Abstract
In a normal population, VWF plasma levels (VWF:Ag) and VWF activity (VWF:RCo) increase by approximately 0.17 and 0.15 IU/ml per decade, but the influence of age is unknown in patients with type 1 von Willebrand disease (VWD). In a retrospective cohort study, the medical records of 31 type 1 VWD patients over the age of 30, who had been followed for ≥5 years, were reviewed for baseline clinical data and previously performed VWF:Ag, VWF:RCo and factor VIII levels (FVIII:C). VWF multimer analysis was normal in 27/31 cases performed. Mean bleeding score was 9.4 (range 2-21). Mean age at diagnosis was 33 (range 16-60 years), and duration of follow-up ranged from 5-26 years (mean 11 years). Patients had 2-10 time points of VWD testing (mean of 5.2). The mean VWF:Ag, VWF:RCo and FVIII:C at time of diagnosis were 0.44 IU/ml 0.34 IU/ml and 0.75 IU/ml. At last follow-up, the mean VWF:Ag, VWF:RCo and FVIII:C were significantly increased to 0.71 IU/L, 0.56 IU/ml and 0.90 IU/ml (p=<0.001, <0.001, and 0.0081, respectively). 18/31 patients had VWF:Ag, VWF:RCo and FVIII:C levels that increased into the normal range. The rate of change in VWF:Ag and VWF:RCo was 0.30 IU/ml (0.214-0.386, CI 95%, p<0.0001) and 0.20 IU/ml per year (0.126-0.274, CI 95%, p=0.0001). Patients with type 1 VWD experience age-related increases to VWF:Ag and VWF:RCo which can result in normalization of VWF levels. Further studies are required to determine if the bleeding phenotype resolves with the increases in VWF:Ag and VWF:RCo levels.
Keywords: von Willebrand Disease, Aging, Hemostasis, Bleeding
Introduction
Aging is associated with significant changes to coagulation proteins. Coagulation factors including fibrinogen, factor VII (FVII), factor VIII (FVIII), factor IX (FIX) and VWF all increase in parallel with increasing age.(1–4) Similarly, anticoagulant proteins, protein C and S, as well as tissue factor pathway inhibitor, also increase with age but to a lesser degree with studies often demonstrating conflicting results.(5–10) The net effect of aging on hemostasis is increased coagulation activation as demonstrated by increase in the markers of coagulation activation prothrombin fragment 1+2, fibrinopeptide A, thrombin-antithrombin complexes, and D-dimer.(9,11–13) Thus, the hemostatic system changes with age, in favor of hypercoagulability.
The effect of these changes in patients with inherited bleeding disorders is not clear. In hemophilia A, FVIII levels increase with age, particularly in mild disease.(14) The clinical significance of this change has not been determined. In one retrospective cohort study, the authors describe increased bleeding in HA patients with increased age.(15) The majority of increased bleeding was seen in severe HA patients, whose FVIII level would not have increased with age. The few mild and moderate HA patients who experienced increased bleeding had associated comorbidities and medications that such as ASA or vitamin K antagonists. Thus, the effect of age related increases to FVIII along with other physiologically normal changes to the coagulation and anticoagulant proteins in HA has not yet been defined.
Increased age has also been associated with higher VWF levels,(16,17) with studies suggesting that the levels may increase by as much as 0.15-0.17 U/ml per decade.(18) In addition, older age is associated with lower ADAMTS13 activity.(19) Age-related increases of VWF may be seen in patients with VWD, depending upon the underlying pathogenic mechanism, and with the exclusion of type 3 VWD where VWF is not produced at all. We investigated the effect of aging on VWF levels in a retrospective cohort study of 32 type 1 VWD patients.
Methods
Patients
We reviewed our local type 1 VWD database which lists all patients seen at the Inherited Bleeding Disorders Clinic, of Southeastern Ontario in Kingston. Ontario, Canada, and identified 31 type 1 VWD patients over the age of 30, who had VWF levels and activity tested at least twice separated by ≥5years. All of the patients had baseline VWF:Ag and/or VWF:RCo levels <0.50 to meet our local diagnostic criteria of type 1 VWD, which also includes RCo:Ag ratios > 0.6. The patients’ charts were reviewed with the following details being extracted: bleeding score using the condensed MCMDM1-VWD bleeding questionnaire (again, at which time point?), (20) ABO blood group, baseline VWF multimer analysis and all previously performed VWF:Ag, VWF:RCo and FVIII levels. The majority of investigations had been performed at the clinical hemostasis laboratory in the Kingston General Hospital. One sample (ID#3) had a 2 VWF:RCo results reported that were below the lower limit of detection and identified as absent. For the purposes of data analysis, the value of 0.05 IU/ml was substituted in these cases. Any investigations that were associated with DDAVP/factor replacement, pregnancy, acute illness, and hospitalization were excluded.
Statistical Analysis
Comparison of baseline vs. last measured VWF:Ag and VWF:RCo was performed using the paired samples t-test. To account for the repeated measures per patient, the rate of change in VWF:Ag and VWF:RCo was estimated by the linear mixed effects model implemented in SAS version 9.2 (SAS Institute Inc, Cary NC, USA).
Results
Patient characteristics
A total of 31 patients were included and summarized in TABLE 1. The median age of the patients at the point of last VWF level measurements was 46 years old (range 30-74 years). Mean duration of follow-up was 11 years, with a range of 5 to 26 years. Patients had VWF levels performed at 2-10 time points (mean 5.2). The mean bleeding score was 9.4 (range 2-21) with ≥ 4 being an abnormal or positive score. The patient cohort was made up of predominately females (21/31, 68%) and had type O blood type (21/26 with known ABO blood group, 81%). VWF multimer analysis was performed and normal in 28 of 31 patients.
Table 1.
Patient Characteristics
| Mean (Range) | %, (n) | |
|---|---|---|
| Age | 46 (30-74) | - |
| < 45 years | 37 (30-44) | 48 (15) |
| ≥ 45 years | 54 (45-74) | 52 (16) |
| Duration of Follow-up (yrs) | 11 (5-26) | - |
| Number of time points | 5.2 (2-10) | - |
| Female | - | 68 (21) |
| Bleeding Score* | 9.4 (2-21) | - |
| O Blood type,** | - | 21 (81) |
Available for 27 of 31 patients,
Available for 26 of 31 patients
Comparison of baseline and last available VWF levels
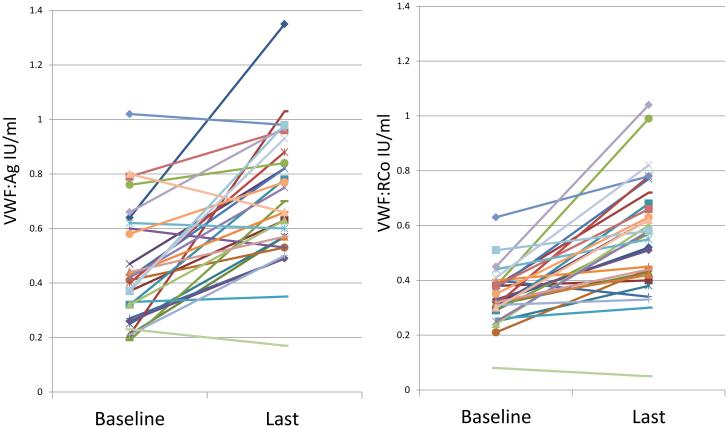
The mean VWF:Ag, VWF:RCo and FVIII:C levels at baseline were 0.44 IU/ml (range 0.19-0.82 IU/ml), 0.34 IU/ml (range 0.08-0.63 IU/ml) and 0.75 IU/ml (range 0.24-1.35 IU/ml), respectively (TABLE 2A). At time of last measurement, the mean VWF:Ag, VWF:RCo and FVIII:C levels had all significantly increased to 0.71 IU/ml (range 0.17-1.35 IU/ml, p<0.0001), 0.56 IU/ml (range 0.05-1.04 IU/ml, p<0.0001) and 0.90 IU/ml (range 0.22-1.41 IU/ml, p=0.0081), respectively. Because this cohort had widely variable baseline VWF levels, we also focused on the more severely affected patients (n=8) with VWF:Ag levels <0.30 IU/ml. This cohort had a mean of 12.7 years of follow-up (range 8.4-21.7 years). Again, VWF:Ag, VWF:RCo and FVIII:C were all significantly increased at point of last follow-up compared to baseline (TABLE 2B).
Table 2.
Comparison of Baseline and Last VWF levels
| Baseline, IU/ml Mean (range) |
Last, IU/ml Mean (range) |
P | |
|---|---|---|---|
| A. All patients (n=31) | |||
| VWF:Ag | 0.44 (0.19-0.82) | 0.71 (0.17-1.35) | <0.0001 |
| VWF:RCo | 0.34 (0.80-0.63) | 0.56 (0.05-1.04) | <0.0001 |
| FVIII:C | 0.75 (0.24-1.350 | 0.90 (0.22-1.41) | 0.0081 |
| B. Patients with VWF:Ag <0.30 IU/ml (n=8) | |||
| VWF:Ag | 0.23 (0.19-0.27) | 0.57 (0.17-1.03) | 0.0071 |
| VWF:RCo | 0.29 (0.08-0.4) | 0.42 (0.05-0.72) | 0.046 |
| FVIII:C | 0.60 (0.24-0.81) | 0.80 (0.26-1.37) | 0.0.21 |
The majority of patients demonstrated an increase of VWF:Ag and VWF:RCo levels within the follow-up time (FIGURE 1) with 58% (n=18) having normalization of both VWF:Ag and VWF:RCO to ≥0.50 IU/ml. Amongst the cohort at patients with baseline VWF:Ag <0.30 IU/ml, 2 of the 8 patients with baseline VWF:Ag levels <0.30 IU/ml had normalization of levels at last follow-up. Only one of the patients, with baseline VWF:Ag 0.23 IU/ml, VWF:RCo 0.08 IU/ml, and a bleeding score 21 demonstrated a decrease of VWF with time. After 22 years of follow-up, her repeat studies demonstrated a VWF:Ag 0.17 IU/ml, and VWF:RCo was below the lower limit of detection (documented as 0.05 IU/ml for analysis purposes).
Figure 1.
Baseline and Last VWF:AG and VWF:RCo for each patient
Rate of Change
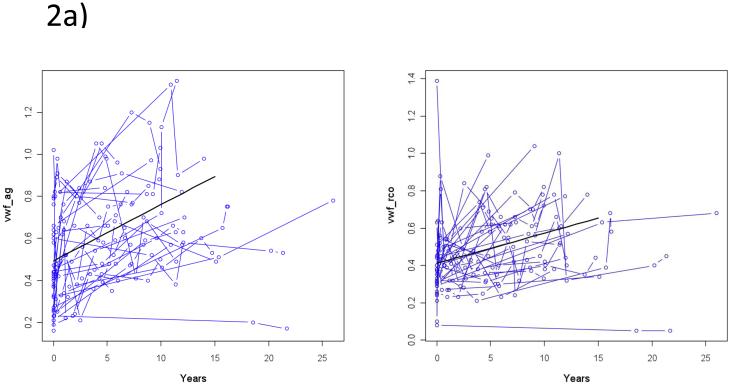
The data available is complicated by variable number of time points measured and duration of follow-up for each patient. In addition, the collected data displayed considerable variability of VWF:Ag and VWF:RCo levels within each individual’s serial measurements. Factors that clearly affect VWF levels, such as hospitalization, pregnancy, and acute illness, were identified within the chart and associated VWF measurements were excluded from the analysis. Nevertheless, spaghetti plots, summarizing each patient’s VWF:Ag and VWF:RCo measurements over time from the point of diagnosis (0 years) (shown in FIGURES 2a and 2b) demonstrate the significant variability seen over time for each patient. To account for the repeated measures per patient, the rate of change in VWF:Ag and VWF:RCo was estimated by the linear mixed effects model. The rate of change of VWF:Ag is 0.30 IU/ml per decade (0.214-0.386, CI 95%, p<0.0001) and VWF:RCo is 0.20 IU/ml per year (0.126-0.274, CI 95%, p=0.0001). The rate of change for both VWF:Ag and VWF:RCo was not significantly different when the following groups were compared: male vs female, age <45 vs ≥45, or baseline VWF:Ag<0.3 vs. ≥0.30.
Figure 2.
Spaghetti Plots for VWF:Ag (2a) and VWF:RCo (2b) levels for each patient
Discussion
Although frequently clinically observed, this small retrospective cohort study represents the first to investigate age-related increase of VWF:Ag and VWF:RCo levels in patients with type 1 VWD. Unfortunately, several significant limitations are present in this study. First, only a small number of patients (n=31) met the criteria of having at least 2 sets of VWD studies performed in the absence of other medical confounders such as illness, or pregnancy, over at least a five year period. Second, a sustained elevation of VWF levels may occur in chronic disease such as hyperthyroidism, renal failure, diabetes, liver disease, atherosclerosis, chronic inflammatory states and cancer.(21) Chronic co-morbidities were not accounted for in our analysis. Finally, our data demonstrated striking intra-patient variability as demonstrated in FIGURES 2a and 2b. Serial measurement of VWF levels within a subject may vary widely as a result of an acute response to ß-adrenergic stimuli, drugs, or more sustained physiological factors, such as pregnancy, hypothyroidism, and use of certain medications.(22,23) Medical charts were reviewed to exclude any such obvious confounders, but these may not have been documented at the time of VWF testing. Another issue is the high degree of assay variability, particularly for VWF:RCo. This variability was minimized in our study by the analysis of samples at a single laboratory at the Kingston General Hospital.
Despite these limitations, we have shown that patients with type 1 VWD experience age-related increases to VWF:Ag and VWF:RCo. Over the period of follow-up (mean 11 years, range 5-26 years), VWF:Ag increased significantly by a mean of 0.27 U/ml (p<0.001) and VWF:RCo by 0.22 U/ml (p<0.001). In a normal population, VWF values are associated with age, with levels being significantly higher in centenarians as compared to younger controls.(17) In addition, sustained elevations of VWF levels occur in chronic diseases such as renal failure, diabetes, and atheroscerlosis.(21) As these diseases increase in frequency with increasing age, we expected a higher rate of change with increasing age, which was not seen. The difference between the rate of change for VWF:Ag and VWF:RCo was not significant (p= 0.96 and 0.92, respectively) when comparing the cohort <45 years (n=15) vs ≥45 years (n=16). In type 1 VWD, approximately 35% of individuals do not have a putative mutation in the coding region, splice junctions or proximal promoter of the VWF gene, suggesting that other genes, regulating VWF secretion or clearance for example, may contribute to the pathophysiology of this disease.(24,25) In the case of mild type 1 VWD (VWF≥0.30 U/ml), only ~50% of individuals will have a mutation identified. We hypothesized that in the more severely affected cohort (VWF<0.30 U/ml) where there is an increased incidence of mutations within the VWF gene, there will be a decreased rate of change with increasing age. However, our analysis was not significant (p= 0.64 and 0.79, respectively) when comparing the cohort VWF:Ag<0.3 U/ml (n=8) vs. ≥0.30 U/ml (n=13) which could be due to the small sample size.
Within the study cohort, a significant portion of mild to moderate type 1 VWD patients self-correct with age and no longer meet our local diagnostic criteria for VWD (n=18, 58%). VWD has been associated with significant bleeding symptoms, resultant iron deficiency and decreased health-related quality of life.(26,27) This emphasizes the importance of appropriate diagnosis and treatment. On the other hand, the erroneous or outdated label of VWD may subject patients to unnecessary medical interventions, such as DDAVP which has been associated with myocardial infarction in the elderly,(28) and hesitancy to institute certain treatments such as anticoagulants or pursue surgical interventions. In the normal population, age-related increases in coagulation factors outweigh the increases seen in coagulation inhibitors and therefore favor increased coagulation activation as demonstrated by increased coagulation activation markers, prothrombin fragment F1 and 2, and thrombin-antithrombin complexes.(6) In VWD, this shift of the hemostatic balance may contribute to coagulation phenotypes closer to normal. Interestingly, Tosetto et al. demonstrated a trend for increasing bleeding score with increasing age in index cases with type 1 VWD and affected family members suggesting that the risk of bleeding in type 1 VWD persists with increasing age.(29) To date, no studies have investigated whether age-related normalization of VWF levels constitutes a normalization of bleeding risk. It seems biologically reasonable to assume that as VWF levels increase into the normal range that the risk of bleeding in type 1 VWD should be reduced or eliminated, however it is also possible that the increased bleeding phenotype will persist. One limitation to the current studies is that we have only determined plasma VWF levels and have not investigated any trend of platelet VWF levels with age. Given the important role of platelet VWF in effecting normal hemostasis it would be informative to determine whether levels of the platelet pool of VWF also rise with age.
The results of this study pose a number of practically important questions. Should the diagnosis of type 1 VWD be rejected when, in older age, the VWF levels have risen to a clearly normal range? Alternatively, should age-adjusted values for VWF:Ag and VWF:RCo be used, a practically challenging proposal that isn’t currently recommended for standardization of VWF measurement? Overall, further studies, with larger patient numbers, laboratory and clinical phenotypic bleeding data are required provide these answers.
Acknowledgments
The authors would to thank Andrew Day, Xiaoqun Sun, and Wilma Hopman from the Clinical Research Centre at Kingston General Hospital, Kingston Ontario for the statistical assistance and Ms. Louise Dwyer of the Clinical Hemostasis Laboratory at the Kingston General Hospital for the technical help. David Lillicrap is the recipient of a Canada Research Chair in Molecular Hemostasis. Natalia Rydz is the recipient of a Bayer Hemophilia Clinical Training Award.
Footnotes
Authorship and Disclosures
P.D.J receives research funding from CSL Behring and honoraria from CSL Behring, Bayer, and Baxter for educational presentations. N.R. performed the analysis and wrote the paper. D.L. and P.D.J. edited the paper.
References
- 1.Franchini M. Hemostasis and aging. Crit Rev Oncol Hemat. 2006 Nov;60(2):144–51. doi: 10.1016/j.critrevonc.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Balleisen L, Bailey J, Epping PH, Schulte H, Van de Loo J. Epidemiological study on factor VII, factor VIII and fibrinogen in an industrial population: I. Baseline data on the relation to age, gender, body-weight, smoking, alcohol, pill-using, and menopause. Thromb Haemost. 1985 Aug 30;54(2):475–9. [PubMed] [Google Scholar]
- 3.Kannel WB, Wolf PA, Castelli WP, D’Agostino RB. Fibrinogen and risk of cardiovascular disease. The Framingham Study. J Am Med Assoc. 1987 Sep 4;258(9):1183–6. [PubMed] [Google Scholar]
- 4.Hager K, Setzer J, Vogl T, Voit J, Platt D. Blood coagulation factors in the elderly. Arch Gerontol Geriat. 1989;9(3):277–82. doi: 10.1016/0167-4943(89)90047-2. [DOI] [PubMed] [Google Scholar]
- 5.Dolan G, Neal K, Cooper P, Brown P, Preston FE. Protein C, antithrombin III and plasminogen: effect of age, sex and blood group. Brit J Haematol. 1994 Apr;86(4):798–803. doi: 10.1111/j.1365-2141.1994.tb04832.x. [DOI] [PubMed] [Google Scholar]
- 6.Lowe GD, Rumley A, Woodward M, Morrison CE, Philippou H, Lane DA, et al. Epidemiology of coagulation factors, inhibitors and activation markers: the Third Glasgow MONICA Survey. I. Illustrative reference ranges by age, sex and hormone use. Brit J Haematol. 1997 Jun;97(4):775–84. doi: 10.1046/j.1365-2141.1997.1222936.x. [DOI] [PubMed] [Google Scholar]
- 7.Stout RW, Crawford VL, McDermott MJ, Rocks MJ, Morris TC. Seasonal changes in haemostatic factors in young and elderly subjects. Age Ageing. 1996 May;25(3):256–8. doi: 10.1093/ageing/25.3.256. [DOI] [PubMed] [Google Scholar]
- 8.Cadroy Y, Daviaud P, Saivin S, Sie P, Boneu B. Distribution of 16 hemostatic laboratory variables assayed in 100 blood donors. Nouv Rev Fr Hematol. 1990 Jan;32(4):259–64. [PubMed] [Google Scholar]
- 9.Bauer K a, Weiss LM, Sparrow D, Vokonas PS, Rosenberg RD. Aging-associated changes in indices of thrombin generation and protein C activation in humans. Normative Aging Study. J Clin Invest. 1987 Dec;80(6):1527–34. doi: 10.1172/JCI113238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tracy RP, Arnold a. M, Ettinger W, Fried L, Meilahn E, Savage P. The Relationship of Fibrinogen and Factors VII and VIII to Incident Cardiovascular Disease and Death in the Elderly?: Results From the Cardiovascular Health Study. Arterioscl Throm Vas. 1999 Jul 1;19(7):1776–83. doi: 10.1161/01.atv.19.7.1776. [DOI] [PubMed] [Google Scholar]
- 11.Mari D, Coppola R, Provenzano R. Hemostasis factors and aging. Exp Gerontol. 2008 Feb;43(2):66–73. doi: 10.1016/j.exger.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Bauer KA, Kass BL, Ten Cate H, Hawiger JJ, Rosenberg RD. Factor IX is activated in vivo by the tissue factor mechanism. Blood. 1990 Aug 15;76(4):731–6. [PubMed] [Google Scholar]
- 13.Cadroy Y, Pierrejean D, Fontan B, Sié P, Boneu B. Influence of aging on the activity of the hemostatic system: prothrombin fragment 1+2, thrombin-antithrombin III complexes and D-dimers in 80 healthy subjects with age ranging from 20 to 94 years. Nouv Rev Fr Hematol. 1992 Jan;34(1):43–6. [PubMed] [Google Scholar]
- 14.Miesbach W, Alesci S, Krekeler S, Seifried E. Age-dependent increase of FVIII:C in mild haemophilia A. Haemophilia? 2009 Sep;15(5):1022–6. doi: 10.1111/j.1365-2516.2009.02051.x. [DOI] [PubMed] [Google Scholar]
- 15.Miesbach W, Alesci S, Krekeler S, Seifried E. Comorbidities and bleeding pattern in elderly haemophilia A patients. Haemophilia? 2009 Jul;15(4):894–9. doi: 10.1111/j.1365-2516.2009.02030.x. [DOI] [PubMed] [Google Scholar]
- 16.Conlan MG, Folsom AR, Finch A, Davis CE, Sorlie P, Marcucci G, et al. Associations of factor VIII and von Willebrand factor with age, race, sex, and risk factors for atherosclerosis. The Atherosclerosis Risk in Communities (ARIC) Study. Thromb Haemost. 1993 Sep 1;70(3):380–5. [PubMed] [Google Scholar]
- 17.Coppola R, Mari D, Lattuada A. von Willebrand factor in Italian centenarians. Haematologica. 2003 [PubMed] [Google Scholar]
- 18.Kadir R a, Economides DL, Sabin C a, Owens D, Lee C a. Variations in coagulation factors in women: effects of age, ethnicity, menstrual cycle and combined oral contraceptive. Thromb Haemost. 1999 Nov;82(5):1456–61. [PubMed] [Google Scholar]
- 19.Kokame K, Sakata T, Kokubo Y, Miyata T. von Willebrand factor-to-ADAMTS13 ratio increases with age in a Japanese population. J Thromb Haemost. 2011 Jul;9(7):1426–8. doi: 10.1111/j.1538-7836.2011.04333.x. [DOI] [PubMed] [Google Scholar]
- 20.Bowman M, Mundell G, Grabell J, Hopman WM, Rapson D, Lillicrap D, et al. Generation and validation of the Condensed MCMDM-1VWD Bleeding Questionnaire for von Willebrand disease. J Thromb Haemost. 2008 Dec;6(12):2062–6. doi: 10.1111/j.1538-7836.2008.03182.x. [DOI] [PubMed] [Google Scholar]
- 21.Sadler JE, Mannucci PM, Berntorp E, Bochkov N, Boulyjenkov V, Ginsburg D, et al. Impact, diagnosis and treatment of von Willebrand disease. Thromb Haemost. 2000 Aug;84(2):160–74. [PubMed] [Google Scholar]
- 22.Van den Burg PJM, Hospers JEHJ, Mosterd WLW, Bouma BNB, Huisveld IIA. Aging, physical conditioning, and exercise-induced changes in hemostatic factors and reaction products. J Appl Physiol; 2000;88(5):1558. doi: 10.1152/jappl.2000.88.5.1558. [DOI] [PubMed] [Google Scholar]
- 23.Ribeiro J, Almeida-Dias A, Ascensão A, Magalhães J, Oliveira a R, Carlson J, et al. Hemostatic response to acute physical exercise in healthy adolescents. J Sci Med Sport. 2007 Jun;10(3):164–9. doi: 10.1016/j.jsams.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Goodeve A, Eikenboom J, Castaman G, Rodeghiero F, Federici AB, Batlle J, et al. Phenotype and genotype of a cohort of families historically diagnosed with type 1 von Willebrand disease in the European study, Molecular and Clinical Markers for the Diagnosis and Management of Type 1 von Willebrand Disease (MCMDM-1VWD) Blood. 2007 Jan 1;109(1):112–21. doi: 10.1182/blood-2006-05-020784. [DOI] [PubMed] [Google Scholar]
- 25.James PD, Notley C, Hegadorn C, Leggo J, Tuttle A, Tinlin S, et al. The mutational spectrum of type 1 von Willebrand disease: results from a Canadian cohort study. Blood. 2007;109(1):145. doi: 10.1182/blood-2006-05-021105.. [DOI] [PubMed] [Google Scholar]
- 26.Barr RD, Sek J, Horsman J, Furlong W, Saleh M, Pai M, et al. Health status and health-related quality of life associated with von Willebrand disease. Am J Hematol. 2003 Jun;73(2):108–14. doi: 10.1002/ajh.10327. [DOI] [PubMed] [Google Scholar]
- 27.De Wee EM, Mauser-Bunschoten EP, Van Der Bom JG, Degenaar-Dujardin MEL, Eikenboom HCJ, Fijnvandraat K, et al. Health-related quality of life among adult patients with moderate and severe von Willebrand disease. J Thromb Haemost. 2010 Jul;8(7):1492–9. doi: 10.1111/j.1538-7836.2010.03864.x. [DOI] [PubMed] [Google Scholar]
- 28.Nichols WL, Hultin MB, James AH, Manco-Johnson MJ, Montgomery RR, Ortel TL, et al. von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA) Haemophilia. 2008 Mar;14(2):171–232. doi: 10.1111/j.1365-2516.2007.01643.x. [DOI] [PubMed] [Google Scholar]
- 29.Tosetto A, Rodeghiero F, Castaman G, Goodeve A, Federici AB, Batlle J, et al. A quantitative analysis of bleeding symptoms in type 1 von Willebrand disease: results from a multicenter European study (MCMDM-1 VWD) J Thromb Haemost. 2006 Apr;4(4):766–73. doi: 10.1111/j.1538-7836.2006.01847.x. [DOI] [PubMed] [Google Scholar]