Abstract
Hepatitis C virus (HCV) replication is associated with the endoplasmic reticulum, where the virus can induce cellular stress. Oxidative cell damage plays an important role in HCV physiopathology. Oxidative stress is triggered when the concentration of oxygen species in the extracellular or intracellular environment exceeds antioxidant defenses. Cells are protected and modulate oxidative stress through the interplay of intracellular antioxidant agents, mainly glutathione system (GSH) and thioredoxin; and antioxidant enzyme systems such as superoxide dismutase, catalase, GSH peroxidase, and heme oxygenase-1. Also, the use of natural and synthetic antioxidants (vitamin C and E, N-acetylcysteine, glycyrrhizin, polyenylphosphatidyl choline, mitoquinone, quercetin, S-adenosylmethionine and silymarin) has already shown promising results as co-adjuvants in HCV therapy. Despite all the available information, it is not known how different agents with antiviral activity can interfere with the modulation of the cell redox state induced by HCV and decrease viral replication. This review describes an evidence-based consensus on molecular mechanisms involved in HCV replication and their relationship with cell damage induced by oxidative stress generated by the virus itself and cell antiviral machinery. It also describes some molecules that modify the levels of oxidative stress in HCV-infected cells.
Keywords: Hepatitis C virus, Oxidative stress, Reactive oxygen species, Vitamin E, Antioxidants, Glycyrrhizin, S-adenosylmethionine, N-acetylcysteine, Silymarin
Core tip: This review focuses on the available findings regarding the relationship between viral and cellular proteins and the resulting regulation of oxidative stress. To understand the liver damage induced by hepatitis C virus and its persistence is important to know how the cell regulatory systems involved in the production and elimination of reactive oxygen species (ROS) benefit the replication of the virus, as well as their participation in the cell defense mechanisms and immune perturbation following the infection. We must also consider the involvement of ROS in signaling pathways that induce viral replication and its implication in antiviral therapies.
INTRODUCTION
The number of deaths as a result of liver cirrhosis and cancer rose by 50 million in the last two decades, according to the first-ever World Health Organization study on liver disease mortality. Liver cancer is largely a problem in developing countries accounting for 83% of the estimated 782000 new cases occurred in 2012. Liver cancer is the fifth most common type of cancer reported in men and the ninth in women. Liver cancer is the second leading cause of death by cancer around the world, and it is considered to be responsible for nearly 746000 deaths in 2012 (9.1% of the total). The prognosis for liver cancer patients is very poor (overall ratio of mortality to incidence of 0.95), and as such, the geographical patterns in incidence and mortality are frighteningly similar[1]. The highest hepatitis C virus (HCV) prevalence worldwide are reported in the Western Pacific, Southeast Asia, and Africa regions, with around 120 million people infected who have limited access to the new anti-HCV drugs. It is important to mention that most of the people with HCV infection do not live in North America, Europe, or Japan (approximately 3.2 million people in the United States are chronically infected with HCV), which are the primary market for current anti-HCV drugs. In addition, it is well known that low- and middle-income countries, like Egypt (22%) and China (3.2%), report the highest prevalence. Although HCV genotypes 1 and 3 are more prevalent in most countries regardless of economic status, the largest proportions of genotypes, 4 and 5 are in low-income countries[2-4]. The total population infected with HCV in Latin America in 2010 was estimated at 7.8 million, of which 4.6 million are infected with genotype 1 (approximately 70% with subtypes 1a and 1b) and genotypes 2a, 2b, 2c and 3a which constitute 25% of the remaining genotypes, whereas the other genotypes 4 to 7 are less common[5]. It is estimated that up to 3% of the global population (approximately 150-170 million persons) is infected with chronic hepatitis C. HCV has been demonstrated to be the leading cause of chronic liver disease[4], cirrhosis and hepatocellular carcinoma (HCC), and is the underlying cause of over 475000 annual deaths, worldwide[6].
There is still no anti-HCV vaccine available and until recently, the only approved treatment, based on a combination of pegylated interferon (PEG-INF) and ribavirin (RBV), was partially effective in treated patients and also had considerable side effects in most of the patients[7]. Recently, after several years of research, new therapies that specifically block the virus have been developed. In 2011, the first anti-HCV specific drugs were approved for clinical use. Since then, a new era began for HCV infected patients with the founding of new direct acting agents (DAAs)[8,9]. However, the availability and accessibility of new protease inhibitors, telaprevir, boceprevir, simeprevir; and the recently approved RNA polymerase inhibitor sofosbuvir, depends on the region where patients are located and their access through government health programs, since the costs of DAAS are high. Increasing response rates are expected in the near future due to the development of numerous new DAAs and host-targeted drugs active against HCV[10-12]. There are constant efforts to identify new cheaper and effective antiviral molecules through other therapeutic approaches. Recent antioxidant compounds reports highlight their antiviral activity against HCV and post them as anti-HCV co-adjuvants that can improve the effectiveness of treatment as well as shorten the period and reduce the overall cost of the therapy.
HCV MOLECULAR BIOLOGY
HCV is a positive-strand RNA virus, classified into the Hepacivirus genus (Flaviviridae family), with a genome around 9600 nucleotides in length. The single strand genome carries a 50 and 30 nt length in non-coding region (NCR) flanking a single open-reading frame, encoding a single polyprotein of around 3009 aminoacid residues. Interestingly, this 50 NCR forms an internal ribosome entry site that leads the translation of the viral polyprotein, which in turn is cleaved by both viral and host proteases, in order to produce the structural (core, E1 and E2) and non-structural (NS2 to NS5B) viral proteins. There are numerous reports about the interaction between viral and cellular proteins to facilitate replication of the virus, however more information is needed to understand their role in the pathophysiology of the disease[13,14].
HCV AND OXIDATIVE STRESS
It is well known that most of the viral replication cycle takes place associated with the endoplasmic reticulum (ER), which encourages cellular stress. This effect has been associated with a specific regulation of the virus replication cycle[15]. It is reported that viral proteins such as HCV-core, E1, and NS3 can modulate and inactivate mitochondrial respiratory chain enzymes and in turn unshackle blockade of the electrons, disruption of mitochondrial transmembrane potential, and electron leakage inducing an increase of intracellular reactive oxygen species (ROS) levels[16,17]. Together, all these molecular events impair mitochondrial and cellular signaling pathways.
The generation of oxygen species in several biological processes exceeds the capacity of antioxidant systems. A greater imbalance in the pro-oxidant and antioxidant ratio in benefit of the pro-oxidant can induces cellular damage[15,18].
Several reports demonstrate that expression of HCV proteins increase ROS levels due to activation of several pathways including the activation of mitogen-activated protein kinase (MAPK), ER response, Nuclear factor κB (NF-κB) and calcium signaling[19]. In the cytoplasm, the ER is actively involved in the induction of ROS, since excessive production of viral proteins induces the unfolded proteins response which is accompanied by calcium release. Then, the mitochondria absorbed the released calcium quickly, giving as a result an elevation of ROS. The constant production of ROS is a challenge that the cell has to overcome to survive. Normally, cells have sufficient capacity to balance the demand for antioxidant compounds and enzymatic antioxidant systems with the production of ROS[20], then modulating or blocking tissue damage. A biological condition that disrupts this balance, can induce accumulation of ROS and oxidative stress. Among the mechanisms involved in cell protection against oxidative stress damage we can found several intracellular antioxidant agents (glutathione, S-adenosil-methionine and thioredoxin), and antioxidant enzymes (superoxide dismutase, catalase, GSH peroxidase and heme oxygenase-1)[21-23].
In fact, there are large amount of data demonstrating that several intracellular signaling pathways are altered by HCV proteins in order to promote replication. The HCV can do accomplish this by finely regulating the oxido-reductive state of the host cell. MAPK and phosphoinositide-3 kinase (PI3K)/Akt signaling pathways are critical controllers of HCV replication and in turn, these pathways are modulated by phosphorylation cascades and oxidative stress. The first evidences that a virus could induce oxidative stress by increasing ROS levels, were published by Peterhans et al[24] in 1979. They demonstrated that the infection of mouse splenocytes with Sendai virus induced an increase of chemiluminescence levels, which it meant that the luminol had been oxidized by ROS in the experimental setting. They demonstrated that virus inactivated with UV light are able to generate ROS too, whereas virus inactivated by heating could not generate ROS, suggesting that viral structure conformation are mediating this action[24].
It has been shown that oxidative damage has a major role in HCV-induced liver damage, via ROS accumulation, produced from HCV infected cells and infiltrating immune cells[25,26]. The high amount of either HCV-structural or non structural proteins induce oxidative stress and disrupt the antioxidant equilibrium into the cells. In addition, a direct interaction of the HCV-core protein with mitochondria is able to decrease the mitochondrial NADPH levels, reducing the activity of the electron transport complex I and then increasing generation of ROS. It has been shown that HCV-core protein over-expression diminished GSH levels and induced GSSG levels. In addition, an expected compensatory response increases the major enzymatic antioxidant elements such as GSH reductasa, catalase, MnSOD and heme oxygenase-1 (HO-1), in infected cells[27].
It is reported that expression of viral core protein induces the expression of p21-waf1, activates the NF-κB, bind to p53 and induces oxidative stress markers (ROS and peroxidated lipids)[28-30]. There is a differential effect on the enzymatic antioxidant systems in response to the presence of different viral proteins. Abdalla et al[31] reported that HCV infection reduced in vivo hepatic expression of HO-1 and in vitro HCV-core protein expression causes a similar effect, but this effect is not detected in superoxide dismutase (SOD) and catalase enzymes. This group also studied the effect of HCV-core expression in regulation of GSH levels. They findings demonstrated that GSH levels were diminished upon HCV-core expression, but at the same time oxidized GSH (GSSG) levels were undetectable. This finding led them to evaluate if thioredoxin (Trx) was also regulated by HCV-core protein, and they found that in fact Trx was also oxidated. As expected there is a compensatory induction of anti-oxidant defenses in cells expressing viral proteins[31]. On the other hand, expression of non-structural protein 5A (NS5A) up-regulates mitochondrial ROS levels because it induces the release of calcium from ER, binds to PI3K and triggers NF-κB signaling[19,32-34]. This event is followed by a translocation of NF-κB to the nucleus, where it binds to DNA and activates several gene promoters. In addition, activation of NF-κB signaling can be blocked by several antioxidants[35]. Figure 1 Possible interactions/mechanisms of antioxidant agents with reported anti-HCV effect.
Figure 1.
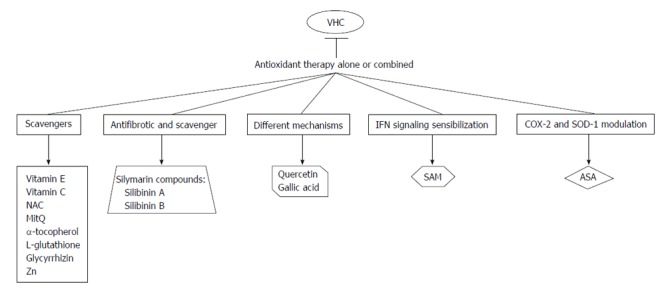
Possible interactions/mechanisms of antioxidant agents with reported anti-hepatitis C virus effect. SOD: Superoxide dismutase; SAM: S-adenosylmethionine; ASA: Acetylsalicylic acid; Zn: Zinc; IFN: Interferon.
On the other hand, twenty years ago, Suematsu et al[36] showed that patients with hepatitis C had increased serum lipid peroxides, and further this increase was also confirmed by Higueras et al[37], but interestingly they reported that lipid peroxides levels were decreased in patients treated with alpha-interferon. Still, it has not been defined whether this effect was due to the decrease of viral replication and inflammation or a combination of induction of antioxidant enzymes by the alpha-interferon treatment[37]. Another important component in this viral redox imbalance is the transcription factor Nrf2, reported by Waris et al[33] in 2010. An independent study by Ivanov et al[38], on Huh7 cells reported that induction of the Nrf2/ARE signaling pathway is triggered at least by five of the HCV viral proteins (core, E1, E2, NS4B, and NS5A). It is reported that virus can affect the cell redox equilibrium by inducing cellular pro-oxidants such as Fe2+/3+ ions, nitric oxide and by decreasing the synthesis of antioxidant enzyme systems. In addition, ROS could regulate virus replication modulating oxidative stress present in the infected cell in order to choose survivors viral mutants, by inducing mutations and also by activating transcription factors such as NF-κB that could participate in viral protein expression[39]. Based on the multiple oxidant and antioxidant activities performed in vivo and in vitro systems during viral infections, and the variable ability of antioxidants to cross cell membranes, the systematic use of antioxidants as antiviral therapies had been limited[40].
On the other hand, HCV is potentially lymphotropic, because it can invade and propagate in cells of the immune system. It is known that processing of HCV proteins are performed in the ER but HCV proteins accumulate at the space between the mitochondrial outer membrane and ER. It has been reported that HCV proteins can move from the ER synthesis site to the mitochondria, therefore, there is an interaction between HCV proteins with the mitochondrial machinery in hepatic and extra-hepatic sites. Impairment of mitochondria-nuclear cross talk through involvement of PI3 kinases has been published by Bhargava et al[17], 2011.
ANTIOXIDANT THERAPY
It has been shown that HCV modifies antioxidant defense mechanisms yielding a cellular oxidative imbalance[29,31,41]. Several authors, including our research group, have demonstrated that HCV effectively modifies gene expression regulatory proteins of oxidative stress as SOD and catalase[26,31,33]. The limitations of interfering with such mechanisms in viral diseases are similar to those when interfering with oxidant generation. This is a result of the association between these pathways with the normal host physiology as well as with host pathology. It is clear that antioxidant therapy could be criticized because it involves a wide variety of drugs, rather than the magic one or two putatively specific ones used in modern pharmacotherapy. The most common antioxidant used in several clinical trials and in vitro experiments are described in Table 1. However, because the symptoms and pathology of viral diseases are ultimately the result of complex host reactions in addition to direct viral effects, there is a scientific basis for this strategy of viral disease therapy. Clearly, this does not obviate the need for further research to identify a drugs that may specifically interfere with viral replication[40,42,43]. The modulation of major signaling pathways by high cell ROS levels are shown in Figure 2.
Table 1.
Most common antioxidant used in several clinical trials and in vitro experiments
| Antioxidant | Dose range |
| Scavengers | |
| Vitamin E[47,48] | 400-544 IU/d or 600 mg1 |
| Vitamin C[48] | 500 mg-10 g |
| N-acetylcysteine[78] | 600 mg or 1800 mg/d1 |
| Mitoquine Q[65,66] | 40 or 80 mg/d |
| α-tocopherol[79] | 600 mg, 500 mg/d, 800 IU/d |
| Glycyrrhizin[40,42,53,54] | 500 mg, 120 mg |
| Different mechanisms | |
| Silymarin (Silibinin A, Silibinin B, etc.)[40,64,80-82] | 250 mg or 5-20 mg/kg1 |
| S-adenosylmethionine[69] | 1600 mg/d1 |
| Acetylsalicylic acid[73] | 4 mmol/L (in vitro) |
| Gallic acid | 300 mg/mL (in vitro) |
Combined treatment with interferon.
Figure 2.
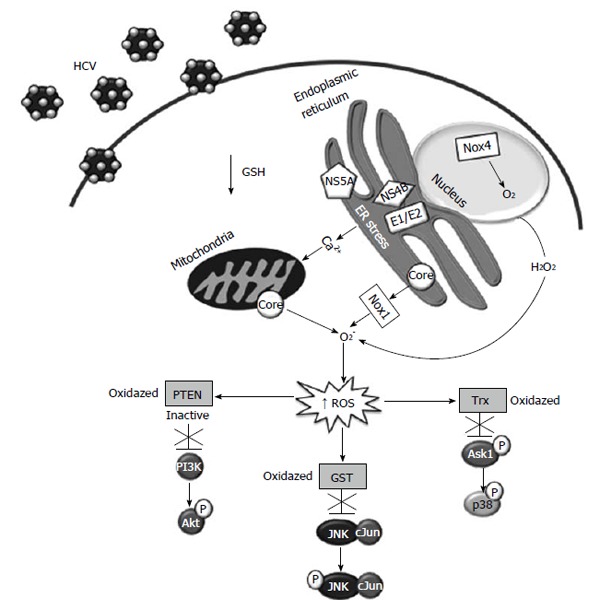
Cell signaling pathways modulated by increased reactive oxygen species levels in hepatitis C virus infected cells. ROS: Reactive oxygen species; HCV: Hepatitis C virus; GSH: Glutathione system; Trx: Thioredoxin; PI3K: Phosphoinositide-3 kinase.
VITAMINS C AND E
Vitamin C (ascorbic acid) is as an electron donor in enzymatic reactions and thus can block free radical chain reactions. Vitamins C, A, and E are the most important natural antioxidants associated with cell-mediated immunity and toxic hepatitis[44]. Vitamin C defends cells from free radical damage by reducing radicals, scavenging lipid-peroxidation-derived radicals, or reducing tocopherol radicals to tocopherol[45]. In cells, vitamin E is the major lipid soluble chain-breaking antioxidant in mitochondria, microsomes, and lipoproteins[46]. von Herbay et al[47] reported that vitamin E decreases the hepatic aminotransferase levels in patients whit chronic HCV infection. Murakami et al[48] observed that antioxidant vitamins (E and C) supplemented during interferon alpha treatment inhibited the decrease in eicosapentaenoic acid of mononuclear cell. They also stated that it might be possible to enhance the efficacy of combination therapy of interferon alfa-2b and RBV[48]. There are other reports, with conflicting or non-reproducible results, so there is still controversy on their usefulness.
ZINC
It has been reported that zinc (Zn) supplementation down-regulated oxidative stress products such as malondialdehyde (MDA), 4-hydroxyalkenals, and 8-hydroxydeoxyguanine in plasma; inhibited induction of tumor necrosis factor-α and interleukin-1β mRNA in mononuclear cells; and protected against NF-κB activation in mononuclear cells[49]. Polaprezinc (zinc plus l-carnosine), has been studied in many studies as an antioxidant adjuvant to IFN as treatment for chronic HCV infection, showing promising results[50]. There are several potential mechanisms supporting the antioxidant effects of zinc, such as the decrease of hepatic fibrosis, antioxidant activity, down-regulation of ferritin, and improvement in hepatic encephalopathy. Zn also is able to decrease HCV replication, then it has been used as an adjuvant for treatment of HCV infection, but further studies are needed to understand the involved mechanisms[51].
N-ACETYLCYSTEINE
On the other hand, Look et al[52] demonstrated that adjuvant antioxidant therapy with N-acetylcysteine/Selenium co-supplementation has not beneficial effect to improve antiviral activity in chronic hepatitis C patients upon a six-month interferon alpha monotherapy. These data suggest that antioxidant compounds could have a beneficial effect on necro-inflammatory variables, but have no effect on the viral cycle reduction.
GLYCYRRHIZIN
Another antioxidant evaluated in several clinical trials to combat HCV is glycyrrhizin, a free radical scavenger. Intravenous administration of glycyrrhizin, diminished transaminase enzyme levels, and decrease cellular damage in patients with chronic HCV infections[53,54]. In the Melhem et al[55] study, 50 HCV-infected patients were treated for 20 wk with a mix of seven oral antioxidants (glycyrrhizin, schisandra, silymarin, lipoic acid, ascorbic acid, L-glutathione, and alpha-tocopherol), along with four different intravenous preparations (glycyrrhizin, ascorbic acid, L-glutathione, B-complex) twice weekly for the first 10 wk. In patients who underwent this treatment, liver enzymes normalization occurred in 44% who had elevated ALT levels previous to treatment. Twenty five percent of the patients showed a decrease in viral load by one log or more. Histological improvement was also noted in 36.1% of patients. There were no major adverse reactions noticed[55]. These data portrays promising results for the use of glycyrrhizin in different liver diseases.
SILYMARIN COMPLEX
Silibinin is a major component of milk thistle (silymarin), which has been demonstrated in several in vitro and clinical trials that is a strong antioxidant and antifibrotic agent. The mechanisms of action of silymarin include different events, such as the stimulation of ribosomal RNA transcription and levels, protecting the cell membrane from oxidative stress damage and blockage of the uptake of toxins. A combination of antioxidant compounds that have been shown to improve cellular and biochemical markers in chronic HCV patients is formed by alpha-lipoic acid, silymarin, and selenium[56,57]. There are conflicting results on the efficacy of silymarin. As an example a phase I trial assessing the efficacy of oral doses of silymarin in non-responders HCV infected patients demonstrated no adverse effects but also showed no antiviral efficacy against HCV[41]. However, in other report the administration of intravenous (iv) silibilin in non-responders HCV infected patients decreased HCV-RNA levels in a dose-dependent manner[58]. It is reported that silibinin monotherapies of 5, 10, 15 or 20 mg/kg for one week decrease viral load (from 0.55 to 3.02 logs). The addition of combination of PEG-IFN/RBV at day 8 resulted in a higher reduction of viremia, but some patients had slight rebound viremia upon iv silibinin treatment was discontinued despite continued standard of care.
In another study, antiviral activity of silymarin complex was evaluated in vitro by using the HCV-RNA polymerase and NS3/4A protease enzyme assays. Results demonstrated that silibinin A, silibinin B, their water-soluble dihydrogen succinate forms and Legalon SIL (a commercially available iv preparation of silibinin), were able to inhibit HCV-RNA polymerase activity (IC50 of the order of 75-100 μmol/L). In addition, Silibinin A and silibinin B also decreased HCV genotype 1b replication and HCV genotype 2a strain JFH1 replication in cell culture. Interestingly, none of these agents affected the HCV protease function[59]. The antioxidant effects of silibinin have been showed in several cell studies. Silibinin is classified as an antioxidant compound because it blocks radical formation, binds many radical species (scavenger), interferes with lipid peroxidation mechanisms of membranes, and then increases the intracellular content of scavengers[60].
Gomez et al[61] in 2010 investigated the role of Viusid as an antioxidant and an immunomodulator in nonresponder HCV infected patients. Authors reported that MDA and 4-hydroxyalkenal levels were significantly decreased in serum of the treated patients when compared with placebo.
GALLIC ACID
Gallic acid (GA) is a phenolic compound present in several natural sources and it has been reported to have various biological effects such as antioxidant, anti-inflammatory, antibiotic, anticancer, antiviral and cardiovascular protection. Recently, our group started to investigate whether GA is able to have an effect on HCV replication. Recently, we examined the effects of GA on HCV expression using a subgenomic HCV replicon cell culture system that expresses HCV-nonstructural proteins (unpublished data). We observed that GA down-regulated NS5A-HCV protein expression (around 55%) and HCV-RNA levels (nearly 50%) in a time-dependent fashion compared with untreated cells. Interestingly, we observed that GA treatment decreased ROS levels at early times of exposure in cells expressing HCV proteins (manuscript in press). Similar results were found upon PDTC exposure. These findings suggest the possibility that antioxidant capacity of GA could contribute to the mechanism(s) involved in the down-regulation of HCV replication in hepatoma cells, however further experiments are needed to confirm these findings.
HEME OXYGENASE REGULATION
There are some reports regarding to modulation of antioxidant enzymes as a HCV therapy, Zhu et al[62] worked with HO which catalyzes the rate-limiting reaction in the catabolism of heme which produces equimolar amounts of biliverdin, carbon monoxide and free iron. They reported that increased expression of HO-1 is associated with diminished HCV replication and also with an increase of the resistance of hepatocytes to oxidative damage. Based on this findings, heme oxygenase regulation could be useful as an adjunctive antiviral therapy[62-64].
MITOQUINONE
Mitoquinone or MitQ (Antipodean Pharmaceuticals, Auckland, New Zeland) is a potent antioxidant that bonds the antioxidant moiety of coenzyme Q10 (known as ubiquinone) to a triphenylphosphonium cation. The cation causes the attached antioxidant to accumulate several-hundred fold within mitochondria in vivo upon oral administration, protecting them from oxidative injury and cell death. A phase-2 study of 28 d of MitQ revealed a decrease in serum aminotransferase in HCV treated patients[65,66].
QUERCETIN
Quercetin is a flavonoid antioxidant. In vitro treatment of HCV infected cells with quercetin diminished viral replication and infectious particle production. It has been shown that quercetin blocks viral protein production independently of viral genome replication; may help to elucidate the replication cycle and has potential use as antiviral agent helping to reduce virus production with low toxicity[67]. Additionally, dihydroquercetin has been shown to be beneficial as a hepatoprotective substance in the treatment of toxic hepatitis and liver fibrosis by enhancing antioxidant enzyme activity and decreasing the pro-oxidant effect[67,68].
S-ADENOSYLMETHIONINE
Further information into the hepatoprotective mechanisms of antioxidant agents might be discovered by analyzing the relationship between glutathione precursor S-adenosylmethionine (SAM) and involved signaling pathways. Administration of SAM to non-responders patients infected with HCV, showed beneficial effects in combination with PEG-IFN and RBV. Feld et al[69] also suggest that this effect is through the methylation of STAT-1, a transcription factor responsible of interferon stimulated gene expression, which enhances its translocation to the nucleus. Our recent results indicate that there is a combination of actions regarding to the molecular mechanism of SAM on HCV replication. Administration of SAM to HCV replicon cells, leads to an increase of glutathione synthesis (unpublished data). There are also reports of SAM benefits in patients with alcoholic liver cirrhosis. The possible mechanisms of action of SAM include: (1) function as a methyl donor compound and restoring of mitochondrial glutathione levels, which is necessary to counterbalance the oxidant environment in cirrhotic liver; and (2) decreasing the hepatic production of nitric oxide (NO), through the modulation of iNOS enzyme. SAM perform these effects in part by accelerating synthesis of inhibitor of κB-alpha and regulating the activation of NF-κB, thereby decreasing the transactivation of iNOS promoter[70]. Nevertheless, further experiments are needed to explore the participation of NO synthase-2 promoter and the effect of SAM in HCV replication.
We are currently investigating the molecular mechanisms of SAM against HCV in a subgenomic replicon cell model. We found that SAM is capable of modulate the antioxidant defense systems at transcriptional and translational level (SOD1, SOD2 and thioredoxin 1); we also found that biosynthesis of GSH in presence of SAM is increased in short periods of time (2-6 h). In addition, MAT1/MAT2 turnover is switched in presence of SAM (unpublished data). MAT1 is the enzyme responsible of the conversion of methionine to s-adenosylmethionine, and it is down-regulated in hepatocarcinoma and liver diseases. Presently, we need more experimental data to understand the role of SAM in the modulation of HCV.
ACETYLSALICYLIC ACID
Several studies including our reported findings demonstrated that sodium salicylate and acetylsalicylic acid (ASA) block the replication of flaviviruses, such as Japanese encephalitis virus, HCV and dengue virus[71,72]. Liao et al[71] demonstrated that salicylates inhibit flavivirus infection through a mechanism including p38-MAPK activity, but not NF-κB activation. In other hand, Mazur et al[72] reported inhibition of influenza virus replication in vitro and in vivo by ASA. This antiviral activity was mediated by a mechanism including expression of proapoptotic factors, decrease of caspase activation, and blocking of the nuclear export of viral ribonucleoproteins[72]. In 2008, our research group demonstrated that ASA presented anti-HCV properties in HCV-replicon cells through inhibition of COX-2 activity and expression, which is mediated in part by the activation of (MEK1/2)/p38 MAPKs[73,74]. Our results suggest that ASA in combination with standard therapy could be an excellent adjuvant in the treatment of chronic HCV infection.
As antiviral agents, antioxidants could be used in four different ways: (1) to deteriorate cellular mechanisms involved in HCV replication; (2) to improve liver enzyme activity and levels; (3) to counteract and protect against liver cell injury; and (4) to improve interferon anti-viral response. Triple antioxidants therapies have been tested in clinical trials, which include alpha-lipoic acid, silymarin and selenium in order to suppress HCV-induced liver damage, when used together with Vitamins C and E, and in a healthy diet and exercise regime[43,75,76].
Several studies are in favor of a positive control of HCV replication by oxidative stress and to search counteracting this effect. Taking all these data together, there is plenty of evidence suggesting that antioxidants can effectively improve the response of HCV infected patients, even if they are non-responders. There are also proofs that antioxidants ameliorate the oxidative and nitrosative stress in liver disease, ultimately decreasing inflammation and fibrosis progression. Some investigators think that although it is important testing these antioxidant compounds in clinical trials, they make emphasis on the need of researching the side effects of different antioxidants on HCV replication before its use as therapy. However, there are conflicting data published by Nakamura et al[77], where they reported an enhanced HCV replication with resveratrol treatment, so further research is needed.
CONCLUSION
It is well established that HCV infection leads to strong cellular oxidative stress, triggering several HCV-associated metabolic disorders including HCC, steatosis, liver fibrosis, cirrhosis and iron overload. Today, several molecular interplays and signaling pathways involving viral proteins, host cell factors, ROS-generating enzymes and cellular antioxidant systems have been elucidated. In fact, several intracellular signaling pathways are altered by the expression of HCV proteins in favor of virus replication and they are finely regulated by the cellular redox state. Additional mechanisms by which HCV induces and modulate oxidative stress still remain to be discovered and require further studies. With the current findings regarding the dual function of the oxidative stress induced by the virus and the host cell, it may be possible to establish new and more effective therapeutic targets for HCV treatment.
Footnotes
P- Reviewer: Kittner JM, Mishra PK, Tsukiyama-Kohara K S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
Supported by The CONACYT, No. CB-2011-1-58781 to Ana M Rivas-Estilla (partially); and Red CA Fisiopatología de Enfermedades Hepáticas 2015.
Conflict-of-interest statement: There is no conflict of interest associated with any of the senior author or other coauthors contributed their efforts in this manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 8, 2015
First decision: September 8, 2015
Article in press: December 2, 2015
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin D, Forman D, Bray F. GLOBOCAN 2012 v1. 0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. [Internet]. Lyon, Fr. Int. Agency Res Cancer; 2013. p. 11. Available from: http://globocan.iarc.fr. [Google Scholar]
- 2.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 3.Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, Barnes E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61:77–87. doi: 10.1002/hep.27259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Hepatitis C Fact sheet N°164 [Internet]. [Updated 2015 Jul; accessed 2015 Aug 20] Available from: http://www.who.int/mediacentre/factsheets/fs164/en/ [Google Scholar]
- 5.Kershenobich D, Razavi HA, Sánchez-Avila JF, Bessone F, Coelho HS, Dagher L, Gonçales FL, Quiroz JF, Rodriguez-Perez F, Rosado B, et al. Trends and projections of hepatitis C virus epidemiology in Latin America. Liver Int. 2011;31 Suppl 2:18–29. doi: 10.1111/j.1478-3231.2011.02538.x. [DOI] [PubMed] [Google Scholar]
- 6.Torresi J, Johnson D, Wedemeyer H. Progress in the development of preventive and therapeutic vaccines for hepatitis C virus. J Hepatol. 2011;54:1273–1285. doi: 10.1016/j.jhep.2010.09.040. [DOI] [PubMed] [Google Scholar]
- 7.El-Shamy A, Hotta H. Impact of hepatitis C virus heterogeneity on interferon sensitivity: an overview. World J Gastroenterol. 2014;20:7555–7569. doi: 10.3748/wjg.v20.i24.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207–1217. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bacon BR FDA. FDA approves Sovaldi for chronic hepatitis C [Internet] US Food Drug Adm: 2013. Available from: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm377888.htm. [Google Scholar]
- 11.Hill A, Khoo S, Fortunak J, Simmons B, Ford N. Minimum costs for producing hepatitis C direct-acting antivirals for use in large-scale treatment access programs in developing countries. Clin Infect Dis. 2014;58:928–936. doi: 10.1093/cid/ciu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tencate V, Sainz B Jr, Cotler SJ, Uprichard SL. Potential treatment options and future research to increase hepatitis C virus treatment response rate. Hepat Med. 2010;2010:125–145. doi: 10.2147/HMER.S7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Z, Choi J, Lu W, Ou JH. Hepatitis C virus f protein is a short-lived protein associated with the endoplasmic reticulum. J Virol. 2003;77:1578–1583. doi: 10.1128/JVI.77.2.1578-1583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ploss A, Evans MJ. Hepatitis C virus host cell entry. Curr Opin Virol. 2012;2:14–19. doi: 10.1016/j.coviro.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oberley LW, Buettner GR. Role of superoxide dismutase in cancer: a review. Cancer Res. 1979;39:1141–1149. [PubMed] [Google Scholar]
- 16.Machida K, McNamara G, Cheng KT, Huang J, Wang CH, Comai L, Ou JH, Lai MM. Hepatitis C virus inhibits DNA damage repair through reactive oxygen and nitrogen species and by interfering with the ATM-NBS1/Mre11/Rad50 DNA repair pathway in monocytes and hepatocytes. J Immunol. 2010;185:6985–6998. doi: 10.4049/jimmunol.1000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhargava A, Raghuram GV, Pathak N, Varshney S, Jatawa SK, Jain D, Mishra PK. Occult hepatitis C virus elicits mitochondrial oxidative stress in lymphocytes and triggers PI3-kinase-mediated DNA damage response. Free Radic Biol Med. 2011;51:1806–1814. doi: 10.1016/j.freeradbiomed.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet. 1994;344:721–724. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- 19.Gong G, Waris G, Tanveer R, Siddiqui A. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-kappa B. Proc Natl Acad Sci USA. 2001;98:9599–9604. doi: 10.1073/pnas.171311298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halliwell B. Oxygen and nitrogen are pro-carcinogens. Damage to DNA by reactive oxygen, chlorine and nitrogen species: measurement, mechanism and the effects of nutrition. Mutat Res. 1999;443:37–52. doi: 10.1016/s1383-5742(99)00009-5. [DOI] [PubMed] [Google Scholar]
- 21.Tsan MF. Superoxide dismutase and pulmonary oxygen toxicity: lessons from transgenic and knockout mice (Review) Int J Mol Med. 2001;7:13–19. doi: 10.3892/ijmm.7.1.13. [DOI] [PubMed] [Google Scholar]
- 22.Immenschuh S, Ramadori G. Gene regulation of heme oxygenase-1 as a therapeutic target. Biochem Pharmacol. 2000;60:1121–1128. doi: 10.1016/s0006-2952(00)00443-3. [DOI] [PubMed] [Google Scholar]
- 23.Guo X, Shin VY, Cho CH. Modulation of heme oxygenase in tissue injury and its implication in protection against gastrointestinal diseases. Life Sci. 2001;69:3113–3119. doi: 10.1016/s0024-3205(01)01417-5. [DOI] [PubMed] [Google Scholar]
- 24.Peterhans E. Sendai virus stimulates chemiluminescence in mouse spleen cells. Biochem Biophys Res Commun. 1979;91:383–392. doi: 10.1016/0006-291x(79)90630-2. [DOI] [PubMed] [Google Scholar]
- 25.Lieber CS. Role of oxidative stress and antioxidant therapy in alcoholic and nonalcoholic liver diseases. Adv Pharmacol. 1997;38:601–628. doi: 10.1016/s1054-3589(08)61001-7. [DOI] [PubMed] [Google Scholar]
- 26.Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, Weinman SA. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122:366–375. doi: 10.1053/gast.2002.30983. [DOI] [PubMed] [Google Scholar]
- 27.Abdalla MY, Ahmad IM, Spitz DR, Schmidt WN, Britigan BE. Hepatitis C virus-core and non structural proteins lead to different effects on cellular antioxidant defenses. J Med Virol. 2005;76:489–497. doi: 10.1002/jmv.20388. [DOI] [PubMed] [Google Scholar]
- 28.Otsuka M, Kato N, Lan K, Yoshida H, Kato J, Goto T, Shiratori Y, Omata M. Hepatitis C virus core protein enhances p53 function through augmentation of DNA binding affinity and transcriptional ability. J Biol Chem. 2000;275:34122–34130. doi: 10.1074/jbc.M000578200. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida H, Kato N, Shiratori Y, Otsuka M, Maeda S, Kato J, Omata M. Hepatitis C virus core protein activates nuclear factor kappa B-dependent signaling through tumor necrosis factor receptor-associated factor. J Biol Chem. 2001;276:16399–16405. doi: 10.1074/jbc.M006671200. [DOI] [PubMed] [Google Scholar]
- 30.Wen F, Abdalla MY, Aloman C, Xiang J, Ahmad IM, Walewski J, McCormick ML, Brown KE, Branch AD, Spitz DR, et al. Increased prooxidant production and enhanced susceptibility to glutathione depletion in HepG2 cells co-expressing HCV core protein and CYP2E1. J Med Virol. 2004;72:230–240. doi: 10.1002/jmv.10567. [DOI] [PubMed] [Google Scholar]
- 31.Abdalla MY, Britigan BE, Wen F, Icardi M, McCormick ML, LaBrecque DR, Voigt M, Brown KE, Schmidt WN. Down-regulation of heme oxygenase-1 by hepatitis C virus infection in vivo and by the in vitro expression of hepatitis C core protein. J Infect Dis. 2004;190:1109–1118. doi: 10.1086/423488. [DOI] [PubMed] [Google Scholar]
- 32.He Y, Nakao H, Tan SL, Polyak SJ, Neddermann P, Vijaysri S, Jacobs BL, Katze MG. Subversion of cell signaling pathways by hepatitis C virus nonstructural 5A protein via interaction with Grb2 and P85 phosphatidylinositol 3-kinase. J Virol. 2002;76:9207–9217. doi: 10.1128/JVI.76.18.9207-9217.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waris G, Livolsi A, Imbert V, Peyron JF, Siddiqui A. Hepatitis C virus NS5A and subgenomic replicon activate NF-kappaB via tyrosine phosphorylation of IkappaBalpha and its degradation by calpain protease. J Biol Chem. 2003;278:40778–40787. doi: 10.1074/jbc.M303248200. [DOI] [PubMed] [Google Scholar]
- 34.Qadri I, Iwahashi M, Capasso JM, Hopken MW, Flores S, Schaack J, Simon FR. Induced oxidative stress and activated expression of manganese superoxide dismutase during hepatitis C virus replication: role of JNK, p38 MAPK and AP-1. Biochem J. 2004;378:919–928. doi: 10.1042/BJ20031587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schreck R, Meier B, Männel DN, Dröge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992;175:1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suematsu T, Kamada T, Abe H, Kikuchi S, Yagi K. Serum lipoperoxide level in patients suffering from liver diseases. Clin Chim Acta. 1977;79:267–270. doi: 10.1016/0009-8981(77)90486-7. [DOI] [PubMed] [Google Scholar]
- 37.Higueras V, Raya A, Rodrigo JM, Serra MA, Romá J, Romero FJ. Interferon decreases serum lipid peroxidation products of hepatitis C patients. Free Radic Biol Med. 1994;16:131–133. doi: 10.1016/0891-5849(94)90250-x. [DOI] [PubMed] [Google Scholar]
- 38.Ivanov AV, Bartosch B, Smirnova OA, Isaguliants MG, Kochetkov SN. HCV and oxidative stress in the liver. Viruses. 2013;5:439–469. doi: 10.3390/v5020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruggieri A, Anticoli S, Nencioni L, Sgarbanti R, Garaci E, Palamara AT. Interplay between Hepatitis C Virus and Redox Cell Signaling. Int J Mol Sci. 2013;14:4705–4721. doi: 10.3390/ijms14034705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsiang CY, Lin LJ, Kao ST, Lo HY, Chou ST, Ho TY. Glycyrrhizin, silymarin, and ursodeoxycholic acid regulate a common hepatoprotective pathway in HepG2 cells. Phytomedicine. 2015;22:768–777. doi: 10.1016/j.phymed.2015.05.053. [DOI] [PubMed] [Google Scholar]
- 41.Hawke RL, Schrieber SJ, Soule TA, Wen Z, Smith PC, Reddy KR, Wahed AS, Belle SH, Afdhal NH, Navarro VJ, et al. Silymarin ascending multiple oral dosing phase I study in noncirrhotic patients with chronic hepatitis C. J Clin Pharmacol. 2010;50:434–449. doi: 10.1177/0091270009347475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gabbay E, Zigmond E, Pappo O, Hemed N, Rowe M, Zabrecky G, Cohen R, Ilan Y. Antioxidant therapy for chronic hepatitis C after failure of interferon: results of phase II randomized, double-blind placebo controlled clinical trial. World J Gastroenterol. 2007;13:5317–5323. doi: 10.3748/wjg.v13.i40.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esrefoglu M. Oxidative stress and benefits of antioxidant agents in acute and chronic hepatitis. Hepat Mon. 2012;12:160–167. doi: 10.5812/hepatmon.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stehbens WE. Oxidative stress, toxic hepatitis, and antioxidants with particular emphasis on zinc. Exp Mol Pathol. 2003;75:265–276. doi: 10.1016/s0014-4800(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 45.Villalba JM, Navarro F, Gómez-Díaz C, Arroyo A, Bello RI, Navas P. Role of cytochrome b5 reductase on the antioxidant function of coenzyme Q in the plasma membrane. Mol Aspects Med. 1997;18 Suppl:S7–13. doi: 10.1016/s0098-2997(97)00015-0. [DOI] [PubMed] [Google Scholar]
- 46.Tappel AL. Lipid peroxidation damage to cell components. Fed Proc. 1973;32:1870–1874. [PubMed] [Google Scholar]
- 47.von Herbay A, Stahl W, Niederau C, Sies H. Vitamin E improves the aminotransferase status of patients suffering from viral hepatitis C: a randomized, double-blind, placebo-controlled study. Free Radic Res. 1997;27:599–605. doi: 10.3109/10715769709097863. [DOI] [PubMed] [Google Scholar]
- 48.Murakami Y, Nagai A, Kawakami T, Hino K, Kitase A, Hara Y, Okuda M, Okita K, Okita M. Vitamin E and C supplementation prevents decrease of eicosapentaenoic acid in mononuclear cells in chronic hepatitis C patients during combination therapy of interferon alpha-2b and ribavirin. Nutrition. 2006;22:114–122. doi: 10.1016/j.nut.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 49.Prasad AS, Bao B, Beck FW, Kucuk O, Sarkar FH. Antioxidant effect of zinc in humans. Free Radic Biol Med. 2004;37:1182–1190. doi: 10.1016/j.freeradbiomed.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 50.Nagamine T, Takagi H, Takayama H, Kojima A, Kakizaki S, Mori M, Nakajima K. Preliminary study of combination therapy with interferon-alpha and zinc in chronic hepatitis C patients with genotype 1b. Biol Trace Elem Res. 2000;75:53–63. doi: 10.1385/BTER:75:1-3:53. [DOI] [PubMed] [Google Scholar]
- 51.Yuasa K, Naganuma A, Sato K, Ikeda M, Kato N, Takagi H, Mori M. Zinc is a negative regulator of hepatitis C virus RNA replication. Liver Int. 2006;26:1111–1118. doi: 10.1111/j.1478-3231.2006.01352.x. [DOI] [PubMed] [Google Scholar]
- 52.Look MP, Gerard A, Rao GS, Sudhop T, Fischer HP, Sauerbruch T, Spengler U. Interferon/antioxidant combination therapy for chronic hepatitis C--a controlled pilot trial. Antiviral Res. 1999;43:113–122. doi: 10.1016/s0166-3542(99)00041-8. [DOI] [PubMed] [Google Scholar]
- 53.van Rossum TG, Vulto AG, Hop WC, Brouwer JT, Niesters HG, Schalm SW. Intravenous glycyrrhizin for the treatment of chronic hepatitis C: a double-blind, randomized, placebo-controlled phase I/II trial. J Gastroenterol Hepatol. 1999;14:1093–1099. doi: 10.1046/j.1440-1746.1999.02008.x. [DOI] [PubMed] [Google Scholar]
- 54.Abe Y, Ueda T, Kato T, Kohli Y. [Effectiveness of interferon, glycyrrhizin combination therapy in patients with chronic hepatitis C] Nihon Rinsho. 1994;52:1817–1822. [PubMed] [Google Scholar]
- 55.Melhem A, Stern M, Shibolet O, Israeli E, Ackerman Z, Pappo O, Hemed N, Rowe M, Ohana H, Zabrecky G, et al. Treatment of chronic hepatitis C virus infection via antioxidants: results of a phase I clinical trial. J Clin Gastroenterol. 2005;39:737–742. doi: 10.1097/01.mcg.0000174023.73472.29. [DOI] [PubMed] [Google Scholar]
- 56.Salmi HA, Sarna S. Effect of silymarin on chemical, functional, and morphological alterations of the liver. A double-blind controlled study. Scand J Gastroenterol. 1982;17:517–521. doi: 10.3109/00365528209182242. [DOI] [PubMed] [Google Scholar]
- 57.Buzzelli G, Moscarella S, Giusti A, Duchini A, Marena C, Lampertico M. A pilot study on the liver protective effect of silybin-phosphatidylcholine complex (IdB1016) in chronic active hepatitis. Int J Clin Pharmacol Ther Toxicol. 1993;31:456–460. [PubMed] [Google Scholar]
- 58.Ferenci P, Scherzer TM, Kerschner H, Rutter K, Beinhardt S, Hofer H, Schöniger-Hekele M, Holzmann H, Steindl-Munda P. Silibinin is a potent antiviral agent in patients with chronic hepatitis C not responding to pegylated interferon/ribavirin therapy. Gastroenterology. 2008;135:1561–1567. doi: 10.1053/j.gastro.2008.07.072. [DOI] [PubMed] [Google Scholar]
- 59.Ahmed-Belkacem A, Ahnou N, Barbotte L, Wychowski C, Pallier C, Brillet R, Pohl RT, Pawlotsky JM. Silibinin and related compounds are direct inhibitors of hepatitis C virus RNA-dependent RNA polymerase. Gastroenterology. 2010;138:1112–1122. doi: 10.1053/j.gastro.2009.11.053. [DOI] [PubMed] [Google Scholar]
- 60.Trouillas P, Marsal P, Svobodová A, Vostálová J, Gazák R, Hrbác J, Sedmera P, Kren V, Lazzaroni R, Duroux JL, et al. Mechanism of the antioxidant action of silybin and 2,3-dehydrosilybin flavonolignans: a joint experimental and theoretical study. J Phys Chem A. 2008;112:1054–1063. doi: 10.1021/jp075814h. [DOI] [PubMed] [Google Scholar]
- 61.Gomez EV, Perez YM, Sanchez HV, Forment GR, Soler EA, Bertot LC, Garcia AY, del Rosario Abreu Vazquez M, Fabian LG. Antioxidant and immunomodulatory effects of Viusid in patients with chronic hepatitis C. World J Gastroenterol. 2010;16:2638–2647. doi: 10.3748/wjg.v16.i21.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu Z, Wilson AT, Mathahs MM, Wen F, Brown KE, Luxon BA, Schmidt WN. Heme oxygenase-1 suppresses hepatitis C virus replication and increases resistance of hepatocytes to oxidant injury. Hepatology. 2008;48:1430–1439. doi: 10.1002/hep.22491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen WC, Wang SY, Chiu CC, Tseng CK, Lin CK, Wang HC, Lee JC. Lucidone suppresses hepatitis C virus replication by Nrf2-mediated heme oxygenase-1 induction. Antimicrob Agents Chemother. 2013;57:1180–1191. doi: 10.1128/AAC.02053-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mehrab-Mohseni M, Sendi H, Steuerwald N, Ghosh S, Schrum LW, Bonkovsky HL. Legalon-SIL downregulates HCV core and NS5A in human hepatocytes expressing full-length HCV. World J Gastroenterol. 2011;17:1694–1700. doi: 10.3748/wjg.v17.i13.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gane EJ, Weilert F, Orr DW, Keogh GF, Gibson M, Lockhart MM, Frampton CM, Taylor KM, Smith RA, Murphy MP. The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver Int. 2010;30:1019–1026. doi: 10.1111/j.1478-3231.2010.02250.x. [DOI] [PubMed] [Google Scholar]
- 66.Gane E, Orr D, Weilert F, Keogh G, Gibson M, Murphy M, Smith R, Lockhart M, Frampton C, Taylor K. Phase II study of the mitochondrial antioxidant mitoquinone for hepatitis C. J Hepatol. 2008:48 Supplement 2: S318. [Google Scholar]
- 67.Gonzalez O, Fontanes V, Raychaudhuri S, Loo R, Loo J, Arumugaswami V, Sun R, Dasgupta A, French SW. The heat shock protein inhibitor Quercetin attenuates hepatitis C virus production. Hepatology. 2009;50:1756–1764. doi: 10.1002/hep.23232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dionisio N, Garcia-Mediavilla MV, Sanchez-Campos S, Majano PL, Benedicto I, Rosado JA, Salido GM, Gonzalez-Gallego J. Hepatitis C virus NS5A and core proteins induce oxidative stress-mediated calcium signalling alterations in hepatocytes. J Hepatol. 2009;50:872–882. doi: 10.1016/j.jhep.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 69.Feld JJ, Modi AA, El-Diwany R, Rotman Y, Thomas E, Ahlenstiel G, Titerence R, Koh C, Cherepanov V, Heller T, et al. S-adenosyl methionine improves early viral responses and interferon-stimulated gene induction in hepatitis C nonresponders. Gastroenterology. 2011;140:830–839. doi: 10.1053/j.gastro.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Majano PL, García-Monzón C, García-Trevijano ER, Corrales FJ, Cámara J, Ortiz P, Mato JM, Avila MA, Moreno-Otero R. S-Adenosylmethionine modulates inducible nitric oxide synthase gene expression in rat liver and isolated hepatocytes. J Hepatol. 2001;35:692–699. doi: 10.1016/s0168-8278(01)00208-2. [DOI] [PubMed] [Google Scholar]
- 71.Liao CL, Lin YL, Wu BC, Tsao CH, Wang MC, Liu CI, Huang YL, Chen JH, Wang JP, Chen LK. Salicylates inhibit flavivirus replication independently of blocking nuclear factor kappa B activation. J Virol. 2001;75:7828–7839. doi: 10.1128/JVI.75.17.7828-7839.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mazur I, Wurzer WJ, Ehrhardt C, Pleschka S, Puthavathana P, Silberzahn T, Wolff T, Planz O, Ludwig S. Acetylsalicylic acid (ASA) blocks influenza virus propagation via its NF-kappaB-inhibiting activity. Cell Microbiol. 2007;9:1683–1694. doi: 10.1111/j.1462-5822.2007.00902.x. [DOI] [PubMed] [Google Scholar]
- 73.Trujillo-Murillo K, Rincón-Sánchez AR, Martínez-Rodríguez H, Bosques-Padilla F, Ramos-Jiménez J, Barrera-Saldaña HA, Rojkind M, Rivas-Estilla AM. Acetylsalicylic acid inhibits hepatitis C virus RNA and protein expression through cyclooxygenase 2 signaling pathways. Hepatology. 2008;47:1462–1472. doi: 10.1002/hep.22215. [DOI] [PubMed] [Google Scholar]
- 74.Rivas-Estilla AM, Bryan-Marrugo OL, Trujillo-Murillo K, Pérez-Ibave D, Charles-Niño C, Pedroza-Roldan C, Ríos-Ibarra C, Ramírez-Valles E, Ortiz-López R, Islas-Carbajal MC, et al. Cu/Zn superoxide dismutase (SOD1) induction is implicated in the antioxidative and antiviral activity of acetylsalicylic acid in HCV-expressing cells. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1264–G1273. doi: 10.1152/ajpgi.00237.2011. [DOI] [PubMed] [Google Scholar]
- 75.Moreno-Otero R, Trapero-Marugán M. Hepatoprotective effects of antioxidants in chronic hepatitis C. World J Gastroenterol. 2010;16:1937–1938. doi: 10.3748/wjg.v16.i15.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Quarato G, Scrima R, Ripoli M, Agriesti F, Moradpour D, Capitanio N, Piccoli C. Protective role of amantadine in mitochondrial dysfunction and oxidative stress mediated by hepatitis C virus protein expression. Biochem Pharmacol. 2014;89:545–556. doi: 10.1016/j.bcp.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 77.Nakamura M, Saito H, Ikeda M, Hokari R, Kato N, Hibi T, Miura S. An antioxidant resveratrol significantly enhanced replication of hepatitis C virus. World J Gastroenterol. 2010;16:184–192. doi: 10.3748/wjg.v16.i2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beloqui O, Prieto J, Suárez M, Gil B, Qian CH, García N, Civeira MP. N-acetyl cysteine enhances the response to interferon-alpha in chronic hepatitis C: a pilot study. J Interferon Res. 1993;13:279–282. doi: 10.1089/jir.1993.13.279. [DOI] [PubMed] [Google Scholar]
- 79.Takagi H, Kakizaki S, Sohara N, Sato K, Tsukioka G, Tago Y, Konaka K, Kabeya K, Kaneko M, Takayama H, et al. Pilot clinical trial of the use of alpha-tocopherol for the prevention of hepatocellular carcinoma in patients with liver cirrhosis. Int J Vitam Nutr Res. 2003;73:411–415. doi: 10.1024/0300-9831.73.6.411. [DOI] [PubMed] [Google Scholar]
- 80.El-Kamary SS, Shardell MD, Abdel-Hamid M, Ismail S, El-Ateek M, Metwally M, Mikhail N, Hashem M, Mousa A, Aboul-Fotouh A, et al. A randomized controlled trial to assess the safety and efficacy of silymarin on symptoms, signs and biomarkers of acute hepatitis. Phytomedicine. 2009;16:391–400. doi: 10.1016/j.phymed.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Loguercio C, Festi D. Silybin and the liver: from basic research to clinical practice. World J Gastroenterol. 2011;17:2288–2301. doi: 10.3748/wjg.v17.i18.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fried MW, Navarro VJ, Afdhal N, Belle SH, Wahed AS, Hawke RL, Doo E, Meyers CM, Reddy KR. Effect of silymarin (milk thistle) on liver disease in patients with chronic hepatitis C unsuccessfully treated with interferon therapy: a randomized controlled trial. JAMA. 2012;308:274–282. doi: 10.1001/jama.2012.8265. [DOI] [PMC free article] [PubMed] [Google Scholar]


