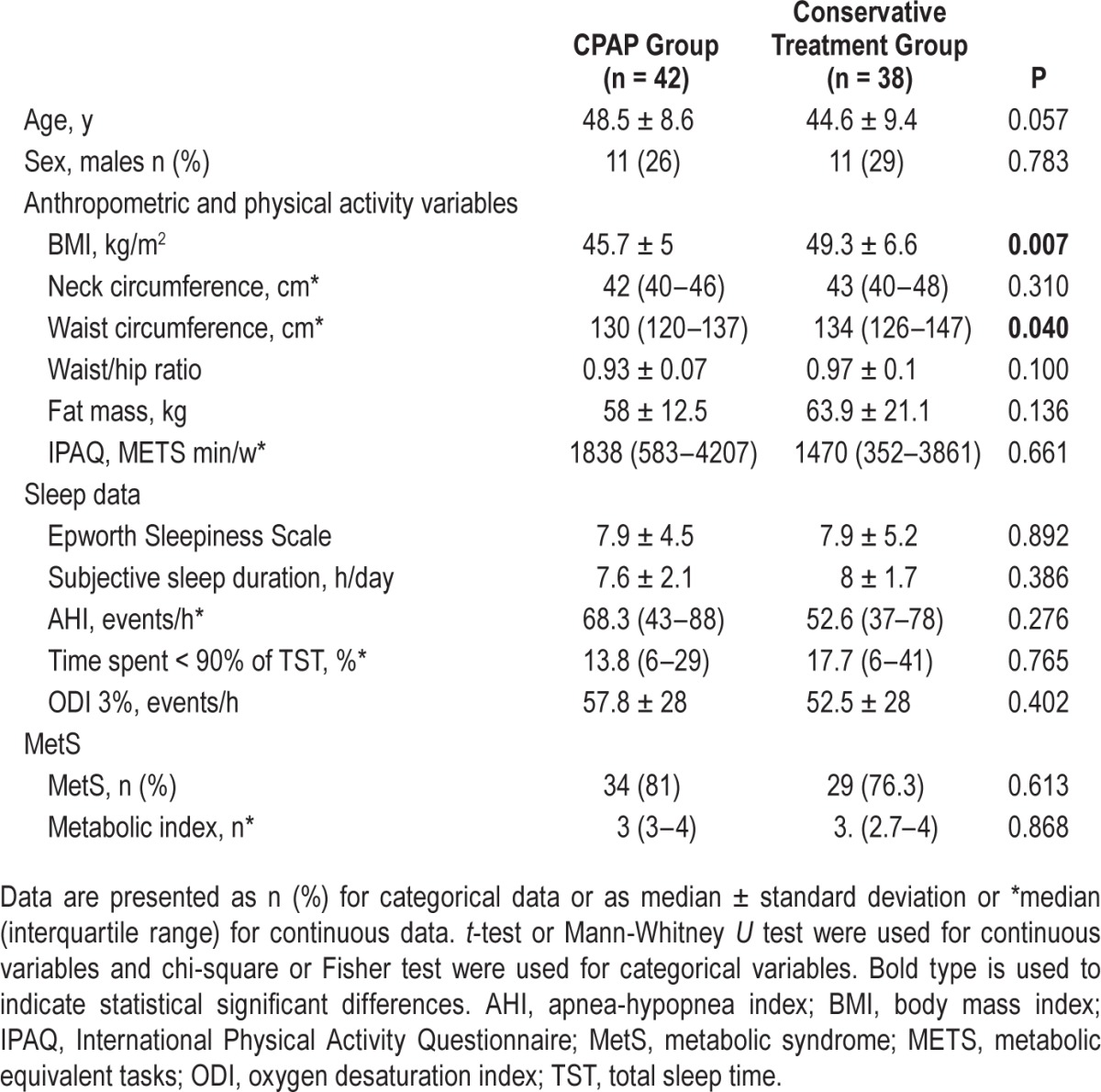
Table 1 shows the baseline characteristics of the CPAP and CT groups. At baseline, patients in the CT group had higher BMI and waist circumference when compared to patients in the CPAP group (Table 1). There were no major differences in age, sex, body fat, treatments, physical activity, or OSA severity between the two groups. Neither sleep efficiency nor sleep structure differed (Table S2, supplemental material) and metabolic variables and prevalence of MetS (Table 1) and its components were comparable at baseline.
Table 1.
Baseline characteristics of the study population.
Effects of Treatment
Objective mean compliance at 12 w in the CPAP group was 5.4 ± 1.6 h per night. Thirty-six patients (86%) used the CPAP machine on average ≥ 4 h at 12 w. Mean CPAP pressure was 10.0 ± 1.6 cmH2O. Patients in the CPAP group presented a significant improvement in sleepiness compared to patients in the CT group (Table 2).
Table 2.
Effects of continuous positive airway pressure versus conservative treatment on clinical variables, glucose metabolism, and metabolic syndrome components.

Table 2 and Figure 2 summarize the changes observed in both groups after 12 w of treatment. Both groups presented a slight but equivalent weight loss with a one-point decrease in BMI (Table 2). Insulin resistance measured by HOMA-IR did not change after treatment. Most of the other variables explored, including FPG or HbA1c, were also unchanged at 12 w in both groups. The percentage of patients in which MetS reversed (19% versus 15.8%) and worsened (2.4% versus 7.9%) was similar in the CPAP and the CT groups.
Figure 2.

Effect of 12 w continuous positive airway pressure and conservative treatment on glucose tolerance in subjects with morbid obesity and severe sleep apnea. Improvement was considered if a patient with glucose intolerance had normal tolerance at the end of the study. Worsened was considered if a patient with normal tolerance turned to intolerant at the end of the study.
Results of OGTT were obtained from 72 patients in both groups at baseline and after 12 w of treatment. Eight patients did not take part in the glucose tolerance test (eight missing values) and an additional three patients had a missing value in the numeric variable because of a data collection error. At baseline 18 of 38 patients (47.4%) patients in the CPAP group and 8 out of 34 (23.5%) in the CT group had IGT (P = 0.036). After 12 w of treatment with CPAP, IGT reverted to normal glucose tolerance in 9 patients and glucose tolerance remained unchanged in 29 patients. In the CT group, IGT reverted to normal glucose tolerance in 5 patients, IGT developed in 5 patients, and 24 remained unchanged (P = 0.039 at the exact Fisher test) (Figure 2). When this result was adjusted by logistic regression, baseline glucose intolerance was a predictor of improved oral glucose tolerance at the end of the study (P = 0.001). Additionally, 2-h PG after a 75-g glucose load decreased more in CPAP patients than in those treated conventionally (Table 2). We observed a significant effect of the treatment group (P = 0.048 ANOVA) in changes in 2-h PG over time (repeated measures). When we adjusted the same analysis for basal BMI (ANCOVA), it continued to have a trend toward significance (P = 0.081) and this covariable did not show significance (BMI, P = 0.571 group P = 0.280).
When the six patients with < 4 h of CPAP compliance were excluded from analysis, the decrease of 2-h PG after 75-g load at 12 w remained significant (CPAP −0.38 ± 1.5 versus CT 0.32 ± 1.9, mean difference −0.71, confidence interval (CI) −1.5 to −0.27, P = 0.020).
Finally, because the CT group presented higher levels of obesity than the CPAP group, the analysis was repeated after excluding five extremely obese patients. The BMI of the two groups became comparable (45.7 ± 5.1 and 47.5 ± 5.1 kg/m2, P = 0.077). Changes in the reversal of IGT, as well as the decrease of 2-h PG in OGTT in the CPAP group, remained significantly different (CPAP −0.94 ± 1.5 versus CT 0.43 ± 2.1, mean difference −0.94, CI −1.86 to −0.043, P = 0.003). After 12 w of treatment with CPAP, IGT reverted to normal glucose tolerance in 9 patients and glucose tolerance remained unchanged in 29 patients. In the CT group, IGT reverted to normal glucose tolerance in 5 patients, IGT developed in 5 patients, and 20 remained unchanged (P = 0.030 at the exact Fisher test).
DISCUSSION
In the current study performed in nondiabetic subjects with morbid obesity and severe sleep apnea, we demonstrated that the effective treatment of OSA with CPAP for 12 w improves glucose tolerance without improvement in insulin resistance as measured by HOMA-IR. Other metabolic variables and MetS prevalence were not significantly different between the CPAP and conservative intervention groups. To the best of our knowledge, this is the first randomized controlled study to evaluate these aspects in this population and it provides new information about the benefits of CPAP therapy on glucose metabolism and its potential mechanisms. These findings are potentially relevant because impaired glucose tolerance, a risk factor for the development of type 2 diabetes, is very prevalent in obese subjects.
Studies addressing the effect of CPAP treatment on glucose metabolism have reported conflicting results.11–16 In a non-controlled study, Harsch et al.11 were the first to observe an improvement in insulin sensitivity, as measured by hyperinsulinemic euglycemic clamp, following 2 nights of CPAP treatment, in a group of nondiabetic Caucasian males with moderate OSA.11 In a crossover controlled study, no change in HOMA-IR was found in a more obese population of Caucasian males with severe OSA after 6 w of CPAP treatment when compared with sham-CPAP.12 More recently, Hoyos et al.14 did not find any difference in HOMA-IR in a 12-w RCT conducted in nonselected obese patients with OSA, but reported significant changes in the Insulin Sensitivity Index, though only in compliant patients. Another RCT conducted in overweight-obese Asian patients with moderate to severe OSA found a significant improvement in insulin sensitivity after only 1 w.22 In addition to the individual negative results in changes in HOMA-IR after CPAP in the aforementioned and other studies,13,23 a recent meta-analysis of RCT showed a modest decrease of HOMA-IR (−0.44) after CPAP compared to sham CPAP, mainly in nondiabetic patients.24 It is noteworthy that in the studies by Hoyos et al.14 and Coughlin et al.,12 mean CPAP use was lower than 4 h per night, suggesting that good adherence to CPAP may be crucial for the achievement of a positive effect of CPAP treatment on insulin resistance.
In spite of the lack of effect on HOMA-IR while fasting, we found that 12 w of CPAP therapy improved glucose tolerance. This apparent discrepancy between HOMA-IR and 2-h PG during OGTT reflects the underlying physiological bases of IGT. IGT is associated with peripheral insulin resistance, most importantly at the level of skeletal muscle.25 The HOMA-IR index is derived from the product of the fasting plasma glucose and insulin concentration and largely reflects the resistance of glucose production by liver and kidney to suppression by insulin, whereas hyperglycemic clamp–determined insulin sensitivity reflects the sensitivity of muscle glucose uptake to stimulation by insulin.26 In this context, the improvement in glucose tolerance observed in our study after 12 w of CPAP treatment may reflect the reduction in muscle insulin resistance. Previously, Weinstock et al.15 did not find any difference in glucose tolerance measured by OGTT after 8 w of CPAP compared to sham CPAP in a moderately obese population, but they reported a positive effect of CPAP on 2-h PG, HOMA-IR, the insulin sensitivity index, and the percentage of normalization of glucose tolerance in severe OSA (AHI > 30).15 Our results agree with data from Weinstock et al., because only patients with severe OSA were included in our study, and suggest that OSA severity is an important factor in studies addressing the effects of CPAP on glucose metabolism.
The positive data obtained at 12 w in the CPAP group regarding the response to glucose load indicate a potentially clinically relevant effect. The number of patients with IGT decreased in the CPAP group, with a shift toward normal response to OGTT, but not in the CT group. This effect could be, in part, overestimated due to a higher proportion of patients with glucose intolerance at baseline in the CPAP group due to random bias. In fact, we have shown that baseline impairment was a significant predictor of improvement, but it is important to note that no patients worsened in the CPAP group and five patients (14.7%) became intolerant in the conservative group. In any case, IGT is a reversible strong risk factor of progression to diabetes and the development of cardiovascular disease.27,28 Therefore, given the particularly high risk of the development of type 2 diabetes in severely obese patients with IGT, our findings suggest a potential role of CPAP treatment as an adjunctive therapy to lifestyle modifications and weight reduction, to prevent or delay the progression from pre-diabetes to type 2 diabetes.
In line with two previous controlled studies, the prevalence of MetS was unchanged after CPAP treatment. Coughlin et al.12 did not find any effect on the whole syndrome after 6 w of CPAP treatment compared to sham CPAP in a population of patients with severe to moderate OSA, detecting changes only in blood pressure. Hoyos et al.29 also observed no improvement in MetS in their unselected population with treated metabolic comorbidities. Taken together, these results suggest that the obesity factor is stronger than OSA in causing this cluster of metabolic abnormalities.
Apart from its randomized controlled design, other strengths of our study include satisfactory CPAP compliance compared with previous studies in which low CPAP compliance could have limited a positive change in glucose metabolism.12–14 In order to control the possible effect of physical activity on metabolism we monitored it by IPAQ questionnaire: this important confounding factor has only been addressed previously by West et al.,13 and our results agree with theirs, because CPAP treatment was not associated with changes in physical activity. Unlike previous studies, which were performed only in men12–14,23 or groups with a low percentage of women,11,24 our population was composed mainly of women, which gives an additional value to our study results.
Our study has a number of potential limitations that should be considered. First, it has to be taken in account that our main positive result, changes in glucose tolerance, was a secondary outcome in which we have some missing values; the study was designed according to insulin resistance defined by HOMA-IR. For that reason the power of the effect of the treatment group in changes in 2-h PG over time was calculated a posteriori and it was 0.946. Second, despite blinded randomization, patients in the CT group were heavier and had higher waist circumferences. We addressed this random bias by performing a post hoc analysis excluding patients with a higher BMI and by using the ANCOVA analysis. The main findings remained unchanged. Additionally, both groups showed a decrease in BMI, probably because of diet and lifestyle counseling. Previously, weight loss programs have been used with positive results in patients with OSA30 and when we applied them equally to the two groups, both had an equivalent weight loss. Consequently, the changes in glucose tolerance can be attributed to the effects of CPAP. Finally, our patients, as a clinical population, underwent active lipid-lowering and antihypertensive treatment that could have conditioned the differences between groups.
In summary, this RCT showed that, in individuals with both morbid obesity and severe sleep apnea, 12 w of CPAP treatment improved glucose tolerance without concomitant changes in the HOMA-IR index and physical activity. As the HOMA-IR index largely reflects hepatic insulin resistance and IGT is associated with peripheral insulin resistance, our study supports an improvement in peripheral insulin resistance as the preferential mechanism involved in the improvement in glucose tolerance after CPAP treatment. This study therefore contributes to the debate on the effect of CPAP treatment on glucose metabolism, and supports the use of CPAP as an adjunct therapy to lifestyle intervention in order to improve glucose metabolism in individuals with morbid obesity and severe sleep apnea.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by: Fondo de Investigación Sanitaria [Grant: FIS PI080800]; Societat Catalana de Pneumologia SOCAP [2052/09]. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Author Contributions: Neus Salord researched data, contributed to the discussion, and wrote and edited the manuscript. Mercedes Mayos researched data, contributed to the discussion, and reviewed and contributed to the manuscript. Ana M Fortuna, Antonio Perez, Carme Monasterio, Mercè Gasa researched data, contributed to the discussion, and reviewed the manuscript. Núria Vilarassa, Josep Maria Montserrat, Maria R. Bonsignore contributed to the discussion, and reviewed the manuscript. Neus Salord is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. David Bridgewater assisted with the English in versions of the manuscript. Advice on the statistical analysis was given by Ignasi Gich (Hospital de la Santa Creu i Sant Pau, Barcelona, Spain) and Cristina Esquinas (Hospital de la Vall d'Hebrón, Barcelona, Spain). The authors thank the sleep unit staff at Hospital Universitari de Bellvitge, Hospitalet de Llobregat, Spain, Tomas Brinquis, Pilar Garriga, Maria Calvo, Neus Martí and Carme Rodríguez and Rosa Miralda, Ariadna Farré and Montserrat Carreras at Hospital de la Santa Creu i Sant Pau, Barcelona, Spain for their inestimable collaboration.
ABBREVIATIONS
- 2-h PG
plasma glucose after 2 hour overload in oral glucose tolerance test
- AHI
apnea-hypopnea index
- BP
blood pressure
- CPAP
continuous positive airway pressure
- CT
conservative treatment
- FPG
fasting plasma glucose
- HbA1c
the percentage of glycosylated hemoglobin
- HDL
high-density lipoprotein
- HOMA-IR
homeostasis model assessment of insulin resistance
- IGT
impaired glucose tolerance
- MI
metabolic index
- MetS
metabolic syndrome
- OGTT
oral glucose tolerance test
- OSA
obstructive apnea-hypopnea syndrome
- RCT
randomized controlled trial
REFERENCES
- 1.Bonsignore MR, McNicholas WT, Montserrat JM, Eckel J. Adipose tissue in obesity and obstructive sleep apnoea. Eur Respir J. 2012;39:746–67. doi: 10.1183/09031936.00047010. [DOI] [PubMed] [Google Scholar]
- 2.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–45. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28:1769–78. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 4.Ho JS, Cannaday JJ, Barlow CE, Mitchell TL, Cooper KH, FitzGerald SJ. Relation of the number of metabolic syndrome risk factors with all-cause and cardiovascular mortality. Am J Cardiol. 2008;102:689–92. doi: 10.1016/j.amjcard.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Lam JC, Mak JC, Ip MS. Obesity, obstructive sleep apnoea and metabolic syndrome. Respirology. 2012;17:223–36. doi: 10.1111/j.1440-1843.2011.02081.x. [DOI] [PubMed] [Google Scholar]
- 6.Lévy P, Bonsignore MR, Eckel J. Sleep, sleep-disordered breathing and metabolic consequences. Eur Respir J. 2009;34:243–60. doi: 10.1183/09031936.00166808. [DOI] [PubMed] [Google Scholar]
- 7.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172:1590–5. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gasa M, Salord N, Fortuna AM, et al. Obstructive sleep apnoea and metabolic impairment in severe obesity. Eur Respir J. 2011;38:1089–97. doi: 10.1183/09031936.00198810. [DOI] [PubMed] [Google Scholar]
- 9.Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol. 2010;7:677–85. doi: 10.1038/nrcardio.2010.145. [DOI] [PubMed] [Google Scholar]
- 10.Sanders MH, Montserrat JM, Farré R, Givelber RJ. Positive pressure therapy: a perspective on evidence-based outcomes and methods of application. Proc Am Thorac Soc. 2008;5:161–72. doi: 10.1513/pats.200709-150MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harsch IA, Schahin SP, Radespiel-Tröger M, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;169:156–62. doi: 10.1164/rccm.200302-206OC. [DOI] [PubMed] [Google Scholar]
- 12.Coughlin SR, Mawdsley L, Mugarza JA, Wilding JP, Calverley PM. Cardiovascular and metabolic effects of CPAP in obese males with OSA. Eur Respir J. 2007;29:720–27. doi: 10.1183/09031936.00043306. [DOI] [PubMed] [Google Scholar]
- 13.West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax. 2007;62:969–74. doi: 10.1136/thx.2006.074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoyos CM, Killick R, Yee BJ, Phillips CL, Grunstein RR, Liu PY. Cardiometabolic changes after continuous positive airway pressure for obstructive sleep apnoea: a randomised sham-controlled study. Thorax. 2012;67:1081–9. doi: 10.1136/thoraxjnl-2011-201420. [DOI] [PubMed] [Google Scholar]
- 15.Weinstock TG, Wang X, Rueschman M, et al. A controlled trial of CPAP therapy on metabolic control in individuals with impaired glucose tolerance and sleep apnea. Sleep. 2012;35:617–25. doi: 10.5665/sleep.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pépin JL, Tamisier R, Lévy P. Obstructive sleep apnoea and metabolic syndrome: put CPAP efficacy in a more realistic perspective. Thorax. 2012;67:1025–7. doi: 10.1136/thoraxjnl-2012-202807. [DOI] [PubMed] [Google Scholar]
- 17.Craig CL, Marchall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 18.Marin R, de la Sierra A, Armario P, Campo C, Banegas JR, Gorostidi M Sociedad Española de Hipertensión-Liga Española para la Lucha contra la Hipertensión Arterial (SEH-LELHA) 2005 Spanish guidelines in diagnosis and treatment of arterial hypertension. Med Clin (Barc) 2005;125:24–34. doi: 10.1157/13076402. [DOI] [PubMed] [Google Scholar]
- 19.Masa JF, Jiménez A, Durán J, et al. Alternative methods of titrating continuous positive airway pressure: a large multicenter study. Am J Respir Crit Care Med. 2004;170:1218–24. doi: 10.1164/rccm.200312-1787OC. [DOI] [PubMed] [Google Scholar]
- 20.Genuth S, Alberti KG, Bennett P, et al. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–7. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 21.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentration in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 22.Lam JC, Lam B, Yao TJ, et al. A randomised controlled trial of nasal continuous positive airway pressure on insulin sensitivity in obstructive sleep apnoea. Eur Respir J. 2010;35:138–45. doi: 10.1183/09031936.00047709. [DOI] [PubMed] [Google Scholar]
- 23.Comondore VR, Cheema R, Fox J, et al. The impact of CPAP on cardiovascular biomarkers in minimally symptomatic patients with obstructive sleep apnea: a pilot feasibility randomized crossover trial. Lung. 2009;187:17–22. doi: 10.1007/s00408-008-9115-5. [DOI] [PubMed] [Google Scholar]
- 24.Iftikhar IH, Khan MF, Das A, Magalang UJ. Meta-analysis: continuous positive airway pressure improves insulin resistance in patients with sleep apnea without diabetes. Ann Am Thorac Soc. 2013;10:115–20. doi: 10.1513/AnnalsATS.201209-081OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycemia: the current status on definition and intervention. Diabetic Med. 2002;19:708–23. doi: 10.1046/j.1464-5491.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- 26.Meyer C, Pimenta W, Woerle HJ, et al. Different mechanisms for impaired fasting glucose and impaired postprandial glucose tolerance in humans. Diabetes Care. 2006;29:1909–4. doi: 10.2337/dc06-0438. [DOI] [PubMed] [Google Scholar]
- 27.Ten-year follow-up report on Birmingham Diabetes Survey of 1961. Report by the Birmingham Diabetes Survey Working Party. Br Med J. 1976;2:35–7. doi: 10.1136/bmj.2.6026.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nathan DM, Davidson MB, DeFronzo RA, et al. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30:753–9. doi: 10.2337/dc07-9920. [DOI] [PubMed] [Google Scholar]
- 29.Hoyos CM, Sullivan DR, Liu PY. Effect of CPAP on the metabolic syndrome: a randomized sham-controlled study. Thorax. 2013;68:588–9. doi: 10.1136/thoraxjnl-2012-203074. [DOI] [PubMed] [Google Scholar]
- 30.Bonsignore MR, Borel AL, Machan E, Grunstein R. Sleep apnoea and metabolic dysfunction. Eur Respir Rev. 2013;22:353–64. doi: 10.1183/09059180.00003413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


