Abstract
Whether internalizing symptoms increase or remain at similar levels throughout childhood is currently not well understood. Moreover, the association between vagal regulation of cardiac activity and internalizing symptoms across childhood needs to be clarified. We used a multilevel conceptual framework to examine how children's vagal regulation of cardiac activity and mothers' internalizing symptoms were jointly associated with children's developmental trajectories of internalizing symptoms from ages 4 to 10 years old. Data came from 384 children who participated in an ongoing longitudinal study. Children and their mothers came to the research laboratory at ages 4, 5, 7, and 10. Mothers reported their children's and their own internalizing symptoms. Children's vagal regulation of cardiac activity was assessed during quiet baseline tasks and also during challenge tasks. Multilevel models revealed that child internalizing symptoms increased from ages 4 to 10 years old, but only in females, and especially between ages 7 and 10. More vagal withdrawal in response to challenge was associated with more internalizing symptoms, particularly with more somatic symptoms. Associations between children's physiological regulation and internalizing symptoms differed by children's age, sex, and presence of maternal internalizing symptoms. Understanding associations between vagal regulation of cardiac activity and internalizing symptoms during childhood calls for fine-grained developmental analyses that take into account the heterogeneity of internalizing symptoms, and also developmental phase, context, and sex.
Keywords: internalizing symptoms, vagal regulation, RSA, somatic symptoms, pre-adolescence
Internalizing symptoms and disorders are among the most costly mental health problems in Western society (Collins et al., 2011). Historically, research on internalizing symptoms has focused on the early adolescent ages and onward (e.g., Angold, Erkanli, Silberg, Eaves, & Costello, 2002; Deković, Buist, & Reitz, 2004; Galambos, Barker, & Almeida, 2003; Kovacs & Paulauskas, 1984; Twenge & Nolen-Hoeksema, 2002). Recent work has shown, however, that rates of internalizing problems during childhood are comparable to those later in life, with 10 to 15% of US preschoolers meeting diagnostic criteria for a DSM anxiety or depressive disorder at any one assessment (Egger & Angold, 2006; Keenan, Shaw, Walsh, Delliquadri, & Giovannelli, 1997). Such early-onset internalizing problems predict more severe and persistent later mental health problems (e.g., Christie et al., 1988; Kasch & Klein, 1996; Kovacs, 1996). Therefore, understanding the development of internalizing symptoms during childhood may be key to preventing high costs associated with internalizing disorders across the life course.
The development of childhood internalizing symptoms can best be understood within a multilevel developmental psychopathology perspective, which draws on multiple levels of analyses in understanding adaptation and maladaptation across development (e.g., Calkins & Fox, 2002; Cicchetti, 2008; Cicchetti & Blender, 2006; Cicchetti & Dawson, 2002; Sroufe & Rutter, 1984). According to this perspective, multiple contributors—from different levels of biological and ecological influence—co-act and interact in the development of psychopathology. Here, we focus on several risk factors at the child's level: sex, vagal regulation of cardiac activity, and also behavioral regulation (externalizing symptoms). And, we test how such individual-level risks interact with contextual risk— including maternal psychopathology—in increasing or alleviating risk for internalizing symptoms. Notably, one of the key constructs in our study—the child's physiological reactivity during challenge—illustrates how developmental influences can both be risky or protective depending on the context in which they occur (Belsky & Pluess, 2009; Ellis & Boyce, 2005; Essex, Armstrong, Burk, Goldsmith, & Boyce, 2011; Obradović, Bush, Stamperdahl, Adler, & Boyce, 2010). Thus, our study will be informative about a key construct in the developmental psychopathology perspective—multifinality of outcomes—for children who display high versus low levels of vagal withdrawal when confronted with challenge (Cicchetti & Rogosch, 1999; Richters, 1997).
Taken together, the present study draws on a multilevel perspective to describe developmental trajectories of internalizing symptoms in children aged 4 to 10 years old in a community sample, and to test sex differences in these trajectories. The study also examines how children's individual-level factors—including sex and vagal regulation of cardiac activity—and maternal risks interact in predicting internalizing symptoms, and whether these associations change across development. Analytically, we use a multilevel modeling approach, which capitalizes on repeated measurements from study participants to extract information about changes within the person and also stable between-persons differences (Curran & Bauer, 2011; Hoffman & Stawski, 2009).
Trajectories of Internalizing Symptoms During the Childhood Years
Overall trajectories
Internalizing-spectrum symptoms primarily include symptoms of depression and anxiety, and also social withdrawal and somatic changes (Achenbach & Edelbrock, 1983). Few studies have examined developmental trajectories of such internalizing symptoms before adolescence. To date, these studies typically report small—if any—overall increases in mother- and also teacher-reported internalizing symptoms from ages 2 to approximately ages 8 to 10 (Colder, Mott, & Berman, 2002; Gazelle & Ladd, 2003; Sterba, Prinstein, & Cox, 2007). Although overall changes appear to be small, evidence points to larger increases in internalizing symptoms among subsets of children, especially among children with individual-level (e.g., temperamental) and/or contextual-level (e.g., poor relationships) risks (Gazelle & Ladd, 2003).
Sex differences in trajectories
Internalizing symptoms are known to be more prevalent in females than in males from mid adolescence (Angold et al., 2002; Twenge & Nolen-Hoeksema, 2002; Zahn-Waxler, Klimes-Dougan, & Slattery, 2000) throughout the reproductive years. Evidence for sex differences before mid adolescence, however, is mixed. A number of reviews suggested that, if anything, there may be a slight male excess in internalizing disorders before puberty (e.g., Angold & Worthman, 1993). Consistent with these suggestions, teacher-reported internalizing symptoms were higher in males than in females in one sample of kindergarteners— but subsequent changes in internalizing symptoms across the elementary school years did not differ by sex (Gazelle & Ladd, 2003). However, other community-based studies found no sex differences in initial levels of or changes in internalizing symptoms during the childhood years (Colder et al., 2002). Finally, in a large community sample that was representative of several communities in the US, a higher proportion of girls than boys had elevated and stable internalizing symptoms (Sterba et al., 2007).
Thus, although internalizing disorders are common in childhood, studies that have explicitly focused on changes in internalizing symptoms over time have generally reported that overall increases and also sex differences in internalizing symptoms during the pre-adolescent years are small—and/or limited to children with particular vulnerabilities. Renewed attention to potential increases in internalizing symptoms during the pre-adolescent period—including sex differences in changes—is warranted, however. Puberty played a central role in doubling rates of internalizing disorders by age 12/13 in previous cohorts (e.g., Angold, Costello, & Worthman, 1998). Secular changes in pubertal onset have since been documented, with pubertal processes in females currently beginning up to two years earlier compared to previous cohorts (Herman-Giddens, 2006). Therefore, we hypothesize that in our more recent cohort, we may observe increases in females' internalizing symptoms during the late middle childhood years.
Cardiac Regulation as A Correlate of Childhood Internalizing Symptoms
Neuroendocrine processes associated with the onset of puberty in females are not the only biological risk factors that have been linked to the development of internalizing symptoms. In this paper, we focus on the role of children's vagal regulation of cardiac activity, and its interaction with maternal risk, in the development of internalizing symptoms. Porges' Polyvagal Theory suggests that vagal regulation of cardiac activity often is involved in deficits in children's emotional regulation and social functioning and thus could serve as one of the biological foundations of problem behaviors in children (Porges, 2003, 2007; Porges & Furman, 2011). Here, we focus on inter-individual differences in activity of the parasympathetic nervous system, using two specific measures.
First, we measure baseline RSA (respiratory sinus arrhythmia) when children are resting in the laboratory without being exposed to environmental challenge. High baseline RSA (here also referred to as baseline vagal tone) is thought to reflect the organism's ability to support physiological and behavioral response when needed, and, thus, to reflect a higher capacity and flexibility in responding to environmental demands, including for regulating emotional reactivity (e.g., Calkins & Keane, 2004; Fortunato, Gatzke-Kopp, & Ram, 2013). For example, high baseline vagal tone has been linked with positive psychological adjustment and more successful regulation of negative emotions in children and adolescents (Beauchaine, 2001; Calkins & Fox, 2002) and also in adults (Kok & Fredrickson, 2010). Indeed, when the sample used here was studied at earlier ages (to age 5), higher levels of baseline vagal tone were associated with fewer internalizing symptoms (Calkins, Blandon, Williford, & Keane, 2007).
Second, we measure changes in vagal activity during times of challenge. Porges' theory suggests that during times of challenge, vagal input to the heart is withdrawn, resulting in decreases in RSA, and a freeing up of physiological resources that will allow the organism to engage with and address challenges encountered (Porges, 2003, 2007; Porges & Furman, 2011). In calculating such vagal withdrawal, we subtract RSA during a laboratory challenge from baseline RSA. The resultant measure is referred to as vagal withdrawal (or change RSA, vagal regulation, suppression, reduction, brake). It is viewed as a marker of parasympathetic-based physiological regulation, and assesses whether RSA decreases from baseline RSA when children encounter a challenge that requires active coping or emotional and behavioral regulation.
Vagal withdrawal during challenge is interpreted to reflect the organism's attention to and mobilization of resources to engage with and address that challenge. Nevertheless, the role of vagal withdrawal in psychopathology is less clear compared to the role of baseline vagal tone (Fortunato et al., 2013). Both, a lack of vagal withdrawal (or “blunted” vagal withdrawal) and RSA augmentation in response to challenge has been associated with greater levels of externalizing-spectrum behaviors (e.g., Calkins, Graziano, & Keane, 2007; Fortunato et al., 2013; Porges, Doussard-Roosevelt, Portales, & Greenspan, 1996). Findings with respect to internalizing-spectrum symptoms and disorders are less consistent, however (Graziano & Derefinko, 2013). Some work suggests that low vagal withdrawal could restrict children's ability to successfully cope with stressors (Graziano & Derefinko, 2013). And indeed, some studies have reported evidence of lower withdrawal scores being associated with more internalizing symptoms (Gentzler, Santucci, Kovacs, & Fox, 2009; Schmidt, Fox, Schulkin, & Gold, 1999). Other work, however, suggests that it may be high levels of vagal withdrawal that are associated with dysregulated affect and internalizing-spectrum problems (Beauchaine, 2001; Fortunato et al., 2013; Friedman, 2007; Gazelle & Druhen, 2009; Hinnant & El-Sheikh, 2009). In fact, the highest levels of vagal withdrawal have been reported in young people with particularly severe (Crowell et al., 2005) and also comorbid forms of internalizing symptoms (Calkins, Graziano, et al., 2007). For example, using just age 5 data from the dataset used here, Calkins and colleagues (2007) reported that children with comorbid internalizing and externalizing symptoms had the highest levels of vagal withdrawal across five different laboratory tasks.
In sum, high baseline RSA is typically universally associated with higher adaptation across different psychopathology outcomes. Evidence for associations between vagal withdrawal in response to challenge and internalizing symptoms is mixed, with some work suggesting that this measure has the potential to discriminate between externalizing and internalizing symptoms (Boyce et al., 2001; Calkins, Blandon, et al., 2007). Considering previous cross-sectional reports from our study, we expected that high vagal withdrawal would be associated with more internalizing symptoms—perhaps especially when high vagal withdrawal is combined with high externalizing symptoms. However, we also took into account several additional considerations, including heterogeneity of internalizing symptoms, contextual risk, and developmental-methodological considerations in further clarifying associations between cardiac regulation and internalizing psychopathology.
Additional Considerations in Understanding Cardiac Regulation-Internalizing Symptoms Associations
Heterogeneity in Childhood Internalizing Symptoms
Much of the work examining children's internalizing symptoms and cardiac regulation has relied on relatively generic summary assessments of child internalizing psychopathology such as the total internalizing symptoms scale of the Child Behavior Checklist Achenbach (Achenbach & Edelbrock, 1983). The internalizing symptoms assessed on this scale—anxious/depressed, somatic, and withdrawal symptoms—are quite heterogeneous, however, and could have distinct physiological correlates (e.g., Antonijevic, 2006; Rottenberg, Chambers, Allen, & Manber, 2007). Notably, the vagus nerve innervates not only the heart, but also the viscera, including the stomach. And, the vagus nerve is involved in immune system-brain communications, including in the development of cytokine-induced somatic symptoms or “sickness behaviors” (e.g., Dantzer, Konsman, Bluthé, & Kelley, 2000; Konsman, Parnet, & Dantzer, 2002; Marsland et al., 2007; Pavlov & Tracey, 2004; Thayer & Sternberg, 2006; Thayer & Sternberg, 2010). Thus, it is possible that inter-individual variation in vagal regulation is particularly closely associated with the somatic symptoms reported on the CBCL—including stomach aches, nausea, feeling sick, and physical problems without a known cause. Indeed, cross-sectional work from the pre-adolescent Tracking Adolescents' Individual Lives Survey sample suggested that the association of autonomic measures and internalizing symptoms could even be in opposite directions for somatic versus cognitive-affective symptoms (Bosch et al., 2009).
Increased Sensitivity to Risky Environments
A second consideration that has been raised in response to inconsistencies in findings regarding associations between vagal withdrawal and psychopathology is the possibility that vagal withdrawal may actually represent a double-edged sword in children's development, because its role may be highly dependent on contexts (Ellis & Boyce, 2005; Essex et al., 2011; Obradović et al., 2010). On the one hand, high vagal withdrawal could create biological sensitivities that allow children to flourish in optimal environments. Furthermore, high vagal withdrawal could reflect adaptive coping with select stressors (e.g., Obradović, 2012). On the other hand, in contexts characterized by overwhelming, “toxic” risks (Shonkoff & Garner, 2012), high vagal withdrawal could increase risk for children's internalizing psychopathology.
Environments created by mothers with high levels of internalizing symptoms have been described as emotionally disengaged from the child, with high levels of negative and depressive affect, depressive behaviors and cognitions, and also increases in stressful life events in the environment of the child (e.g., Goodman & Gotlib, 1999). Furthermore, anxious and depressed mothers have sometimes been described as over-intrusive and controlling (e.g., Lovejoy, Graczyk, O'Hare, & Neuman, 2000; Wood, McLeod, Sigman, Hwang, & Chu, 2003). When coupled with a child's high capacity to engage with environmental challenge, maternal depression and anxiety could become overwhelming to the child (Beauchaine, 2001; Crowell et al., 2005), and, thus, further enhance risks for child internalizing symptoms (e.g., Goodman & Gotlib, 1999; Sterba et al., 2007). Therefore, we hypothesize that high levels of vagal withdrawal will be particularly associated with internalizing symptoms in children whose mother suffers from high levels of internalizing symptomatology.
Changing Associations Across Development
A third consideration is that the nature of the association between cardiac regulation and internalizing symptoms could change in late childhood/early adolescence with the onset of pubertal processes. One set of empirical findings has shown that risk factors that are linked with internalizing symptoms during other developmental periods (e.g., childhood, adulthood) may temporarily cease to be associated with internalizing symptoms during times of dramatic biopsychosocial change (e.g., Shanahan, Copeland, Angold, & Costello, 2011), including during adolescence when biological risk factors such as pubertal hormones and low birth weight may become more prominent in etiological pathways (e.g., Costello, Worthman, Erkanli, & Angold, 2007). Indeed, during this time, stress systems could at least partially be recalibrated (Del Giudice, Ellis, & Shirtcliff, 2011), perhaps accounting for at least some of the temporary suspensions of associations between risk factors and internalizing outcomes. Other sets of findings suggest that during later parts of adolescence, stress-sensitivity increases, especially in girls (e.g., Ge, Conger, & Elder, 2001; Ge, Natsuaki, & Conger, 2006). In either scenario, the changing biological milieu and stress sensitivity could contribute to (potentially sex-differentiated) changes in associations between vagal regulation of cardiac activity and internalizing symptoms across development.
Developmental-Methodological Considerations
Inconsistencies in findings regarding vagal withdrawal and psychopathology have stimulated not only conceptual, but also methodological thinking about how to best study vagal regulation of cardiac activity. Young children's baseline vagal tone demonstrates considerable stability across assessments—even when spaced years apart (e.g., Bornstein & Suess, 2000; Perry et al., 2013). Thus, interindividual differences in vagal tone are detectable even at a young age. Measures of vagal withdrawal, however, are much less stable. Such low stability may be adaptive considering that highly stable stress reactivity could prevent children from responding adaptively to changing environments and times. Indeed, during the childhood years, correlations of vagal withdrawal from one year to the next are moderate in size at best (e.g., Calkins & Keane, 2004; Perry et al., 2013), but often, these correlations are small and non-significant (e.g., Bornstein & Suess, 2000; Perry et al., 2013), even across two-week intervals (e.g., Doussard-Roosevelt, Montgomery, & Porges, 2003).
Such instability poses a conundrum to developmental psychopathologists. On the one hand, the field strives to understand developmental changes in context. Therefore, changes in vagal withdrawal across time and contexts and their associations with psychopathology are often interpreted to reflect meaningful changes and adaptations. On the other hand, the field is also interested in stable inter-individual differences in self-regulatory capabilities that protect individuals in the face of adversity or increase their susceptibility to the development of psychopathology—but such inter-individual differences may not be well-captured with any single vagal withdrawal score. Indeed, it is possible that when we consider only single time-, situation-, or even task-specific measurements of vagal withdrawal, our understanding of any stable inter-individual differences in vagal withdrawal and their role in the development of psychopathology will be incomplete.
Therefore, recent work has suggested capitalizing on multiple measurements of vagal withdrawal to differentiate stable inter-individual differences in vagal withdrawal from within-person changes and fluctuations that could be situation specific—and to test how each of these aspects of vagal withdrawal are associated with psychopathology (Burt & Obradović, 2013). Consistent with this suggestion, we use a multilevel analytic strategy that capitalizes on four measurement points, allowing us to disaggregate within-person changes and fluctuations from between-persons stable differences in vagal withdrawal in order to better understand the signal between vagal withdrawal and internalizing psychopathology. We hypothesize that when we disaggregate vagal withdrawal observations over time into within-person changes/fluctuations versus more stable, trait-like between-persons differences, it will be the between-persons differences that are most strongly associated with children's psychopathology—a finding that has also been reported for other highly reactive physiological systems involved in stress regulation (e.g., Shirtcliff, Granger, Booth, & Johnson, 2005).
The Present Study
Taken together, the first goal of our study was to chart the developmental course of mother-reported internalizing symptoms from ages 4 to 10 in a relatively recent cohort of children, and to test sex differences in these trajectories. A second goal was to shed light on the role of baseline vagal tone and vagal withdrawal in children's internalizing symptoms. We expected that higher levels of vagal tone would be associated with lower internalizing symptoms, but that higher vagal withdrawal would be associated with higher internalizing symptoms. A third goal was to test whether associations between vagal regulation of cardiac activity and children's internalizing symptoms would be modified by the presence of maternal internalizing psychopathology. In conducting our analyses, we acknowledge the heterogeneity of internalizing symptoms. We also test whether associations differ by age and by sex. We used data from the long-term longitudinal ongoing Right Track Study. Internalizing symptoms in this study had previously been studied to age 5 (Calkins, Blandon, et al., 2007); the present study incorporates assessments at ages 7 and 10.5 years old and applies a multilevel modeling methodology in analyzing associations between vagal activity and trajectories of internalizing symptoms to late middle childhood.
Method
Participants
The current study used data from three cohorts of children who are part of an ongoing longitudinal study. The goal for recruitment was to obtain a sample of children who were at risk for developing future externalizing behavior problems that was representative of the surrounding community in terms of race and socioeconomic status (SES). All cohorts were recruited through child day care centers, the County Health Department, and the local Women, Infants, and Children program. Potential participants for Cohorts 1 and 2 were recruited at two years of age (Cohort 1, 1994-1996; Cohort 2, 2000-2001) and screened using the Child Behavior Checklist (CBCL 2-3, Achenbach, 1992) completed by the mother in order to oversample for externalizing behavior problems. Children were identified as being at risk for future externalizing behaviors if they received an externalizing T score of 60 or above. Efforts were made to obtain approximately equal numbers of males and females. This recruitment effort resulted in a total of 307 children. Cohort 3 was initially recruited when infants were 6 months of age (in 1998) for their level of frustration based on laboratory observation and parent report and were followed through the toddler period (for more information, see Calkins, Dedmon, Gill, Lomax, & Johnson, 2002). Children from Cohort 3 whose mothers completed the CBCL at two-years of age (N=140) were then included in the larger study. Of the entire sample (N = 447), 37% of the children were identified as being at risk for future externalizing problems at age 2. There were no significant demographic differences between cohorts with regard to sex, χ2 (2, N = 447) = 0.63, p = .73, race, χ2 (2, N = 447) = 1.13, p = .57, or two-year SES, F (2, 444) = 0.53, p = .59.
Of the 447 originally selected participants, six were dropped because they did not participate in any data collection at 2 years old. An additional 12 families participated at recruitment, did not participate at two-year, but did participate at later years. At 4 years of age, 399 families participated. Families lost to attrition included those who could not be located, moved out of the area, declined participation, or did not respond to phone and letter requests to participate. There were no significant differences between families who did and did not participate at age four in terms of sex, χ2 (1, N = 447) = 3.27, p = .07, race, χ2 (1, N = 447) = .65, p = .42, two-year SES, t (432) = -.92, p = .36, or 2-year externalizing T score, t (445) = .45, p = .65. At age 5, 365 families participated, including four who did not participate in the four-year assessment. Again, there were no significant differences between families who did and did not participate in terms of sex, χ2 (1, N = 447) = .76, p = .38, race, χ2 (1, N = 447) = .14, p = .71, 2-year SES, t (432) = -1.93, p = .06, and 2-year externalizing T score, t (445) = 1.39, p = .17.
At 7 years of age, 350 families participated, including 19 who did not participate in the 5-year assessment. Again, there were no significant differences between families who did and did not participate in terms of sex, χ2 (1, N = 447) = 2.12, p = .15, race, χ2 (3, N = 447) = .19, p = .67, and two-year externalizing T score, t (445) = 1.30, p = .19. Families with lower 2-year SES, t (432) = -2.61, p < .01, were less likely to participate in the 7-year assessment. At age 10, 357 families participated, including 31 families who did not participate in the 7-year assessment. No significant differences were noted between families who did and did not participate in the 10-year assessment in terms of child sex, χ2 (1, N = 447) = 3.31, p = .07; race, χ2 (3, N = 447) = 3.12, p = .08; 2-year SES, t(432) = .02, p = .98; or 2-year externalizing T score, t (445) = -.11, p = .91.
At the 4 year laboratory visit, 67% of the sample was European American, 27% African American, 4% biracial, and 2% Hispanic, and families were economically diverse. Hollingshead (Hollingshead, 1975) socioeconomic status (SES) scores for our sample ranged from 14-66 (M = 42.43, SD = 10.64), meaning that families from each level of social strata captured by this scale were represented in the current sample. Scores ranging from 40 to 54 are representative of the middle class. Averaged across all ages, maternal internalizing symptoms were at M = 0.36, SD = 3.55 for the raw-scores and M = 48.11, SD = 7.64 for the T-scores. In the current analyses, the sample was limited to children who had at least one valid assessment of physiological data between ages 4 to 10 and who also had data on internalizing symptoms (N=384 children, 176 male, 208 female).
Procedures
Children and their mothers came to our university-based laboratory to participate in the study. Consent from mothers and, beginning at age 4, verbal assents from children were obtained before data collection began. Mothers completed questionnaires in a private setting. Mother-child dyads participated in a number of tasks to measure emotional, behavioral, and psychophysiological regulation. Although laboratory procedures were not identical across time points, analogous age-appropriate tasks for children and their mothers were conducted at each time point as a way to maintain measurement equivalence. Mothers received an honorarium of $50 for every assessment that they participated in across the years of the study, and children received small age-appropriate toys for their participation.
Measures
Internalizing Symptoms
Mothers reported their children's internalizing symptoms on the Child Behavior Checklist (CBCL) at each age (Achenbach & Edelbrock, 1983). Here, we focused on assessments at ages 4, 5, 7, and 10, which used the CBCL for 4- to 18-year-olds. The CBCL has been found to be a reliable index of behavior problems across childhood. The internalizing subscale consisted of 33 items that included the anxious/depressed (13 items), somatic complaints (12 items), and withdrawn subscales (8 items). Examples of anxious/depressed symptoms included “feels worthless or inferior,” “worries,” and “feels or complains that no one loves him/her.” Example somatic symptoms included “nausea, feels sick,” “headaches,” and “stomachaches.” Example withdrawn symptoms included “would rather be alone than with others,” “too shy or timid,” and “withdrawn, does not get involved with others.” Mothers rated their child on a 3-point scale (not true, sometimes true, often true). Because our goal was to model change over time, we used the raw scores, and not the age-adjusted T-scores. Higher scores indicated higher internalizing symptoms. Across observations, bivariate correlations among the subscales were as follows: anxious/depressed-somatic: r = .50, p < .01; anxious/depressed-withdrawn: r = .60, p < .01; somatic-withdrawn: r = .36, p < .01. Because the overall internalizing score and also the scores representing each subscale were positively skewed, we used square-root transformed versions of the variables summarizing these scales.
Assessment of Baseline Vagal Tone and Vagal Withdrawal
For the 4, 5, 7 and 10 year assessments, vagal tone was assessed via an electrocardiogram (EKG) recording of children's Respiratory Sinus Arrhythmia (RSA) and Heart Rate (HR) was obtained throughout the course of the visits. Three disposable pediatric electrodes were placed on the child's chest in an inverted triangle pattern. The electrodes were connected to a preamplifier, the output of which was processed through a vagal tone monitor (VTM-I, Delta Biometrics, Inc, Bethesda, MD) for R-wave detection. At the 10 year assessment for cohorts 2 and 3, updated equipment was used to collect heart rate in which heart rate-EKG collection varied slightly. For these cohorts, application of three disposable electrodes were placed on the child using Fetrode ® leads in a similar inverted triangle pattern (right collarbone, lower left rib, and lower right side of the stomach). The Fetrode lead is less sensitive to gross motor movement compared to regular electrode leads. The electrodes were connected to a preamplifier, the output of which was processed through a vagal tone monitor (Biolog 399×; UFI; Morro Bay, CA) for R-wave detection. A data file containing the interbeat intervals (IBIs) for the entire period of HR collection was transferred to a laptop computer for later artifact editing (resulting from child movement) and analysis. Analyses were conducted with MXEDIT program for the 4, 5, 7 and 10 year cohort 1 assessments. For the 10 year cohorts 2 and 3 assessments, Cardio Batch/Edit software (Brain-Body Center, University of Illinois at Chicago, Chicago) was used.
Both software programs used the Porges (2003) method for deriving RSA values. Editing the files consisted of scanning the data for outlier points. Identified outliers were replaced by dividing or summing them with the adjacent data so that the outliers would be consistent with the surrounding data. Only data files in which less than 10% of the data required editing were included in the current study. The Porges method of analyzing IBI data applies an algorithm to the sequential heart period (HP) data. The algorithm uses a moving 21-point polynomial to detrend periodicities in HP that are slower than RSA. Next, a bandpass filter extracts variance in HP within the frequency band of spontaneous respiration in young children, 0.24-1.04 Hz. The natural log of this variance is taken and reported in units of ln (msec)2. For each of the laboratory tasks, RSA was calculated every 30 seconds and the average across the 30-second epochs for each episode was used in subsequent analyses. Data were excluded if the standard deviation for an episode was over 1.0.
For the purposes of our data analysis, at each assessment, we used the baseline measurement of vagal tone and also measurements of vagal tone taken during challenge tasks. Baseline RSA was generally obtained at the beginning of the laboratory visit while the child was resting in a chair or engaged in a quiet activity that would limit motion artifacts in the data. For example, during ages 4 and 5, children watched a 5-min segment of the videotape “Spot.” At the 7 and 10-year assessments, children were asked to sit still without talking for 2 and 4 minutes, respectively.
RSA withdrawal was calculated as the difference between baseline RSA and average RSA during challenge tasks. Positive change scores represented a decrease in RSA from the baseline to the task, reflecting attempts at vagal regulation. Specifically, we focused on the average vagal withdrawal measured during frustration and teaching tasks here—which both were administered at ages 4, 5, 7, and 10. High irritability/low frustration tolerance is considered a common characteristic of children with internalizing disorders (Stoddard et al., 2013; Stringaris, 2011; Stringaris, Zavos, Leibenluft, Maughan, & Eley, 2012); thus, biological regulation in response to frustration tasks could be highly informative for understanding the development of internalizing symptoms. Example frustration tasks included instructing the child to draw perfect circles and then telling him/her in what ways the circles were not perfect, asking the child to sort candy for themselves but then taking the candy, and playing games that were “rigged” in a way that would elicit frustration in the child. Example teaching tasks included asking the mother to work on puzzles of increasing difficulty with the child and to assist the child when needed. Given our interest in mothers with internalizing symptoms, vagal withdrawal in these teaching tasks spent with mothers was also of particular interest here. Another approach to assessing vagal withdrawal at each age would be to average vagal withdrawal across all tasks administered during a given laboratory visit. Yet another approach would be to analyze vagal withdrawal observed in different tasks separately, which would have exponentiated the number of analyses conducted here.
Hollingshead Socioeconomic Status scores were computed using a weighted average of parental education and employment (Hollingshead, 1975). The range observed in the current sample indicated that families from each level of social strata captured by this scale were represented in the current sample.
Maternal Internalizing Symptoms
The Symptom Checklist-90—Revised (Derogatis, 1986) was used to measure self-reported maternal psychopathology symptoms at each assessment. The 90 items were rated on how much distress they caused over the previous 7 days using a 5-point scale ranging from 0 to 4 (not at all, a little bit, moderately, quite a bit, and extremely). The correlation of the maternal depressive and anxious symptoms subscales across all assessments was at r = .72, p < .01. Thus, for the present analyses, mothers' scores on the depressive and anxious symptoms subscales were averaged.
Child Externalizing Symptoms
The Child Behavior Checklist (CBCL) maternal report was used to assess externalizing symptoms (Achenbach & Edelbrock, 1983) at each age. The externalizing subscale consisted of 33 items that included the minor subscales of aggression and delinquency.
Analytic Strategy
The analytic strategy was designed to examine trajectories of internalizing symptoms, including normative mean trajectories, sex differences in these trajectories, variation in trajectories, and sources of within- and between-persons variation in internalizing symptoms. Participants could have up to four observations between ages 4 to 10, creating a multilevel nested-within-persons data structure. Multilevel modeling (MLM), also known as hierarchical linear, general linear mixed, or random coefficient models (Laird & Ware, 1982) extends multiple regression to address nested data structures, such as the repeated measures structure of the current study (Bryk & Raudenbush, 1987, 1992).
Therefore, multilevel modeling (MLM) using PROC MIXED in SAS (SAS Institute Inc, 2008) was performed to conduct growth curve analyses on repeated measures of internalizing symptoms, specifying the covariance matrix as unstructured. Level 1 here represents within-person change and Level 2 represents stable between-persons differences. Accordingly, at Level 1, age, and time-varying covariates (e.g., within-person change in baseline vagal tone) were included to model within-person change over time. At Level 2, time-invariant, covariates (e.g., race/ethnicity, sex, person-mean of baseline vagal tone across time) were used to model between-persons stable differences in trajectories of internalizing symptoms.
A series of increasingly complex models were run in three steps. First, we tested unconditional means models with random intercepts but no predictors in order to establish baseline values for fit indices and to estimate the percent variance of internalizing symptoms at the between-versus within-person levels via the intraclass correlation (ICC = intercept variance/(intercept variance + residual variance). This unconditional means model allows for an estimation of how much variation in internalizing symptoms from ages 4 to 10 is attributable to within- versus between-persons differences.
Next, an overall growth model was selected by testing a series of models with fixed and random age and age2 effects, and with two-way interactions between sex and age. The statements SUBJECT=ID and TYPE=UN were used to specify the nested/repeated data structure. The fit of other covariance structures, including autoregressive ones were also tested, but provided a worse fit to the data. Growth models presented in Table 2 were selected by comparing goodness-of fit indices across models. Results from these models show the basic developmental course of internalizing symptoms, and also indicate whether it varies by sex.
Table 2.
Fixed effects, random effects, and model fits for unconditional means model (Model 1) and basic growth curve models (Model 2) of square-root-transformed internalizing symptoms from ages 4 to 10. Model 3 is a basic growth model that uses a dichotomized age term (1 = 10.5 yrs old, 0 = 4 to 7 yrs old). N = 1,311 observations from 384 children.
|
Model 1 Unconditional Model |
Model 2 Basic Growth Curve |
Model 3 Dichotomous Age10 Term |
||||
|---|---|---|---|---|---|---|
| Estimate | SE | Estimate | SE | |||
| Fixed Effects | ||||||
| Intercept | 1.814*** | 0.052 | 1.946** | 0.304 | 1.781*** | 0.099 |
| Race (1=Caucasian) | 0.071 | 0.107 | 0.070 | 0.108 | ||
| Age | -0.085 | 0.090 | 0.349*** | 0.073 | ||
| Age2 | 0.010 | 0.006 | ||||
| Sex (1 = Male) | -0.920* | 0.422 | -0.144 | 0.103 | ||
| Age by Sex | 0.265* | 0.127 | -0.330** | 0.102 | ||
| Age2 by Sex | -0.021* | 0.008 | ||||
| Random Effects | ||||||
| Intercept | 0.848** | 0.074 | 1.050** | 0.164 | 0.825*** | 0.074 |
| Age | 0.011** | 0.003 | 0.213** | 0.079 | ||
| Residual | 0.539** | 0.025 | 0.440** | 0.025 | 0.474*** | 0.027 |
| ICC | .61 | |||||
| Model Fit | ||||||
| REML Deviance | 3608.6 | 3579.6 | 3559.3 | |||
| AIC | 3612.6 | 3587.6 | 3567.3 | |||
| BIC (smaller is better) | 3620.5 | 3603.4 | 3583.1 | |||
p < .001
p < .01
p < .05,
p < .10
Acronyms: REML = Restricted Maximum Likelihood; AIC = Akaike's information criterion; BIC = Bayesian information criterion; ICC = Intraclass correlation.
ICC was calculated as follows: Intercept random effect/(Intercept random effect + Residual Random Effect)
Next, we entered covariates into the model. We began by entering RSA baseline and RSA withdrawal variables. Because these variables were measured at each time-point, they can be considered time-varying covariates. Some elaborations regarding the use of time-varying covariates are necessary here. As with internalizing symptoms, these time-varying covariates contain multilevel information about between- and within-persons differences (Curran & Bauer, 2011; Hoffman & Stawski, 2009). For example, the baseline vagal tone variable contains information about how a person's typical baseline vagal tone deviates from other people's typical baseline vagal tones (a between-persons, Level 2 effect) and also how a person's baseline vagal tone at any one point deviates from the person's own typical or expected baseline vagal tone (a within-person, Level 1 effect).
Therefore, we created Level 1 within-person and Level 2 between-persons differences variables for both vagal tone and vagal withdrawal. The latter was created as the person-mean of vagal tone across all assessments. The former was created to indicate how much a person's vagal tone at the current assessment deviated from that person's typical vagal tone (i.e., their person-mean). These within- and between persons variables can also be created with regression analyses techniques that take into account expected mean changes in these time-varying covariates over time (Curran & Bauer, 2011; Hoffman & Stawski, 2009). When we conducted such analyses, results were similar to the pattern of results reported here. In our results section, we also refer to Level 2 between-persons effects as “trait-like” and within-person Level 1 effects as “state-like” effects, because the ideas of trait and state are useful corollaries in understanding the multilevel models used here (Curran & Bauer, 2011; Nezlek, 2007). In order to facilitate interpretation and to simplify the highly complicated analyses, we focused on disaggregating within- and between-persons effects for the physiological variables, but treated maternal internalizing symptoms, child externalizing symptoms, and SES as time-invariant variables—using the person means across all observations for these variables as each person's value on these variables.
Taken together, multilevel analytic models allow one to contextualize a person's time-varying physiological covariates—such as baseline vagal tone—by comparing it to other people's typical levels (e.g., other people's typical baseline vagal tone), but also to each individual's own typical, or expected level (e.g., a person's typical baseline vagal tone). MLM can disaggregate these between- and within-person effects for continuous time-varying covariates (Curran & Bauer, 2011; Hoffman & Stawski, 2009). The use of MLM is also ideal because individuals with missing at random data can be incorporated (Kuo, Mohler, Raudenbush, & Earls, 2000). Thus, all available data could be included here, improving statistical estimation (Little & Rubin, 1987) and the robustness of estimates (Schafer, 1997).
Results
Descriptive statistics for all study variables are shown in Table 1.
Table 1.
Descriptive statistics of main study variables across all ages.
| Age 4 | Age 5 | Age 7 | Age 10 | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Internalizing Symptoms | 3.96 | 4.23 | 4.63 | 5.13 | 4.52 | 5.00 | 5.65 | 6.16 |
| Square Root (Internalizing) | 1.66 | 1.10 | 1.82 | 1.16 | 1.78 | 1.17 | 2.00 | 1.28 |
| Anxiety/Depression | 1.91 | 2.47 | 2.62 | 3.18 | 2.48 | 3.09 | 2.82 | 3.19 |
| Somatic Symptoms | 0.77 | 1.31 | 0.77 | 1.42 | 1.09 | 1.65 | 1.67 | 2.36 |
| Withdrawal Symptoms | 1.33 | 1.55 | 1.31 | 1.64 | 1.03 | 1.50 | 1.19 | 1.75 |
| Baseline Vagal Tone | 5.85 | 1.18 | 6.08 | 1.15 | 6.53 | 1.21 | 6.44 | 1.21 |
| Vagal Withdrawal | 0.28 | 0.60 | 0.25 | 0.72 | 0.79 | 0.78 | 0.57 | 0.82 |
| Socioeconomic Status | 42.42 | 10.67 | 42.99 | 10.58 | 44.56 | 11.66 | 44.15 | 11.98 |
| Maternal Internalizing | 49.13 | 9.12 | 49.04 | 8.75 | 46.74 | 8.27 | 47.00 | 8.68 |
| Child Externalizing | 10.53 | 6.79 | 10.20 | 7.60 | 7.67 | 6.40 | 5.82 | 6.23 |
Unconditional Means and Basic Growth Curve Models
A first set of models established unconditional means models and growth curves of internalizing symptoms from ages 4 to 10 for children who had data on vagal withdrawal at least once between ages 4 and 10. The intra-class correlation (ICC) calculated from Model 1 in Table 2 was .61 for the overall sample (.59 for females, .64 for males). Thus, approximately 61% of the total variation in children's internalizing symptoms from ages 4 to 10 was due to between-persons variation (i.e., stable inter-individual differences), whereas approximately 39% was due to within-person variation (i.e., within-person changes/fluctuations over time).
Fixed Effects
Next, growth curve models established the basic trajectory of internalizing symptoms (Model 2 in Table 2). Because of the possibility that trajectories of internalizing symptoms could become sex-differentiated during late childhood, interactions of the age and age2 coefficients with sex were tested. The highest-order significant coefficient was the age2 by sex interaction. Figure 1 shows that there were essentially no increases in females' internalizing symptoms from ages 4 to 7, followed by relatively steep increases between ages 7 and 10. In comparison, the age and age2 terms were not significant when the analyses were conducted for males only.
Figure 1.
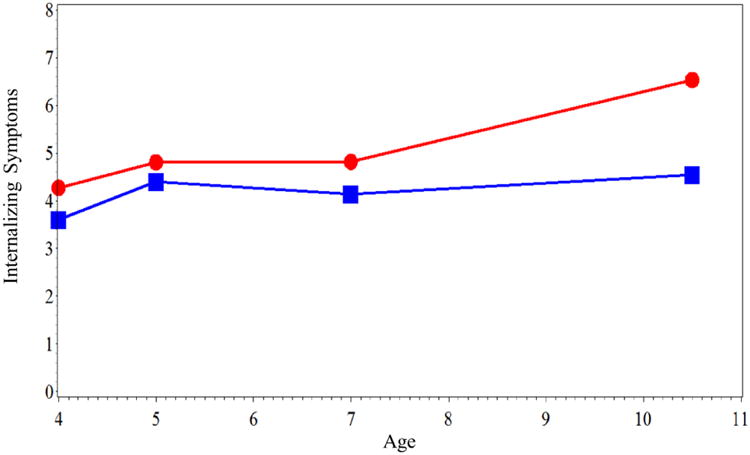
Mean internalizing symptoms in females (red line with circles) and males (blue line with squares) from ages 4 to 10.5 years old.
Additional analyses for illuminating patterns of change
An inspection of Figure 1 suggests that that the steepest increases occurred between ages 7 and 10. Therefore, we created a dichotomous age10 term to better quantify change associated with this spline point (coded 0 at ages < 10 and 1 at age 10.5). The age10 term was used in Model 3. Results showed that the age10 by sex interaction was significant at b = -.33, p < .01. When analyses were conducted separately by sex, the age10 term was significant at b = .35, SE = .07, p < .001 in females, but not in males (b = .02, SE = .07, p = .78).
Additional analyses examined patterns of change for each subscale of internalizing symptoms. ICCs were at .54, .42, and .49 for the anxious/depressed, somatic symptoms, and withdrawn subscales, respectively. For anxious depressive symptoms, the age10 by sex interaction was at b = -.20, SE = .09, p = .02. For somatic symptoms, the interaction was at b = -.15, SE = .09, p = .09. Finally, for withdrawal, the interaction was at b = -.21, SE = .08, p = .007. Taken together, mother-reported internalizing symptoms during childhood were relatively stable. The model using the dichotomous age10 term (Model 3) was the most parsimonious model for capturing sex-differentiated changes in internalizing symptoms from ages 4 to 10—for the overall scale and also each of the subscales. Therefore, all subsequent analyses expand on Model 3 when including additional covariates.
Clarifying Associations between Cardiac Regulation and Internalizing Symptoms
A next set of analyses (shown in Table 3) added: 1) the between-persons vagal regulation variables and 2) the within-person vagal regulation variables to Model 3, controlling also for SES. Most associations with internalizing symptoms that were significant or marginally significant were with between-persons stable as opposed to the within-persons change variables. Among the three internalizing symptoms subscales, the strongest association was observed between higher levels of vagal withdrawal and more somatic symptoms. There were also significant association between higher baseline vagal tone and fewer somatic and withdrawal symptoms.
Table 3.
Associations of between- and within-person vagal variables with overall internalizing scale and internalizing subscales.
| Overall | Anx/dep | Somatic | Withdrawn | |||||
|---|---|---|---|---|---|---|---|---|
| Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | |
| Intercept | 2.735*** | 0.422 | 1.521*** | 0.323 | 1.326*** | 0.276 | 1.674*** | 0.260 |
| Controls | ||||||||
| Race (1 = Caucasian) | 0.050 | 0.111 | 0.131 | 0.088 | -0.095+ | 0.072 | 0.049 | 0.070 |
| Age10 (1 = 10.5 yrs old) | 0.348*** | 0.073 | 0.237*** | 0.062 | 0.358** | 0.067 | 0.015 | 0.055 |
| Sex (1 = Male) | -0.136 | 0.102 | -0.117 | 0.084 | -0.107+ | 0.061 | 0.007 | 0.066 |
| Age10 × Sex | -0.327** | 0.103 | -0.198* | 0.087 | -0.150+ | 0.090 | -0.209** | 0.078 |
| Socioeconomic Status | -0.011 | 0.006 | -0.006 | 0.004 | -0.006+ | 0.003 | -0.011** | 0.004 |
| Cardiac Regulation | ||||||||
| Step 1 – Between-Persons Variables | ||||||||
| BP Vagal Tone | -0.093 | 0.059 | -0.024 | 0.045 | -0.079* | 0.038 | -0.075* | 0.036 |
| BP Vagal Withdrawal | 0.220* | 0.110 | 0.128 | 0.087 | 0.183** | 0.066 | 0.059 | 0.075 |
| Step 2 – Within-Person Variables | ||||||||
| WP Vagal Tone | 0.030 | 0.033 | 0.039 | 0.031 | 0.021 | 0.031 | -0.008 | 0.026 |
| WP Vagal Withdrawal | -0.039 | 0.044 | -0.038 | 0.043 | 0.039 | 0.038 | -0.071* | 0.034 |
p < .001
p < .01
p < .05,
p < .10
Acronyms: BP = between-persons; WP = within-person; SE = standard error of estimate; Anx/dep = anxiety/depression
Because significant associations were primarily identified with the between-persons variables, and the within-persons variables also did not further interact with sex or age in the prediction of internalizing symptoms, the within-persons vagal tone and withdrawal variables were subsequently removed from all models. We should also note that no significant squared effects for either squared baseline vagal tone or squared vagal withdrawal emerged. Furthermore, the between-persons baseline vagal tone and withdrawal variables did not interact in the prediction of internalizing symptoms.
Interactions with Sex and Age
Next, we tested whether associations between the between-persons vagal variables and internalizing symptoms differed by age and sex or both. A three-way interaction between vagal withdrawal, age10, and sex emerged in the prediction of overall internalizing symptoms (b = .58 SE = .23, p < .05), and also in the prediction of the anxious/depressed subscale (b = .38, SE = .19, p = .05). Follow-up analyses, illustrated in Figure 2, showed that higher levels of vagal withdrawal were associated with internalizing symptoms only in girls aged 4 to 7, but not in older girls or in boys (b = 0.36, SE=.14, p = .02 for the association between vagal withdrawal and overall internalizing symptoms in young girls, and b = 0.01, SE = .23, p = .95 for the association between vagal withdrawal and overall internalizing symptoms in older girls). Figure 2 also illustrates that girls with high vagal withdrawal already had relatively high levels of internalizing symptoms in early middle childhood, which stay high, increasing only slightly into late middle childhood/early adolescence. On the other hand, females with low vagal withdrawal had lower rates of internalizing symptoms in early middle childhood, but then increased more steeply, reaching levels that were comparable to those of girls with high vagal withdrawal. Indeed, in girls with low vagal withdrawal, the age10 term was significant at b = .57, SE = .10, p < .001, but in girls with high vagal withdrawal, the age10 term was not significant in predicting internalizing symptoms (b = .13, SE = .10, p = .18).
Figure 2.
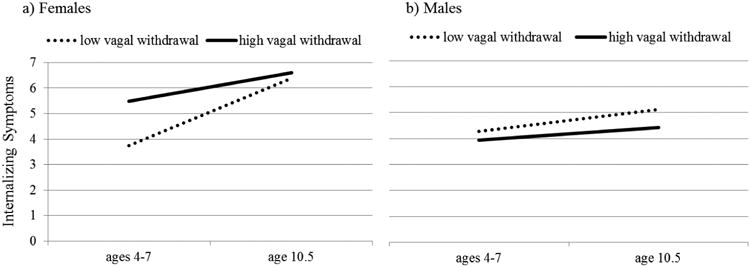
Mean internalizing symptoms in females and males with low versus high vagal withdrawal before and at age 10.5.
Interactions with Maternal Psychopathology
In a next step, we conducted analyses testing whether high vagal withdrawal confers a high risk for internalizing symptoms especially—or only—in the context of maternal internalizing psychopathology, and also whether these processes change with age. There was a main effect of maternal internalizing symptoms on child internalizing symptoms at b = .68, SE = .15, p < .01; maternal internalizing symptoms was also associated with each subscale of internalizing symptoms. Results also revealed a three-way interaction among vagal withdrawal, maternal internalizing symptoms, and the age10 term in the prediction of overall internalizing symptoms (b = -.76, SE = .33, p = .02), and also anxious/depressive symptoms (b = -.75, SE = .35, p = .03), and withdrawal symptoms (b = -.68, SE = .26, p < .01). Follow-up analyses showed that associations between vagal withdrawal and internalizing symptoms were especially strong in children whose mothers scored above the median on internalizing symptoms—but only between ages 4 to 7 and not thereafter (b = .38, SE = .18, p = .04 for the association between vagal withdrawal and child internalizing symptoms in the context of high maternal internalizing symptoms at ages 4-7, and b = .17, SE = .29, p = .55 for the same association after age 7). In contrast, when maternal internalizing symptoms were below the median, vagal withdrawal and child internalizing symptoms were not associated (b = .06, SE = .11, p = .56). Thus, it is mostly in the high-risk context of maternal psychopathology that high vagal withdrawal is associated with increased risk for child internalizing symptoms— anxious/depressed and withdrawal symptoms in particular.
Testing the Moderating Role of Externalizing Symptoms
Finally, previous work had reported that vagal withdrawal is highest in individuals with co-occurring internalizing and externalizing symptoms. Therefore, we tested whether the role of vagal withdrawal would be further modified by externalizing symptoms. There was a main effect of child externalizing symptoms on child internalizing symptoms (b = .09, SE = .01, p < .01). Furthermore, a two-way interaction between vagal withdrawal and externalizing symptoms emerged (b = .03, SE = .01, p < .05). Follow-up analyses indicated that high vagal withdrawal was only associated with increased risk for internalizing symptoms in the context of high externalizing symptoms, but not otherwise. Taking the vagal withdrawal by child externalizing symptoms into account did not change the significance of any of the previously reported interactions.
Discussion
Whether internalizing symptoms increase or remain at similar levels throughout childhood is currently not well understood. Moreover, the association of vagal regulation of cardiac activity and internalizing symptoms during these ages needs to be clarified. Here, we used a multilevel modeling analytic strategy to examine developmental trajectories of internalizing symptoms from ages 4 to 10 and to test sex differences in these trajectories. Furthermore, we drew on a multilevel conceptual framework to test whether changes in childhood internalizing symptoms would be predicted by interactions between children's physiological regulation and maternal internalizing symptomatology. We also tested whether associations between child vagal withdrawal and internalizing symptoms would differ by sex and age.
Results indicated that internalizing symptoms increased after age 7, but only in females. It is possible that with the earlier onset of pubertal processes in recent cohorts, sex-differentiation in internalizing symptoms will be observed before the 12/13 year mark that had previously been established as a benchmark for sex-differentiated changes with earlier cohorts. Our sample consisted of approximately 30% African American children—in whom puberty has been found to begin at earlier ages compared to Caucasian children (Herman-Giddens et al., 1997). And, parts of the sample were initially over-recruited for externalizing symptoms. Thus, the racial composition of the sample and also the higher percentage of children with early externalizing symptoms could have contributed to the earlier emergence of sex differences compared to previous cohorts and other studies. Maternal-reported child internalizing symptoms were quite stable from ages 4 to 10. Stability of maternal reports of child behavior are not a new finding. In fact, stability coefficients for mother-reported externalizing behaviors from ages 4 to 10 in this sample were similarly high. More work is needed to test whether internalizing symptoms assessed from other reporters (e.g., teachers, fathers, the child—to the extent possible) are similarly stable or whether high stability is, in part, a function of “generic” stability of maternal reports of child behavior.
The Role of Cardiac Regulation in Children's Internalizing Symptoms
For vagal regulation of cardiac activity, the strongest “signal” with respect to internalizing symptoms emerged when considering stable between-persons differences. The literature on vagal withdrawal and psychopathology had been characterized by a number of inconsistent findings—drawing attention to both methodological and conceptual refinements that may be necessary in understanding the role of parasympathetic reactivity in psychopathology. Our findings lend support to recent methodological suggestions that testing both stable and also change aspects of vagal withdrawal in relation to psychopathology is worthwhile (Burt & Obradović, 2013).
Our results suggested that it was the stable, more trait-like aspects of vagal withdrawal that were associated with increased risk for internalizing symptoms during preadolescence. It is possible, however, that with respect to other outcomes, including more in-the-moment and approach-type disruptive and behavioral symptoms, it will be deviations from these typical vagal withdrawal levels that will be most strongly associated with psychopathology. Any single score of vagal withdrawal represents a mixture of more stable and the more in-the-moment aspects. Only longitudinal designs with multiple measurements can tease these aspects apart. In addition to the multilevel analytic techniques used here, state-trait models and other latent variable approaches reviewed by Burt and Obradović (2013) are also promising approaches for disaggregating the different types of information contained within single scores.
Additional Nuances in Studying Cardiac Regulation-Internalizing Symptoms Associations
Our findings illustrated that when studying cardiac regulation-internalizing symptoms associations, the heterogeneous nature of internalizing symptoms should be considered and a disaggregation into the more somatic and the more cognitive-affective internalizing symptoms may be warranted (Bosch et al., 2009). Given that the vagus nerve not only connects the brain with the heart, but also with viscera, it was not surprising to see that it was the somatic symptoms that were most strongly associated with the vagal variables assessed here. Such somatic symptoms have been found to be promising markers for identifying risk for psychopathology even decades later (Shelby et al., 2013). The associations detected here could, in part, be indicative of the role of the vagus nerve in immune system-brain communications (e.g., Dantzer et al., 2000; Konsman et al., 2002; Marsland et al., 2007; Pavlov & Tracey, 2004; Thayer & Sternberg, 2006; Thayer & Sternberg, 2010) that may also play a role in the development of some internalizing disorders (Raison, Capuron, & Miller, 2006). A better understanding is needed of whether children with low baseline vagal tone, high vagal withdrawal and high somatic symptoms go on to meet thresholds for internalizing disorders and associated impairments at later points in time, and, thus, whether vagal regulation, in conjunction with high somatic symptoms could be a promising risk marker for later internalizing disorders.
Our findings further suggest that the developmental story of the role of vagal regulation of cardiac activity may differ for somatic symptoms versus anxious/depressive symptoms. High vagal withdrawal was relatively robustly associated with somatic symptoms across development, and no differences in association were identified across ages and males versus females. On the other hand, associations between high vagal withdrawal and anxious/depressive symptoms were conditional upon developmental stage and sex. Only in the pre-adolescent girls was high vagal withdrawal significantly associated with anxious/depressive symptoms (and also the overall internalizing scale), but not in the older girls or boys. Previous research had suggested that the onset of puberty brings along new biological factors that contribute to rising rates of internalizing symptoms in females during their reproductive years. The emergence of these new biological factors may at least temporarily suspend associations between other risk factors and internalizing symptoms (Shanahan et al., 2011). Furthermore, it is possible that stress systems are at least partially being recalibrated during this time (Del Giudice et al., 2011). The Right Track sample will be assessed again at ages 15 and 17 years old; thus, future work with this sample will test whether associations between vagal withdrawal and anxious/depressive symptoms come back online in females at later points in development or whether they have been permanently altered during puberty.
Cardiac Regulation-Internalizing Symptoms Associations in the Context of Maternal Risk
During pre-adolescence, some findings were generally consistent with sensitivity to context hypotheses (Ellis & Boyce, 2005; Essex et al., 2011; Obradović et al., 2010). Specifically, pre-adolescent girls with high vagal withdrawal were only at risk for higher internalizing symptoms in the context of high maternal internalizing symptoms. Parenting behaviors of mothers with high levels of depressive and anxious symptoms have long been characterized as emotionally disengaged with high levels of negative and depressive affect, behaviors and cognitions. Maternal internalizing symptoms are also associated with parenting behaviors that increase stressful life events in the environment of the child (e.g., Goodman & Gotlib, 1999). If a child has high capacity to direct attention to such stressors—but at the same time is unable to change the stressors or successfully cope with them—such stressors could become overwhelming and “toxic” (Shonkoff & Garner, 2012). Thus, consistent with sensitivity to context ideas, it may be that children with high vagal withdrawal thrive only in contexts of environmental support, but are especially negatively affected in contexts of overwhelming risks (Beauchaine, 2001; Crowell et al., 2005). Despite the extensive documentation of risk environments created by mothers with high internalizing symptomatology, it is, of course, also possible that the effects of maternal internalizing psychopathology identified here at least partially represent genetic risk.
Taken together, our findings added even more layers to thinking about vagal regulation and psychopathology during childhood. A recent meta-analysis reported that the overall effect size of associations between vagal withdrawal and psychopathology tends to be small (Graziano & Derefinko, 2013). Recent reviews have also pointed out that associations between vagal variables and psychopathology could differ depending on a) the psychopathology outcome considered, b) the task used to assess vagal withdrawal, c) the typical contextual risk in the child's rearing environment, d) the developmental phase that the child is currently in (Fortunato et al., 2013), e) whether the sample is community-based or clinical (Graziano & Derefinko, 2013), and f) the methodology used in assessing vagal activity (Graziano & Derefinko, 2013). Our results further suggest that these associations could depend on g) whether it is the stable aspects of vagal withdrawal or measurement-point-specific fluctuations that are being considered, h) which domain of internalizing symptoms is being considered, and i) the sex of the person.
The possibility that the nature of the association between RSA and psychopathology may depend on 9 or more facets makes this a conceptually rich field for developmental psychopathologists embracing multilevel and contextual perspectives. At the same time, the potential complexities contribute to difficulties in distinguishing between what is a finding indicative of an actual signal rather than a chance-finding or “noise” in RSA-psychopathology associations. The present study is not immune to this criticism. Indeed, an important task for the field in moving forward is to have an ongoing discussion about how to acknowledge and incorporate contingencies and complexities while also having appropriate safeguards against over-interpretation of chance findings.
Multilevel Analytic Techniques and Multilevel Conceptual Approaches
One aim of our study was to take a multilevel approach to understanding the development of internalizing symptoms during the earlier parts of childhood. It is important to note that in the field of developmental psychopathology the term “multilevel” is used to refer to both conceptual and also to methodological/statistical approaches—with different meanings in each context. Conceptually, the term “multi-level” is used to characterize models that incorporate different domains of influence on development and also examine how these levels of influence combine in shaping the development of the person—leaning, for example, on Bronfenbrenner's ecological theory (Bronfenbrenner, 1979) and also person-oriented frameworks by Magnusson, Cairns and others (Cairns & Cairns, 1994; Cairns, Elder, & Costello, 1996; Magnusson, 1999a, 1999b).
Analytically, multilevel modeling refers to techniques that can model nested data structures such as repeated-measures data. Such analytic approaches could, however, be devoid of conceptual multilevel thinking. For example, had we used our multilevel modeling analytical approach, but drawn on variables from one domain only and also not considered interactions across domains, our analyses would not have reflected multilevel conceptual thinking—despite using multilevel modeling techniques in SAS. Thus, a clear distinction between conceptual multilevel thinking and multilevel analytic techniques is important—as well as the awareness that multilevel analytic techniques cannot replace multilevel conceptual thinking (see also Sterba & Bauer, 2010).
Limitations and Future Directions
The current study had several limitations. First, the measurement of internalizing symptoms was assessed via maternal reports, and results could differ when using different reporters of internalizing symptoms. Nevertheless, it is notable that measures of child physiological regulation were associated with maternal reports of children's internalizing emotions, cognitions, behaviors, and somatic symptoms. Second, our measurement of internalizing symptoms did not allow us to distinguish between children's anxious and depressive symptoms. Past research has primarily reported sex-differentiated changes in depressive symptoms around the time of puberty; future research with recent cohorts should test whether sex-differentiated changes in anxious/depressive symptoms late middle childhood are primarily driven by depressive symptoms or to what extent anxiety symptoms contribute to these emerging sex differences. Third, although the racial/ethnic composition of the sample corresponded to that of the counties from which it was drawn, the sample was limited mostly to Caucasian and African American participants, and the processes studied here should also be examined in other racial/ethnic groups including Latino and Asian groups. Fourth, we did not assess puberty at age 10; thus we cannot verify whether increases in females' internalizing symptoms between ages 7 and 10 were driven by an early puberty group.
Fifth, we used complex analytic techniques here that allowed us to test whether developmental trajectories of internalizing symptoms differ in subgroups characterized by sex, vagal regulation, and maternal internalizing psychopathology. Other types of statistical techniques, such as growth mixture models can also capture such heterogeneity (as, for example, in Sterba et al., 2007). Given our interest in sex differences, subscales of internalizing symptoms, and the moderate overall sample size, such techniques could have been difficult to implement and they could have been prone to local solutions, misclassification, and over-extraction (Bauer, 2007; Bauer & Curran, 2003; Hipp & Bauer, 2006). Nevertheless, our findings and the many contingencies of the effects of vagal regulation of cardiac activity on psychopathology suggest that the use of person-oriented models remains a worthwhile pursuit in this area of research. Sixth, as with any developmental-observational work, the nature of the laboratory-based tasks changed across assessment waves to be age-appropriate. It is possible that changes in tasks contributed to findings of changing associations. However, had we kept age four tasks identical over time, we surely would have failed to measure the same constructs over time.
Despite these limitations, our findings contribute to clarifying the developmental course of early internalizing symptoms in a recent cohort, and also to clarifying and contextualizing associations between vagal regulation of cardiac activity and internalizing symptoms. Future work should extend these types of analytic approaches to investigating externalizing symptoms. Future work should also test whether there are specific aspects of the environment created by mothers with high internalizing symptoms that might help account for the development of internalizing symptoms in children with high vagal withdrawal.
Figure 3.
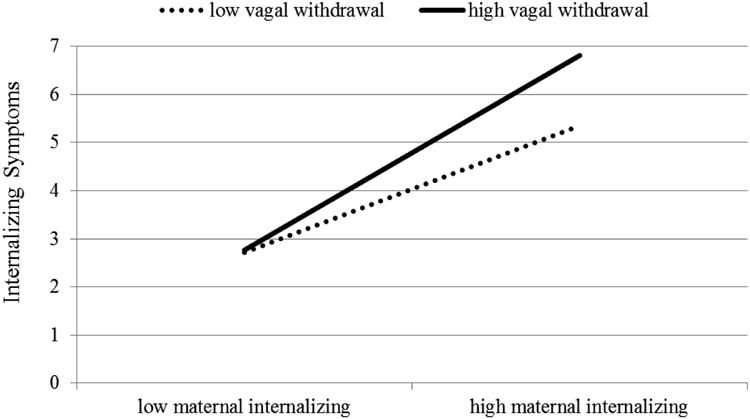
Between ages 4 and 7, high vagal withdrawal is associated with more internalizing symptoms in children in the context of high maternal internalizing symptoms.
Acknowledgments
This research was supported by National Institute of Mental Health (NIMH) Behavioral Science Track Award for Rapid Transition MH 55625 and NIMH FIRST award MH 55584 (to S.D.C.) and by NIMH Grant MH 58144. We thank the parents and children who have repeatedly given their time and effort to participate in this research and are grateful to the entire RIGHT Track staff for their help collecting, entering, and coding data.
References
- Achenbach TM. Manual for the Child Behavior Checklist/2-3. Burlington, VT: University of Vermont Department of Psychiatry; 1992. [Google Scholar]
- Achenbach TM, Edelbrock CS. Manual for the Child Behavior Checklist and Child Behavior Profile. Burlington, VT: University of Vermont; 1983. [Google Scholar]
- Andersen SL. Trajectories of brain development: Point of vulnerability or window of opportunity? Neuroscience & Biobehavioral Reviews. 2003;27(1-2):3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Worthman CM. Puberty and depression: The roles of age, pubertal status, and pubertal timing. Psychological Medicine. 1998;28(1):51–61. doi: 10.1017/s003329179700593x. [DOI] [PubMed] [Google Scholar]
- Angold A, Erkanli A, Silberg J, Eaves L, Costello EJ. Depression scale scores in 8-17-year-olds: Effects of age and gender. Journal of Child Psychology and Psychiatry. 2002;43(8):1052–1063. doi: 10.1111/1469-7610.00232. [DOI] [PubMed] [Google Scholar]
- Angold A, Worthman CW. Puberty onset of gender differences in rates of depression: A developmental, epidemiologic and neuroendocrine perspective. Journal of Affective Disorders. 1993;29(2):145–158. doi: 10.1016/0165-0327(93)90029-j. [DOI] [PubMed] [Google Scholar]
- Antonijevic IA. Depressive disorders -- is it time to endorse different pathophysiologies. Psychoneuroendocrinology. 2006;31(3):1–15. doi: 10.1016/j.psyneuen.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Bauer DJ. Observations on the use of growth mixture models in psychological research. Multivariate Behavioral Research. 2007;42(4):757–786. [Google Scholar]
- Bauer DJ, Curran PJ. Distributional assumptions of growth mixture models: Implications for over-extraction of latent trajectory classes. Psychological Methods. 2003;8(3):338–363. doi: 10.1037/1082-989X.8.3.338. [DOI] [PubMed] [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray's motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13(2):183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychological Bulletin. 2009;135(6):885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, Suess PE. Child and mother cardiac vagal tone: Continuity, stability, and concordance across the first 5 years. Developmental Psychology. 2000;36(1):54–65. [PubMed] [Google Scholar]
- Bosch NM, Riese H, Dietrich A, Ormel J, Verhulst FC, Oldehinkel AJ. Preadolescents' somatic and cognitive-affective depressive symptoms are differentially related to cardiac autonomic function and cortisol: the TRAILS study. Psychological Medicine. 2009;71(9):944–950. doi: 10.1097/PSY.0b013e3181bc756b. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Quas J, Alkon A, Smider NA, Essex MJ, Kupfer DJ The Macarthur Assessment Battery Working Group of the Macarthur Foundation Research Network on Psychopathology and Development. Autonomic reactivity and psychopathology in middle childhood. British Journal of Psychiatry. 2001;179(2):144–150. doi: 10.1192/bjp.179.2.144. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U. The Ecology of Human Development: Experiment by Nature and Design. Cambridge, MA: Harvard University Press; 1979. [Google Scholar]
- Bryk AS, Raudenbush SW. Application of hierarchical linear models to assessing change. Psychological Bulletin. 1987;101(1):147–158. [Google Scholar]
- Bryk AS, Raudenbush SW. Hierarchical Linear Models: Applications and Data Analysis Methods. Vol. 1. Newbury Park, NJ: Sage Publications; 1992. [Google Scholar]
- Burt KB, Obradović J. The construct of psychophysiological reactivity: Statistical and psychometric issues. Developmental Review. 2013;33(1):29–57. [Google Scholar]
- Cairns RB, Cairns BD. Lost and found: I Recovery of subjects in longitudinal research. In: Cairns RB, Cairns BD, editors. Lifelines and Risks: Pathways of Youth in Our Time. 1st. New York, NY: Cambridge University Press; 1994. [Google Scholar]
- Cairns RB, Elder GHJ, Costello EJ. The making of developmental science. In: Cairns RB, Elder GHJ, Costello EJ, editors. Developmental Science. Vol. 20. New York, NY: Cambridge University Press; 1996. pp. 223–234. [Google Scholar]
- Calkins SD, Blandon AY, Williford AP, Keane SP. Biological, behavioral, and relational levels of resilience in the context of risk for early childhood behavior problems. Development and Psychopathology. 2007;19(3):675–700. doi: 10.1017/S095457940700034X. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Dedmon SE, Gill KL, Lomax LE, Johnson LM. Frustration in infancy: Implications for emotion regulation, physiological processes, and temperament. Infancy. 2002;3:175–197. doi: 10.1207/S15327078IN0302_4. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Fox NA. Self-regulatory processes in early personality development: A multilevel approach to the study of childhood social withdrawal and aggression. Development and Psychopathology. 2002;14(3):477–498. doi: 10.1017/s095457940200305x. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Graziano PA, Keane SP. Cardiac vagal regulation differentiates among children at risk for behavior problems. Biological Psychology. 2007;74(2):144–153. doi: 10.1016/j.biopsycho.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, Keane SP. Cardiac vagal regulation across the preschool period: Stability, continuity, and implications for childhood adjustment. Developmental Psychobiology. 2004;45(3):101–112. doi: 10.1002/dev.20020. [DOI] [PubMed] [Google Scholar]
- Christie KA, Burke JD, Regier DA, Rae DS, Boyd JH, Locke BZ. Epidemiologic evidence for early onset of mental disorders and higher risk of drug abuse in young adults. American Journal of Psychiatry. 1988;145(8):971–975. doi: 10.1176/ajp.145.8.971. [DOI] [PubMed] [Google Scholar]
- Cicchetti D. A multiple-levels-of-analysis perspective on research in development and psychopathology. In: Beauchaine T, Hinshaw S, editors. Child and adolescent psychopathology. New York: Wiley; 2008. pp. 27–57. [Google Scholar]
- Cicchetti D, Blender JA. A multiple-levels-of-analysis perspective on resilience: Implications for the developing brain, neural plasticity, and preventive interventions. Annals of the New York Academy of Sciences. 2006;1094:248–258. doi: 10.1196/annals.1376.029. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Dawson G. Multiple levels of analysis. Development and Psychopathology. 2002;14(3):417–420. doi: 10.1017/s0954579402003012. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Psychopathology as risk for adolescent substance use disorders: A developmental psychopathology perspective. Journal of Clinical Child Psychology. 1999;28:355–365. doi: 10.1207/S15374424jccp280308. [DOI] [PubMed] [Google Scholar]
- Colder C, Mott J, Berman A. The interactive effects of infant activity level and fear on growth trajectories of early childhood behavior problems. Development and Psychopathology. 2002;14(1):1–23. doi: 10.1017/s0954579402001013. [DOI] [PubMed] [Google Scholar]
- Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS Scientific Advisory Board and the Executive Committee of the Grand Challenges on Global Mental Health. Grand challenges in global mental health. Nature. 2011;475(7354):27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Worthman C, Erkanli A, Angold A. Prediction from low birthweight to female adolescent depression: A test of competing hypotheses. Archives of General Psychiatry. 2007;64(3):338–344. doi: 10.1001/archpsyc.64.3.338. [DOI] [PubMed] [Google Scholar]
- Crowell SE, Beauchaine TP, McCauley E, Smith CJ, Stevens AL, Sylvers P. Psychological, autonomic, and serotonergic correlates of parasuicide among adolescent girls. Development and Psychopathology. 2005;17(4):1105–1127. doi: 10.1017/s0954579405050522. [DOI] [PubMed] [Google Scholar]
- Curran PJ, Bauer DJ. The disaggregation of within-person and between-person effects in longitudinal models of change. Annual Review of Psychology. 2011;62:583–619. doi: 10.1146/annurev.psych.093008.100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Konsman JP, Bluthé RM, Kelley KW. Neural and humoral pathways of communication from the immune system to the brain: parallel or convergent? Autonomic Neuroscience. 2000;85(1-3):60–65. doi: 10.1016/S1566-0702(00)00220-4. [DOI] [PubMed] [Google Scholar]
- Deković M, Buist KL, Reitz E. Stability and changes in problem behavior during adolescence: Latent growth analysis. Journal of Youth and Adolescence. 2004;33(1):1–12. [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA. The Adaptive Calibration Model of stress responsivity. Neuroscience & Biobehavioral Reviews. 2011;35(7):1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR. Manual for the Symptom Checklist 90—Revised (SCL-90R) Baltimore, MC: 1986. [Google Scholar]
- Doussard-Roosevelt JA, Montgomery LA, Porges SW. Short-term stability of physiological measures in kindergarten children: Respiratory sinus arrhythmia, heart period, and cortisol. Developmental Psychobiology. 2003;43(3):230–242. doi: 10.1002/dev.10136. [DOI] [PubMed] [Google Scholar]
- Egger HL, Angold A. Common emotional and behavioral disorders in preschool children: Presentation, nosology, and epidemiology. Journal of Child Psychiatry and Psychology. 2006;47(3/4):313–337. doi: 10.1111/j.1469-7610.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- Ellis B, Boyce WT. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development & Psychopathology. 2005;17(2):271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Armstrong JM, Burk LR, Goldsmith HH, Boyce WT. Biological sensitivity to context moderates the effects of the early teacher-child relationship on the development of mental health by adolescence. Development and Psychopathology. 2011;23(1):149–161. doi: 10.1017/S0954579410000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortunato CK, Gatzke-Kopp LM, Ram N. Associations between respiratory sinus arrhythmia reactivity and internalizing and externalizing symptoms are emotion specific. Cognitive, Affective, & Behavioral Neuroscience. 2013;13(2):238–251. doi: 10.3758/s13415-012-0136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman BH. An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biological Psychology. 2007;74(2):185–199. doi: 10.1016/j.biopsycho.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Galambos NL, Barker ET, Almeida DM. Parents do matter: Trajectories of change in externalizing and internalizing problems in early adolescence. Child Development. 2003;74(2):578–594. doi: 10.1111/1467-8624.7402017. [DOI] [PubMed] [Google Scholar]
- Gazelle H, Druhen MJ. Anxious solitude and peer exclusion predict social helplessness, upset affect, and vagal regulation in response to behavioral rejection by a friend. Dev Psychol. 2009;45(4):1077–1096. doi: 10.1037/a0016165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazelle H, Ladd GW. Anxious solitude and peer exclusion: A diathesis-stress model of internalizing trajectories in childhood. Child Development. 2003;74(1):257–278. doi: 10.1111/1467-8624.00534. [DOI] [PubMed] [Google Scholar]
- Ge X, Conger R, Elder G. Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Developmental Psychology. 2001;37(3):404–417. doi: 10.1037//0012-1649.37.3.404. [DOI] [PubMed] [Google Scholar]
- Ge X, Natsuaki MN, Conger RD. Trajectories of depressive symptoms and stressful life events among male and female adolescents in divorced and nondivorced families. Development and Psychopathology. 2006;18(1):253–273. doi: 10.1017/S0954579406060147. [DOI] [PubMed] [Google Scholar]
- Gentzler AL, Santucci AK, Kovacs M, Fox NA. Respiratory sinus arrhythmia reactivity predicts emotion regulation and depressive symptoms in at-risk and control children. [Research Support, N.I.H., Extramural] Biol Psychol. 2009;82(2):156–163. doi: 10.1016/j.biopsycho.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SH, Gotlib IH. Risk for psychopathology in the children of depressed mothers: A developmental model for understanding mechanisms of transmission. Psychological Review. 1999;106(3):485–490. doi: 10.1037/0033-295x.106.3.458. [DOI] [PubMed] [Google Scholar]
- Graziano P, Derefinko K. Cardiac vagal control and children's adaptive functioning: A meta-analysis. Biological Psychology. 2013;94(1):22–37. doi: 10.1016/j.biopsycho.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman-Giddens ME. Recent data on pubertal milestones in United States children: The secular trend toward earlier development. International Journal of Andrology. 2006;29(1):241–246. doi: 10.1111/j.1365-2605.2005.00575.x. [DOI] [PubMed] [Google Scholar]
- Herman-Giddens ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, Koch GG, Hasemeier CM. Secondary sexual characteristics and menses in young girls seen in office practice: A study from the Pediatric Research in Office Settings Network. Pediatrics. 1997;99:505–512. doi: 10.1542/peds.99.4.505. [DOI] [PubMed] [Google Scholar]
- Hinnant JB, El-Sheikh M. Children's externalizing and internalizing symptoms over time: the role of individual differences in patterns of RSA responding. J Abnorm Child Psychol. 2009;37(8):1049–1061. doi: 10.1007/s10802-009-9341-1. [DOI] [PubMed] [Google Scholar]
- Hipp JR, Bauer DJ. Local solutions in the estimation of growth mixture model. Psychological Methods. 2006;11(1):36–53. doi: 10.1037/1082-989X.11.1.36. [DOI] [PubMed] [Google Scholar]
- Hoffman L, Stawski RS. Persons as contexts: Evaluating between-person and within-person effects in longitudinal analysis. Research in Human Development. 2009;6(2-3):97–120. [Google Scholar]
- Hollingshead AB. Four factor index of social status. Yale University; New Haven, CT: 1975. [Google Scholar]
- Kasch KL, Klein DN. The relationship between age at onset and comorbidity in psychiatric disorders. Journal of Nervous and Mental Disease. 1996;184(11):703–707. doi: 10.1097/00005053-199611000-00008. [DOI] [PubMed] [Google Scholar]
- Keenan K, Shaw DS, Walsh B, Delliquadri E, Giovannelli J. DSM-III-R disorders in preschool children from low-income families. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(5):620–627. doi: 10.1097/00004583-199705000-00012. [DOI] [PubMed] [Google Scholar]
- Kok BE, Fredrickson BL. Upward spirals of the heart: Autonomic flexibility, as indexed by vagal tone, reciprocally and prospectively predicts positive emotions and social connectedness. Biological Psychology. 2010;85(3):432–436. doi: 10.1016/j.biopsycho.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: Mechanisms and implications. [Review] Trends Neurosci. 2002;25(3):154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- Kovacs M. Presentation and course of major depressive disorder during childhood and later years of the life span. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35(6):705–715. doi: 10.1097/00004583-199606000-00010. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Paulauskas SL. Developmental stage and the expression of depressive disorders in children: An empirical analysis. In: Cicchetti D, Schneider-Rosen K, editors. Childhood Depression. San Francisco: New Directions for Child Development; 1984. pp. 59–80. [Google Scholar]
- Kuo M, Mohler B, Raudenbush SL, Earls FJ. Assessing exposure to violence using multiple informants: application of hierarchical linear model. The Journal of Child Psychology and Psychiatry. 2000;41(8):1049–1056. [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(12):963–974. [PubMed] [Google Scholar]
- Little R, Rubin D. Statistical Analysis with Missing Data. New York, NY: Wiley and Sons; 1987. [Google Scholar]
- Lovejoy MC, Graczyk PA, O'Hare E, Neuman G. Maternal depression and parenting behavior: A meta-analytic review. Clinical Psychology Review. 2000;20(5):561–592. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- Magnusson D. Holistic interactionism: A perspective for research on personality development. In: Pervin LA, John OP, editors. Handbood of Personality: Theory and Research. 2nd. New York: The Guilford Press; 1999a. pp. 219–247. [Google Scholar]
- Magnusson D. On the individual: A person-oriented approach to developmental research. European Psychologist. 1999b;4(4):205–218. [Google Scholar]
- Marsland AL, Gianaros PJ, Prather AA, Jennings JR, Neumann SA, Manuck SB. Stimulated production of proinflammatory cytokines covaries inversely with heart rate variability. Psychosomatic Medicine. 2007;69(8):709–716. doi: 10.1097/PSY.0b013e3181576118. [DOI] [PubMed] [Google Scholar]
- Nezlek JB. A multilevel framework for understanding relationships among traits, states, situations and behaviors. European Journal of Personality. 2007;21(6):789–810. [Google Scholar]
- Obradović J. How can the study of physiological reactivity contribute to our understanding of adversity and resilience processes in development? Development and Psychopathology. 2012;24(2):371–387. doi: 10.1017/S0954579412000053. [DOI] [PubMed] [Google Scholar]
- Obradović J, Bush NR, Stamperdahl J, Adler NE, Boyce WT. Biological sensitivity to context: The interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Development. 2010;81(1):270–289. doi: 10.1111/j.1467-8624.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ. Neural regulators of innate immune responses and inflammation. Cellular and Molecular Life Sciences. 2004;61(18):2322–2331. doi: 10.1007/s00018-004-4102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry NB, Nelson JA, Swingler MM, Leerkes EM, Calkins SD, Marcovitch S, O'Brien M. The relation between maternal emotional support and child physiological regulation across the preschool years. Developmental Psychobiology. 2013;55(4):382–394. doi: 10.1002/dev.21042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: Phylogenetic contributions to social behavior. Physiology & Behavior. 2003;79(3):503–513. doi: 10.1016/s0031-9384(03)00156-2. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, Greenspan SI. Infant regulation of the vagal “brake” predicts child behavior problems: a psychobiological model of social behavior. Developmental Psychobiology. 1996;29(8):697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Porges SW, Furman SA. The early development of the autonomic nervous system provides a neural platform for social behavior: A polyvagal perspective. Infant and Child Development. 2011;20(1):106–118. doi: 10.1002/icd.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends in Immunology. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richters JE. The Hubble hypothesis and the developmentalist's dilemma. Development and Psychopathology. 1997;9:193–229. doi: 10.1017/s0954579497002022. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Chambers AS, Allen JJ, Manber R. Cardiac vagal control in the severity and course of depression: the importance of symptomatic heterogeneity. Journal of Affective Disorders. 2007;103(1-3):173–179. doi: 10.1016/j.jad.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS/STAT® Software: Version 9.2. Cary, NC: SAS Institute, Inc; 2008. [Google Scholar]
- Schafer JL. Analysis of incomplete multivariate data. London: Chapman & Hall; 1997. [Google Scholar]
- Schmidt L, Fox N, Schulkin J, Gold PW. Behavioral and psychophysiological correlates of self-presentation in tempermentally shy children. Developmental Psychobiology. 1999;35:119–135. [PubMed] [Google Scholar]
- Shanahan L, Copeland WE, Angold A, Costello EJ. Child-, adolescent, and young adult-onset depressions: Differential risk factors in development? Psychological Medicine. 2011;41(11):2265–2274. doi: 10.1017/S0033291711000675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelby GD, Shirkey KC, Sherman AL, Beck JE, Haman K, Shears AR, et al. Walker LS. Functional abdominal pain in childhood and long-term vulnerability to anxiety disorders. Pediatrics. 2013;132(3):475–482. doi: 10.1542/peds.2012-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Development and Psychopathology. 2005;17(1):167–184. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1):e232–246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- Sroufe LA, Rutter M. The domain of developmental psychopathology. Child Development. 1984;55:17–29. [PubMed] [Google Scholar]
- Sterba SK, Bauer DJ. Matching method with theory in person-oriented developmental psychopathology research. Development and Psychopathology. 2010;22(2):239–254. doi: 10.1017/S0954579410000015. [DOI] [PubMed] [Google Scholar]
- Sterba SK, Prinstein MJ, Cox MJ. Trajectories of internalizing problems across childhood: Heterogeneity, external validity, and gender differences. Development and Psychopathology. 2007;19(2):345–366. doi: 10.1017/S0954579407070174. [DOI] [PubMed] [Google Scholar]
- Stoddard J, Stringaris A, Brotman MA, Montville D, Pine DS, Leibenluft E. Irritability in child and adolescent anxiety disorders. Depression and Anxiety. 2013 doi: 10.1002/da.22151. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A. Irritability in children and adolescents: a challenge for DSM-5. European Child and Adolescent Psychiatry. 2011;20(2):61–66. doi: 10.1007/s00787-010-0150-4. [DOI] [PubMed] [Google Scholar]
- Stringaris A, Zavos H, Leibenluft E, Maughan B, Eley TC. Adolescent irritability: phenotypic associations and genetic links with depressed mood. American Journal of Psychiatry. 2012;169(1):47–54. doi: 10.1176/appi.ajp.2011.10101549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Sternberg E. Beyond heart rate variability: Vagal regulation of allostatic systems. Annals of the New York Academy of Sciences. 2006;1088:361–372. doi: 10.1196/annals.1366.014. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Sternberg EM. Neural aspects of immunomodulation: Focus on the vagus nerve. Brain, Behavior, and Immunity. 2010;24(8):1223–12228. doi: 10.1016/j.bbi.2010.07.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twenge JM, Nolen-Hoeksema SK. Age, gender, race, SES, and birth cohort differences on the Children's Depression Inventory. Journal of Abnormal Psychology. 2002;111(4):578–588. doi: 10.1037//0021-843x.111.4.578. [DOI] [PubMed] [Google Scholar]
- Wood JJ, McLeod BD, Sigman M, Hwang WC, Chu BC. Parenting and childhood anxiety: Theory, empirical findings, and future directions. Journal of Child Psychology and Psychiatry. 2003;44(1):134–151. doi: 10.1111/1469-7610.00106. [DOI] [PubMed] [Google Scholar]
- Zahn-Waxler C, Klimes-Dougan B, Slattery MJ. Internalizing problems of childhood and adolescence: Prospects, pitfalls, and progress in understanding the development of anxiety and depression. Development and Psychopathology. 2000;12(3):443–466. [PubMed] [Google Scholar]


