Abstract
Backgound
Thrombocytopenia is, after anemia, the second most common abnormality of the complete blood count in pregnancy, with a reported frequency of 6.6% to 11.2%. It has many causes. Thrombocytopenia should be diagnostically evaluated as early as possible in pregnancy, so that the obstetrical management can be accordingly planned to minimize harm to the mother and child. As the various underlying diseases share clinical features and laboratory findings, the differential diagnosis is often a difficult interdisciplinary challenge.
Method
In this article, we review pertinent literature (2000–January 2015) retrieved by a selective search in PubMed.
Results
Gestational thrombocytopenia is the most common type, accounting for 75% of cases, followed by severe pre-eclampsia/HELLP syndrome (hemolysis, elevated liver enzymes, low platelet count) in 15–22% and autoimmune thrombocytopenia (ITP) in 1–4%. Gestational thrombocytopenia and ITP differ in the bleeding history, the severity of thrombocytopenia, the frequency of neonatal thrombocytopenia, and the rate of normalization of the platelet count after delivery. The HELLP syndrome and rarer microangiopathic hemolytic anemias (e.g., thrombotic thrombocytopenic purpura) can be differentiated on the basis of their main clinical features, such as hypertension/proteinuria and upper abdominal pain, the severity of hemolysis and thrombocytopenia, the degree of transaminase elevation, and the rapidity of postpartum remission of the clinical and laboratory findings. A stepwise diagnostic procedure should be followed to distinguish further causes, e.g., to differentiate thrombocytopenia due to infection, autoimmune disease, or drugs from thrombocytopenia due to a rare hereditary disease.
Conclusion
The early interdisciplinary evaluation of thrombocytopenia in pregnancy is a prerequisite for the optimal care of the mother and child. The development of evidence-based recommendations for interdisciplinary management should be a goal for the near future.
Thrombocytopenia (defined as a platelet count below 150 G/L) is the second most common abnormality of the complete blood count in pregnancy among European women, with a prevalence of 6.6–11.6% in the third trimester; only anemia is more common (18.7%) (1– 3, e1– e4). Its cause can be specific to pregnancy (e.g., gestational thrombocytopenia), pregnancy-associated but nonspecific (e.g., thrombotic thrombocytopenic purpura), or independent of pregnancy (e.g., autoimmune thrombocytopenia) (Box 1).
Box 1. Causes of thrombocytopenia in pregnancy and their relative frequencies (4–6).
-
Pregnancy-associated causes
gestationa thrombocytopenia, 70–80%: isolated thrombocytopenia
-
thrombocytopenia in systemic disease with additional manifestations
pre-eclampsia (severe), 15–20%
HELLP syndrome, < 1%
acute fatty liver of pregnancy, < 1%
-
Causes that are independent of pregnancy (partly in association with other systemic disease, partly pre-existing but with first clinical manifestation in pregnancy):
-
congenital:
von Willebrand syndrome type 2B, < 1%
hereditäary thrombocytopenia (e.g., MYH9 disease), < 1%
-
acquired
-
immune-mediated:
autoimmune thrombocytopenia, 1–4%: isolated thrombocytopenia
systemic lupus erythematosus < 1%
antiphospholipid syndrome < 1%
drug-induced thrombocytopenia < 1%
thrombotic thrombocytopenic purpura*, hemolytic-uremic syndrome < 1%
-
non-immune-mediated:
secondary thrombocytopenia associated with infectious disease (e.g., HIV, HCV, EBV)
bone marrow diseases (acute leukemia, PNH) < 1%
poor nutrition, folate or vitamin B12 deficiency, < 1%
hypersplenism, < 1%
-
-
*congenital ADAMTS13 deficiency also possible
EBV, Epstein-Barr virus; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immune deficiency virus; MYH9, myosin heavy polypeptide 9; PNH, paroxysmal nocturnal hemoglobinuria
The differential diagnosis of thrombocytopenia is highly important, as the risk of bleeding for both mother and child and the risk of severe maternal complications vary from one underlying disease to another, as does the required treatment. The most common type of thrombocytopenia in pregnancy, gestational thrombocytopenia, poses no danger to either the mother or the child; in contrast, autoimmune thrombocytopenia can cause both maternal bleeding, mainly in the peripartum period, and severe hemorrhage in the neonate, because antiplatelet antibodies cross the placenta. To avoid life-threatening complications (hemolysis, elevated liver enzymes, low platelet count), HELLP syndrome must be differentiated from other, rarer types of microangiopathic hemolytic anemia (thrombotic thrombocytopenic purpura, hemolytic-uremic syndrome) so that targeted treatment can be delivered and life-threatening complications, such as cerebral hemorrhage and organ failure, prevented. The variable clinical and laboratory findings of the diseases that cause thrombocytopenia, and the frequently ensuing difficulties in differential diagnosis, pose a challenge for specialists in multiple disciplines.
We will, therefore, approach this topic from three different points of view, first examining the laboratory features of the different underlying diseases and then addressing the relevant hematologic and obstetrical considerations.
Method
We searched PubMed for articles published from January 2000 to January 2015 that contained the key words “thrombocytopenia” and “pregnancy.” The current recommendations of specialist societies from Germany and other countries were also considered, as were important publications from before the year 2000.
The diagnostic algorithm
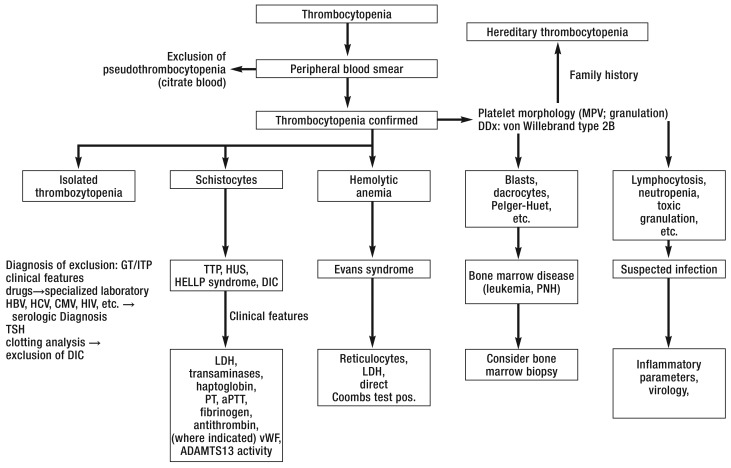
The differential diagnosis of thrombocytopenia in pregnancy is based on the patient’s personal and family history, drug history, and dietary habits, the physical findings (e.g., hematomas, petechiae), and the findings of basic laboratory tests (Box 2). A rational diagnostic algorithm (Figure 1) has been developed on the basis of the relative frequencies of the causes of thrombocytopenia in pregnancy (Box 1) and the abnormalities that accompany pregnancy in each individual case.
Box 2. The differential diagnosis of thrombocytopenia in pregnancy.
-
Ruling out pseudothrombocytopenia
parallel platelet counts in blood anticoagulated with EDTA and citrate; no centrifugation; use a special blood-collecting system if necessary
a platelet count in citrate blood that is normal, or markedly higher than the platelet count in EDTA blood, indicates pseudothrombocytopenia; this can be further verified by peripheral blood smear.
-
Interpreting the peripheral blood smear
Platelets: giant platelets (e.g., in Bernhard-Soulier syndrome); pathological staining of granules, or abnormally few granules within platelets, in rare hereditary types of thrombocytopathy and thrombocytopenia (e.g., gray platelet syndrome); platelet aggregation in EDTA and citrate blood; if indictated, measure platelets directly from native blood in laboratory (e.g., in suspected von Willebrand syndrome type 2B).
Leukocytes: Döhle inclusion bodies in granulocytes: May-Hegglin anomaly and other MYH9 diseases.
Erythrocytes: Fragmentocytes in microangiopathic hemolytic anemia (e.g., in HELLP syndrome: fraction < 1%, in thrombotic thrombocytopenic purpura 2–5% [12]).
-
Basic laboratory testing
complete blood count and differential with reticulocyte count
direct antiglobulin/Coombs test
liver and thyroid function tests
Virology tests (e.g., for HIV, HBV, HCV, CMV)
-
Further diagnostic testing
antiphospholipid antibodies (including lupus anticoagulant)
antinuclear antibodies
exclusion of von Willebrand syndrome type 2B or defects of the vWF-splitting protease
CMV, cytomegalovirus; EDTA, ethylene diamine tetra-acetic acid; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immune deficiency virus; MYH9, myosin heavy polypeptide; vWF, von Willebrand factor
Figure 1.
Algorithm for the differential diagnosis of thrombocytopenia in pregnancy
CMV, cytomegalovirus; DDx, differential diagnosis; DIC, disseminated intravascular coagulation; GT, gestational thrombocytopenia; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HUS, hemolytic-uremic syndrome; ITP, autoimmune thrombocytopenia; LDH, lactate dehydrogenase; MPV, mean platelet volume; PNH, paroxysmal nocturnal hemoglobinuria; TSH, thyroid-stimulating hormone; TTP, thrombotic thrombocytopenic purpura; vWF, von Willebrand factor; modified from (4).
Thrombocytopenia must be evaluated in any of the following situations:
it appeared before pregnancy or has been known since childhood,
it appeared in the first or second trimester,
the platelet count is lower than 80 G/L at any time in pregnancy, or
the patient or any close relative is known to have a bleeding tendency, regardless of the gestational age and current platelet count.
Laboratory testing
Platelet counting by automated hematology instrumentation can yield falsely low values (e5); thus, pseudothrombocytopenia (< 1% of complete blood count analyses with EDTA) should be ruled out by parallel platelet counting in blood that has been anticoagulated with ethylene diamine tetra-acetic acid (EDTA) and with citrate. A blood smear (e6) should be made as part of the initial diagnostic testing and differentiation of thrombocytopenia (e6); it should be evaluated by an experienced medical-technical laboratory assistant (MTA) or by a physician specialized in laboratory medicine (Box 2).
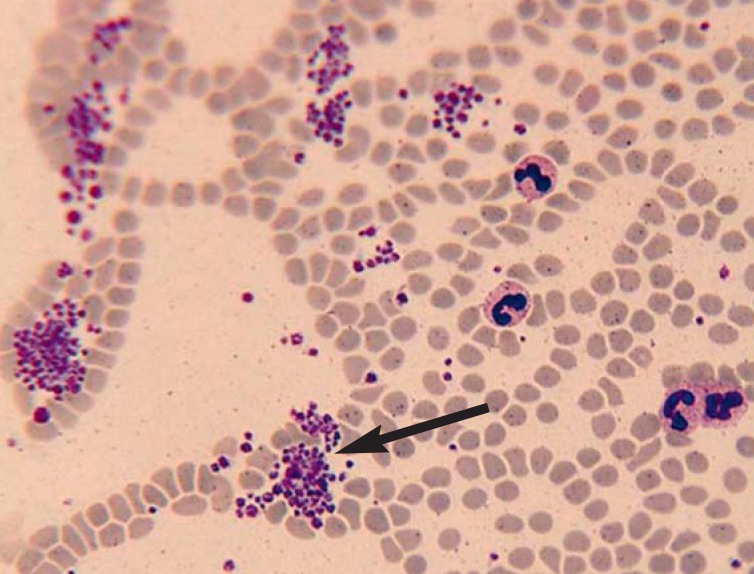
Platelet aggregates in a peripheral blood smear in a classic case of pseudothrombocytopenia are shown in Figure 2.
Figure 2.
Platelet aggregation (arrow) in the peripheral blood smear of a patient with classic pseudothrombocytopenia. This laboratory artefact is due to the release of cryptantigens and the binding of antiplatelet autoantibodies when calcium is removed.
The differential diagnosis of gestational thrombocytopenia
Gestational thrombocytopenia arises in 5–8% of all pregnancies and is the most common form of thrombocytopenia in pregnancy, accounting for 75% of cases (7, e7, e8). It is caused by pregnancy-induced hemodilution and increased platelet turnover (8). The platelet count is above 130–150 G/L in 75% of pregnancies and below 100 G/L in only 10% (7, e9). Gestational thrombocytopenia is asymptomatic; it does not increase the risk of bleeding for either the mother or the child (5, 6, 9). The neonate’s platelet count is normal, not low, as in autoimmune thrombocytopenia (10). Gestational thrombocytopenia does not affect the mode of delivery (6). In the authors’ experience, the platelet count normalizes within two weeks after delivery. No treatment is needed; it suffices to check the platelet count as part of routine care for pregnancy (11).
The most important differential diagnosis of gestational thrombocytopenia is autoimmune thrombocytopenia (with a prevalence of 1 in 1000 to 10 000 pregnancies), which accounts for 1–4% of all cases of thrombocytopenia in pregnancy (13, e10) (Table 1). Primary autoimmune thrombocytopenia is defined as an isolated thrombocytopenia below 100 G/L, without any clinically evident accompanying illnesses or causes (14, 15, e11), which is due to the generation of specific IgG antibodies against glycoprotein complexes of the platelet membrane that cause the sequestration of circulating platelets in the spleen. According to McCrae (8), a platelet count below 100 G/L in the first trimester with a progressive decline over the course of pregnancy indicates autoimmune thrombocytopenia.
Table 1. The differential diagnosis of gestational versus autoimmun thrombocytopenia.
| Criteria | Gestational thrombocytopenia | Autoimmune thrombocytopenia |
|---|---|---|
| % of thrombocytopenia in pregnancy | ca. 75% | ca. 3% |
| Usual time of onset during pregnancy | Late 2nd to 3rd trimester | 1st to early 2nd trimester |
| Clinical course during pregnancy | Asymptomatic | Elevated rate of spontaneous bleeding if the platelet count is below 20 G/L |
| History | No prior history of bleeding | Bleeding tendency that manifested itself before pregnancy (e.g., petechiae) |
| Platelet count indicating diagnosis | ≥ 100 G/L | < 100 g/l |
| Fetal thrombocytopenia | None | Possible*1 |
| Course of platelet count after birth | Normalization within 2 weeks | Rise possible |
| Treatment | None | Initially: prednisone or prednisolone, 20–30 mg/day*2 |
The diagnosis is known before pregnancy because of the typical bleeding history in two-thirds of cases; in the remaining third, it is only recognized during pregnancy, or just before delivery (16). Most pregnant women with autoimmune thrombocytopenia are asymptomatic, need no treatment, and have an uncomplicated course of pregnancy (4, 6). Thus, this entity can be difficult to tell apart from gestational thrombocytopenia, and the diagnosis is sometimes only made retrospectively from the course of the platelet count after delivery (5, 6).
In retrospective studies, estimates of the frequency of severe thrombocytopenia in pregnant women with autoimmune thrombocytopenia have ranged from 8.6% to 42.1%; the wide variation is due to varying inclusion criteria (16, 17, e12).
Gestational thrombocytopenia and autoimmune thrombocytopenia are diagnoses of exclusion. Special laboratory tests for the definitive confirmation of these diagnoses are not routinely available.
The measurement of antiplatelet antibodies is not recommended as a routine test (grade B recommendation [11]), because the frequencies of detection of antiplatelet antibodies in these two diagnoses may overlap depending on the particular test system used (18, e13), and because a negative test still does not rule out autoimmune thrombocytopenia (11). The demonstration of glycoprotein-specific antibodies supports the presumptive diagnosis of autoimmune thrombocytopenia (specificity ca. 80%, sensitivity ca. 60% [e14]). The distinction between gestational thrombocytopenia and autoimmune thrombocytopenia is important for the following reasons:
In autoimmune thrombocytopenia, antiplatelet antibodies cross the placenta and cause thrombocytopenia, with a platelet count below 100 G/L in 15–50% of neonates, below 50 G/L in 8–30%, and below 20 G/L in 1–9% (14– 16, e1, e15). Neonates whose mothers previously underwent splenectomy for the treatment of autoimmune thrombocytopenia are in danger of developing thrombocytopenia, regardless of whether the mother has a normal platelet count, a platelet count below 50 G/L at some point in pregnancy, and/or a platelet count below 100 G/L at term (19). The rate of intracranial hemorrhage in such cases is less than 1.5% (19, e15, e16), in contrast to the 6% to 25% rate (e17, resp. (20) in the rarer entity of severe fetal/neonatal alloimmune thrombocytopenia acquired during pregnancy, which arises in 5 per 10000 pregnancies (20, e17). In this disease, as opposed to autoimmune thrombocytopenia, the mother has a normal platelet count.
The treatment of autoimmune thrombocytopenia includes the following:
Invasive measures: scalpel-electrode and vaginal surgical delivery should be avoided in view of the risk of hemorrhage in the neonate (11, 14, 15); autoimmune thrombocytopenia is not an indication for cesarean section; the platelet count should be above 50 G/L before any cesarean section or spinal anesthesia and above 80 G/L before any peridural anesthesia [12]).
The neonate’s platelet count should be measured in cord blood immediately after delivery and rechecked over the following week (with an expected nadir from the second to the fifth day after birth); if it is below 50 G/L, transcranial ultrasound is recommended; if it is below 20 G/L or there is any evidence of bleeding, intravenous immunoglobulins (19) or steroids (11) should be given. Neither the maternal platelet count nor the demonstration of antiplatelet antibodies is correlated with the neonate’s platelet count (6).
Unlike gestational thrombocytopenia, autoimmune thrombocytopenia elevates the risk of postpartum hemorrhage in the first 24–48 hours after delivery (17). Analgesic drugs that inhibit platelet function, such as ibuprofen, should be avoided.
31–49 % of women with autoimmune thrombocytopenia (unlike those with gestational thrombocytopenia) need to be treated with glucocorticoids, such as prednisone, and/or immunoglobulin G to raise the platelet count above 20–30 G/L, either during pregnancy or just before or just after delivery (16, 17, e13). Treatment recommendations for various indications are found in the current guidelines (11, 15).
Differential diagnosis: pre-eclampsia, HELLP syndrome, and others
Pre-eclampsia (2–3% of all pregnancies) and HELLP syndrome (0.5–0.9% of all pregnancies) together account for 15–22% of all cases of thrombocytopenia in pregnancy (4, 6, e18). 15–50% of women with pre-eclampsia have thrombocytopenia, depending on the severity of the condition (7, 8).
In HELLP syndrome, thrombocytopenia (below 100 G/L) is an obligate component of the triad of laboratory findings. This syndrome is due to cytokine-mediated endothelial dysfunction leading to systemic activation of the clotting system and intravascular consumption of clotting factors and platelets.
In over 90% of cases, HELLP syndrome can be diagnosed on the basis of its typical clinical features, i.e., upper abdominal pain, hypertension, and proteinuria (> 300 mg/24 hr) in a previously normotensive woman after the 20th week of gestation; in 15–20% of the affected patients, however, hypertension and/or proteinuria is absent (21, e19). The severity of thrombocytopenia is correlated with maternal morbidity and perinatal mortality (22). If the platelet count is below 50 G/L, the rate of maternal complications is 64%, and the perinatal mortality is 16.4%; the corresponding figures for platelet counts in the range of 50–100 G/L are 54% and 14.4%, respectively, and, for platelet counts in the range of 100–150 G/L, 40% and 11.7% (22, e20). The platelet count reaches a nadir 23–29 hours after delivery (23) and normalizes in 6–11 days. In 10–30% of cases, HELLP syndrome only arises postpartum, up to 72 hours after delivery (23, e21). HELLP syndrome does not cause neonatal thrombocytopenia (e22).
The specific clinical features of HELLP syndrome make it easy to distinguish from gestational thrombocytopenia and autoimmune thrombocytopenia, but it can be cumbersome to tell apart from rare types of microangiopathic hemolytic anemia, such as thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome (1 in 25000 to 100000 pregnancies [24, (25]).
According to current concepts, the presumptive diagnosis in Coombs-negative hemolytic anemia (i.e., lack of demonstration of incomplete anti-erythrocyte IgG antibodies) and thrombocytopenia without any other identifiable cause is thrombotic thrombocytopenic purpura, regardless of the level of activity of ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin type 1 domains, no.13), a cleavage protease of von Willebrand factor (26, 27, e23). A severe ADAMTS13 deficiency (activity < 5%) confirms the diagnosis (28, 29). The clinical condition arises through generalized platelet aggregation with thrombosis of the microcirculation and consecutive end-organ failure.
Congenital thrombotic thrombocytopenic purpura (the Upshaw-Schulman syndrome [25]) is a pathophysiologically related condition. This disease is caused by mutations of the ADAMTS13 gene, rather than by antibodies that inactivate ADAMTS13, as is the case in acquired thrombotic thrombocytopenic purpura.
Pregnancy is thought to trigger acute episodes of both congenital and acquired thrombotic thrombocytopenic purpura (27, 30) by inducing a rise in the concentration of von Willebrand factor (vWF) and an absolute increase in uncleaved high-molecular-weight vWF multimers (27, e24). In the largest cohort study of pregnancy-associated thrombotic thrombocytopenic purpura that has been carried out to date, 35 of 47 patients had their first clinical manifestation of thrombotic thrombocytopenic purpura during pregnancy, usually from the 30 week of gestation onward, or after delivery; 23 of these women had previously unrecognized congenital thrombotic thrombocytopenic purpura (31).
The classic pentad of thrombotic thrombocytopenic purpura, consisting of microangiopathic hemolytic anemia, marked thrombocytopenia, neurologic deficits, fever, and renal dysfunction, is present in less than 40% of cases (26, e23, e25).
The distinction between HELLP syndrome and thrombotic thrombocytopenic purpura is important for further management. There is a consensus-based recommendation (level IV evidence) for rapid delivery after the 34th week of gestation in HELLP syndrome (21). In acute, acquired thrombotic thrombocytopenic purpura, the guideline recommendation (based on registry studies; level III evidence, [28]) is for plasma exchange within 4–8 hours of the clinical onset of microangiopathic hemolytic anemia and thrombocytopenia, if possible (28). This procedure has lowered the maternal mortality in this condition from 90% (in untreated cases) to 10–20% (28, e25).
The main distinguishing features of thrombotic thrombocytopenic purpura, as opposed to HELLP syndrome, are the following: more severe hemolysis and thrombocytopenia; fever; a lower frequency of upper abdominal pain, hypertension, and proteinuria; the mainly neurologic manifestations; a lower (or entirely absent) increase in transaminase levels; the usually normal clotting status (PT, aPTT); and the persistence of symptoms for more than 72 hours after delivery (4, 6, 32, e25) (Table 2).
Table 2. The differential diagnosis of microangiopathia and thrombocytopenia in pregnancy*.
| Parameter | Pre-eclampsia | HELLP syndrome | TTP | aHUS | AFLP | APS | SLE |
|---|---|---|---|---|---|---|---|
| Hypertension | +++ | +++ | + | ++ | + | +/- | ++ |
| Proteinuria | +++ | +++ | +/- | +++ | +/- | +/- | +++ |
| Upper abdominal pain | +/- | +++ | +/- | +/- | ++ | +/- | +/- |
| Neurologic deficits | + | + | ++ | +/- | + | + | + |
| Thrombocytopenia | + | +++ | +++ | +++ | + | + | + |
| Hemolysis | +/- | +++ | +++ | +++ | + | +/- | + |
| Renal dysfunction | +/- | + | + | +++ | ++ | +/- | ++ |
| Elevated transaminases | + | +++ | +/- | +/- | +++ | +/- | + |
| Disseminated intravascular coagulation | +/- | + | +/- | +/- | +++ | +/- | +/- |
| Peak incidence | 3rd trimester | 3rd trimester, post partum | 2nd/3rd trimester | post partum | 3rd trimester | at any time | at any time |
| Management | if severe: rapid delivery | rapid delivery | plasma exchange | (plasma exchange / infusion) ecuzulimab | supportiverapid delivery | ASA,low-molecular-weight heparin | hydroxychloroquine, corticosteroids, other immune suppressants |
TTP, thrombotic thrombocytopenic purpura; AFLP, acute fatty liver of pregnancy; aHUS, atypical hemolytic uremic syndrome; APS, antiphospholipid syndrome; ASA, N-acetylsalicylic acid; SLE, systemic lupus erythematosus; +/- sometimes (0–20%); + moderately frequent (20–50%); ++ frequent (50–80%); +++ very frequent or constant (80–100%)
The lower ADAMTS13 activity in thrombotic thrombocytopenic purpura (28) compared to HELLP syndrome (e26) is irrelevant to rapid clinical decision-making. ADAMTS13 activity can only be measured in special laboratories, and only with a long turnaround time (24–48 hours) that make these tests unsuitable for acute diagnosis (e23, e27).
It has been reported that, if the ratio of the lactate dehydrogenase level to the aspartate aminotransferase level in the third trimester of pregnancy is higher than 22, this can help distinguish thrombotic thrombocytopenic purpura from HELLP syndrome in patients with hematuria and a very low platelet count (33, e28).
The main clinical feature of atypical hemolytic-uremic syndrome without diarrhea, arising a few days or up to ten weeks after delivery, is primary renal dysfunction (oliguria, anuria) with acute renal failure and a need for hemodialysis, leading to end-stage renal failure in 76% of cases (29, 34, 35). Nonetheless, the differential diagnosis of hemolytic-uremic syndrome from thrombotic thrombocytopenic purpura is often difficult in the acute setting (36).
Hemolytic-uremic syndrome is thought to be caused by genetically determined dysregulation of the complement system, whose uncontrolled activation leads to endothelial cell damage and to thrombotic microangiopathy, which manifests itself mainly in the kidneys (29, 35). A severe deficiency of ADAMTS13 is absent in hemolytic-uremic syndrome, as opposed to thrombotic thrombocytopenic purpura (36). The differential diagnosis of hemolytic-uremic syndrome from HELLP syndrome and thrombotic thrombocytopenic purpura is clinically relevant, because an effective treatment for hemolytic-uremic syndrome is available: in a prospective trial, 26 weeks of treatment with the terminal complement inhibitor eculizumab normalized the platelet count in 82% of cases and significantly improved renal function in 47% (37).
The clinical features that enable the diagnosis of another rare condition, acute fatty liver of pregnancy (1 in 5000 to 10000 deliveries), are listed in Table 2. This life-threatening condition (maternal mortality ca. 10%, [e29]) manifests itself with marked nausea and vomiting, severe hypoglycemia, leukocytosis, hyperbilirubinemia, consumption coagulopathy, and encephalopathy (3, 38, e29), and as many as 50% of the affected women also have manifestations of pre-eclampsia. The thrombocytopenia and hemolysis that accompany this condition are, however, less severe than in HELLP syndrome, thrombotic thrombocytopenic purpura, and hemolytic-uremic syndrome (4, e30). The treatment consists of supportive measures (fluid and glucose administration, correction of coagulopathy) and immediate delivery (level III evidence, [3, e29); the putative benefit of plasma exchange is currently debated (e29, e31).
Thrombocytopenia of other causes
Secondary thrombocytopenia due to systemic lupus erythematosus or antiphospholipid syndrome can be differentiated from the above conditions on the basis of the clinical history, the different degree of severity of certain individual manifestations (Table 2), and, above all, the specific laboratory findings. It should be borne in mind that the risk of pre-eclampsia is 15% in systemic lupus erythematosus, up to 60% in lupus nephritis, and up to 50% in antiphospholipid syndrome (32).
Hereditary thrombocytopenia is often not diagnosed until young adulthood (39) if it is mild and causes little or no functional deficit. According to a recent retrospective analysis of 339 pregnancies with 13 different types of hereditary thrombocytopenia, pregnancy exacerbates neither thrombocytopenia nor the risk of bleeding. The risk of post-partum hemorrhage is elevated in women with hereditary thrombocytopenia (40), but not in those with the May-Hegglin anomaly. The main diagnostic clues to these conditions are derived from the patient’s history and family history and from the peripheral blood smear.
Drug-induced thrombocytopenia will not be discussed any further here (for more information, see www.ouhsc.edu/platelets/ditp.html). The risk of heparin-induced thrombocytopenia after the administration of low-molecular-weight heparin is less than 1 % (e32); therefore, routinely checking the platelet count in the first two weeks after exposure is no longer recommended (e33).
Key Messages.
The differential diagnostic workup of thrombocytopenia in pregnancy is essential so that the proper treatment can be given and the risk of bleeding can be minimized for both mother and child.
This evaluation is based on the clinical history, the findings of the peripheral blood smear (including the exclusion of pseudothrombocytopenia), and a diagnostic algorithm that has been constructed to take account of the different incidences of the conditions causing thrombocytopenia in pregnancy.
Gestational thrombocytopenia affects 5–8% of all pregnant women and poses no risk to either the mother or the child. This condition must be distinguished from autoimmune thrombocytopenia (frequency: 1 in 1000 to 10000 pregnancies), which increases the risk of bleeding in the neonate.
The differential diagnosis of HELLP syndrome (0.5–0.8% of all pregnancies) and rarer types of microangiopathic hemolytic anemia is important for treatment planning.
The diagnosis and treatment of thrombocytopenia in pregnancy require optimal interdisciplinary collaboration.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Dr. Bergmann has served as a paid consultant for Instrumentation Laboratories and has received payment from the Roche and Siemens companies for preparing continuing medical education events.
Prof. Rath has received lecture honoraria from the CSL Behring and Ferring Pharmaceuticals companies.
References
- 1.Abbassi-Ghanavati M, Greer LG, Cummingham FG. Pregnancy and laboratory adminisrative data, a reference table for clinicians. Obstet Gynecol. 2006;114:1326–1331. doi: 10.1097/AOG.0b013e3181c2bde8. [DOI] [PubMed] [Google Scholar]
- 2.Boehlen F, Hohlfeld H, Extermann P, Perneger T, Moerloose de P. Platelet count at term pregnancy: a reappraisal of the threshold. Obstet Gynecol. 2000;95 doi: 10.1016/s0029-7844(99)00537-2. [DOI] [PubMed] [Google Scholar]
- 3.Burrows RF, Kelton JG. Thrombocytopenia at delivery. Am J Obstet Gynecol. 1990;162:731–734. doi: 10.1016/0002-9378(90)90996-k. [DOI] [PubMed] [Google Scholar]
- 4.Gernsheimer T, James AH, Stasi R. How I treat thrombocytopenia in pregnancy. Blood. 2013;121:38–47. doi: 10.1182/blood-2012-08-448944. [DOI] [PubMed] [Google Scholar]
- 5.Adams TM, Allaf MB, Vintzileos AM. Maternal thrombocytopenia in pregnancy: diagnosis and management. Clin Lab Med. 2013;33:327–341. doi: 10.1016/j.cll.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Myers B. Diagnosis and management of maternal thrombocytopenia in pregnancy. Br J Haematol. 2012;158:3–15. doi: 10.1111/j.1365-2141.2012.09135.x. [DOI] [PubMed] [Google Scholar]
- 7.Burrows RF, Kelton JG. Current Obstetric Medicine. Platelet and pregnancy. In: Lee RV, editor. St. Louis. Vol. 2. Mosby-Year Book.; 1993. pp. 83–94. [Google Scholar]
- 8.McCrae KR. Thrombocytopenia in pregnancy: differential diagnosis, pathogenesis and management. Blood Rev. 2003;17:7–14. doi: 10.1016/s0268-960x(02)00056-5. [DOI] [PubMed] [Google Scholar]
- 9.Gernsheimer TB. Thrombocytopenia in pregnancy: is this immune thrombocytopenia or ….? Hematology. 2012;2012:198–202. doi: 10.1182/asheducation-2012.1.198. [DOI] [PubMed] [Google Scholar]
- 10.Kamphuis MM, Oepkes D. Fetal and neonatal alloimmune thrombocytopenia: prenatal interventions. Prenat Diagn. 2011;31:712–719. doi: 10.1002/pd.2779. [DOI] [PubMed] [Google Scholar]
- 11.Matzdorf A, Eberl W, Giagounidis A, Imbach P, Pabinger I, Wörmann B. Immunthrombozytpenie (ITP) 2013. www.dgho-onkopedia/leitlinien/immunthrombozytopenie-itp. doi: 10.1159/000356910. (last accessed on 10 January 2015) [DOI] [PubMed] [Google Scholar]
- 12.Stella CL, Dacus J, Guzman E, et al. the diagnostic dilemma of thrombotic thrombocytopenic purpura/hemostatic uremic syndrome in the obstetric triage and emergency department: lessons from 4 tertiary hospitals. Am J Obstet Gynecol. 2009;381:e1–e6. doi: 10.1016/j.ajog.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 13.Segal JB, Powe NR. Prevalence of immune thrombocytopenia: analysis of adminisrative data. J Thromb Haemost. 2006;4:2377–2383. doi: 10.1111/j.1538-7836.2006.02147.x. [DOI] [PubMed] [Google Scholar]
- 14.Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115:168–186. doi: 10.1182/blood-2009-06-225565. [DOI] [PubMed] [Google Scholar]
- 15.Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Crowther MA. the American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190–4207. doi: 10.1182/blood-2010-08-302984. [DOI] [PubMed] [Google Scholar]
- 16.Webert KE, Mittal R, Sigouin C, Heddle NM, Kelton JG. A retrospective 11-year analysis of obstetric patients with idiopathic thrombocytopenic purpura. Blood. 2003;102:4306–4311. doi: 10.1182/blood-2002-10-3317. [DOI] [PubMed] [Google Scholar]
- 17.Loustou V, Debouverie O, Canoui-Poitrine F, et al. Effect of pregnancy on the course of immune thrombocytopenia: a retrospective study of 118 pregnancies in 52 women. Br J Haematol. 2014;166:929–935. doi: 10.1111/bjh.12976. [DOI] [PubMed] [Google Scholar]
- 18.Lakshmanan S, Cuker A. Contemporary management of primary immune thrombocytopenia in adults. J Thromb Haemost. 2012;10:1988–1998. doi: 10.1111/j.1538-7836.2012.04876.x. [DOI] [PubMed] [Google Scholar]
- 19.Koyama S, Tomimatsu T, Kanagawa T, Kumasawa K, Tsutsui T, Kimura T. Reliable predictors of neonatal immune thrombocytopenia in pregnant women with idiopathic thrombocytopenic purpura. Am J Hematol. 2012;87:15–21. doi: 10.1002/ajh.22178. [DOI] [PubMed] [Google Scholar]
- 20.Kamphuis MM, Paridaans NP, Porcelijn L, Lopriore E, Oepkes D. Incidence and consequences of neonatal alloimmune thrombocytopenia: a systematic review. Pediatrics. 2014;133:715–721. doi: 10.1542/peds.2013-3320. [DOI] [PubMed] [Google Scholar]
- 21.AWMF-Leitlinie 015/018. Diagnostik and therapie hypertensiver Schwangerschaftserkrankungen. www.awmf.org/uploads/tx_szleitlinien/015-018l_S1_Diagnostik_therapie_hypertensiver_Schwangerschaftserkrankungen_2014-01.pdf. (last accessed on 10 January 2015)
- 22.Martin JN, Jr., Rinehart BK, May WL, Magann EF, Terrone DA, Blake PG. the spectrum of severe preeclampsia: comparative analysis by HELLP syndrome classification. Am J Obstet Gynecol. 1999;180:1373–1384. doi: 10.1016/s0002-9378(99)70022-0. [DOI] [PubMed] [Google Scholar]
- 23.Rath W, Faridi A, Dudenhausen JW. HELLP syndrome. J Perinat Med. 2000;28:249–260. doi: 10.1515/JPM.2000.033. [DOI] [PubMed] [Google Scholar]
- 24.Dashe JS, Ramin SM, Cunningham FG. the long-term consequences of thrombotic microangiopathy in pregnancy. Obstet Gynecol. 1998;91:662–668. doi: 10.1016/s0029-7844(98)00031-3. [DOI] [PubMed] [Google Scholar]
- 25.Galbusera M, Noris M, Remuzzi G. Thrombotic thrombocytopenic purpura—then and now. Semin Thromb Haemost. 2006;32:81–89. doi: 10.1055/s-2006-939763. [DOI] [PubMed] [Google Scholar]
- 26.George JN. How I treat patients with thrombotic thrombocytopenic purpura. Blood. 2010;116:4060–4069. doi: 10.1182/blood-2010-07-271445. [DOI] [PubMed] [Google Scholar]
- 27.Scully M, Hunt BJ, Benjamin S, et al. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol. 2012;158:323–335. doi: 10.1111/j.1365-2141.2012.09167.x. [DOI] [PubMed] [Google Scholar]
- 28.George JN, Nester CM. syndrome of thrombotic microangiopathy. N Engl J Med. 2014;371:654–666. doi: 10.1056/NEJMra1312353. [DOI] [PubMed] [Google Scholar]
- 29.Moatti-Cohen M, Garrec C, Wolf M, et al. Unexpected frequency of Upshaw-Schulman syndrome in pregnancy-onset thrombotic thrombocytopenic purpura. Blood. 2012;119:5888–5897. doi: 10.1182/blood-2012-02-408914. [DOI] [PubMed] [Google Scholar]
- 30.von Auer C, von Krogh AS, Kremer Hovinga JA, Lämmle B. Current insights into thrombotic microangiopathies: Thrombotic thrombocytopenic purpura and pregnancy. Thromb Res. 2015;135(Suppl.1):30–33. doi: 10.1016/S0049-3848(15)50437-4. [DOI] [PubMed] [Google Scholar]
- 31.Scully M, Thomas M, Underwood M, et al. Thrombotic thrombocytopenic purpura and pregnancy: presentation, management, and subsequent pregnancy outcomes. Blood. 2014;124:211–219. doi: 10.1182/blood-2014-02-553131. [DOI] [PubMed] [Google Scholar]
- 32.Rath W. Das HELLP-Syndrom - eine interdisziplinäre Herausforderung. Dtsch Arztebl. 1998;95:A2997–A3002. [Google Scholar]
- 33.Keiser SD, Boyd KW, Rehberg JF, et al. A high LDH to AST ratio helps to differentiate pregnancy-associated thrombotic thrombocytopenic purpura (TTP) from HELLP syndrome. J Matern-Fetal Neonatal Med. 2012;25:1059–1063. doi: 10.3109/14767058.2011.619603. [DOI] [PubMed] [Google Scholar]
- 34.Fakhouri F, Roumenina I, Provot E, et al. Pregnancy-associated hemolytic uremic syndrome revisited in the era of complement gene mutations. J Am Soc Nephrol. 2010;21:859–867. doi: 10.1681/ASN.2009070706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noris M, Remuzzi G. Atypical hemolytic uremic syndrome. N Engl J Med. 2009;361:1676–1687. doi: 10.1056/NEJMra0902814. [DOI] [PubMed] [Google Scholar]
- 36.Cataland SR, Wu HM. How I treat: the clinical differentiation and initial treatment of adult patients with hemolytic uremic syndrome. Blood. 2014;123:2476–2484. doi: 10.1182/blood-2013-11-516237. [DOI] [PubMed] [Google Scholar]
- 37.Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor ecuzulimab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169–2181. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- 38.Boregowada G. Gastrointestinal and liver diseases in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2013;27:835–853. doi: 10.1016/j.bpobgyn.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Balduini CL, Savoia A, Seri M. Inherited thrombocytopenias frequently diagnosed in adults. J Thromb Haemost. 2013;11:1006–1009. doi: 10.1111/jth.12196. [DOI] [PubMed] [Google Scholar]
- 40.Noris P, Schlegel N, Kiersy C, et al. Analysis of 339 pregnancies in 181 women with 13 different forms of inherited thrombocytopenia. Haematologica. 2014;99:1387–1394. doi: 10.3324/haematol.2014.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e1.Jensen JF, Wiedmeier SE, Henry E, Silver R, Christensen RD. Linking maternal platelet counts with neonatal platelet counts and outcomes using the data repositories of a multi-hospital health care system. Am J Perinatol. 2011;28:597–604. doi: 10.1055/s-0031-1276733. [DOI] [PubMed] [Google Scholar]
- e2.World Health Organization. WHO Global Database of Anaemia, WHO. 2008. Worldwide prevalence of Anaemia, Report 1993-2005. [Google Scholar]
- e3.Sullivan CA, Martin JN., Jr. Management of obstetric patient with thrombocytopenia. Clin Obstet Gynecol. 1995;35:521–534. doi: 10.1097/00003081-199509000-00011. [DOI] [PubMed] [Google Scholar]
- e4.Saino S, Kekomaki R, Rijkonen S, Terano K. Maternal thrombocytopenia at term: a population-based study. Acta Obstet Gynecol Scand. 2000;79:744–749. [PubMed] [Google Scholar]
- e5.Solanki DL, Blackburn BC. Spurious leukocytosis and thrombocytopenia. A dual phenomenon caused by clumping of platelets in vitro. JAMA. 1983;250:2514–2515. doi: 10.1001/jama.250.18.2514. [DOI] [PubMed] [Google Scholar]
- e6.Shalev O, Lotman A. Pseudothrombocytopenia. New Engl J Med. 1993;329 doi: 10.1056/NEJM199311113292006. [DOI] [PubMed] [Google Scholar]
- e7.Mc Crae KR, Samuels P, Schreiber AD. Pregnancy-associated thrombocytopenia pathogenesis and management. Blood. 1992;80:2697–2714. [PubMed] [Google Scholar]
- e8.Crowther MA, Burrows RF, Ginsberg J, Kelton JG. Thrombocytopenia in pregnancy: diagnosis, pathogenesis and management. Blood Rev. 1996;10:8–18. doi: 10.1016/s0268-960x(96)90015-6. [DOI] [PubMed] [Google Scholar]
- e9.Win N, Rowley M, Pollard C, Beard J, Hamley H, Broker M. Severe gestational (incidental) thrombocytopenia to treat or not to treat. Haemotologica. 2005;10:69–72. doi: 10.1080/10245330400020421. [DOI] [PubMed] [Google Scholar]
- e10.Gill KK, Kelton JG. Management of idiopathic thrombocytopenic purpura in pregnancy. Semin Hematol. 2000;37:275–289. doi: 10.1016/s0037-1963(00)90106-9. [DOI] [PubMed] [Google Scholar]
- e11.Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definition, and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113:2386–2393. doi: 10.1182/blood-2008-07-162503. [DOI] [PubMed] [Google Scholar]
- e12.Jahromi BN, Shiravani Z, Salarian L. Perinatal outcome of pregnancies complicated by immune thrombocytopenia. Iran Rev Crescent Med J. 2012;14:430–435. [PMC free article] [PubMed] [Google Scholar]
- e13.Lescale KB, Eddleman KA, Cines DB, et al. Antiplatelet antibody testing in thrombocytopenic pregnant women. Am J Obstet Gynecol. 1996;174:1014–1019. doi: 10.1016/s0002-9378(96)70342-3. [DOI] [PubMed] [Google Scholar]
- e14.McMillian R, Wang L, Tani P. Prospective evaluation of the immunobead assay for the diagnosis of adult chronic immune thrombocytopenic purpura (ITP) J Thromb Haemost. 2003;3:485–491. doi: 10.1046/j.1538-7836.2003.00091.x. [DOI] [PubMed] [Google Scholar]
- e15.van der Lugt NM, van Kampen A, Walther FJ, Brand A, Lopriore E. Outcome and management in neonatal thrombocytopenia due to maternal idiopathic thrombocytopenic purpura. Vox Sang. 2013;105:236–243. doi: 10.1111/vox.12036. [DOI] [PubMed] [Google Scholar]
- e16.Fujimura K, Harada Y, Fujimoto T, et al. Nationwide study of idiopathic thrombocytopenic purpura in pregnant women and the clinical influence on neonates. Int J Hematol. 2002;75:426–433. doi: 10.1007/BF02982137. [DOI] [PubMed] [Google Scholar]
- e17.Kjeldsen-Kragh J, Killie MK, Tomter G, et al. A screening and intervention program aimed to reduce mortality and serious morbidity associated with severe neonatal alloimmune thrombozytopenia. Blood. 2007;110:833–839. doi: 10.1182/blood-2006-08-040121. [DOI] [PubMed] [Google Scholar]
- e18.Onisai M, Vladaraneanu AM, Delcea C, et al. Perinatal outcome for pregnancies complicated with thrombocytopenia. J Matern Fetal Neonatal Med. 2012;25:1622–1626. doi: 10.3109/14767058.2011.648245. [DOI] [PubMed] [Google Scholar]
- e19.Rath W, Fischer T. The diagnosis and treatment of hypertensive disorders of pregnancy: new findings for antenatal and inpatient care. Dtsch Arztebl Int. 2009;106:733–738. doi: 10.3238/artebl.2009.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e20.Martin JN., Jr. Milestones in the quest for best management of patients with HELLP syndrome (microangiopathic hemolytic anemia, hepatic dysfunction, thrombocytopenia) Int J Gynecol Obstet. 2013;121:202–207. doi: 10.1016/j.ijgo.2013.02.001. [DOI] [PubMed] [Google Scholar]
- e21.Kirkpatrick CA. The HELLP syndrome. Acta Clinica. 2010;65:91–97. doi: 10.1179/acb.2010.020. [DOI] [PubMed] [Google Scholar]
- e22.Harms K, Rath W, Hertig E, Kuhn W. Maternal hemolysis, elevated liver enzymes, low platelet count, and neonatal outcome. Am J Perinatol. 1995;12:1–6. doi: 10.1055/s-2007-994387. [DOI] [PubMed] [Google Scholar]
- e23.George JN, Charania RS. Evaluation of patients with microangiopathic hemolytic anemia and thrombocytopenia. Semin Thromb Hemost. 2013;39:153–160. doi: 10.1055/s-0032-1333538. [DOI] [PubMed] [Google Scholar]
- e24.Furlan M, Lämmle B. Aetiology and pathogenesis of thrombotic thrombocytopenic purpura and haemolytic uraemic syndrome: the role of von Willebrand factor-cleaving protease. Best Pract Res Clin Haematol. 2001;14:437–454. doi: 10.1053/beha.2001.0142. [DOI] [PubMed] [Google Scholar]
- e25.Kappler S, Ronan-Bentle S, Graham A. Thrombotic microangiopathies (TTP, HUS, HELLP) Emerg Med Clin N Am. 2014;32:649–671. doi: 10.1016/j.emc.2014.04.008. [DOI] [PubMed] [Google Scholar]
- e26.Hulstein JJJ, van Runnard Heimel PJ, Franx A, et al. Acute activation of the endothelium results in increased levels of active von Willebrand factor in hemolysis, elevate liver enzymes and low platelets (HELLP) syndrome. J Thromb Haemost. 2006;4:2569–2575. doi: 10.1111/j.1538-7836.2006.02205.x. [DOI] [PubMed] [Google Scholar]
- e27.Owens MY, Martin JN, Jr., Wallace K, et al. Postpartum thrombotic microangiopathic syndrome. Transfus Apher Sci. 2013;48:51–57. doi: 10.1016/j.transci.2012.05.016. [DOI] [PubMed] [Google Scholar]
- e28.Martin JN, jr, Bailey AP, Rehberg JP, Owens MT, Keiser SD, May WL. Thrombotic thrombocytopenic purpura in 166 pregnancies. Am J Obstet Gynecol. 2008;199:96–104. doi: 10.1016/j.ajog.2008.03.011. [DOI] [PubMed] [Google Scholar]
- e29.Nelson DB, Yost NP, Cunningham FG. Acute fatty liver or pregnancy: clinical outcomes and expected duration of recovers. AJOG. 2013;209(456):e1–e7. doi: 10.1016/j.ajog.2013.07.006. [DOI] [PubMed] [Google Scholar]
- e30.Papafragkakis H, Singhal S, Anand S. Acute fatty liver of pregnancy. South Med J. 2013;106:588–593. doi: 10.1097/SMJ.0000000000000007. [DOI] [PubMed] [Google Scholar]
- e31.Jin F, Cao M, Bai Y, et al. Therapeutic effects of plasma exchange for the treatment of 39 patients with acute fatty liver of pregnancy. Discov Med. 2012;13:369–373. [PubMed] [Google Scholar]
- e32.Greer JA, Nelson-Piercy C. Low-molecular-weight heparins for thromboprophylaxis and treatment of venous thromboembolism in pregnancy: a systematic review of safety and efficacy. Blood. 2005;106:401–407. doi: 10.1182/blood-2005-02-0626. [DOI] [PubMed] [Google Scholar]
- e33.Watson H, Davidson S, Keeling D. Guidelines on the diagnosis and management of heparin-induced thrombocytopenia: second edition. Br J Haematol. 2012;159:528–540. doi: 10.1111/bjh.12059. [DOI] [PubMed] [Google Scholar]