Abstract
In the West in particular, the vast majority of gastric cancer (GC) patients present with advanced-stage disease. Although combination chemotherapy is still the most important component of treatment for these patients, it confers a modest survival advantage. Recently, increased knowledge of the key molecular signaling pathways involved in gastric carcinogenesis has led to the discovery of specific molecular-targeted therapeutic agents. Some of these agents such as trastuzumab and ramucirumab have changed the treatment paradigm for this disease. In this paper, we will summarize the current clinical status of targeted drug therapy in the management of GC.
Keywords: Gastric cancer, Targeted therapy, Angiogenesis, Epidermal growth factor, Treatment
Core tip: Systemic chemotherapy confers a modest survival advantage in patients with advanced gastric cancer. The new therapeutic agents that target various inter- and intracellular signaling transduction pathways offer the promise of improved clinical outcomes in selected patients. The success of some of these agents has changed the treatment paradigm for advanced gastric cancer. We herein discuss the current and potential future roles of targeted therapy in the management of this malignancy.
INTRODUCTION
Gastric cancer (GC) is a very aggressive tumor and is currently the third leading cause of cancer-related deaths in both sexes at the world level (8.8% of the total)[1,2]. At initial diagnosis, a significant proportion of Western GC patients (65%) are found to have unresectable disease or distant metastases. In Japan and South Korea, where nationwide government-sponsored screening programs have been established, still up to 80% of patients who undergo a curative resection for GC develop locoregional or distant recurrence[2,3].
The clinical management of patients with advanced GC remains one of the most challenging tasks in clinical oncology. Until recently, systemic chemotherapy alone has been the mainstay of treatment for these patients[4]. However, the disease often exhibits relative resistance to chemotherapeutic agents, and a satisfactory response is achieved only in a minority of the patients[5,6]. In addition, although systemic chemotherapy can substantially increase symptom control and improve the patient’s quality of life, its long-term results are still not satisfactory and unfortunately many patients die less than a year after starting therapy[5,6].
Thus, there is undoubtedly a need to develop more effective treatment strategies for this formidable disease. As in other solid tumors, the uses of targeted agents that block vital inter- and intracellular signaling pathways have recently emerged as a strategy for the treatment of advanced GC[7-12]. Significant advances in our understanding of the underlying biologic processes of GC have recently expanded the number and range of potential therapeutic targets. Targeted agents may be used either alone or in combination with anti-neoplastic agents for patients with both chemotherapy-naïve and chemotherapy-refractory disease. Some of these, such as trastuzumab and ramucirumab have been shown to have significant therapeutic activity and a good safety profile, have changed the treatment paradigm, and are therefore currently licensed in the United States and Europe as part of the management of patients with GC.
In this review, we will outline well-established targeted treatments for GC and discuss novel agents currently in development as well as some directions for future research.
Anti-epidermal growth factor receptor therapies
The epidermal growth factor receptor (EGFR) belongs to the ErbB family of receptor tyrosine kinases (RTK), which contains four closely related members: ErbB1 (HER1 or EGFR), ErbB2 (Her2/neu), ErbB3 and ErbB4[13,14]. EGFR activation by one of its ligands initiates diverse downstream signaling pathways including the RAS/RAF/MAP kinase and PI3K/Akt/mTOR signaling networks. Both pathways play a vital role in several critical cellular processes including proliferation, growth, survival, motility, and tissue invasion[13,14].
EGFR overexpression has been correlated with more aggressive tumor behavior and a worse clinical results in patients with GC, suggesting that EGFR is therapeutic target for this aggressive malignancy[13,14]. The current therapeutic strategies targeting EGFR include neutralizing monoclonal antibodies (moAbs) directed against the extracellular receptor domain and small molecule tyrosine kinase inhibitors (TKIs) of the intracellular tyrosine kinase domain (Figure 1).
Figure 1.
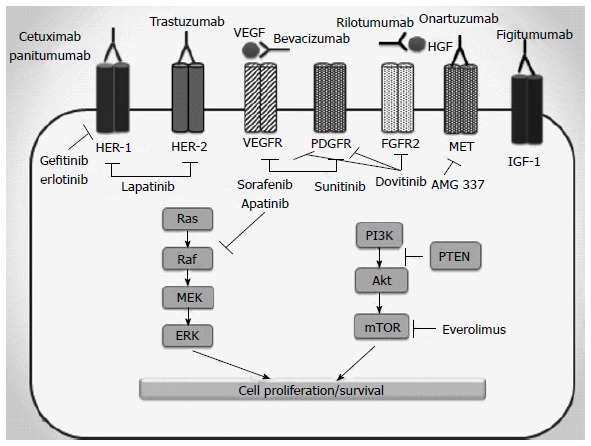
Molecular targets and relevant drugs in metastatic gastric cancer. HER: Human epidermal growth factor receptor; VEGF: Vascular endothelial growth factor; VEGFR: Vascular endothelial growth factor receptor; PDGFR: Platelet-derived growth factor receptor; HGF: Hepatocyte growth factor; FGFR2: Fibroblast growth factor receptor 2; IGF-1: Insulin-like growth factor 1; Raf: GTPase Raf; Ras: GTPase Ras; MEK: MAP kinase; ERK: Extracellular-signal-regulated kinase; PI3K: Phosphatidylinositol 3-kinase; PTEN: Phosphatase and tensin homolog; AKT: Protein kinase B; mTOR: Mammalian target of rapamycin.
Cetuximab and panitumumab are engineered antibodies that bind to EGFR with higher affinity compared to its natural ligands[15,16]. Several phase II clinical trials have tested the feasibility of adding cetuximab to different chemotherapy regimens including 5-FU/folinic acid (LV)/irinotecan, 5-FU/LV/oxaliplatin (FOLFOX), docetaxel/cisplatin, capecitabine/cisplatin, and capecitabine/oxaliplatin for chemotherapy-naïve advanced GC patients[17-20]. In these trials, overall response rates ranged from 41% to 69%, median progression-free survival (PFS) varied from 5 to 8.5 mo, and median overall survival (OS) varied from 9 to 16.6 mo. A randomized phase II clinical study (CALGB 80403/ECOG 1206) evaluated three different conventional chemotherapy regimens (Epirubicin, cisplatin and 5-FU vs irinotecan and cisplatin vs FOLFOX) in combination with cetuximab. Response rates were 58%, 38%, and 51%, respectively, and median OS was 8.6 and 10 mo, respectively. Cetuximab combined with FOLFOX was found to be the least toxic of the three[21].
Unfortunately, these promising initial outcomes were not verified in the phase III EXPAND trial[22]. In this study, 904 previously untreated metastatic GC and gastro-esophageal junction (GEJ) cancer patients were randomly allocated to receive chemotherapy (cisplatin and capecitabine) with or without cetuximab[22]. No differences in clinical outcome were found between treatment groups, and the primary and secondary efficacy endpoints were not met; the median PFS and OS were 4.4 mo (95%CI: 4.2 to 5.5 mo) and 9.4 mo (95%CI: 8.3 to 10.6 mo), respectively in the combined therapy group compared with 5.6 mo (95%CI: 5.1 to 5.7 mo) and 10.7 mo (95%CI: 9.4 to 11.3 mo), respectively in the chemotherapy-alone group (P = 0.32 and P = 0.95 for PFS and OS, respectively). The addition of cetuximab also did not increase the overall response rate, which was 30% and 29% with or without cetuximab, respectively (Table 1).
Table 1.
Summary of completed phase III trials of targeted agents in the treatment of advanced gastric and gastroesophageal adenocarcinoma
| Author/trial | Line of treatment | Target | Agent | Treatment | ORR (%) | PFS (mo) | OS (mo) |
| Lordick et al[22] (2013)/ EXPAND | First | EGFR | Cetuximab | Cisplatin/capecitabine ± cetuximab | 30 vs 29 P = 0.77 | 4.4 vs 5.6 P = 0.32 | 9.4 vs 10.7 P = 0.95 |
| Waddell et al[23] (2013)/REAL-3 | First | EGFR | Panitumumab | EOX ± panitumumab | 46 vs 42 P = 0.42 | 6.0 vs 7.4 P = 0.068 | 8.8 vs 11.3 P = 0.013 |
| Bang et al[32] (2010)/ ToGA | First | HER2 | Trastuzumab | Cisplatin/capecitabine or 5-FU ± trastuzumab | 47 vs 35 P = 0.0017 | 6.7 vs 5.5 P = 0.0002 | 13.8 vs 11.1 P = 0.0046 |
| Hecht et al[34] (2013)/ LoGIC | First | EGFR/HER2 | Lapatinib | CAPOX ± lapatinib | 53 vs 40 P = NA | 6.0 vs 5.4 P = 0.1 | 12.2 vs 10.5 P = 0.35 |
| Ohtsu et al[37] (2011)/ AVAGAST | First | VEGF-A | Bevacizumab | Cisplatin/capecitabine ± bevacizumab | 46 vs 37.4 P = 0.03 | 6.7 vs 5.3 P = 0.037 | 12.1 vs 10.1 P = 0.1002 |
| Shen et al[39] (2015)/ AVATAR | First | VEGF-A | Bevacizumab | Cisplatin/capecitabine ± bevacizumab | 40.7 vs 33.7 P = 0.348 | 6.3 vs 6.0 P = 0.47 | 11.4 vs 10.5 P = 0.55 |
| Bang et al[35] (2014)/TyTAN | Second | EGFR/HER2 | Lapatinib | Paclitaxel ± lapatinib | 27 vs 9 P < 0.001 | 5.4 vs 4.4 P = 0.13 | 11.0 vs 8.9 P = 0.1044 |
| Fuchs et al[41] (2014)/ REGARD | Second | VEGFR-2 | Ramucirumab | BSC + ramucirumab or placebo | 3.4 vs 2.6 P = 0.76 | 2.1 vs 1.3 P < 0.0001 | 5.2 vs 3.8 P = 0.0473 |
| Wilke et al[43] (2014)/ RAINBOW | Second | VEGFR-2 | Ramucirumab | Paclitaxel + ramucirumab or placebo | 28 vs 16 P = 0.0001 | 4.4 vs 2.9 P < 0.0001 | 9.6 vs 7.4 P = 0.017 |
| Ohtsu et al[52] (2013)/ GRANITE-1 | Second or third | mTOR | Everolimus | Everolimus or placebo | 4.5 vs 2.1 P = NA | 1.7 vs 1.4 P < 0.001 | 5.4 vs 4.3 P = 0.124 |
ORR: Overall response rate; PFS: Progression-free survival; OS: Overall survival; EGFR: Epidermal growth factor receptor; EOX: Epirubicin, oxaliplatin and capecitabine; HER2: Human epidermal growth factor receptor 2; 5-FU: 5-fluorouracil; CAPOX: Capecitabine and oxaliplatin; NA: Not available; VEGF-A: Vascular endothelial growth factor A; VEGFR-2: Vascular endothelial growth factor receptor 2; mTOR: Mammalian target of rapamycin.
Similarly, the phase III REAL-3 trial was performed to determine the effects of adding panitumumab to a combination chemotherapy regimen of epirubicin, oxaliplatin, and capecitabine (EOX) in patients with advanced esophago-gastric adenocarcinoma[23]. In this trial, patients were randomly allocated to receive EOX or a modified EOX plus panitumumab. Disappointingly, adding panitumumab to EOX chemotherapy resulted in worsened OS [8.8 mo compared with 11.3 mo for the EOX regimen (HR = 1.37; P = 0.013)]. A trend toward a shorter PFS was also seen in patients receiving panitumumab (6.0 mo vs 7.4 mo, HR = 1.22; P = 0.068). The panitumumab-containing arm was associated with an increased rate of grade 3-4 diarrhea (17% vs 11%), rash (11% vs 1%), mucositis (5% vs none), and hypomagnesaemia (5% vs none) but reduced rate of neutropenia (13% vs 28%).
Lastly, other novel humanized IgG1 anti-EGFR moAbs including matuzumab and nimotuzumab have also been investigated as first- or second-line treatment for advanced GC, and have also failed to generate a strong efficacy signal[24-26]. The small molecule EGFR TKIs have not been extensively studied in the treatment of advanced GC largely due to their limited activity in this setting[27,28]. Why EGFR-targeting strategies have failed to be successful in this disease in spite of lack of activating KRAS mutations and in spite of good biologic rationale remains a mystery.
Anti-HER2 (ERBB2) therapy
As previously mentioned HER2 is another member of the ERB family of receptor tyrosine kinases[29]. Overexpression and amplification of the HER2 is detected in 10%-38% of GC patients[30]. Although the association between HER2 status and prognosis in GC still controversial, the results of some clinical studies have suggested that patients with HER2 negative disease have a more favorable prognosis than those with HER2 positive disease[29,31]. Perhaps one of the most convincing data supporting the clinical benefits of targeted therapy in advanced GC come from the phase III ToGA study[32]. This landmark study investigated the addition of trastuzumab, a moAb that binds to the extracellular ligand binding domain of the HER2 receptor, to combination chemotherapy (cisplatin and either capecitabine or 5-FU) in patients with previously untreated HER2 overexpressing [defined as HER2 fluorescence in situ hybridization (FISH) positive or immunohistochemistry (IHC) 3 positive], and advanced gastric or GEJ cancer. Over 3000 patients were screened for the study. Among the 594 enrolled patients, 296 received chemotherapy alone and 298 received chemotherapy plus trastuzumab. Patients receiving the combined therapy achieved improvement in all measures of efficacy including OS (13.8 mo vs 11.1 mo; HR = 0.74, P = 0.0046), PFS (6.7 mo vs 5.5 mo; HR = 0.71, P = 0.0002), and overall response rate (47% vs 35%, P = 0.0017). A post hoc subgroup analysis of the study demonstrated that the patients with strongly HER-2 positive tumors (defined as IHC2+/FISH+ or IHC3+) derived significant OS benefit from the addition of trastuzumab to chemotherapy (16 mo vs 11.8 mo, HR = 0.68). Moreover, the tolerability of the combination was good and there was no significant difference in the incidence of grade 3 or 4 side effects between the treatment groups. Based on these results, trastuzumab was approved in the Unites States and European Union for use in the first-line treatment of HER2-overexpressing locally advanced or metastatic GC.
Pertuzumab is a new moAb that binds to the extracellular ligand binding domain of HER2 and blocks its dimerization with other HER-family receptors[31]. When used together, the combination of pertuzumab plus trastuzumab provide a more comprehensive blockade of HER signalling than either agent alone. Therefore, the JACOB phase III study is currently recruiting participants to evaluate the effectiveness of pertuzumab in addition to trastuzumab plus chemotherapy (cisplatin plus capecitabine or 5-FU) in chemo-naïve patients with HER2-overexpressing advanced gastric or GEJ cancer (NCT01774786).
Trastuzumab emtansine (T-DM1) is a newly developed HER2-targeted antibody–drug conjugate that links trastuzumab to a highly potent maytansine-derived anti-microtubule drug (DM1)[33]. After binding the trastuzumab moiety to HER2 receptors on the tumor surface, T-DM1 is internalized by endocytosis and degraded in lysosomes, resulting in release of DM1-containing cytotoxic catabolites[33]. A phase II-III trial (NCT01641939) is now investigating the effectiveness of T-DM1 compared with taxanes (docetaxel or paclitaxel) in patients with metastatic HER2-positive GC who develop progression of disease following first-line chemotherapy.
Lapatinib is an oral small-molecule tyrosine kinase inhibitor of EGFR and HER2 that blocks their tyrosine kinase activities. Two phase III trials were performed to explore the effectiveness of lapatinib in first- and second-line treatment of advanced GC. The LoGIC III trial investigated the efficacy of lapatinib when administered in combination with capecitabine plus oxaliplatin (CAPOX) as first-line therapy[34]. In total, 545 patients whose tumors overexpressed HER-2 were assigned to receive CAPOX plus lapatinib or placebo. No significant difference in survival between the two treatment arms was detected. Median OS and PFS in the chemotherapy + lapatinib group were 12.2 and 6 mo, respectively, compared to 10.5 and 5.4 mo in the control group. Similarly, in the phase III TyTan trial conducted in Asia, 430 patients with advanced GC who had experienced failure of fluoropyrimidine and cisplatin-based chemotherapy and exhibited FISH-confirmed HER2 amplification received lapatinib plus weekly paclitaxel or weekly paclitaxel alone[35]. Although, the addition of lapatinib to paclitaxel extended the primary endpoint of OS from a median of 8.9 mo to 11.0 mo, this improvement failed to reach statistical significance (P = 0.1044). The further subgroup analysis revealed a statistically significant benefit in both OS and PFS from the addition of lapatinib to chemotherapy in patients with HER2 IHC3+ tumors and in Chinese patients.
Targeting angiogenesis pathways
Angiogenesis is necessary for tumors to grow beyond a certain size, survive or spread. Vascular endothelial growth factor (VEGF) and its receptors (VEGFR1, VEGFR2 and VEGFR3) are important players in the development of this process. Binding of the ligand VEGF-A to VEGFR-2 triggers a signaling cascade leading to endothelial cell proliferation, migration, new vessel formation, and sustained angiogenesis[24]. Therefore, inhibition of the VEGF signaling has become a useful clinical maneuver in the treatment of several types of cancer.
Anti-VEGF moAb: Bevacizumab is a fully human moAb targeting VEGF-A[36]. The potential role of this drug in the management of patients with metastatic GC was evaluated in the phase III AVAGAST and AVATAR trials. The AVAGAST trial was global, randomized, placebo-controlled trial conducted for evaluation of the benefits of bevacizumab when added to first-line capecitabine and cisplatin chemotherapy in 774 metastatic GC patients[37]. The trial did not show any significant improvement in OS in the bevacizumab cohort. Median OS was 12.1 mo with bevacizumab plus chemotherapy and 10.1 mo with placebo plus chemotherapy (HR = 0.87; 95%CI: 0.73 to 1.03; P = 0.1002). Despite this, both median PFS (6.7 mo vs 5.3 mo; HR = 0.80; 95%CI: 0.68 to 0.93; P = 0.0037) and overall response rate (46.0% vs 37.4%; P = 0.0315) were significantly increased by the addition of bevacizumab vs placebo. Preplanned subgroup analysis of the study also demonstrated geographical differences in the therapeutic effectiveness of bevacizumab treatment. A survival benefit for bevacizumab was demonstrated in patients recruited from North America and Latin America centers (median, 11.5 mo vs 6.8 mo for placebo-chemotherapy; HR = 0.63; 95%CI: 0.43 to 0.94), whereas patients recruited from Asia centers seemed to have no obvious benefit (HR = 0.97; 95%CI: 0.75 to 1.25). Subsequently, the study investigators identified plasma VEGF-A levels and degree of tumor neuropilin-1, a co-receptor for VEGF-A, expression as potential predictive biomarkers of bevacizumab efficacy[38]. A negative OS correlation was found in patients with low expression of tumor neuropilin-1 (HR = 0.75; 95%CI: 0.59 to 0.97) compared to those with high expression (HR = 1.07; 95%CI: 0.81 to 1.40; interaction P = 0.06). Of note, these findings were significant only in non-Asian patients.
AVATAR, a study similar in design to AVAGAST, was performed in Chinese patient population with advanced GC[39]. It was again demonstrated that the addition of bevacizumab to chemotherapy consisting capecitabine and cisplatin in this specific patient population did not improve OS (11.4 mo in the placebo arm vs 10.5 mo in the bevacizumab arm, HR = 1.11; P = 0.55).
Ramucirumab is a novel humanized IgG1 moAb that selectively binds to the extracellular ligand binding domain of VEGFR-2 and blocks VEGF-induced angiogenic signaling[40]. In theory, this has the advantage of blocking signaling from VEGF isoforms other than VEGF-A. Its efficacy and safety in advanced GC was evaluated in two international, phase III, randomized, double-blinded and placebo-controlled studies. In the REGARD trial, a total 355 advanced gastric or GEJ cancer patients progressing after first-line platinum- or fluoropyrimidine-based combination chemotherapy were randomized to receive best supportive care (BSC) plus either ramucirumab or placebo[41]. Ramucirumab was given intravenously every 2 wk at 8 mg/kg and the median treatment duration was 8 wk. Patients receiving ramucirumab had a significantly improved median OS (5.2 mo vs 3.8 mo; HR = 0.776; P = 0.0473) and PFS (2.1 mo vs 1.3 mo; HR = 0.483; P < 0.0001) than patients receiving placebo. The 12-wk PFS rate was 40% for ramucirumab group and 16% for placebo group. Additionally, the overall response rate (3.4% vs 2.6%) and disease control rate (49% vs 23%) were also higher in the ramucirumab group compared to the placebo group (P < 0.0001). Ramucirumab had an acceptable toxicity profile. The most frequently recorded grade 3 or higher side effects in patients receiving ramucirumab were hypertension, anemia, abdominal pain, ascites, fatigue and hyponatremia. After presentation of these results, ramucirumab was approved for the second-line therapy advanced GC in the United States. Interestingly, these results are quite similar to those achieved with chemotherapy in the second-line setting[42].
The RAINBOW study tested ramucirumab in combination with paclitaxel in metastatic GEJ or gastric adenocarcinoma patients who experienced disease progression after first-line platinum- and fluoropyrimidine-based chemotherapy[43]. In this study, 665 patients were randomly assigned to receive ramucirumab or placebo plus paclitaxel. OS was defined again primary endpoint for efficacy. Median OS for patients received ramucirumab plus paclitaxel was 9.6 mo, compared to 7.4 mo for those received paclitaxel alone (HR = 0.807; 95%CI: 0.678-0.962; P = 0.0169). Median PFS was 4.4 mo and 2.9 mo, respectively (HR = 0.635; 95%CI: 0.536-0.752; P < 0.0001). The objective response rate was higher in the combination arm compared to paclitaxel alone arm (28% vs 16%, P = 0.0001). Ramucirumab was relatively well tolerated. As expected, grade 3 or higher side effects were somewhat more frequent among patients receiving ramucirumab plus paclitaxel greater with combination treatment and included neutropenia, leukopenia, hypertension and fatigue. The RAINBOW study showed that an effective second-line treatment may improve the duration of survival in metastatic GC, and it is the only study to date to demonstrate a 2-mo improvement in OS in this setting. Therefore, ramucirumab is the first anti-angiogenic agent to demonstrate activity for advanced GC, and now approved both as monotherapy and in combination with paclitaxel for this malignancy.
Anti-VEGF TKI: Apatinib is an orally administered TKI that selectively binds to VEGFR-2 and inhibits VEGF-induced endothelial cell proliferation and migration. As a result, it leads to a significant decrease in tumor microvessel density[44]. In a phase II trial conducted in China, apatinib was shown to increase PFS and OS in patients with metastatic GC progressing after 2 or more previous lines of chemotherapy[45]. Data from a phase III trial presented at the 2014 ASCO Annual Meeting confirmed the effectiveness of apatinib in this setting[46]. This trial included 273 patients with advanced GC who experienced disease progression after second-line treatment. Patients were randomly assigned to receive apatinib or placebo. The primary endpoint, median OS, was significantly longer in the apatinib group than in the placebo group (195 d vs 140 d; HR = 0.71; 95%CI: 0.54-0.94; P < 0.016). The apatinib group also had a better median PFS than the placebo group; 78 d vs 53 d, respectively (HR = 0.44; 95%CI: 0.33-0.61; P < 0.0001). Therefore, apatinib provides a new promising treatment option for advanced GC, although one which overlaps with ramucirumab in both degree of activity and mechanism.
Two multi-targeted kinase inhibitors that share VEGF receptors as targets are sunitinib and sorafenib. Both of these agents have been tested in GC and have shown some signs of efficacy, but have not progressed to advanced trials[47-49]. Given the modest activity and the toxicity profiles of these two agents, it is unlikely that they would supplant ramucirumab at this time and are no longer being studied in GC.
The mTOR pathway: The mTOR (mammalian target of rapamycin) is an essential cellular signaling pathway that has a crucial role in the regulation of cell growth, survival, proliferation, metabolism, and angiogenesis[50]. Everolimus, an orally administered rapamycin analog, is the only mTOR inhibitor that has been evaluated in advanced GC[51]. Phase II trials documented that it can produce stabile disease in a significant portion of patients with chemo-refractory advanced GC. Despite these promising data, in the phase III GRANITE-1 trial, everolimus failed to demonstrate any significant improvement in OS compared to BSC alone[52]. In this study, advanced GC patients who had progressive disease after first- or second-line cytotoxic chemotherapy were randomized to receive everolimus treatment (10 mg/d) or matching placebo in conjunction with BSC. Median OS was 5.4 mo for patients receiving everolimus and 4.3 mo for patients receiving placebo (HR = 0.90; 95%CI: 0.75 to 1.08; P = 0.124). Another phase III trial (AIO-STO-0111) is now investigating the efficacy of everolimus when given in combination with paclitaxel in GC patients progressing following prior chemotherapy regimen.
Targeting the hepatocyte growth factor/c-MET signaling pathway
A transmembrane protein-tyrosine kinase receptor c-MET and its ligand, hepatocyte growth factor (HGF) control many vital cellular events such as cell proliferation, survival, motility, invasion and angiogenesis[53]. C-MET overexpression has been detected in 18%-82% of GC patients, with genetic amplification of the CMET occurring in only 2%-3% of cases[54]. Patients with c-Met overexpressing tumors may have poorer survival, and the prognostic effect of overexpression seems to be independent of disease stage[53]. Therefore, c-MET has been recognized as potentially significant therapeutic target in GC.
Rilotumumab is a fully humanized IgG2 moAb that selectively binds HGF and prevents its binding to the MET receptor[53]. The results of a phase Ib/II study of rilotumumab in combination with platinum-based chemotherapy in patients with locally advanced or metastatic GC have demonstrated the potential therapeutic value of drugs that target the c-MET pathway in this disease[55]. In the phase II part of this study, 121 patients were randomized to ECX regimen plus placebo (n = 39) or ECX plus either 7.5 mg/kg (n = 42) or 15 mg/kg (n = 40) rilotumumab. Median PFS was 5.1 mo (2.9-7.0) in the rilotumumab 15 mg/kg group, 6.8 mo (4.5-7.5) in the rilotumumab 7.5 mg/kg group, 5.7 mo (4.5-7.0) in both rilotumumab groups combined, and 4.2 mo (2.9-4.9) in the placebo group. The HR for PFS compared with placebo was 0.69 (80%CI: 0.49-0.97; P = 0.164) for rilotumumab 15 mg/kg, 0.53 (80%CI: 0.38-0.73; P = 0.009) for rilotumumab 7.5 mg/kg, and 0.60 (80%CI: 0.45-0.79; P = 0.016) for combined rilotumumab. Rilotumumab was generally well tolerated by patients, with common side effects including neutropenia, anemia, thrombocytopenia, peripheral edema, and deep vein thrombosis. The association between MET expression and clinical outcomes was also evaluated in this trial. MET expression was found to be prognostic for shortened OS in the placebo group (5.7 mo vs 11.5 mo). In the subgroup of patients with increased MET expression, median OS was longer in patients receiving rilotumumab than in those receiving placebo (10.6 mo vs 5.7 mo). However, no survival benefit was seen with the addition of rilotumumab to chemotherapy among MET-negative patients.
Based on these data, the RILOMET-1 [a multicenter, randomized, double-blind, placebo-controlled phase III study of rilotumumab (15 mg/kg) plus ECX regimen as first-line therapy for metastatic MET-positive gastric or GEJ adenocarcinoma] and the RILOMET-2 trial (a multicenter, randomized, double-blind, placebo controlled phase III study of rilotumumab plus cisplatin and capecitabine regimen as first-line therapy for Asian patients with metastatic MET-positive gastric or GEJ cancer) have been conducted. Unfortunately, the RILOMET-1 study has been reported as negative via press release (AMGEN press release), with final presentation of data pending at an upcoming meeting.
Onartuzumab is an Escherichia coli-derived humanized monovalent moAb against MET that specifically binds to the MET receptor and blocks HGF-MET binding[56]. Shah et al[57] have presented the results of a phase II trial that compared FOLFOX plus onartuzumab vs FOLFOX plus placebo in patients with metastatic gastroesophageal adenocarcinoma. The primary endpoint of the trial was not met (6.77 mo in onartuzumab arm vs 6.97 mo in the placebo arm, HR = 1.08; 95%CI: 0.71-1.63). In MET-positive patients, PFS was 5.95 mo for patients receiving onartuzumab vs 6.8 mo for those in the placebo arm (HR = 1.38; 95%CI: 0.60-3.20). Serious adverse events, including neutropenia, thrombocytopenia, peripheral edema, and pulmonary embolism also occurred more frequently in patients on onartuzumab (55% vs 40%).
The phase III MetGastric trial will assess the effectiveness and toxicity of onartuzumab in combination with modified-FOLFOX6 chemotherapy in patients with metastatic HER2-negative and MET-positive gastric or GEJ adenocarcinoma[58]. In this study, enrolled patients will receive the chemotherapy with either onartuzumab or placebo, and patients who have not progressed after 12 cycles of treatment will continue with either onartuzumab or placebo until evidence of disease progression or intolerable toxicity.
Targeting programmed cell death-1 receptor and its ligand
Programmed cell death-1 (PD-1) is a cell surface and immune inhibitory receptor expressed on a variety of immune cells, especially cytotoxic T cells. Two distinct ligands for PD-1 were identified: Programmed death ligand 1 (PD-L1) and PD-L2[59]. While PD-L2 is expressed mainly on macrophages and dendritic cells, PDL-1 is expressed exclusively by tumor cells and their microenvironment[60]. Tumors that express PD-L1 often tend to be aggressive and carry a poor prognosis[61]. Tumor cells utilize the PD-1/PD-L1 pathway to evade immune-cell attack. Activation of this pathway in tumor cells blocks T-cell-mediated cytotoxicity and allows tumor cells to continue to proliferate[59-61]. Drugs targeting PD-L1 pathway may stimulate antitumor immunity, especially (although not exclusively) in PD-L1 positive tumors.
At the 2014 European Society for Medical Oncology meeting, data on safety and tolerability, and preliminary anti-tumor efficacy of pembrolizumab in advanced GC patients were presented by Muro et al[62] (KEYNOTE-012 study). This drug is a selective and humanized moAb that blocks interaction between PD-1 and its ligands PD-L1 and PD-L2. Muro et al[62] enrolled 39 patients with PD-L1 positive advanced GC: 19 from Asia-Pacific, 20 from rest of world. Sixty-seven percent of these patients had received more than 2 chemotherapy lines. Pembrolizumab was administered 10 mg/kg once every 2 wk for up to 24 mo in the absence of intolerable toxicity or disease progression. The overall response rate was 31.6% in patients in the Asia-Pacific region and 30% in patients from rest the world. Median duration of response has not yet been reached at the time of initial presentation, but ranged from 8+ to 20+ wk. Four patients developed grade 3-5 drug-related adverse events including peripheral sensory neuropathy, fatigue, decreased appetite, hypoxia, and pneumonitis (n = 1 each). One treatment-related death was recorded due to hypoxia. The authors of the study have concluded that pembrolizumab treatment seems to have and acceptable safety and tolerability profile and it provides encouraging clinical antitumor activity in chemo-refractory disease. On the basis of these promising preliminary data, phase II KEYNOTE-059 study will be initiated to evaluate pembrolizumab as single agent or in combination with cisplatin and 5-FU in patients with metastatic PD-L1 positive gastric or GEJ adenocarcinoma.
Recent analysis from the Gastric Cancer Genome Atlas Project: The Cancer Genome Atlas is a large-scale effort coordinated by the United States National Cancer Institute to extensively characterize the genetic and epigenetic landscape of human cancers. The group has reported on the analysis of 259 untreated primary gastric cancers. This analysis proposed dividing gastric cancer into 4 molecular subtypes: EBV driven, microsatellite unstable (MSI high), genomic stable and chromosomal unstable tumors. This molecular subtyping highlights important targets within these groups for further study, and potentially allows for patient enrichment that could result in higher chance of positive trial results. For example, EBV driven tumors are characterized by high rate of PIK3CA mutations, where drugs targeting the Pi3K pathway are available in clinical trials[63]. Additionally, EBV-positive gastric cancers preferentially overexpress CD274 and PDCD1LG2 (PD-L1 and PD-L2) that were discussed above[64]. These are currently being evaluated as predictive biomarkers for immune checkpoint inhibitor activity[65,66]. In addition, this subgroup has significant promoter hypermethylation, such that evaluating hypomethylating agents such as azacitidine, decitabine and others in clinical development might represent a promising strategy.
The MSI-high genotype is associated with high mutational rate, representing a wealth of antigens that could be recognized by the immune system[67,68]. This genotype has been proposed to be responsive to checkpoint inhibitors, and clinical trials are ongoing (NCT01876511, NCT02060188) addressing response to checkpoint inhibitors in MSI high gastrointestinal cancers.
Other mutations that have been reported (KRAS, P53, APC, and CTNNB1) are still challenging to target and are the subject of numerous reviews. Knowledge of frequency of mutation of these genes, however, provides impetus for further basic research. For example, cell cycle regulators could have better chance of activity in P53 mutant tumors[69,70]. Lastly, the WNT/beta catenin pathway is currently a focus of much preclinical and clinical research[71].
CONCLUSION
Gastric cancer has long represented one of the most difficult gastrointestinal malignancies to treat. Encouragingly, recent progress with targeted therapies offers hope for patients with advanced GC, and expands the therapeutic armamentarium considerably against this formidable disease. As these therapies continue to be developed, we must focus on determination of predictive markers, and preferably co-develop drugs with these markers. The mechanisms underlying primary or acquired resistance to targeted agents also should be clarified in order to help further drug development.
We propose a treatment algorithm that is consistent with current National Cancer Center Network guidelines (version 3, 2015) and that integrates targeted therapies into the management of advanced GC (Figure 2). The addition of trastuzumab to a first-line chemotherapy doublet (cisplatin and capecitabine or 5-FU) is now considered standard of care for patients with HER2 positive advanced GC. The results of the phase III JACOB trial are awaited with great interest to see if the combined use of trastuzumab and pertuzumab can improve clinical outcome. Anti-angiogenic therapy has failed to meet the expectations as first-line treatment. But second-line treatment with ramucirumab or apatinib now represents a good alternative for chemo-refractory GC patients for whom the options are still are quite limited. Other targeted agents currently under evaluation in clinical trials including inhibitors of m-TOR, c-MET, IGFR, and FGFR pathways can help expand our treatment repertoire in the future against advanced GC. Lastly, knowledge gained from detailed molecular profiling of gastric cancers gives us a roadmap for future basic and clinical research.
Figure 2.
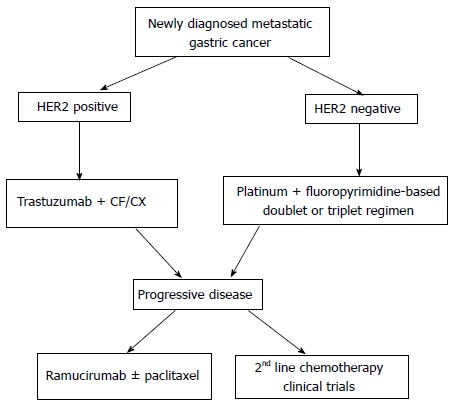
Proposed targeted therapy algorithm for advanced gastric cancer. CF: Cisplatin plus 5-Fluorouracil; HER: Human epidermal growth factor receptor; CX: Cisplatin plus capecitabine.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interest for this article.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 22, 2015
First decision: July 1, 2015
Article in press: October 27, 2015
P- Reviewer: Lee TY S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.De Vita F, Di Martino N, Fabozzi A, Laterza MM, Ventriglia J, Savastano B, Petrillo A, Gambardella V, Sforza V, Marano L, et al. Clinical management of advanced gastric cancer: the role of new molecular drugs. World J Gastroenterol. 2014;20:14537–14558. doi: 10.3748/wjg.v20.i40.14537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyerhardt JA, Fuchs CS. Adjuvant therapy in gastric cancer: can we prevent recurrences? Oncology (Williston Park) 2003;17:714–721, 728; discussion 728-729, 732-733. [PubMed] [Google Scholar]
- 4.Kanat O, O’Neil BH. Metastatic gastric cancer treatment: a little slow but worthy progress. Med Oncol. 2013;30:464. doi: 10.1007/s12032-013-0464-4. [DOI] [PubMed] [Google Scholar]
- 5.Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903–2909. doi: 10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]
- 6.Oba K, Paoletti X, Bang YJ, Bleiberg H, Burzykowski T, Fuse N, Michiels S, Morita S, Ohashi Y, Pignon JP, et al. Role of chemotherapy for advanced/recurrent gastric cancer: an individual-patient-data meta-analysis. Eur J Cancer. 2013;49:1565–1577. doi: 10.1016/j.ejca.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Schinzari G, Cassano A, Orlandi A, Basso M, Barone C. Targeted therapy in advanced gastric carcinoma: the future is beginning. Curr Med Chem. 2014;21:1026–1038. doi: 10.2174/0929867321666131129124054. [DOI] [PubMed] [Google Scholar]
- 8.Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Gastric cancer. Crit Rev Oncol Hematol. 2009;71:127–164. doi: 10.1016/j.critrevonc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Lordick F, Allum W, Carneiro F, Mitry E, Tabernero J, Tan P, Van Cutsem E, van de Velde C, Cervantes A. Unmet needs and challenges in gastric cancer: the way forward. Cancer Treat Rev. 2014;40:692–700. doi: 10.1016/j.ctrv.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Yang W, Raufi A, Klempner SJ. Targeted therapy for gastric cancer: molecular pathways and ongoing investigations. Biochim Biophys Acta. 2014;1846:232–237. doi: 10.1016/j.bbcan.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Zagouri F, Papadimitriou CA, Dimopoulos MA, Pectasides D. Molecularly targeted therapies in unresectable-metastatic gastric cancer: a systematic review. Cancer Treat Rev. 2011;37:599–610. doi: 10.1016/j.ctrv.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Kasper S, Schuler M. Targeted therapies in gastroesophageal cancer. Eur J Cancer. 2014;50:1247–1258. doi: 10.1016/j.ejca.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Martinelli E, De Palma R, Orditura M, De Vita F, Ciardiello F. Anti-epidermal growth factor receptor monoclonal antibodies in cancer therapy. Clin Exp Immunol. 2009;158:1–9. doi: 10.1111/j.1365-2249.2009.03992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Tomas A, Futter CE, Eden ER. EGF receptor trafficking: consequences for signaling and cancer. Trends Cell Biol. 2014;24:26–34. doi: 10.1016/j.tcb.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall J. Clinical implications of the mechanism of epidermal growth factor receptor inhibitors. Cancer. 2006;107:1207–1218. doi: 10.1002/cncr.22133. [DOI] [PubMed] [Google Scholar]
- 17.Wong H, Yau T. Targeted therapy in the management of advanced gastric cancer: are we making progress in the era of personalized medicine? Oncologist. 2012;17:346–358. doi: 10.1634/theoncologist.2011-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meza-Junco J, Sawyer MB. Metastatic gastric cancer - focus on targeted therapies. Biologics. 2012;6:137–146. doi: 10.2147/BTT.S23917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smyth EC, Cunningham D. Targeted therapy for gastric cancer. Curr Treat Options Oncol. 2012;13:377–389. doi: 10.1007/s11864-012-0192-6. [DOI] [PubMed] [Google Scholar]
- 20.Cidon EU, Ellis SG, Inam Y, Adeleke S, Zarif S, Geldart T. Molecular targeted agents for gastric cancer: a step forward towards personalized therapy. Cancers (Basel) 2013;5:64–91. doi: 10.3390/cancers5010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enzinger PC, Burtness B, Hollis D, Niedzwiecki D, Ilson D, Benson AB, Mayer RJ, Goldberg RM. CALGB 80403/ECOG 1206: A randomized phase II study of three standard chemotherapy regimens (ECF, IC, FOLFOX) plus cetuximab in metastatic esophageal and GE junction cancer. J Clin Oncol. 2010;28:15. doi: 10.1200/JCO.2015.65.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490–499. doi: 10.1016/S1470-2045(13)70102-5. [DOI] [PubMed] [Google Scholar]
- 23.Waddell T, Chau I, Cunningham D, Gonzalez D, Okines AF, Okines C, Wotherspoon A, Saffery C, Middleton G, Wadsley J, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:481–489. doi: 10.1016/S1470-2045(13)70096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shum H, Rajdev L. Multimodality management of resectable gastric cancer: A review. World J Gastrointest Oncol. 2014;6:393–402. doi: 10.4251/wjgo.v6.i10.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao S, Starling N, Cunningham D, Sumpter K, Gilligan D, Ruhstaller T, Valladares-Ayerbes M, Wilke H, Archer C, Kurek R, et al. Matuzumab plus epirubicin, cisplatin and capecitabine (ECX) compared with epirubicin, cisplatin and capecitabine alone as first-line treatment in patients with advanced oesophago-gastric cancer: a randomised, multicentre open-label phase II study. Ann Oncol. 2010;21:2213–2219. doi: 10.1093/annonc/mdq247. [DOI] [PubMed] [Google Scholar]
- 26.Satoh T, Lee KH, Rha SY, Sasaki Y, Park SH, Komatsu Y, Yasui H, Kim TY, Yamaguchi K, Fuse N, et al. Randomized phase II trial of nimotuzumab plus irinotecan versus irinotecan alone as second-line therapy for patients with advanced gastric cancer. Gastric Cancer. 2015;18:824–832. doi: 10.1007/s10120-014-0420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dragovich T, Campen C. Anti-EGFR-Targeted Therapy for Esophageal and Gastric Cancers: An Evolving Concept. J Oncol. 2009;2009:804108. doi: 10.1155/2009/804108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wainberg ZA, Lin LS, DiCarlo B, Dao KM, Patel R, Park DJ, Wang HJ, Elashoff R, Ryba N, Hecht JR. Phase II trial of modified FOLFOX6 and erlotinib in patients with metastatic or advanced adenocarcinoma of the oesophagus and gastro-oesophageal junction. Br J Cancer. 2011;105:760–765. doi: 10.1038/bjc.2011.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chua TC, Merrett ND. Clinicopathologic factors associated with HER2-positive gastric cancer and its impact on survival outcomes--a systematic review. Int J Cancer. 2012;130:2845–2856. doi: 10.1002/ijc.26292. [DOI] [PubMed] [Google Scholar]
- 30.Morishita A, Gong J, Masaki T. Targeting receptor tyrosine kinases in gastric cancer. World J Gastroenterol. 2014;20:4536–4545. doi: 10.3748/wjg.v20.i16.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Won E, Janjigian YJ, Ilson DH. HER2 directed therapy for gastric/esophageal cancers. Curr Treat Options Oncol. 2014;15:395–404. doi: 10.1007/s11864-014-0292-6. [DOI] [PubMed] [Google Scholar]
- 32.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 33.Barok M, Joensuu H, Isola J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Res. 2014;16:209. doi: 10.1186/bcr3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hecht JR, Bang YJ, Qin S, Chung HC, Xu JM, Park JO, Jeziorski K, Shparyk Y, Hoff PM, Sobrero AF, et al. Lapatinib in combination with capecitabine plus oxaliplatin (CapeOx) in HER2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma (AC): The TRIO-013/LOGiC Trial. J Clin Oncol. 2013;31:LBA4001. doi: 10.1200/JCO.2015.62.6598. [DOI] [PubMed] [Google Scholar]
- 35.Bang YJ. A randomized, open-label, phase III study of lapatinib in combination with weekly paclitaxel versus weekly paclitaxel alone in the second-line treatment of HER2 amplified advanced gastric cancer (AGC) in Asian population: Tytan study. J Clin Oncol. 2013;31:11. doi: 10.1200/JCO.2013.53.6136. [DOI] [PubMed] [Google Scholar]
- 36.O’Neil BH, McCarthy T. Angiogenesis inhibitors in gastric cancer. Orphan Drugs: Research and Reviews. 2014;4:55–61. [Google Scholar]
- 37.Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968–3976. doi: 10.1200/JCO.2011.36.2236. [DOI] [PubMed] [Google Scholar]
- 38.Van Cutsem E, de Haas S, Kang YK, Ohtsu A, Tebbutt NC, Ming Xu J, Peng Yong W, Langer B, Delmar P, Scherer SJ, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol. 2012;30:2119–2127. doi: 10.1200/JCO.2011.39.9824. [DOI] [PubMed] [Google Scholar]
- 39.Shen L, Li J, Xu J, Pan H, Dai G, Qin S, Wang L, Wang J, Yang Z, Shu Y, et al. Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: randomized, double-blind, phase III study (AVATAR study) Gastric Cancer. 2015;18:168–176. doi: 10.1007/s10120-014-0351-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fontanella C, Ongaro E, Bolzonello S, Guardascione M, Fasola G, Aprile G. Clinical advances in the development of novel VEGFR2 inhibitors. Ann Transl Med. 2014;2:123. doi: 10.3978/j.issn.2305-5839.2014.08.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry DR, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 42.Ford HE, Marshall A, Bridgewater JA, Janowitz T, Coxon FY, Wadsley J, Mansoor W, Fyfe D, Madhusudan S, Middleton GW, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 2014;15:78–86. doi: 10.1016/S1470-2045(13)70549-7. [DOI] [PubMed] [Google Scholar]
- 43.Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 44.Geng R, Li J. Apatinib for the treatment of gastric cancer. Expert Opin Pharmacother. 2015;16:117–122. doi: 10.1517/14656566.2015.981526. [DOI] [PubMed] [Google Scholar]
- 45.Li J, Qin S, Xu J, Guo W, Xiong J, Bai Y, Sun G, Yang Y, Wang L, Xu N, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol. 2013;31:3219–3225. doi: 10.1200/JCO.2013.48.8585. [DOI] [PubMed] [Google Scholar]
- 46.Qin S. Phase III study of apatinib in advanced gastric cancer: A randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2014;32:5. [Google Scholar]
- 47.Bang YJ, Kang YK, Kang WK, Boku N, Chung HC, Chen JS, Doi T, Sun Y, Shen L, Qin S, et al. Phase II study of sunitinib as second-line treatment for advanced gastric cancer. Invest New Drugs. 2011;29:1449–1458. doi: 10.1007/s10637-010-9438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yi JH, Lee J, Lee J, Park SH, Park JO, Yim DS, Park YS, Lim HY, Kang WK. Randomised phase II trial of docetaxel and sunitinib in patients with metastatic gastric cancer who were previously treated with fluoropyrimidine and platinum. Br J Cancer. 2012;106:1469–1474. doi: 10.1038/bjc.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun W, Powell M, O’Dwyer PJ, Catalano P, Ansari RH, Benson AB. Phase II study of sorafenib in combination with docetaxel and cisplatin in the treatment of metastatic or advanced gastric and gastroesophageal junction adenocarcinoma: ECOG 5203. J Clin Oncol. 2010;28:2947–2951. doi: 10.1200/JCO.2009.27.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 51.Doi T, Muro K, Boku N, Yamada Y, Nishina T, Takiuchi H, Komatsu Y, Hamamoto Y, Ohno N, Fujita Y, et al. Multicenter phase II study of everolimus in patients with previously treated metastatic gastric cancer. J Clin Oncol. 2010;28:1904–1910. doi: 10.1200/JCO.2009.26.2923. [DOI] [PubMed] [Google Scholar]
- 52.Ohtsu A, Ajani JA, Bai YX, Bang YJ, Chung HC, Pan HM, Sahmoud T, Shen L, Yeh KH, Chin K, et al. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol. 2013;31:3935–3943. doi: 10.1200/JCO.2012.48.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hack SP, Bruey JM, Koeppen H. HGF/MET-directed therapeutics in gastroesophageal cancer: a review of clinical and biomarker development. Oncotarget. 2014;5:2866–2880. doi: 10.18632/oncotarget.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sotoudeh K, Hashemi F, Madjd Z, Sadeghipour A, Molanaei S, Kalantary E. The clinicopathologic association of c-MET overexpression in Iranian gastric carcinomas; an immunohistochemical study of tissue microarrays. Diagn Pathol. 2012;7:57. doi: 10.1186/1746-1596-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iveson T, Donehower RC, Davidenko I, Tjulandin S, Deptala A, Harrison M, Nirni S, Lakshmaiah K, Thomas A, Jiang Y, et al. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: an open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. Lancet Oncol. 2014;15:1007–1018. doi: 10.1016/S1470-2045(14)70023-3. [DOI] [PubMed] [Google Scholar]
- 56.Merchant M, Ma X, Maun HR, Zheng Z, Peng J, Romero M, Huang A, Yang NY, Nishimura M, Greve J, et al. Monovalent antibody design and mechanism of action of onartuzumab, a MET antagonist with anti-tumor activity as a therapeutic agent. Proc Natl Acad Sci USA. 2013;110:E2987–E2996. doi: 10.1073/pnas.1302725110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shah MA, Cho JY, Huat ITB, Tebbutt NC, Yen CJ, Kang A, Shames DS, Bu L, Kang YK. Randomized phase II study of FOLFOX /- MET inhibitor, onartuzumab (O), in advanced gastroesophageal adenocarcinoma (GEC) J Clin Oncol. 2015;33:2. doi: 10.1634/theoncologist.2016-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cunningham D, Bang YJ, Tabernero J, Shah MA, Lordick F, Hack SP. MetGastric: A randomized phase III study of onartuzumab (MetMAb) in combination with mFOLFOX6 in patients with metastatic HER2-negative and MET-positive adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2015;33:TPS4155. [Google Scholar]
- 59.Momtaz P, Postow MA. Immunologic checkpoints in cancer therapy: focus on the programmed death-1 (PD-1) receptor pathway. Pharmgenomics Pers Med. 2014;7:357–365. doi: 10.2147/PGPM.S53163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim JW, Eder JP. Prospects for targeting PD-1 and PD-L1 in various tumor types. Oncology (Williston Park) 2014;28 Suppl 3:15–28. [PubMed] [Google Scholar]
- 61.Dolan DE, Gupta S. PD-1 pathway inhibitors: changing the landscape of cancer immunotherapy. Cancer Control. 2014;21:231–237. doi: 10.1177/107327481402100308. [DOI] [PubMed] [Google Scholar]
- 62.Muro K, Bang Y, Shankaran V, Geva R, Catenacci DVT, Gupta S, Eder JP, Berger R, Gonzalez EJ, Pulini J, et al. LBA15 - A phase 1b study of pembrolizumab (Pembro; MK-3475) in patients (Pts) with advanced gastric cancer. Ann Oncol. 2014;25:1–41. [Google Scholar]
- 63.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28:1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gulley ML. Genomic assays for Epstein-Barr virus-positive gastric adenocarcinoma. Exp Mol Med. 2015;47:e134. doi: 10.1038/emm.2014.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther. 2015;14:847–856. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 67.Xiao Y, Freeman GJ. The microsatellite instable subset of colorectal cancer is a particularly good candidate for checkpoint blockade immunotherapy. Cancer Discov. 2015;5:16–18. doi: 10.1158/2159-8290.CD-14-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43–51. doi: 10.1158/2159-8290.CD-14-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hirai H, Iwasawa Y, Okada M, Arai T, Nishibata T, Kobayashi M, Kimura T, Kaneko N, Ohtani J, Yamanaka K, et al. Small-molecule inhibition of Wee1 kinase by MK-1775 selectively sensitizes p53-deficient tumor cells to DNA-damaging agents. Mol Cancer Ther. 2009;8:2992–3000. doi: 10.1158/1535-7163.MCT-09-0463. [DOI] [PubMed] [Google Scholar]
- 70.Rajeshkumar NV, De Oliveira E, Ottenhof N, Watters J, Brooks D, Demuth T, Shumway SD, Mizuarai S, Hirai H, Maitra A, et al. MK-1775, a potent Wee1 inhibitor, synergizes with gemcitabine to achieve tumor regressions, selectively in p53-deficient pancreatic cancer xenografts. Clin Cancer Res. 2011;17:2799–2806. doi: 10.1158/1078-0432.CCR-10-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Madan B, Virshup DM. Targeting Wnts at the source--new mechanisms, new biomarkers, new drugs. Mol Cancer Ther. 2015;14:1087–1094. doi: 10.1158/1535-7163.MCT-14-1038. [DOI] [PubMed] [Google Scholar]


