Abstract
AIM: To examine survival outcomes of perihilar cholangiocarcinoma (PCCA) resection including mortality, morbidity and prognostic factors.
METHODS: Multivariate analyses were carried out based on the survival data of all patients with histologically confirmed PCCA who underwent curative resection at Srinagarind Hospital from January 2006 to December 2011.
RESULTS: There were 29 (19%) cases of intrahepatic CCA that involved hilar and 124 (81%) with hilar bile-duct cancer. R0 resection was carried out on 66 (43.1%) patients of whom 50 (32.7%) also had lymph node metastasis. The other patients underwent R1 resection. The overall 5-year survival rate was 20.6% (95%CI: 13.8-28.4) and median survival time was 19.9 mo. Postoperative mortality was 2%, and 30% of patients had complications. Patients without lymph node metastasis were 60% less likely to die than those with metastasis. Achieving R0 led to a 58% reduction in the chance of mortality as compared to R1.
CONCLUSION: To achieve a better survival outcome, focus should center on performing radical surgery and detection of patients with early stage cancer.
Keywords: Perihilar cholangiocarcinoma, Thailand, Curative resection, Five-year survival, Prognostic factors
Core tip: Cholangiocarcinoma is usually fatal because detection most commonly occurs during late stage disease. Early detection leads to a substantially better survival outcome Thus, priority should be placed on early stage detection allowing curative radical surgery.
INTRODUCTION
Cholangiocarcinoma (CCA) is the most common primary liver cancer in the northeast of Thailand where it has its highest incidence worldwide[1,2] and where it is one of the major causes of death. CCA is divided into three types according to the location of the primary tumor. Perihilar cholangiocarcinoma (PCCA) is the most common type. It occurs when the tumor originates or includes the confluence of the hepatic duct and accounts for 67% of all cases. It is followed by distal cholangiocarcinoma and intrahepatic cholangiocarcinoma which account for 27% and 6% of cases, respectively[3].
PCCA requires a major hepatectomy with bile duct resection, caudate lobectomy and regional lymphadenectomy to achieve a free margin resection and the best survival outcome[4-6]. Due to the anatomical complexity of the hepatic hilum, early lymphatic metastasis and vascular invasion, various techniques have been used to achieve margin negative resection (R0) to improve survival outcome and to reduce operative mortality[7].
Because caudate bile ducts are open to hepatic duct confluence, most PCCA have microscopic tumor invasion to the caudate lobe[8]. Caudate lobectomy is necessary to achieve margin negative resection and better survival without increasing post-operative mortality[9,10]. Preoperative biliary drainage (PBD) is performed to relieve suffering from jaundice or cholangitis as it reduces postoperative morbidity and mortality in patients who undergo right hepatectomy, who have a future liver remnant volume of ≤ 30%[11,12] and a preoperative total bilirubin of ≥ 170 μmol/L (10 mg/dL)[13]. It is not recommended that PBD be performed systemically due to potential complications such as sepsis, vascular injuries, and tumor seeding. Preoperative portal vein embolization (PVE) is widely used for pre-operative preparation to induce hypertrophy of remnant liver. It has resulted in minimized post-operative complications especially in cirrhotic patients[14] or future liver remnant volume (FLR) < 25% in normal liver[15]. Right or left trisectionectomy is performed for Bismuth IV PCCA and patients show an increasing number of margin negative resections and also improved long-term survival[16-18]. Combined major hepatectomy with portal vein and hepatic artery resection have high R0 resection with acceptable mortality[19,20].
Although outcomes of curative resection for PCCA have continuously been improving over the last decade, the 5-year survival rate is unsatisfactorily low. This has also been the case for Thailand where there are few studies on the outcome of curative resection for PCCA and 5-year survival, even though advances have been made in early stage diagnosis and surgical procedures. This study addresses this limitation in Thailand. It is based on a large cohort size and examines survival outcomes of PCCA liver resection including mortality, morbidity and prognostic factors.
MATERIALS AND METHODS
Between January 2006 and December 2011, 153 patients from northeast Thailand received curative hepatic resection for PCCA at Srinagarind Hospital, Khon Kaen, the tertiary referral center for northeast Thailand. Data relating to patient survival are examined and compared. We excluded patients who had gross residual tumor and required palliative procedures. Patient survival was measured from the day of operation until death or the end of the study on 19 September 2014 so that there was a minimum follow-up of 33 mo. This study was approved by Institutional Ethics Committee, Khon Kaen University, No. 571283.
We report continuous data as mean ± SD, and range. Categorical variables were reported as percentages. Median survival time and the 5-year survival were estimated using Kaplan-Meier methods. Log-rank test was used to compare survival experience between selected groups of patients. Cox proportional hazard model was used to determine factors affecting the overall survival. Initially we explored the effect of each factor, independently, on the survival. Based on the results from these bivariate models, all variables with a p-value of less than 0.2 were included into the full model as the starting of the model fitting using backward elimination processes. The least significant factor was removed from the full model, one at a time, where its effect on the model was assessed using a likelihood ratio test. In the final model that contained all factors that were statistically significant survival was used as the basis to estimate the hazard ratios and their 95%CI. Tests for proportional hazard model assumptions and model’s goodness-of-fit were also implemented. A P value of £ 0.05 was considered statistically significant. All statistical analyses were done using Stata version 13 (Stata Corp, College Station, TX, United States).
RESULTS
Of the 153 patients included in the analysis, 113 (73.9%) were male and 40 (26.1%) were female with a combined mean age of 56.8 ± 8.2 years (Table 1). Most of the patients presented with abdominal discomfort 92 (60.1%) and jaundice 90 (58.8%).Thirty seven patients showed weight loss (24.3%) and 18 had fever (11.8%).The median duration of symptoms was 60 d (range 3-300 d). Thirty two patients had co-morbidity that included hypertension, diabetes, renal failure, pulmonary disease and hepatitis.
Table 1.
Characteristics of the 153 of patients with perihilar cholangiocarcinoma presented as number and percentage unless specified otherwise
| Characteristics | n (%) |
| Gender | |
| Male | 113 (73.9) |
| Female | 40 (26.1) |
| Mean ± SD of age (yr) | 56.8 ± 8.22 |
| Symptoms | |
| Median (min-max) duration (d) | 60 (3-300) |
| Abdominal discomfort | 92 (60.1) |
| Jaundice | 90 (58.8) |
| Weight loss | 37 (24.2) |
| Fever | 18 (11.8) |
| Co-morbidity | 32 (20.9) |
| Hypertension | 14 (9.1) |
| Diabetes mellitus | 15 (9.8) |
| Renal failure | 2 (1.3) |
| Chronic obstructive pulmonary disease | 4 (2.6) |
| Hepatitis | 3 (1.9) |
| Others | 6 (3.9) |
Classification of resected tumors
Most tumors originated from the hepatic hilum, namely, 5 patients (3.3%) with Bismuth type I, 9 (5.9%) Bismuth type II, 65 (42.5%) Bismuth type IIIa, 41 (26.8%) Bismuth type IIIb, and 4 (2.6%) Bismuth type IV. There were 29 patients who had intrahepatic mass with hilar invasion (Table 2).
Table 2.
Type and staging of perihilar cholangiocarcinoma
| Characteristics | n (%) |
| Type | |
| Bismuth I | 5 (3.3) |
| Bismuth II | 9 (5.9) |
| Bismuth IIIa | 65 (42.5) |
| Bismuth IIIb | 41 (26.8) |
| Bismuth IV | 4 (2.6) |
| Right intrahepatic mass involving hilar | 22 (14.4) |
| Left intrahepatic mass involving hilar | 7 (4.6) |
| Staging | |
| Stage 0 | 9 (5.9) |
| Stage I | 9 (5.9) |
| Stage II | 65 (42.5) |
| Stage IIIa | 17 (11.1) |
| Stage IIIb | 47 (30.7) |
| Stage IVa | 4 (2.6) |
| Stage IVb | 2 (1.3) |
According to the American Joint Cancer Committee (AJCC) staging for perihilar bile duct tumors 7th edition patients were group as carcinoma in situ (n = 9, 5.9%), stage I (n = 9, 5.9%), stage II (n = 65, 42.5%), stage IIIa (n = 17, 11.1%), stage IIIb (n = 47, 30.7%), stage IVa (n = 4, 2.6%), and stage IVb (n = 2, 1.3%), patients who had intrahepatic tumor were also included in this staging system. Four patients with stage IVa, Bismuth type IV were classified as T4, and all underwent curative trisectionectomy. Two patients with stage IVb had celiac lymph node metastasis all of whom underwent curative resection with extensive lymphadenectomy.
Patients who had co-morbidity were group as carcinoma in situ (n = 3, 9.4%), stage I (n = 1, 3.1%), stage II (n = 18, 56.2%), stage IIIa (n = 3, 9.4%), stage IIIb (n = 7, 21.8%) and none were in stage IV.
Histological findings showed 91 papillary adenocarcinoma (59.4%), 58 tubular adenocarcinoma (37.9%), 1 adenocarcinoma with squamous metaplasia (0.6%), 2 poorly differentiated adenocarcinoma (1.3%), and 1 undifferentiated carcinoma (0.6%).
Preoperative preparation
The 90 patients who presented with jaundice had a mean total bilirubin level of 17.6 mg/dL. A total of 37 patients (41%) underwent preoperative percutaneous biliary drainage with a decrease in total serum bilirubin to 3.9 mg/dL on the day before surgery. Fifty-three patients (59%) underwent surgery without preoperative drainage. These had a total serum bilirubin of 17.1 on the day before surgery (Table 3).
Table 3.
Preoperative biliary drainage in jaundice patients (n = 90)
| Biliary drainage 37 (41%) | Non-biliary drainage 53 (59%) | P value | |
| TB at presentation (mean ± SD) | 19.4 ± 10.5 | 12.1 ± 15.8 | 0.06 |
| TB at day before surgery (mean ± SD) | 3.9 ± 4.1 | 17.1 ± 14.3 | 0.04 |
| Post-operative complication | 35% | 37.70% | 0.80 |
| Length of hospital stay (mean ± SD) | 15.6 ± 9.7 | 16.3 ± 7.6 | 0.56 |
| Mortality (%) | 0 | 2 (3.7) | - |
| Median survival time, mo (95%CI) | 30.4 (16.3-58.7) | 17 (12.2-26.6) | 0.02 |
| 5-year survival rate, %(95%CI) | 29.5 (14.1-46.7) | 7.3 (0.9-22. 4) | 0.02 |
TB: Serum total bilirubin.
Combined preoperative portal vein embolization (PVE) was performed on 10 patients (6.5%) who had an estimated small FLR, right hepatectomy on 4 patients, extended right hepatectomy on 5 patients and one patient underwent left hepatectomy.
Operative procedure
Major hepatectomy was carried out on 145 patients comprising 63 patients who underwent right hepatectomy, 35 patients who underwent extended right hepatectomy including trisectionectomy and 47 patients who had a left hepatectomy. Extrahepatic bile duct resection alone was performed in 8 patients due to limited tumor involvement. Mean operative time was 326 ± 125 min, blood loss was 1274.2 ± 1312.5 mL and length hospital stay was 15 ± 8.2 d.
Post-operative complications occurred in 70.3%, 37.1%, 29.8% of right hepatectomy, right extended hepatectomy and left hepatectomy patients, respectively. There were no significant differences in operative time, blood loss or length of hospital stay between the major hepatectomy procedures.
Combined portal vein resection was performed in 12 patients (7.8%). Mean operative time was 463.3 ± 101.1 min, blood loss was 1804.2 ± 1664.6 mL and length hospital stay was 17.7 ± 8.7 d.
R0 resection was achieved 31.7%, 37.5%, 51.4% and 53.2% of right hepatectomy, bile duct resection, right extended hepatectomy and left hepatectomy patients, respectively. Overall R0 resection was achieved in 66 (43.1%) of patients and R1 (indicated by microscopic residual tumor) in 87 (56.6%) of patients (Table 4).
Table 4.
Operative procedure
| Procedures | n (%) | Operative time; min (mean ± SD) | Blood loss; mL (mean ± SD) | Hospital stay; d (mean ± SD) | Morbidity | R0 |
| Overall | 153 | 326 ± 125 | 1274.2 ± 1312.5 | 15 ± 8.2 | 30% | 43.1% |
| Right hepatectomy | 63 (41.2) | 334.3 ± 118.6 | 1480.2 ± 1644.9 | 15.4 ± 9 | 70.3% | 31.7% |
| Right extended hepatectomy1 | 35 (22.9) | 365.4 ± 133.7 | 1200.6 ± 881.5 | 15.6 ± 8.2 | 37.1% | 51.4% |
| Left hepatectomy | 47 (30.7) | 319.4 ± 112 | 1180.5 ± 1082.9 | 15.2 ± 7.2 | 29.8% | 53.2% |
| Extra hepatic duct resection | 8 (5.2) | 150.6 ± 55.4 | 481.2 ± 380.7 | 8.6 ± 5 | 0 | 37.5% |
| Portal vein resection | 12 (7.8) | 463.3 ± 101.1 | 1804.2 ± 1664.6 | 17.7 ± 8.7 | 50% | 50% |
Included right trisectionectomy.
Operative morbidity and mortality
Mortality at 30 d was 2% with 1 patient dying of postoperative bleeding, renal failure and myocardial infarction, and 2 patients dying after being discharged from hospital. Post-operative complications occurred in 46 patients (30%). Four patients had post-operative bleeding (2.6%) and all underwent re-operation. There were 13 patients who had bile leakage (8.5%) all of whom received conservative treatment. Eleven patients had intra-abdominal collection, 7 of whom underwent percutaneous drainage. There were pleural effusions in 12 patients (7.8%), 9 (5.9%) had wound infection, 7 (1.2%) developed pneumonia, and 2 (1.3%) had urinary tract infection. Jaundice patients who underwent preoperative biliary drainage had comparable post-operative complications, mortality and length of hospital stay to non-biliary drainage patients (Table 3).
Survival analysis
Median survival time after curative resection was 19.9 mo (95%CI: 14.2-23.8; Figures 1 and 2A). Patients with R0 had the longest median survival time of 40.2 (95%CI: 22.4-57.9) mo. The overall survival rate was 68% (95%CI: 60%-74.7%) after 1 year, 33.7% (95%CI: 26.3%-41.2%) after 3 years, and 20.6% (95%CI: 13.8%-28.4%) after 5 years (Figure 3).
Figure 1.
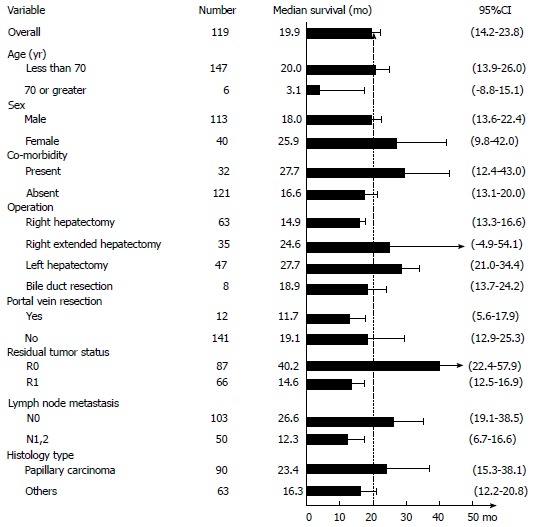
Median survival (mo) of clinico-pathological parameters.
Figure 2.
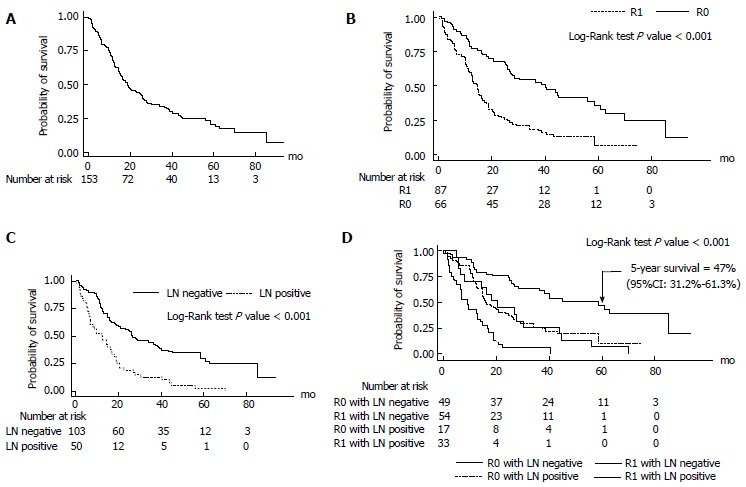
Kaplan-Meier survival curve. A: Kaplan-Meier survival curve of perihilar cholangiocarcinoma; B: Kaplan-Meier survival curve of perihilar cholangiocarcinoma according to residual tumor status; C: Kaplan-Meier survival curve of perihilar cholangiocarcinoma according to lymph node metastasis; D: Kaplan-Meier survival curve of perihilar cholangiocarcinoma according to residual tumor status and lymph node metastasis.
Figure 3.
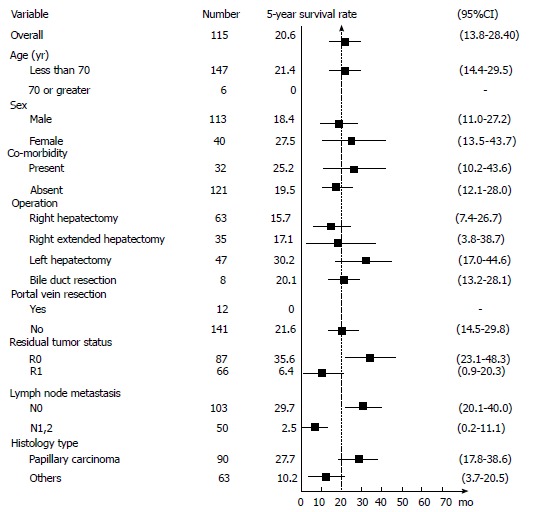
Five-year survival rate of clinico-pathological parameters.
Survival according to stage
Following are survival rates according to the AJCC staging for perihilar bile duct tumor 7th edition 2010, including all 153 patients. Stage 0, 5-year survival rate was 71.1% (95%CI: 23.3%-92.3%); Stage I, 5-year survival rate was 51.8% (95%CI: 16.4%-78.8%); Stage II, 5-year survival rate was 25.3% (95%CI: 15% -37%); Stage IIIa, 5-year survival rate was 17.6% (95%CI: 4.3%-38.3%); Stage IIIb, 5-year survival rate was 2.68% (95%CI: 0.2%-11.8%); Stage IVa and stage IVb had no 5-year survival rate.The patient groups with an early stage showed significantly better long-term survival (P < 0.001).
Twenty-nine patients who had intrahepatic tumor all had tumor with periductal invasion or nodal metastasis, according to AJCC staging for intrahepatic bile duct tumor 7th edition 2010, they were in stage IVa. The 5-year survival rate was 20.1%, median survival time was 14.9 mo comparable to overall survival (P = 0.39)
Survival according to residual tumor status
R0 resection resulted in a significantly better survival with 5-year survival rate of 35.6% (95%CI: 23.1%-48.3%) compared to R1 resection which resulted in a 5-year survival rate of 6.4% (95%CI: 0.9%-20.3%; P < 0.001) (FigureS 2B and 3).
Survival according to histopathology
Lymph node metastasis had a major influence on the patient’s survival. The 5-year survival rate of lymph node negative (N0) patients was 29.7% (95%CI: 20%-40%), while for lymph node metastasis (N1) patients it was 2.5% (95%CI: 0.2%-11.1%; P < 0.001) (Figures 2C and 3).
There was very good long-term survival in the lymph node negative patients within the R0 group. The 5-year survival rate was significantly higher in the R0N0 group 47% (95%CI: 31.2%-61.3%) compared with the R1 or N1groups 6.9% (95%CI: 2%-16.3%; P < 0.001) (Figure 2D).
Bivariate and multivariable survival analyses
The results of our bivariate analysis are shown in Table 5. There are 4 significant parameters associated with long-term survival: age < 70 (P = 0.003), residual tumor status (P < 0.001), lymph node metastasis (P < 0.001), and papillary histology type (P = 0.012). In the multivariable model, where the effect of age was taken into account, however, only the 3 factors excluding age were significantly associated with overall survival (Table 6). Achieving R0 resulted in a 58% (HR = 0.42; 95%CI: 0.28-0.62; P < 0.001) reduction in the chance of mortality as compared to R1. Likewise, patients without lymph node metastasis were 60% (HR = 0.40; 95%CI: 0.27-0.59; P < 0.001) less likely to die than those who had metastasis.
Table 5.
Bivariate analysis of clinico-pathological parameters
| Variable | Number (person-months) | IRR/100 | HR | 95%CI | P value |
| Age (10 yr added) | 153 (4026.8) | 2.96 | 0.99 | (0.97-1.01) | 0. 743 |
| Age (yr) | 0.003 | ||||
| Less than 70 | 147 (3984.4) | 2.84 | 1 | ||
| 70 or greater | 6 (42.4) | 14.15 | 4.71 | (2.02-10.97) | |
| Sex | 0.066 | ||||
| Male | 113 (2729.4) | 3.33 | 1 | ||
| Female | 40 (1297.4) | 2.16 | 0.68 | (0.44-1.04) | |
| Co-morbidity | 0.281 | ||||
| Present | 32 (1009.3) | 2.48 | 0.79 | (0.51-1.23) | |
| Absent | 121 (3017.5) | 3.12 | 1 | ||
| Operation | 0.061 | ||||
| Right hepatectomy | 63 (1389.1) | 3.89 | 1 | ||
| Right extended hepatectomy | 35 (1059.3) | 2.36 | 0.63 | (0.39-1.03) | |
| Left hepatectomy | 47 (1412.6) | 2.27 | 0.6 | (0.39-0.93) | |
| Bile duct resection | 8 (165.8) | 4.83 | 1.12 | (0.53-2.36) | |
| Portal vein resection | 0.259 | ||||
| Yes | 12 (3909.8) | 2.92 | 1.49 | (0.77-2.86) | |
| No | 141 (117.0) | 4.27 | 1 | ||
| Residual tumor status | < 0.001 | ||||
| R1 | 87 (2386.8) | 4.63 | 1 | ||
| R0 | 66 (1640.0) | 1.8 | 0.40 | (0.27-0.59) | |
| Lymph node metastasis | < 0.001 | ||||
| N1, 2 | 103 (3248.6) | 6.17 | 1 | ||
| N0 | 50 (778.2) | 2.19 | 0.38 | (0.26-0.55) | |
| Histology type | |||||
| Papillary carcinoma | 90 (2687.4) | 2.38 | 1 | 0.012 | |
| Others | 63 (1339.4) | 4.11 | 1.61 | (1.11-2.33) |
IRR: Incidence rate per 100 person-months.
Table 6.
Multivariable analysis of clinico-pathological parameters
| Variable | IRR/100 | Unadjusted HR | Adjusted HR1 | 95%CI | P value |
| Residual tumor status | < 0.001 | ||||
| R1 | 4.63 | 1 | 1 | ||
| R0 | 1.8 | 0.40 | 0.42 | (0.28-0.62) | |
| Lymph node metastasis | < 0.001 | ||||
| N1, 2 | 6.17 | 1 | 1 | ||
| N0 | 2.19 | 0.38 | 0.4 | (0.27-0.59) | |
| Histology type | |||||
| Papillary carcinoma | 2.38 | 1 | 1 | 0.018 | |
| Others | 4.11 | 1.61 | 1.58 | (1.08-2.30) |
Each hazard ratio was adjusted for age and other factors listed in the table. IRR: Incidence rate per 100 person-months.
Patients with other types of invasive cholangiocarcinoma had a 58% (HR = 1.58; 95%CI: 1.08-2.30; P = 0.018) higher chance of mortality as compare to papillary carcinoma.
DISCUSSION
Results from this study showed that curative resection of PCCA in Srinagarind hospital, Khon Kaen, Thailand had low perioperative morbidity, mortality and a 5-year survival rate comparable to recent studies. Five-year survival after curative resection for PCCA in Asia ranges from 0% to 64%[7,10,17-19,21-26] and in North America and Europe from 10% to 38%[4,27-29], post-operative morbidity was 26.3%-75% and mortality rates were 0%-11% (Table 7). There are only two previous studies from Thailand[23,26], and one review article by Khuntikeo et al[30], which found a 5-year survival of 0%-10.8%. The substantial improvements shown in our northeast Thailand cohort are most likely to be due to better, more radical surgical procedures, patient selection, and preoperative care.
Table 7.
Five-year survival after curative resection for perihilar cholangiocarcinoma in previous studies
| Ref. | Place | Year | Resections | Morbidity | Mortality | R0 | 5-year survival rate |
| Present study | Thailand | 2014 | 153 | 30% | 2.00% | 43.1% | 20.6% |
| Nimura et al[21] | Japan | 2000 | 100 | 49% | 9.00% | 61.0% | 26.0% |
| Kondo et al[22] | Japan | 2004 | 40 | 48% | 0 | 95.0% | NA |
| Dinant et al[27] | Netherlands | 2005 | 54 | 59% | 11.00% | 46.0% | 38.00% |
| Jarnagin et al[28] | United States | 2005 | 106 | 62.3% | 7.50% | 77.0% | NA |
| DeOliveira et al[29] | United States | 2007 | 173 | 61% | 5.00% | 19.0% | 10.00% |
| Ito et al[4] | United States | 2008 | 38 | 32% | 0 | 63.0% | 33.00% |
| Paik et al[18] | Korea | 2008 | 16 | 75% | 0 | 81.2% | 64.00% |
| Khuntikeo et al[23] | Thailand | 2008 | 30 | 76.70% | 6.70% | NA | 0% |
| Hirano et al[24] | Japan | 2010 | 146 | 44% | 3.40% | 87.0% | 35.5% |
| Igami et al[25] | Japan | 2010 | 298 | 43% | 2.00% | 74.0% | 42.0% |
| Nagino et al[19] | Japan | 2010 | 50 | 54% | 2.00% | 66.0% | 30.3% |
| Cheng et al[10] | China | 2012 | 176 | 26.3% | 2.90% | 78.4% | 13.5% |
| Natsume et al[17] | Japan | 2012 | 201 | 44.2% | 1.00% | 84.9% | 35.2% |
| de Jong et al[6] | United States | 2012 | 224 | NA | 6.70% | 66.5% | 20.2% |
| Nagino et al[7] | Japan | 2013 | 574 | 57.30% | 4.70% | 76.5% | 32.5% |
| Pattanathien et al[26] | Thailand | 2013 | 58 | NA | NA | 46.6% | 10.8% |
NA: Not available.
This study did not show that preoperative biliary drainage in jaundice patients reduced post-operative complications, mortality or length of hospital stay. Preoperative biliary drainage patients showed a better rate of survival, but this was not significant in multivariate analysis.
A small number of patients underwent preoperative PVE because CT was not used to estimate remnant liver volume. The benefit of PVE could not be evaluated due to lack of clear indications.
We found that patients who had co-morbidity had a better survival outcome because these patients regularly had medical attention that increased the chance of detecting an early stage tumor. Early stages (stages 0, I and II) were found in 68.7% of patients who had co-morbidity compare to 50.4% of patients who did not.
This study validates John Hopkin’s definition of perihilar cholangiocarcinoma[3]. i.e., that any tumor involving hepatic duct bifurcation should be treated and staged like perihilar tumor, because there are no differences in the prognosis and treatment strategy. Lymph node metastasis has been identified as a strong factor indicating a very poor prognosis[4,7,9,10,29]. This is also the case in our study since of the 32.7% patients who had lymph node metastasis only 2.5% had a 5-year survival.
Papillary carcinoma associated with intraductal tumor and less aggressive behavior resulted in a significantly longer median survival after resection of 55.7 mo compared to the nodular sclerosing type of 33.5 mo[28,31]. This study shows that papillary carcinoma is an independent prognostic factor with a 5-year survival rate of 27.7%, whereas other CCA types resulted in only a 10.2% survival rate.
Recent studies showed that R0 resection was the factor indicating a good prognosis resulting in a 40.7%-52% 5-year survival compared to R1 resection with 5-year survival of 7.9%-32%[7,17,19,25]. More radical surgery had to be considered in order to achieve a more negative margin, such as combined vascular resection where studies have reported a 5-year survival of 47.6%-58% with mortality of 2%-8.8%[19,20], and left or right trisectionectomy resulting in a 5-year survival of 36.8%-64.2% with an acceptable mortality of 0%-1.2%[16-18,32,33].
Our study, which was based on current surgical techniques, showed a 5-year survival in the R0 group of 35.6%, which was much greater than in the R1 group with a survival of 6.4%. Right hepatectomy was the procedure that achieved the lowest rate of R0 resection, probably due to the preservation of segment 4 liver parenchyma which increased the chance of a histologically positive margin. Appropriately designed studies are needed to investigate this finding (Table 4). R0 resection was carried out in 43.1% of all patients which compares favorably with previous studies where the R0 resection ranged from 19% to 95% (Table 6).
There is one randomized control trial showing the benefit of chemotherapy in advanced biliary tract cancer[34], but there are no randomized trials to prove the benefit of chemotherapy in an adjuvant setting. This study was not designed to prove the benefit of chemotherapy but there is one retrospective study from our institute from the years 2009-2011 that may imply benefit of adjuvant chemotherapy. The study included 263 patients who underwent curative resection for all types of cholangiocarcinoma. Patients who received adjuvant chemotherapy had a significantly longer median survival time of 21.6 mo compared to those with no adjuvant chemotherapy of 13.4 mo. Benefit was also found in lymph node metastasis, R1 resection, higher carbohydrate antigen 19-9 and higher stage[35].
Based on the information from this study, we can suggest measures to improve long-term survival outcome. Focus should center on: (1) screening tools and screening policy to detect early lymph node negative cases; and (2) radical surgical techniques and perioperative care to improve the R0 resection rate and to minimize post-operative morbidity and mortality.
In conclusion, curative resection in PCCA is possible with current surgical procedures resulting in a two-fold greater survival outcome compared to previous studies from Thailand. Independent factors that were associated with good survival outcome were R0 resection, no lymph node metastasis, and papillary histology.
ACKNOWLEDGMENTS
We thank Professor Trevor N. Petney for editing the manuscript via the Publication Clinic KKU, Thailand.
COMMENTS
Background
The highest incidence worldwide of perihilar cholangiocarcinoma (PCCA) occurs in the northeast of Thailand. Major hepatectomy with bile duct resection is the standard curative procedure; however, to date the 5-year survival rate in Thailand of 0%-10.8%, has been unsatisfactorily low.
Research frontiers
Curative resection in PCCA is safe with improved surgical procedures resulting in a two-fold greater survival outcome compared to previous studies from Thailand. Independent factors that were associated with good survival outcome were R0 resection, no lymph node metastasis, and papillary histology.
Innovation and breakthroughs
Advances in surgical technique combined with early stage diagnosis can lead to a substantially improved prognosis and 5-year survival in PCCA patients.
Applications
To improve long-term survival outcome focus should center on (1) radical surgical techniques and perioperative care to improve the R0 resection rate and to minimize post-operative morbidity and mortality; and (2) screening tools and screening policy to detect early lymph node negative cases.
Terminology
Perihilar cholangiocarcinoma is defined as “tumors that are located in the extrahepatic biliary tree proximal to the origin of the cystic duct”.
Peer-review
The present study has been performed in the place that the highest incidence of cholangiocarcinoma is reported. The study was well designed and conducted with great invention.
Footnotes
Supported by Khon Kaen University through CASCAP and the National Research Council of Thailand through the Medical Research Network of the Consortium of Thai Medical Schools.
Institutional review board statement: The entire study was carried out in strict accordance with protocols approved by Institutional Ethics Committee, Khon Kaen University (Ethics Permit Number: 571283).
Clinical trial registration statement: There are no randomized trials to prove the benefit of chemotherapy in an adjuvant setting. This study was not designed to prove the benefit of chemotherapy but there is one retrospective study from our institute from the years 2009-2011 that may imply benefit of adjuvant chemotherapy.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Data sharing statement: Technical appendix, statistical code, and dataset available from the corresponding author at nkhuntikeo@gmail.com. Participants gave informed consent for data sharing.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 4, 2015
First decision: September 14, 2015
Article in press: October 27, 2015
P- Reviewer: Baiocchi GL, Kukongviriyapan V, Leardkamolkarn V S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
References
- 1.Srivatanakul P, Sriplung H, Deerasamee S. Epidemiology of liver cancer: an overview. Asian Pac J Cancer Prev. 2004;5:118–125. [PubMed] [Google Scholar]
- 2.Green A, Uttaravichien T, Bhudhisawasdi V, Chartbanchachai W, Elkins DB, Marieng EO, Pairqjkul C, Dhiensiri T, Kanteekaew N, Haswell-Elkins MR. Cholangiocarcinoma in north east Thailand. A hospital-based study. Trop Geogr Med. 1991;43:193–198. [PubMed] [Google Scholar]
- 3.Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, Hruban RH, Lillemoe KD, Yeo CJ, Cameron JL. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463–473; discussion 473-475. doi: 10.1097/00000658-199610000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ito F, Agni R, Rettammel RJ, Been MJ, Cho CS, Mahvi DM, Rikkers LF, Weber SM. Resection of hilar cholangiocarcinoma: concomitant liver resection decreases hepatic recurrence. Ann Surg. 2008;248:273–279. doi: 10.1097/SLA.0b013e31817f2bfd. [DOI] [PubMed] [Google Scholar]
- 5.Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BS J, Youssef BA M, Klimstra D, Blumgart LH. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–517; discussion 517-519. doi: 10.1097/00000658-200110000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Jong MC, Marques H, Clary BM, Bauer TW, Marsh JW, Ribero D, Majno P, Hatzaras I, Walters DM, Barbas AS, et al. The impact of portal vein resection on outcomes for hilar cholangiocarcinoma: a multi-institutional analysis of 305 cases. Cancer. 2012;118:4737–4747. doi: 10.1002/cncr.27492. [DOI] [PubMed] [Google Scholar]
- 7.Nagino M, Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, Nimura Y. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg. 2013;258:129–140. doi: 10.1097/SLA.0b013e3182708b57. [DOI] [PubMed] [Google Scholar]
- 8.Nimura Y, Hayakawa N, Kamiya J, Kondo S, Shionoya S. Hepatic segmentectomy with caudate lobe resection for bile duct carcinoma of the hepatic hilus. World J Surg. 1990;14:535–543; discussion 544. doi: 10.1007/BF01658686. [DOI] [PubMed] [Google Scholar]
- 9.Kow AW, Wook CD, Song SC, Kim WS, Kim MJ, Park HJ, Heo JS, Choi SH. Role of caudate lobectomy in type III A and III B hilar cholangiocarcinoma: a 15-year experience in a tertiary institution. World J Surg. 2012;36:1112–1121. doi: 10.1007/s00268-012-1497-0. [DOI] [PubMed] [Google Scholar]
- 10.Cheng QB, Yi B, Wang JH, Jiang XQ, Luo XJ, Liu C, Ran RZ, Yan PN, Zhang BH. Resection with total caudate lobectomy confers survival benefit in hilar cholangiocarcinoma of Bismuth type III and IV. Eur J Surg Oncol. 2012;38:1197–1203. doi: 10.1016/j.ejso.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Farges O, Regimbeau JM, Fuks D, Le Treut YP, Cherqui D, Bachellier P, Mabrut JY, Adham M, Pruvot FR, Gigot JF. Multicentre European study of preoperative biliary drainage for hilar cholangiocarcinoma. Br J Surg. 2013;100:274–283. doi: 10.1002/bjs.8950. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy TJ, Yopp A, Qin Y, Zhao B, Guo P, Liu F, Schwartz LH, Allen P, D’Angelica M, Fong Y, et al. Role of preoperative biliary drainage of liver remnant prior to extended liver resection for hilar cholangiocarcinoma. HPB (Oxford) 2009;11:445–451. doi: 10.1111/j.1477-2574.2009.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiong JJ, Nunes QM, Huang W, Pathak S, Wei AL, Tan CL, Liu XB. Preoperative biliary drainage in patients with hilar cholangiocarcinoma undergoing major hepatectomy. World J Gastroenterol. 2013;19:8731–8739. doi: 10.3748/wjg.v19.i46.8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdalla EK, Hicks ME, Vauthey JN. Portal vein embolization: rationale, technique and future prospects. Br J Surg. 2001;88:165–175. doi: 10.1046/j.1365-2168.2001.01658.x. [DOI] [PubMed] [Google Scholar]
- 15.Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, Denys A, Sauvanet A. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208–217. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumoto N, Ebata T, Yokoyama Y, Igami T, Sugawara G, Shimoyama Y, Nagino M. Role of anatomical right hepatic trisectionectomy for perihilar cholangiocarcinoma. Br J Surg. 2014;101:261–268. doi: 10.1002/bjs.9383. [DOI] [PubMed] [Google Scholar]
- 17.Natsume S, Ebata T, Yokoyama Y, Igami T, Sugawara G, Shimoyama Y, Nagino M. Clinical significance of left trisectionectomy for perihilar cholangiocarcinoma: an appraisal and comparison with left hepatectomy. Ann Surg. 2012;255:754–762. doi: 10.1097/SLA.0b013e31824a8d82. [DOI] [PubMed] [Google Scholar]
- 18.Paik KY, Choi DW, Chung JC, Kang KT, Kim SB. Improved survival following right trisectionectomy with caudate lobectomy without operative mortality: surgical treatment for hilar cholangiocarcinoma. J Gastrointest Surg. 2008;12:1268–1274. doi: 10.1007/s11605-008-0503-1. [DOI] [PubMed] [Google Scholar]
- 19.Nagino M, Nimura Y, Nishio H, Ebata T, Igami T, Matsushita M, Nishikimi N, Kamei Y. Hepatectomy with simultaneous resection of the portal vein and hepatic artery for advanced perihilar cholangiocarcinoma: an audit of 50 consecutive cases. Ann Surg. 2010;252:115–123. doi: 10.1097/SLA.0b013e3181e463a7. [DOI] [PubMed] [Google Scholar]
- 20.Neuhaus P, Thelen A, Jonas S, Puhl G, Denecke T, Veltzke-Schlieker W, Seehofer D. Oncological superiority of hilar en bloc resection for the treatment of hilar cholangiocarcinoma. Ann Surg Oncol. 2012;19:1602–1608. doi: 10.1245/s10434-011-2077-5. [DOI] [PubMed] [Google Scholar]
- 21.Nimura Y, Kamiya J, Kondo S, Nagino M, Uesaka K, Oda K, Sano T, Yamamoto H, Hayakawa N. Aggressive preoperative management and extended surgery for hilar cholangiocarcinoma: Nagoya experience. J Hepatobiliary Pancreat Surg. 2000;7:155–162. doi: 10.1007/s005340050170. [DOI] [PubMed] [Google Scholar]
- 22.Kondo S, Hirano S, Ambo Y, Tanaka E, Okushiba S, Morikawa T, Katoh H. Forty consecutive resections of hilar cholangiocarcinoma with no postoperative mortality and no positive ductal margins: results of a prospective study. Ann Surg. 2004;240:95–101. doi: 10.1097/01.sla.0000129491.43855.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khuntikeo N, Pugkhem A, Bhudhisawasdi V, Uttaravichien T. Major hepatic resection for hilar cholangiocarcinoma without preoperative biliary drainage. Asian Pac J Cancer Prev. 2008;9:83–85. [PubMed] [Google Scholar]
- 24.Hirano S, Kondo S, Tanaka E, Shichinohe T, Tsuchikawa T, Kato K, Matsumoto J, Kawasaki R. Outcome of surgical treatment of hilar cholangiocarcinoma: a special reference to postoperative morbidity and mortality. J Hepatobiliary Pancreat Sci. 2010;17:455–462. doi: 10.1007/s00534-009-0208-1. [DOI] [PubMed] [Google Scholar]
- 25.Igami T, Nishio H, Ebata T, Yokoyama Y, Sugawara G, Nimura Y, Nagino M. Surgical treatment of hilar cholangiocarcinoma in the “new era”: the Nagoya University experience. J Hepatobiliary Pancreat Sci. 2010;17:449–454. doi: 10.1007/s00534-009-0209-0. [DOI] [PubMed] [Google Scholar]
- 26.Pattanathien P, Khuntikeo N, Promthet S, Kamsa-Ard S. Survival rate of extrahepatic cholangiocarcinoma patients after surgical treatment in Thailand. Asian Pac J Cancer Prev. 2013;14:321–324. doi: 10.7314/apjcp.2013.14.1.321. [DOI] [PubMed] [Google Scholar]
- 27.Dinant S, Gerhards MF, Busch OR, Obertop H, Gouma DJ, Van Gulik TM. The importance of complete excision of the caudate lobe in resection of hilar cholangiocarcinoma. HPB (Oxford) 2005;7:263–267. doi: 10.1080/13651820500372376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jarnagin WR, Bowne W, Klimstra DS, Ben-Porat L, Roggin K, Cymes K, Fong Y, DeMatteo RP, D’Angelica M, Koea J, et al. Papillary phenotype confers improved survival after resection of hilar cholangiocarcinoma. Ann Surg. 2005;241:703–12; discussion 712-4. doi: 10.1097/01.sla.0000160817.94472.fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, Choti MA, Yeo CJ, Schulick RD. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–762. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khuntikeo N, Pugkhem A, Titapun A, Bhudhisawasdi V. Surgical management of perihilar cholangiocarcinoma: a Khon Kaen experience. J Hepatobiliary Pancreat Sci. 2014;21:521–524. doi: 10.1002/jhbp.74. [DOI] [PubMed] [Google Scholar]
- 31.Hoang MP, Murakata LA, Katabi N, Henson DE, Albores-Saavedra J. Invasive papillary carcinomas of the extrahepatic bile ducts: a clinicopathologic and immunohistochemical study of 13 cases. Mod Pathol. 2002;15:1251–1258. doi: 10.1097/01.MP.0000036450.61830.8E. [DOI] [PubMed] [Google Scholar]
- 32.Esaki M, Shimada K, Nara S, Kishi Y, Sakamoto Y, Kosuge T, Sano T. Left hepatic trisectionectomy for advanced perihilar cholangiocarcinoma. Br J Surg. 2013;100:801–807. doi: 10.1002/bjs.9099. [DOI] [PubMed] [Google Scholar]
- 33.Nagino M, Kamiya J, Arai T, Nishio H, Ebata T, Nimura Y. “Anatomic” right hepatic trisectionectomy (extended right hepatectomy) with caudate lobectomy for hilar cholangiocarcinoma. Ann Surg. 2006;243:28–32. doi: 10.1097/01.sla.0000193604.72436.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 35.Wirasorn K, Ngamprasertchai T, Khuntikeo N, Pakkhem A, Ungarereevittaya P, Chindaprasirt J, Sookprasert A. Adjuvant chemotherapy in resectable cholangiocarcinoma patients. J Gastroenterol Hepatol. 2013;28:1885–1891. doi: 10.1111/jgh.12321. [DOI] [PubMed] [Google Scholar]


