Abstract
Colorectal cancer (CRC) is a major health problem in the Western world. The diagnostic process is a challenge in all health systems for many reasons: There are often no specific symptoms; lower abdominal symptoms are very common and mostly related to non-neoplastic diseases, not CRC; diagnosis of CRC is mainly based on colonoscopy, an invasive procedure; and the resource for diagnosis is usually scarce. Furthermore, the available predictive models for CRC are based on the evaluation of symptoms, and their diagnostic accuracy is limited. Moreover, diagnosis is a complex process involving a sequence of events related to the patient, the initial consulting physician and the health system. Understanding this process is the first step in identifying avoidable factors and reducing the effects of diagnostic delay on the prognosis of CRC. In this article, we describe the predictive value of symptoms for CRC detection. We summarize the available evidence concerning the diagnostic process, as well as the factors implicated in its delay and the methods proposed to reduce it. We describe the different prioritization criteria and predictive models for CRC detection, specifically addressing the two-week wait referral guideline from the National Institute of Clinical Excellence in terms of efficacy, efficiency and diagnostic accuracy. Finally, we collected information on the usefulness of biomarkers, specifically the faecal immunochemical test, as non-invasive diagnostic tests for CRC detection in symptomatic patients.
Keywords: Colorectal cancer, Colonoscopy, Primary health care, Faecal immunochemical test, Diagnostic yield, Diagnostic accuracy, Risk stratification, Open endoscopy unit, Practice guidelines, Health plan implementation
Core tip: In this review, we summarize the pitfalls in the diagnostic procedure for colorectal cancer (CRC) in symptomatic patients. We collected the available information concerning the value of symptoms as predictors of CRC and the factors involved in the delay of CRC diagnosis, including those related to the patient, to the physicians and to hospital delay. In this way, we review the currently available sets of appropriateness criteria for colonoscopy in symptomatic patients, the prioritization criteria and predictive models for CRC detection and, finally, the role of available biomarkers in the evaluation of symptomatic patients.
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer worldwide and the second leading cause of cancer-related death[1]. In Western Europe, it is the seventh leading cause of death, the fourth leading cause of loss of life expectancy, and it is associated with an elevated consumption of resources[2,3]. The stage of the tumour at the time of treatment is considered the most important predictor of survival. Thus, in Europe, survival is 93% after 3 years for Duke stage A tumours, but it is only 16% after 3 years for stage D tumours[4]. Two strategies are widely used to improve CRC prognosis and to optimize the health resources consumed: Population-based CRC screening programs and early diagnosis strategies in symptomatic patients[5-7]. Population-based screening programs in asymptomatic patients have been demonstrated to reduce the incidence and mortality rates of CRC in two ways: Removing preneoplastic lesions with polypectomy and diagnosing a higher proportion of CRCs at an early stage[8-10].
On the other hand, the early diagnosis of CRC in symptomatic patients remains a problem. It is a complex process that begins when the patient detects the first symptoms until a diagnostic procedure is performed, undergoing a consultation with a general practitioner, a referral to the specialist, and the waiting period for diagnostic procedures, such as colonoscopy. All this contributes to the perception that delay in CRC diagnosis is a multifactorial problem[11]. In the general population, lower abdominal symptoms are very common and are a frequent cause of visits to the general practitioner. The issue is that symptoms are usually very vague and non-specific, with a poor sensitivity for CRC. In most cases, these symptoms are produced by benign, self-limiting illness, contributing to the patient’s delay in seeking help and the practitioner’s delay in referring the patient to a specialist. Moreover, the growing demand for colonoscopy has become a significant problem, as endoscopic resources are limited; these waiting periods also delay the diagnosis of CRC. Computed tomography (CT) colonography could be an alternative, especially in elderly patients with poor specific symptoms such abdominal pain or weight loss[12]. However, the referral rate for additional tests after CT-colonography must be reduced to avoid the potential to increase anxiety and overall cost[13]. For these reasons, as colonoscopy is the gold standard for CRC investigation, several risk classification scores based on symptoms have been developed to determine which patients are most at risk of having CRC and thus to reduce the delay between the initial consultation and the colonoscopy[6,7,14-16].
The objective of this article was to review the pitfalls and missed opportunities in the process of CRC diagnosis in symptomatic patients. First, we evaluated the evidence concerning the value of symptoms as predictors of colorectal neoplasia. We showed the effect of delayed diagnosis on CRC prognosis as well as the factors related to this delay. This includes factors related to the patient, to the first attending physician (most likely in a primary setting), and, finally, to the hospital delay as a result of the waiting period before colonoscopy. We analysed the available sets of criteria for colonoscopy diagnosis in symptomatic patients, along with the prioritization criteria and the predictive scores for CRC diagnosis and their diagnostic yield for CRC. Finally, we explored the usefulness of the available biomarkers to determine the types of patients who can benefit the most from a colonoscopy.
VALUE OF SYMPTOMS
In the general population, abdominal symptoms account for up to 10% of consultations with general practitioners[17]. Most of these symptoms are related to chronic functional conditions (irritable bowel syndrome, chronic constipation) or anorectal benign lesions that do not benefit from colonoscopy evaluation[18,19]. In clinical practice, it is common to perform a colonoscopy in patients with bowel symptoms due to the suspicion of CRC[20]. In fact, many practice guidelines suggest that colonoscopy should be performed for bowel symptoms, but the importance and value of symptoms as indicators of CRC is not well established. While some reports suggest that symptoms may be useful in identifying CRC, others have found no such association[21-24]. Moreover, few of these studies are recent and the perception of symptoms may have changed since the early studies were conducted.
Recently, several meta-analyses have analysed the risk of detecting CRC according to the symptoms reported. Ford et al[22] performed a systematic review and meta-analysis to assess the diagnostic accuracy of alarm features in predicting CRC. They included fifteen studies evaluating 19443 patients, with a pooled 6% CRC prevalence. CRC diagnosis was based either on colonoscopy (8), sigmoidoscopy (1), double contrast barium enema (1), or both lower endoscopy or barium enema (5). They included 1 population-based study, 11 secondary healthcare level studies, 2 primary healthcare level studies and 1 mixed levels study. In summary, the pooled sensitivity of the symptoms was poor (5% to 64%), but specificity was 95% for dark red rectal bleeding and abdominal mass (Figure 1). It is remarkable that both positive and negative likelihood ratios (PLR and NLR) lie close to 1; thus, the presence or absence of symptoms does not significantly modify the probability of CRC detection.
Figure 1.
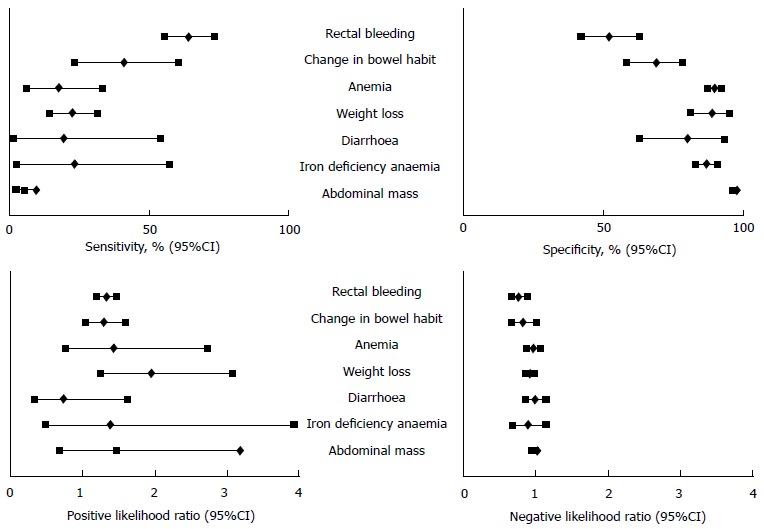
Diagnostic accuracy of symptoms for colorectal cancer detection. Adapted from Ford et al[22].The results are expressed as the median (%) and 95%CI.
Astin et al[23] performed an additional systematic review and meta-analysis to identify the risk of CRC in patients reporting a symptom to a primary care provider. They included 23 studies that recruited 81464 participants. They analysed both single and paired symptoms. Positive predictive values (PPVs) for rectal bleeding from 13 papers ranged from 2.2% to 16% with a pooled estimate of 8.1%, and PLR ranged between 1.09 and 10.13 with a pooled estimate of 5.31. Pooled PPV estimates for other symptoms were: Abdominal pain (three studies), 3.3%; and anaemia (four studies), 9.7%. For rectal bleeding accompanied by weight loss or change in bowel habits, pooled PLRs were 1.9 and 1.8, respectively. Conversely, the PLR was one or less for abdominal pain, diarrhoea, or constipation accompanying rectal bleeding. The authors concluded that the investigation of rectal bleeding or anaemia in primary care patients is warranted, irrespective of whether other symptoms are present.
Additionally, Jellema et al[24] performed a meta-analysis to summarize the available evidence concerning diagnostic tests that might help primary care physicians to identify patients with an increased risk for CRC among those consulting for non-acute lower abdominal symptoms. The tests evaluated included signs, symptoms, referral criteria, blood and faecal tests. With respect to symptoms (Table 1), sensitivity ranged between 13% and 51% and specificity ranged between 59% and 89%. As a result, the risk of detecting a CRC was not modified significantly between those patients with symptoms (PPV ranging between 6% and 14%) and those without any of the symptoms evaluated (1- negative predictive value, NPV, ranging between 3% and 10%). In contrast, the variable age (> 50 years) was more sensitive than any of the symptoms (91%), although the specificity was lower (36%), significantly modifying the risk of CRC detection between patients older and younger than 50 years (10%, 2%).
Table 1.
Summary of findings (sensitivity, specificity, predictive values) for diagnostic tests for colorectal cancer detection evaluated by at least four primary diagnostic studies
| Index test | Sensitivity | Specificity | PPV | 1-NPV |
| Age (> 50) | 91% | 36% | 10% | 2% |
| Sex (male) | 62% | 55% | 13% | 3% |
| Family history | 16% | 91% | 6% | 4% |
| Weight loss | 20% | 89% | 9% | 6% |
| Abdominal pain | 35% | 59% | 5% | 7% |
| Rectal bleeding | 44% | 66% | 7% | 4% |
| All bleeding, dark blood | 35% | 85% | 14% | 5% |
| All bleeding, mixed with stool | 51% | 71% | 6% | 3% |
| Change in bowel habits | 52% | 61% | 9% | 4% |
| Diarrhoea present | 20% | 73% | 6% | 10% |
| Constipation | 13% | 72% | 6% | 9% |
| Two week rule positive | 92% | 42% | 14% | 3% |
| Iron deficiency anaemia | 13% | 92% | 13% | 8% |
| Faecal occult blood test positive | ||||
| Chemical | 75% | 86% | 28% | 1% |
| Immunological | 95% | 84% | 21% | 0% |
The results are expressed as medians or pooled estimates. Adapted from Jellema et al[24]. PPV: Positive predictive value; NPV: Negative predictive value.
Therefore, the value of symptoms for CRC detection is very poor. Symptoms alone are not adequate to establish a suspicion of CRC and they must be synthesized with other variables, such as demographic variables and analytical data.
DELAY IN CRC DIAGNOSIS
The period of time from the first symptoms until a final diagnosis is achieved can vary. In a recently published study, the median interval between symptoms and diagnosis was 128 d with a wide interquartile range (57.5-257.5). This interval was due to the delay from the first symptom until the initial consultation (19 d, interquartile range 3-83) and the delay in health service (66 d, interquartile range 25-159) (Figure 2)[25]. There is a controversy regarding the association between diagnostic and therapeutic delay and the prognosis of CRC. In fact, there seems to be a lack of association between diagnostic delay, CRC survival and stage[26], suggesting that, in CRC, the symptomatic phase is only a small component of the natural history of the disease. When colon and rectal cancer are analysed separately, an opposite association exists. For the colon, a greater delay is associated with an earlier stage at diagnosis, and for the rectum, a smaller delay is associated with an earlier stage[26]. This could be explained because rectal cancer has well-defined symptoms, such as rectal bleeding with or without changes in bowel habits, while colon cancer-related symptoms are very vague at the onset, and when the seriousness of symptoms require investigation, the disease is more advanced[27].
Figure 2.
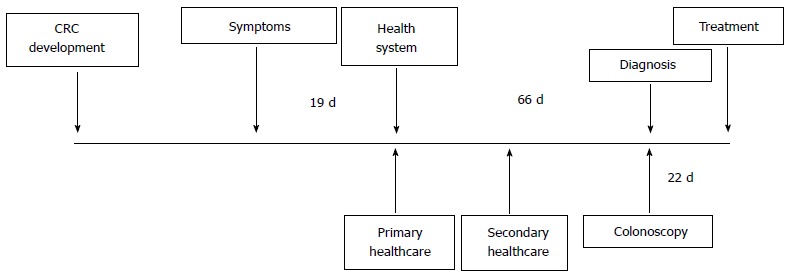
Distribution of delay intervals in colorectal cancer diagnosis (in days). Adapted from Esteva et al[25]. CRC: Colorectal cancer.
Factors related to patient delay
As previously mentioned, lower abdominal symptoms are very common and mostly due to benign, self-limited conditions. Moreover, most are very vague and patients do not relate them to a serious illness. In the complexity of the process of cancer diagnosis, Andersen’s Model of Total Patient Delay[28,29], a theoretical framework, defines five time intervals in the decision-making process: Appraisal delay (time between the detection of symptoms and inferring illness); illness delay (period when the patient contemplates between consulting a medical practitioner or self-treating the illness); behavioural delay [delay in making an appointment with the general practitioner (GP)], scheduling delay (time elapsed between making an appointment and the first medical consultation) and treatment delay (until the initiation of treatment). Factors related to the patient are encompassed in the first four time intervals.
Many studies have focused on determining the causes that lead to a delay in seeking medical help once the patient notices the first symptoms, including factors that would increase the delay and others that would reduce it. These factors are listed in Table 2. Most of the studies show that the main factors associated with patient delay are the lack of knowledge and concern about potential risks associated with the symptoms as well as non-recognition of the seriousness of the symptoms[30,31], suggesting that appraisal delay is the main contributor to the global delay related to the patient[28]. This situation entails a misinterpretation of symptoms, attributing them to a benign disease or assuming that they are part of the ageing process. In this way, non-recognition of the seriousness of symptoms will also lead to self-diagnosis, “wait and see” strategies and self-treatment.
Table 2.
Main factors associated with patient delay
| Increases delay | Reduces delay |
| Appraisal delay | |
| Symptoms attributed to minor illness Lack of knowledge or failed to recognize symptom severity Assumed to be part of the ageing process Non-specific symptoms (altered bowel habits, unexplained weight loss) Self-treatment | Specific symptoms (rectal bleeding, abdominal pain) Symptoms frequent, severe or affect the person’s daily life Pain, vomiting and intestinal obstruction as initial symptoms |
| Illness delay | |
| Younger patients Low socioeconomic status Lower educational level Rural areas Lack of additive private health insurance Family history of cancer | Comorbidity High educational level Retirement |
| Behavioural delay | |
| Fear of pain Fear of cancer Fear of unpleasant or embarrassing investigations Denial of symptoms | Social support Disclosure of symptoms to someone close Knowing a person with CRC |
| Scheduling delay | |
| Too busy to visit Unpleasant or embarrassing visit | Trust in GP |
CRC: Colorectal cancer; GP: General practitioner.
Other important factors described in studies are those related to denial and fear of symptoms[32]. They include fear and denial of cancer, fear of poor prognosis, or fear of embarrassing and unpleasant investigations, which are all related to a lack of adequate information. With respect to the symptoms, patients who suffer from persistent or more serious symptoms affecting the person’s daily life, such as pain, vomiting or obstruction, delayed seeking treatment less often. In contrast, more common symptoms, such as changes in bowel habits, rectal bleeding, or nonspecific symptoms were associated with more prolonged delays. A recent study that examined medical-advice-seeking behaviours showed that one in five persons experiencing rectal bleeding or changes in bowel habits did not seek medical advice. Moreover, among those seeking help for rectal bleeding or changes in bowel habits, up to 18% and 37%, respectively, delayed seeking treatment by more than 1 mo[33]. There is no clear evidence of the way in which factors such as age, gender, marital status, socioeconomic status and education level impact delay. Some studies have shown that males and younger people tend to delay more often. Additionally, low socioeconomic status and low educational level seem to be associated, but results are not consistent. In contrast, social support and a trustful relationship with the general practitioner are strongly associated with less delay[25,34]. Finally, increased knowledge about CRC improves timely help-seeking for symptoms, reducing negative perceptions[35].
In sum, the main factors related to patient delay are caused by the lack of knowledge about symptoms, the importance and implications of CRC diagnosis at an early stage, and the diagnostic tools available. Therefore, an effort to educate the general population about CRC is warranted and may help to reduce delay.
Factors related to practitioner delay
One of the steps in the complex process of CRC diagnosis involves the physician that first sees the patient, usually the general practitioner. He must suspect that the symptoms are due to CRC and refer the patient for further investigations. Many factors are associated with practitioner delay (Table 3). Mitchell et al[30] performed a systematic review including twenty-nine papers that considered factors that influenced practitioner delay. He described two main factors associated with an increase in delay, as both were considered to be factors in most of the studies included (≥ 75%)[30]. The first was initial misdiagnosis, either through prescribing symptomatic treatment or attributing symptoms to other benign conditions. In fact, missed opportunities to diagnose CRC before endoscopic referral occur in 31%-34% of patients presenting with symptoms, entailing an average delay from the first visit > 200 d. Among those patients, there was a mean of 2.41-4.2 missed opportunities. Those patients tended to be older and with more co-morbidities, including congestive heart failure or coronary artery disease. The main diagnostic key was iron- deficiency anaemia, which was associated with the longer delay to referral (> 300 d)[36,37].
Table 3.
Main factors associated with practitioner delay
| Increases delay | Reduces delay |
| Lack of continuity of care Frequent attendance Patient’s socioeconomic status (lower) Initial misdiagnosis Failure to examine or investigate Inaccurate or inadequate tests Co-morbidities Elderly patients Psychiatric co-morbidities | Site (rectum) Experience Use of referral guidelines Suspected CRC diagnosis in the referral Urgent referral to hospital |
CRC: Colorectal cancer.
The second main factor was failure to examine or investigate. Studies showed a frequent lack of physical examination among patients with lower abdominal symptoms, especially digital rectal examination[30]. In two recent studies, only 25% of patients with rectal carcinoma had a digital rectal examination at their first visit[11], and GPs only performed a physical examination of one in three patients[25]. These results are in accordance with previous studies that showed no improvement over time[38,39].
The available results on the effect of age and comorbidities on delay are conflicting. Although previous studies have noted that elderly patients and those with co-morbidities are referred earlier[30], recent studies suggest the opposite, with more missed opportunities and more delay[36,37,40]. Moreover, psychiatric diseases are also associated with referral delay by the GP[40,41]. Regarding the consultation pattern, a greater interval to diagnosis was observed for patients with an increasing number of visits to the GP due to symptoms related to CRC and those lacking continuity of care[25,42]. Inaccurate or inadequate tests and a negative or a false negative test result increased the delay time[30].
Another important aspect is how the physician performs the request or referral. When the referral is urgent, includes three diagnostic clues, mentions the suspicion of CRC or contains documentation of verbal contact, the delay decreases[25,43]. Furthermore, the use of referral guidelines and the appropriate use of urgent referrals seems to reduce delay[44,45]. Studies have shown that strategies based on training primary care physicians to evaluate patients with digestive symptoms and allowing them open access to endoscopy units reduces waiting time and increases the diagnostic yield[19,46]. Educational programs also increase the appropriateness rate of referrals[47]. Furthermore, a Cochrane review concluded that active local training involving secondary health care specialists and structured referral applications are the only interventions that have an impact on outpatient referral rates[48].
Therefore, it seems important to improve educational programs to reduce initial misdiagnosis. CRC is usually first detected at primary healthcare settings, but each GP diagnoses very few CRC patients each year[49]. Additionally, it is mandatory to generalize digital rectal examinations in patients with lower abdominal symptoms as well as to use referral guidelines and open access endoscopy units to increase the appropriateness of referrals and thus reduce delays.
Factors related to hospital delay: Waiting lists and prioritization
The evaluation of symptoms is one of the most important reasons to perform a colonoscopy, ranging between 38.8% and 57.3% of all referrals for colonoscopy[50-53]. However, most of the colonoscopies performed in symptomatic patients are normal or do not yield changes in the therapeutic approach, so the benefit to most of the patients is scarce[18,23,24,54,55]. This, added to the growing demand of colonoscopy requests related to screening programs, establish the need for prioritization criteria and objective tools with the aim of reducing delay in patients with a high suspicion of CRC, preventing them from being affected by waiting lists. The appropriateness criteria for colonoscopy indications proposed by the American Society for Gastrointestinal Endoscopy[56] and the European Panel on the Appropriateness of Gastrointestinal Endoscopy (EPAGE)[57-63] have shown its limited value as a diagnostic tool in symptomatic patients. Although appropriateness is associated with a high sensitivity for CRC and a fair sensitivity for relevant findings, its specificity and positive predictive values are poor. This is related to their high positivity rate (70%-81.4%). In fact, the rate of appropriateness in colonoscopies due to symptom evaluation ranged between 73% and 95.1%, limiting its use in this scenario. In the next section, we will focus on two of the most promising strategies to reduce diagnostic delay due to waiting time for colonoscopy: Prioritization criteria or predictive indexes and diagnostic biomarkers.
PRIORITIZATION CRITERIA AND CRC PREDICTIVE INDEXES
Strategies for the early diagnosis of CRC in symptomatic patients may improve prognosis[64,65]. In this regard, several risk classification scores based on symptoms have been developed. These classification criteria are intended to determine which patients are most at risk of having CRC, and thus to reduce the delay between the initial consultation in primary care settings and the colonoscopy[6,7,14,15]. The two-week wait (TWW) referral guideline was introduced by the National Health System (NHS)[66] and updated to its most recent version in 2011 (Table 4) by the National Institute of Clinical Excellence (NICE)[7]. It has been the most widely used and evaluated diagnostic criteria. Some other referral guidelines have recently been proposed and validated[67,68]. Moreover, several CRC predictive indexes based on clinical symptoms have been proposed[14,15,69-71].
Table 4.
National Institute for Health and care excellence referral criteria[7]
| High risk referral criteria (any) |
| Patients ≥ 40 yr with rectal bleeding and a change of bowel habits persisting ≥ 6 wk |
| Patients ≥ 60 yr with rectal bleeding persisting ≥ 6 wk without a change in bowel habits and without anal symptoms |
| Patients ≥ 60 yr with a change in bowel habits persisting ≥ 6 wk without rectal bleeding |
| Patients presenting with a right lower abdominal mass consistent with involvement of the large bowel |
| Patients presenting with a palpable rectal mass |
| Patients with unexplained iron deficiency anaemia (< 11 g/100 mL in men, < 10 g/100 mL in non-menstruating women) |
The TWW emerged in 2000 in response to the low survival rate at 5 years for CRC in Britain compared to other European countries with similar economic resources. The NHS established a prioritization system based on signs and symptoms associated with a high probability of detecting a CRC. Those patients who met any of these criteria should be assessed within 14 d of their referral. The NHS expected that up to 90% of incident CRC would be diagnosed through the TWW. It has been widely implemented across the NHS. Several articles have been published evaluating the efficacy, efficiency and diagnostic accuracy of the TWW[24,54,72-77]. The TWW was implemented in most NHS centres; however, compliance with the guidelines has been poor. This, coupled with the poor specificity of the system, has resulted in a poor cancer detection rate and a steadily growing volume of hospital referrals. The system has been shown to have an adverse impact on the waiting times for routine colorectal referrals[73]. In fact, only 24% of incident CRC cases were diagnosed through the TWW, and no evidence was found that CRC was diagnosed at an earlier stage[75]. Jellema et al[24] evaluated the diagnostic accuracy of the TWW. The sensitivity and specificity for CRC detection was 92% and 42% with a 14% PPV and a 3% NPV[24]. In sum, it is a diagnostic tool with low specificity and variable sensitivity and its use is subjected to local circumstances[24,54,72-77].
Two additional sets of prioritization criteria have been recently evaluated. The Scottish Intercollegiate Guidelines Network (SIGN) referral criteria are based on modifications of the TWW[6]. In a recently published article that aimed to compare the faecal immunochemical test (FIT) with the TWW and the SIGN referral criteria, the SIGN referral criteria produced a greater number of referrals (60.1% vs 38.1%) and increased the sensitivity for CRC detection (82.5% vs 61.9%), but the specificity was inferior when compared with the TWW (42.7% vs 65.2%)[54].
Additionally, the Galician Health Service in Spain established indications and priority levels (I = fast track, II = preferential, III = normal) for colonoscopy according to the risk of CRC and significant colonic lesion detection in primary health care settings. These criteria consisted of symptoms, imaging abnormalities and analytical data. Therefore, patients with any of the following situations were stratified to priority level I: Palpable right lower abdominal mass; palpable rectal mass; or unexplained iron deficiency anaemia (< 11 g/100 mL in men, < 10 g/100 mL in non-menstruating women). Patients with the following criteria were excluded: NSAID consumption; suspected CRC in imaging studies; rectal bleeding and a change in bowel habits (> 6 wk); patients ≥ 50 years with a change in bowel habits (preferably more frequent stools), persisting ≥ 6 wk without rectal bleeding and patients ≥ 50 years with rectal bleeding persisting ≥ 3 wk without anal symptoms. If the patient met any of the following conditions, they were stratified to priority level II: Faecal haemoglobin concentration > 20 mg/mL or equivalent in the absence of rectal bleeding; high suspicion of inflammatory bowel disease in imaging studies (ultrasound or abdominal CT scan); chronic diarrhoea (> 4 wk evolution), with clinical and laboratory evidence of an inflammatory process after ruling out infectious causes; unexplained iron deficiency anaemia (> 11 g/100 mL in men, > 10 g/100 mL in non-menstruating women); patients < 50 years with persistent rectal bleeding with a negative digital rectal examination < 50 years, with anuscopy/rectoscopy that does not justify the symptoms and, finally, persistent rectal bleeding after medical treatment (2-4 wk) of a benign anal lesion. Finally, priority level III consisted of referrals to colonoscopy that did not meet any of the previous conditions but were adequate according to EPAGE II criteria. These indications and priority levels were evaluated in symptomatic patients after the implementation of the criteria. They were significantly associated with CRC (I = 20.1%, II = 19.1%, III = 4.8%; P < 0.001) and significant colonic lesion (I = 35.3%, II = 34%, III = 19%; P = 0.002) detection rates. Additionally, the diagnostic yield for CRC (OR = 2.41; 95%CI: 1.31-4.42) and detection of significant colonic lesions (OR = 1.88; 95%CI: 1.13-3.15) increased when colonoscopies were referred directly from primary care providers[68].
Several studies have been performed to develop predictive indexes for CRC detection in recent years. The aim was to establish objective criteria that are more accurate for CRC and to detect relevant findings, thus reducing the number of referrals to colonoscopy. Selvachandran et al[14] developed one of the first predictive systems: The Weighted Numerical Score (WNS). The WNS is derived from the weighting of primary symptoms and symptom complexes and is automatically derived from a patient consultation questionnaire linked to a computerized record[14]. In the validation study, the sensitivity of the WNS for CRC at a 40-point threshold reached 99%. In addition to having similar cancer detection rates as the TWW system, the specificity of the WNS cut-off of 70 was significantly better than that of the TWW system (82.7% vs 66.1%; P < 0.001)[78]. Thus, the WNS was subsequently validated, both internally and externally, showing similar detection rates with greater specificity. Unfortunately, it has only been validated for the detection of distal tumours and requires licensed software.
Adelstein et al[15] published a predictive model based on symptoms collected using a validated questionnaire, demographic variables and medical history. On the basis of a range of symptoms (anaemia, rectal bleeding, abdominal pain and mucus passage to the rectum), age, sex, colonoscopy in the past 10 years, use of nonsteroidal anti-inflammatory drugs or aspirin, and history of irritable bowel syndrome, they obtained a predictive model with an area under the curve (AUC) of 0.83 for CRC detection[15]. The Cancer Prediction in Exeter (CAPER) and the Bristol-Birmingham (BB) equation are two additional CRC scoring systems[70,71]. The CAPER score is derived from a primary care case-control study and the BB equation from a large primary care dataset. Their discrimination characteristics were investigated in two datasets (BB and CAPER dataset) and its diagnostic accuracy for CRC detection was compared with the TWW guideline. Both multivariable symptom scoring systems performed significantly better than NICE referral guidelines: AUC of the BB equation: 0.83 (95%CI: 0.82-0.84) and 0.92 (95%CI: 0.91-0.94), respectively; AUC of the CAPER score: 0.79 (95%CI: 0.79-0.80) and 0.91 (95%CI: 0.89-0.93), respectively; and AUC of the TWW rule: 0.65 (95%CI: 0.64-0.66) and 0.75 (95%CI: 0.72-0.79), respectively[70].
Therefore, prioritization criteria based on symptoms and signs seem to have poor diagnostic accuracy for CRC, while predictive indexes that add demographic variables and/or analytical data worked better. This highlights the need to develop more objective tools to reduce CRC delay due to waiting lists.
BIOMARKERS
Currently, there are several biomarkers available for the evaluation of symptomatic patients. They include blood and faecal tests, such as serum and faecal haemoglobin (FOBT), serum carcinoembryonic antigen and faecal calprotectin.
Although serum haemoglobin is not a biomarker, its association with the risk of CRC detection and other colorectal diseases is clearly described. As shown previously (Table 1 and Figure 1), iron deficiency anaemia is highly specific for CRC detection (92%), although it lacks sensitivity[22-24]. Other available serum biomarkers, such as carcinoembryonic antigen (CEA), have been evaluated. However, lack of specificity and sensitivity preclude the use of all existing serum markers for the early detection of CRC. CEA determination is limited to surveillance after CRC resection with a curative intent[79].
Faecal calprotectin has recently emerged as a candidate biomarker for intestinal inflammation with a potential clinical application as a diagnostic adjunct in IBD and other pathologies of the gastrointestinal tract[55,80-82]. Calprotectin levels have been found to be significantly elevated in patients with inflammatory and neoplastic conditions[80]. Despite this, the meta-analysis performed by von Roon et al[80], which included 7 studies with 2661 patients to evaluate CRC detection, did not show significant differences among patients with CRC and controls. Patients with colorectal neoplasia had non-significantly higher calprotectin levels (132.2 μg/g higher) compared with non-cancer controls (P = 0.18). The sensitivity and specificity of calprotectin for the diagnosis of CRC were 36% and 71%, respectively, with an AUC of 0.66.
Multiple studies have demonstrated that CRC screening with chemical FOBT in average-risk populations significantly reduces CRC mortality[83]. To date, no data are available regarding the effect of FIT on CRC mortality or incidence. However, several studies on diagnostic tests have compared chemical FOBT and FIT for the detection of CRC and advanced adenomas. These studies have shown that FIT is more sensitive and specific for the detection of CRC and advanced adenomas and is a cost-effective screening test[84]. Current CRC screening programs are based mainly on FIT. In contrast, the information available on the evaluation of symptomatic patients is scarce. In the meta-analysis published by Jellema et al[24], FIT had a 95% sensitivity and a 84% specificity for CRC detection with a 21% PPV and a 100% NPV (Table 1). However, the studies included in this meta-analysis mixed asymptomatic and symptomatic patients and were performed in secondary care settings. However, the authors concluded that FIT showed good diagnostic performance for CRC.
Four additional studies have recently evaluated the diagnostic accuracy of FIT for CRC detection in symptomatic patients[54,85-87]. In these studies, FIT at different thresholds (10 ng/mL and 20 ng/mL) had an adequate diagnostic accuracy for CRC detection. The ranges of sensitivity, specificity, PPV and NPV were 74.7%-100%, 77.4%-93.9%, 7.6%-53% and 97.8%-100%, respectively. Moreover, in our recently published article, we compared FIT (20 ng/mL cut-off point) with the NICE criteria[7]. Among 787 patients referred for colonoscopy, we detected 97 cases of CRC. FIT had a higher sensitivity (87.6%, 61.9%; P < 0.001) and specificity (77.4%, 42.7%; P < 0.001) for CRC detection than the NICE criteria. Moreover, while the NICE referral criteria was modified according to the CRC location (rectum 76.7%, distal colon 61.4%, proximal colon 43.5%; P = 0.01), FIT sensitivity was not modified by its location (rectum 90%, distal 75%, proximal 87%; P = 0.2)[54]. Finally, McDonald et al[85] also evaluated the diagnostic accuracy of FIT for the detection of significant colonic lesions (CRC, advanced adenoma, IBD) in symptomatic patients. They also exhibited good results (sensitivity, 57%; specificity, 99%; PPV, 62% and NPV, 81.6%). These results are concordant with the results obtained in our series (not published). We found that the sensitivity and specificity of FIT for the detection of significant colonic lesions were 60.2% and 82.4%, respectively, and PPV and NPV were 60.2% and 82.4%, respectively.
In summary, biomarkers appear to be a promising tool for the prioritization of CRC in symptomatic patients. Currently, FIT has demonstrated its accuracy as a prioritization tool alone, and its use should be increased. In the coming years, we should see the emergence of new biomarkers.
CONCLUSION
In conclusion, the value of symptoms as predictors of CRC or relevant colonic findings is poor. In the complexity of the cancer diagnosis, delays can occur in the different phases from the appearance of symptoms until final diagnosis (patient-related, physician-related and hospital-related factors). Understanding the factors that produce the delay is the first step to improving the diagnostic process and reducing the time interval from the first symptoms until diagnosis, improving CRC prognosis. The appropriateness criteria for colonoscopy can be a basis to control the quality of referrals, identifying unnecessary tests, but its value as a diagnostic tool is limited, especially in symptomatic patients. Several prioritization criteria and predictive indexes have been developed. All of them have insufficient sensitivity for CRC detection, so CRC cannot be ruled out in those patients who do not meet these criteria. Moreover, these criteria and indexes are nonspecific and are based mainly on the subjective evaluation of symptoms, thus yielding unnecessary colonoscopies. Finally, the use of biomarkers in symptomatic patients is promising. Adding available biomarkers, especially FIT, to risk classification scores and predictive indexes may increase both the sensitivity and specificity of CRC detection, thus reducing the number of patients referred for colonoscopy to evaluate symptoms and increasing the diagnostic yield of colonoscopy in this setting.
Footnotes
Supported by A grant from Instituto de Salud Carlos III (PI11/00094).
Conflict-of-interest statement: None.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 17, 2015
First decision: July 1, 2015
Article in press: September 28, 2015
P- Reviewer: Milot L S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lansdorp-Vogelaar I, Knudsen AB, Brenner H. Cost-effectiveness of colorectal cancer screening. Epidemiol Rev. 2011;33:88–100. doi: 10.1093/epirev/mxr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciccolallo L, Capocaccia R, Coleman MP, Berrino F, Coebergh JW, Damhuis RA, Faivre J, Martinez-Garcia C, Møller H, Ponz de Leon M, et al. Survival differences between European and US patients with colorectal cancer: role of stage at diagnosis and surgery. Gut. 2005;54:268–273. doi: 10.1136/gut.2004.044214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zauber AG, Knudsen AB, Rutter CM, Lansdorp-Vogelaar I, Savarino JE, van Ballegooijen M, Kuntz KM. Cost-Effectiveness of CT Colonography to Screen for Colorectal Cancer. Technology Assessment Report Project ID: CTCC0608; 2009. pp. 1–92. [PubMed] [Google Scholar]
- 6.Scottish Intercollegiate Guidelines Network (SIGN) Diagnosis and management of colorectal cancer. Edinburgh: SIGN; 2011. (SIGN publication no. 126). [; 2011. p. Dec]. Available from: http://www.sign.ac.uk. [Google Scholar]
- 7. Available from: http: //www.nice.org.uk.
- 8.Garborg K, Holme Ø, Løberg M, Kalager M, Adami HO, Bretthauer M. Current status of screening for colorectal cancer. Ann Oncol. 2013;24:1963–1972. doi: 10.1093/annonc/mdt157. [DOI] [PubMed] [Google Scholar]
- 9.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotterchio M, Manno M, Klar N, McLaughlin J, Gallinger S. Colorectal screening is associated with reduced colorectal cancer risk: a case-control study within the population-based Ontario Familial Colorectal Cancer Registry. Cancer Causes Control. 2005;16:865–875. doi: 10.1007/s10552-005-2370-3. [DOI] [PubMed] [Google Scholar]
- 11.Langenbach MR, Schmidt J, Neumann J, Zirngibl H. Delay in treatment of colorectal cancer: multifactorial problem. World J Surg. 2003;27:304–308. doi: 10.1007/s00268-002-6678-9. [DOI] [PubMed] [Google Scholar]
- 12.Laghi A. Computed tomography colonography in 2014: an update on technique and indications. World J Gastroenterol. 2014;20:16858–16867. doi: 10.3748/wjg.v20.i45.16858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atkin W, Dadswell E, Wooldrage K, Kralj-Hans I, von Wagner C, Edwards R, Yao G, Kay C, Burling D, Faiz O, et al. Computed tomographic colonography versus colonoscopy for investigation of patients with symptoms suggestive of colorectal cancer (SIGGAR): a multicentre randomised trial. Lancet. 2013;381:1194–1202. doi: 10.1016/S0140-6736(12)62186-2. [DOI] [PubMed] [Google Scholar]
- 14.Selvachandran SN, Hodder RJ, Ballal MS, Jones P, Cade D. Prediction of colorectal cancer by a patient consultation questionnaire and scoring system: a prospective study. Lancet. 2002;360:278–283. doi: 10.1016/s0140-6736(02)09549-1. [DOI] [PubMed] [Google Scholar]
- 15.Adelstein BA, Macaskill P, Turner RM, Katelaris PH, Irwig L. The value of age and medical history for predicting colorectal cancer and adenomas in people referred for colonoscopy. BMC Gastroenterol. 2011;11:97. doi: 10.1186/1471-230X-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hippisley-Cox J, Coupland C. Identifying patients with suspected colorectal cancer in primary care: derivation and validation of an algorithm. Br J Gen Pract. 2012;62:e29–e37. doi: 10.3399/bjgp12X616346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones R. Primary care research and clinical practice: gastroenterology. Postgrad Med J. 2008;84:454–458. doi: 10.1136/pgmj.2008.068361. [DOI] [PubMed] [Google Scholar]
- 18.Dacal Rivas A, Quintas Lorenzo P, Francisco González M, Cubiella Fernández J, Alonso Docampo MN, Fernández Seara J. [Effect of the implementation of a program to improve referrals by primary care on appropriateness and wait times in endoscopic examinations] Gastroenterol Hepatol. 2011;34:254–261. doi: 10.1016/j.gastrohep.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Quintas Lorenzo P, Dacal Rivas A, González MF, Cubiella Fernández J, López Sánchez L, García García MJ, Fernández Seara J. Referrals to a gastroenterology outpatient clinic from primary care: evaluation of two programs. Gac Sanit. 2011;25:468–473. doi: 10.1016/j.gaceta.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Lieberman DA, Holub J, Eisen G, Kraemer D, Morris CD. Utilization of colonoscopy in the United States: results from a national consortium. Gastrointest Endosc. 2005;62:875–883. doi: 10.1016/j.gie.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 21.Adelstein BA, Irwig L, Macaskill P, Turner RM, Chan SF, Katelaris PH. Who needs colonoscopy to identify colorectal cancer? Bowel symptoms do not add substantially to age and other medical history. Aliment Pharmacol Ther. 2010;32:270–281. doi: 10.1111/j.1365-2036.2010.04344.x. [DOI] [PubMed] [Google Scholar]
- 22.Ford AC, Veldhuyzen van Zanten SJ, Rodgers CC, Talley NJ, Vakil NB, Moayyedi P. Diagnostic utility of alarm features for colorectal cancer: systematic review and meta-analysis. Gut. 2008;57:1545–1553. doi: 10.1136/gut.2008.159723. [DOI] [PubMed] [Google Scholar]
- 23.Astin M, Griffin T, Neal RD, Rose P, Hamilton W. The diagnostic value of symptoms for colorectal cancer in primary care: a systematic review. Br J Gen Pract. 2011;61:e231–e243. doi: 10.3399/bjgp11X572427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jellema P, van der Windt DA, Bruinvels DJ, Mallen CD, van Weyenberg SJ, Mulder CJ, de Vet HC. Value of symptoms and additional diagnostic tests for colorectal cancer in primary care: systematic review and meta-analysis. BMJ. 2010;340:c1269. doi: 10.1136/bmj.c1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esteva M, Leiva A, Ramos M, Pita-Fernández S, González-Luján L, Casamitjana M, Sánchez MA, Pértega-Díaz S, Ruiz A, Gonzalez-Santamaría P, et al. Factors related with symptom duration until diagnosis and treatment of symptomatic colorectal cancer. BMC Cancer. 2013;13:87. doi: 10.1186/1471-2407-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramos M, Esteva M, Cabeza E, Llobera J, Ruiz A. Lack of association between diagnostic and therapeutic delay and stage of colorectal cancer. Eur J Cancer. 2008;44:510–521. doi: 10.1016/j.ejca.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Korsgaard M, Pedersen L, Sørensen HT, Laurberg S. Reported symptoms, diagnostic delay and stage of colorectal cancer: a population-based study in Denmark. Colorectal Dis. 2006;8:688–695. doi: 10.1111/j.1463-1318.2006.01014.x. [DOI] [PubMed] [Google Scholar]
- 28.Andersen BL, Cacioppo JT. Delay in seeking a cancer diagnosis: delay stages and psychophysiological comparison processes. Br J Soc Psychol. 1995;34(Pt 1):33–52. doi: 10.1111/j.2044-8309.1995.tb01047.x. [DOI] [PubMed] [Google Scholar]
- 29.Walter F, Webster A, Scott S, Emery J. The Andersen Model of Total Patient Delay: a systematic review of its application in cancer diagnosis. J Health Serv Res Policy. 2012;17:110–118. doi: 10.1258/jhsrp.2011.010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell E, Macdonald S, Campbell NC, Weller D, Macleod U. Influences on pre-hospital delay in the diagnosis of colorectal cancer: a systematic review. Br J Cancer. 2008;98:60–70. doi: 10.1038/sj.bjc.6604096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oberoi DV, Jiwa M, McManus A, Hodder R. Colorectal cancer--applying a gender lens. Qual Prim Care. 2014;22:71–79. [PubMed] [Google Scholar]
- 32.Smith LK, Pope C, Botha JL. Patients’ help-seeking experiences and delay in cancer presentation: a qualitative synthesis. Lancet. 2005;366:825–831. doi: 10.1016/S0140-6736(05)67030-4. [DOI] [PubMed] [Google Scholar]
- 33.Courtney RJ, Paul CL, Sanson-Fisher RW, Macrae F, Attia J, McEvoy M. Current state of medical-advice-seeking behaviour for symptoms of colorectal cancer: determinants of failure and delay in medical consultation. Colorectal Dis. 2012;14:e222–e229. doi: 10.1111/j.1463-1318.2012.02881.x. [DOI] [PubMed] [Google Scholar]
- 34.Mariscal M, Llorca J, Prieto D, Delgado-Rodríguez M. Determinants of the interval between the onset of symptoms and diagnosis in patients with digestive tract cancers. Cancer Detect Prev. 2001;25:420–429. [PubMed] [Google Scholar]
- 35.McCaffery K, Wardle J, Waller J. Knowledge, attitudes, and behavioral intentions in relation to the early detection of colorectal cancer in the United Kingdom. Prev Med. 2003;36:525–535. doi: 10.1016/s0091-7435(03)00016-1. [DOI] [PubMed] [Google Scholar]
- 36.Singh H, Daci K, Petersen LA, Collins C, Petersen NJ, Shethia A, El-Serag HB. Missed opportunities to initiate endoscopic evaluation for colorectal cancer diagnosis. Am J Gastroenterol. 2009;104:2543–2554. doi: 10.1038/ajg.2009.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Domínguez-Ayala M, Díez-Vallejo J, Comas-Fuentes A. Missed opportunities in early diagnosis of symptomatic colorectal cancer. Rev Esp Enferm Dig. 2012;104:343–349. doi: 10.4321/s1130-01082012000700002. [DOI] [PubMed] [Google Scholar]
- 38.Young CJ, Sweeney JL, Hunter A. Implications of delayed diagnosis in colorectal cancer. Aust N Z J Surg. 2000;70:635–638. doi: 10.1046/j.1440-1622.2000.01916.x. [DOI] [PubMed] [Google Scholar]
- 39.Slisów W, Marx G, Krüger J. Iatrogenic diagnostic delay in rectal cancer. Zentralbl Chir. 1992;117:73–76. [PubMed] [Google Scholar]
- 40.Wahls TL, Peleg I. Patient- and system-related barriers for the earlier diagnosis of colorectal cancer. BMC Fam Pract. 2009;10:65. doi: 10.1186/1471-2296-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Hout AM, de Wit NJ, Rutten FH, Peeters PH. Determinants of patient’s and doctor’s delay in diagnosis and treatment of colorectal cancer. Eur J Gastroenterol Hepatol. 2011;23:1056–1063. doi: 10.1097/MEG.0b013e32834c4839. [DOI] [PubMed] [Google Scholar]
- 42.Bain NS, Campbell NC, Ritchie LD, Cassidy J. Striking the right balance in colorectal cancer care--a qualitative study of rural and urban patients. Fam Pract. 2002;19:369–374. doi: 10.1093/fampra/19.4.369. [DOI] [PubMed] [Google Scholar]
- 43.Singh H, Petersen LA, Daci K, Collins C, Khan M, El-Serag HB. Reducing referral delays in colorectal cancer diagnosis: is it about how you ask? Qual Saf Health Care. 2010;19:e27. doi: 10.1136/qshc.2009.033712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Debnath D, Dielehner N, Gunning KA. Guidelines, compliance, and effectiveness: a 12 months’ audit in an acute district general healthcare trust on the two week rule for suspected colorectal cancer. Postgrad Med J. 2002;78:748–751. doi: 10.1136/pmj.78.926.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eccersley AJ, Wilson EM, Makris A, Novell JR. Referral guidelines for colorectal cancer--do they work? Ann R Coll Surg Engl. 2003;85:107–110. doi: 10.1308/003588403321219885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Villaamil PV, Bouzo MS, Cubiella J, Gómez FV, Sánchez E, Gómez I, Fernández J. Evaluación de la implantación de las indicaciones y niveles de prioridad del Servizo Galego de Saude para la colonoscopia en pacientes sintomáticos: estudio prospectivo y transversal. Rev Esp Enferm Dig. 2013;105:600–608. [Google Scholar]
- 47.Grassini M, Verna C, Battaglia E, Niola P, Navino M, Bassotti G. Education improves colonoscopy appropriateness. Gastrointest Endosc. 2008;67:88–93. doi: 10.1016/j.gie.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 48.Akbari A, Mayhew A, Al-Alawi MA, Grimshaw J, Winkens R, Glidewell E, Pritchard C, Thomas R, Fraser C. Interventions to improve outpatient referrals from primary care to secondary care. Cochrane Database Syst Rev. 2008;(4):CD005471. doi: 10.1002/14651858.CD005471.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamilton W. Five misconceptions in cancer diagnosis. Br J Gen Pract. 2009;59:441–445, 447; discussion 446. doi: 10.3399/bjgp09X420860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Argüello L, Pertejo V, Ponce M, Peiró S, Garrigues V, Ponce J. The appropriateness of colonoscopies at a teaching hospital: magnitude, associated factors, and comparison of EPAGE and EPAGE-II criteria. Gastrointest Endosc. 2012;75:138–145. doi: 10.1016/j.gie.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 51.Gimeno García AZ, González Y, Quintero E, Nicolás-Pérez D, Adrián Z, Romero R, Alarcón Fernández O, Hernández M, Carrillo M, Felipe V, et al. Clinical validation of the European Panel on the Appropriateness of Gastrointestinal Endoscopy (EPAGE) II criteria in an open-access unit: a prospective study. Endoscopy. 2012;44:32–37. doi: 10.1055/s-0031-1291386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carrión S, Marín I, Lorenzo-Zúñiga V, Moreno De Vega V, Boix J. [Appropriateness of colonoscopy indications according to the new EPAGE II criteria] Gastroenterol Hepatol. 2010;33:484–489. doi: 10.1016/j.gastrohep.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 53.Eskeland SL, Dalén E, Sponheim J, Lind E, Brunborg C, de Lange T. European panel on the appropriateness of gastrointestinal endoscopy II guidelines help in selecting and prioritizing patients referred to colonoscopy--a quality control study. Scand J Gastroenterol. 2014;49:492–500. doi: 10.3109/00365521.2014.886715. [DOI] [PubMed] [Google Scholar]
- 54.Cubiella J, Salve M, Díaz-Ondina M, Vega P, Alves MT, Iglesias F, Sánchez E, Macía P, Blanco I, Bujanda L, et al. Diagnostic accuracy of the faecal immunochemical test for colorectal cancer in symptomatic patients: comparison with NICE and SIGN referral criteria. Colorectal Dis. 2014;16:O273–O282. doi: 10.1111/codi.12569. [DOI] [PubMed] [Google Scholar]
- 55.Jellema P, van Tulder MW, van der Horst HE, Florie J, Mulder CJ, van der Windt DA. Inflammatory bowel disease: a systematic review on the value of diagnostic testing in primary care. Colorectal Dis. 2011;13:239–254. doi: 10.1111/j.1463-1318.2009.02131.x. [DOI] [PubMed] [Google Scholar]
- 56.Appropriate use of gastrointestinal endoscopy. American Society for Gastrointestinal Endoscopy. Gastrointest Endosc. 2000;52:831–837. [PubMed] [Google Scholar]
- 57.Terraz O, Wietlisbach V, Jeannot JG, Burnand B, Froehlich F, Gonvers JJ, Harris JK, Vader JP. The EPAGE internet guideline as a decision support tool for determining the appropriateness of colonoscopy. Digestion. 2005;71:72–77. doi: 10.1159/000084522. [DOI] [PubMed] [Google Scholar]
- 58.Juillerat P, Peytremann-Bridevaux I, Vader JP, Arditi C, Schusselé Filliettaz S, Dubois RW, Gonvers JJ, Froehlich F, Burnand B, Pittet V. Appropriateness of colonoscopy in Europe (EPAGE II). Presentation of methodology, general results, and analysis of complications. Endoscopy. 2009;41:240–246. doi: 10.1055/s-0028-1119643. [DOI] [PubMed] [Google Scholar]
- 59.Schusselé Filliettaz S, Gonvers JJ, Peytremann-Bridevaux I, Arditi C, Delvaux M, Numans ME, Lorenzo-Zúñiga V, Dubois RW, Juillerat P, Burnand B, et al. Appropriateness of colonoscopy in Europe (EPAGE II). Functional bowel disorders: pain, constipation and bloating. Endoscopy. 2009;41:234–239. doi: 10.1055/s-0028-1119625. [DOI] [PubMed] [Google Scholar]
- 60.Schusselé Filliettaz S, Juillerat P, Burnand B, Arditi C, Windsor A, Beglinger C, Dubois RW, Peytremann-Bridevaux I, Pittet V, Gonvers JJ, et al. Appropriateness of colonoscopy in Europe (EPAGE II). Chronic diarrhea and known inflammatory bowel disease. Endoscopy. 2009;41:218–226. doi: 10.1055/s-0028-1119627. [DOI] [PubMed] [Google Scholar]
- 61.Arditi C, Gonvers JJ, Burnand B, Minoli G, Oertli D, Lacaine F, Dubois RW, Vader JP, Schusselé Filliettaz S, Peytremann-Bridevaux I, et al. Appropriateness of colonoscopy in Europe (EPAGE II). Surveillance after polypectomy and after resection of colorectal cancer. Endoscopy. 2009;41:209–217. doi: 10.1055/s-0028-1119646. [DOI] [PubMed] [Google Scholar]
- 62.Arditi C, Peytremann-Bridevaux I, Burnand B, Eckardt VF, Bytzer P, Agréus L, Dubois RW, Vader JP, Froehlich F, Pittet V, Schusselé Filliettaz S, Juillerat P, Gonvers JJ. Appropriateness of colonoscopy in Europe (EPAGE II). Screening for colorectal cancer. Endoscopy. 2009;41:200–208. doi: 10.1055/s-0028-1119626. [DOI] [PubMed] [Google Scholar]
- 63.Peytremann-Bridevaux I, Arditi C, Froehlich F, O’Malley J, Fairclough P, Le Moine O, Dubois RW, Gonvers JJ, Schusselé Filliettaz S, Vader JP, et al. Appropriateness of colonoscopy in Europe (EPAGE II). Iron-deficiency anemia and hematochezia. Endoscopy. 2009;41:227–233. doi: 10.1055/s-0028-1119644. [DOI] [PubMed] [Google Scholar]
- 64.Alonso-Abreu I, Alarcón Fernández O, González-Méndez V, Nicolas-Pérez D, Romero-Garcia R, Gimeno-garcía AZ, Carrillo-Palau M, Jimenez A QE. Impacto de un programa de cita rápida de colonoscopia en el pronóstico de pacientes con cáncer colorrectal sintomatico. Gastroenterol Hepatol. 2011;34:186–190. [Google Scholar]
- 65.Teng CL, Yu JT, Chen YH, Lin CH, Hwang WL. Early colonoscopy confers survival benefits on colon cancer patients with pre-existing iron deficiency anemia: a nationwide population-based study. PLoS One. 2014;9:e86714. doi: 10.1371/journal.pone.0086714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Available from: http://nww.doh.nhsweb.nhs.uk/cancer.
- 67.Scottish Intercollegiate Guidelines Network (SIGN) Diagnosis and management of colorectal cancer. Edinburgh: SIGN; 2011. (SIGN publication no. 126). [; 2011. p. Dec]. Available from: http://www.sign.ac.uk. [Google Scholar]
- 68.Vega-Villaamil P, Salve-Bouzo M, Cubiella J, Valentín-Gómez F, Sánchez-Hernández E, Gómez-Fernández I, Fernández-Seara J. Evaluation of the implementation of Galician Health Service indications and priority levels for colonoscopy in symptomatic patients: prospective, cross-sectional study. Rev Esp Enferm Dig. 2013;105:600–608. doi: 10.4321/s1130-01082013001000005. [DOI] [PubMed] [Google Scholar]
- 69.Ballal MS, Selvachandran SN, Maw A. Use of a patient consultation questionnaire and weighted numerical scoring system for the prediction of colorectal cancer and other colorectal pathology in symptomatic patients: a prospective cohort validation study of a Welsh population. Colorectal Dis. 2010;12:407–414. doi: 10.1111/j.1463-1318.2009.01984.x. [DOI] [PubMed] [Google Scholar]
- 70.Marshall T, Lancashire R, Sharp D, Peters TJ, Cheng KK, Hamilton W. The diagnostic performance of scoring systems to identify symptomatic colorectal cancer compared to current referral guidance. Gut. 2011;60:1242–1248. doi: 10.1136/gut.2010.225987. [DOI] [PubMed] [Google Scholar]
- 71.Hamilton W. The CAPER studies: five case-control studies aimed at identifying and quantifying the risk of cancer in symptomatic primary care patients. Br J Cancer. 2009;101 Suppl 2:S80–S86. doi: 10.1038/sj.bjc.6605396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zafar A, Mak T, Whinnie S, Chapman MA. The 2-week wait referral system does not improve 5-year colorectal cancer survival. Colorectal Dis. 2012;14:e177–e180. doi: 10.1111/j.1463-1318.2011.02826.x. [DOI] [PubMed] [Google Scholar]
- 73.Rai S, Kelly MJ. Prioritization of colorectal referrals: a review of the 2-week wait referral system. Colorectal Dis. 2007;9:195–202. doi: 10.1111/j.1463-1318.2006.01107.x. [DOI] [PubMed] [Google Scholar]
- 74.Chohan DP, Goodwin K, Wilkinson S, Miller R, Hall NR. How has the “two-week wait” rule affected the presentation of colorectal cancer? Colorectal Dis. 2005;7:450–453. doi: 10.1111/j.1463-1318.2005.00821.x. [DOI] [PubMed] [Google Scholar]
- 75.Thorne K, Hutchings HA, Elwyn G. The effects of the Two-Week Rule on NHS colorectal cancer diagnostic services: a systematic literature review. BMC Health Serv Res. 2006;6:43. doi: 10.1186/1472-6963-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shabbir J, Vijayan V, Silavant M, Fowler AL, Cook TA, Lucarotti ME. Two week rule referral for patients with colorectal cancer below the age of 50; are we being ageist? Surgeon. 2009;7:276–281. doi: 10.1016/s1479-666x(09)80004-8. [DOI] [PubMed] [Google Scholar]
- 77.Harrison AJ, Foot CS. Targets and prioritization: the case of cancer in the English NHS. Qual Prim Care. 2012;20:125–129. [PubMed] [Google Scholar]
- 78.Rai S, Ballal M, Thomas WM, Miller AS, Jameson JS, Steward WP. Assessment of a patient consultation questionnaire-based scoring system for stratification of outpatient risk of colorectal cancer. Br J Surg. 2008;95:369–374. doi: 10.1002/bjs.5981. [DOI] [PubMed] [Google Scholar]
- 79.Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandalà M, Cervantes A, Arnold D. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi64–vi72. doi: 10.1093/annonc/mdt354. [DOI] [PubMed] [Google Scholar]
- 80.von Roon AC, Karamountzos L, Purkayastha S, Reese GE, Darzi AW, Teare JP, Paraskeva P, Tekkis PP. Diagnostic precision of fecal calprotectin for inflammatory bowel disease and colorectal malignancy. Am J Gastroenterol. 2007;102:803–813. doi: 10.1111/j.1572-0241.2007.01126.x. [DOI] [PubMed] [Google Scholar]
- 81.Daperno M, Castiglione F, de Ridder L, Dotan I, Färkkilä M, Florholmen J, Fraser G, Fries W, Hebuterne X, Lakatos PL, et al. Results of the 2nd part Scientific Workshop of the ECCO. II: Measures and markers of prediction to achieve, detect, and monitor intestinal healing in inflammatory bowel disease. J Crohns Colitis. 2011;5:484–498. doi: 10.1016/j.crohns.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 82.Waugh N, Cummins E, Royle P, Kandala NB, Shyangdan D, Arasaradnam R, Clar C, Johnston R. Faecal calprotectin testing for differentiating amongst inflammatory and non-inflammatory bowel diseases: systematic review and economic evaluation. Health Technol Assess. 2013;17:xv–xix, 1-211. doi: 10.3310/hta17550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008;103:1541–1549. doi: 10.1111/j.1572-0241.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- 84.Guittet L, Bouvier V, Mariotte N, Vallee JP, Arsène D, Boutreux S, Tichet J, Launoy G. Comparison of a guaiac based and an immunochemical faecal occult blood test in screening for colorectal cancer in a general average risk population. Gut. 2007;56:210–214. doi: 10.1136/gut.2006.101428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McDonald PJ, Digby J, Innes C, Strachan JA, Carey FA, Steele RJ, Fraser CG. Low faecal haemoglobin concentration potentially rules out significant colorectal disease. Colorectal Dis. 2013;15:e151–e159. doi: 10.1111/codi.12087. [DOI] [PubMed] [Google Scholar]
- 86.Oono Y, Iriguchi Y, Doi Y, Tomino Y, Kishi D, Oda J, Takayanagi S, Mizutani M, Fujisaki T, Yamamura A, et al. A retrospective study of immunochemical fecal occult blood testing for colorectal cancer detection. Clin Chim Acta. 2010;411:802–805. doi: 10.1016/j.cca.2010.02.057. [DOI] [PubMed] [Google Scholar]
- 87.Parente F, Marino B, Ilardo A, Fracasso P, Zullo A, Hassan C, Moretti R, Cremaschini M, Ardizzoia A, Saracino I, et al. A combination of faecal tests for the detection of colon cancer: a new strategy for an appropriate selection of referrals to colonoscopy? A prospective multicentre Italian study. Eur J Gastroenterol Hepatol. 2012;24:1145–1152. doi: 10.1097/MEG.0b013e328355cc79. [DOI] [PubMed] [Google Scholar]


