Abstract
Manganese (Mn) and zinc (Zn) are essential divalent cations used by cells as protein cofactors; various human studies and animal models have demonstrated the importance of Mn and Zn for development. Here we describe an autosomal-recessive disorder in six individuals from the Hutterite community and in an unrelated Egyptian sibpair; the disorder is characterized by intellectual disability, developmental delay, hypotonia, strabismus, cerebellar atrophy, and variable short stature. Exome sequencing in one affected Hutterite individual and the Egyptian family identified the same homozygous variant, c.112G>C (p.Gly38Arg), affecting a conserved residue of SLC39A8. The affected Hutterite and Egyptian individuals did not share an extended common haplotype, suggesting that the mutation arose independently. SLC39A8 is a member of the solute carrier gene family known to import Mn, Zn, and other divalent cations across the plasma membrane. Evaluation of these two metal ions in the affected individuals revealed variably low levels of Mn and Zn in blood and elevated levels in urine, indicating renal wasting. Our findings identify a human Mn and Zn transporter deficiency syndrome linked to SLC39A8, providing insight into the roles of Mn and Zn homeostasis in human health and development.
Main Text
It has long been recognized that various divalent cations are important for human health; however, for many cations we still lack a comprehensive understanding of the biochemical roles within cells and the consequences of deficiency. For manganese (Mn, Mn2+) and zinc (Zn, Zn2+), these cations act as cofactors to modulate the activity of proteins, such as enzymes and transcription factors. Studies in various animal models have demonstrated the importance of Mn and Zn for development, highlighting a number of dependent biological systems including metabolism, endocrine control, immunological response, brain development and function, blood clotting, bone and connective tissue growth, systemic growth, and reproduction.1, 2, 3 Parallels between these models and human development remain to be fully elucidated.
In humans, low Mn levels in blood have been documented in individuals with epilepsy, Mseleni joint disease, Down syndrome, osteoporosis, and Perthes disease,4 though the role of Mn, if any, in these conditions is unknown. Similarly, Zn homeostasis has been implicated in pathogenesis of sickle cell disease, diabetes mellitus, alcoholism, and Alzheimer, Parkinson, and chronic kidney disease.2 Interestingly, there are a number of rare genetic diseases resulting from abnormal levels of Zn or Mn, including acrodermatitis enteropathica (SLC39A4 [OMIM: 201100]),5 spondylocheiro dysplastic Ehlers-Danlos syndrome (SLC39A13 [OMIM: 612350]),6 transient neonatal zinc deficiency (SLC30A2 [OMIM: 608118]),7 and a syndrome of hepatic cirrhosis, dystonia, polycythemia, and hypermanganesemia (SLC30A10 [OMIM: 613280]).8 Rare genetic diseases resulting from Mn deficiency have not yet been described. Overall, it is clear we are just beginning to appreciate the biological significance of these essential cations.
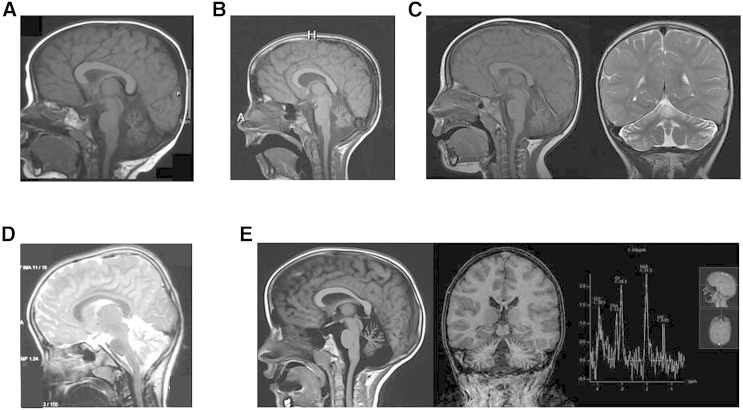
The Hutterite Brethren is an ethno-religious population of ∼40,000 individuals that is divided into three essentially endogamous groups termed Schmiedeleut, Dariusleut, and Lehrerleut.9, 10 This community is uniquely suited for genetic studies due to a small founding population, continued genetic isolation due to sociocultural practices, well-kept genealogical records, and participation in modern health care.9, 11 Six Hutterite individuals were identified as sharing a syndrome characterized by profound intellectual disability, developmental delay, hypotonia, strabismus, and cerebellar atrophy. Additional features included variable short stature, osteopenia, and recurrent infections (Table 1). Hypotonia was evident from birth and is profound; head control was achieved only in early childhood and one affected individual achieved sitting at 7 years of age. Occipital frontal circumference was always within the normal range. Intellectual disability was judged to be severe in all affected individuals; the older individuals are able to communicate with a few words and signs. MRI studies demonstrated a small cerebellum with widened interfoliate sulci and major fissures consistent with mild to diffuse cerebellar atrophy in a normally sized posterior fossa (Figure 1). After unremarkable cytogenetic and metabolic investigations, the affected families were enrolled in the Care4Rare Canada research project based on their shared phenotype and ancestry. Informed consent was obtained from the parents and study design was approved by the Institutional Research Ethics boards at the University of Calgary and the Children’s Hospital of Eastern Ontario.
Table 1.
Clinical Features of Individuals with the SLC39A8 Homozygous Mutation c.112G>C (p.Gly38Arg)
|
Family |
Family A |
Family B |
Family C |
Family D |
Family E |
Family F |
||
|---|---|---|---|---|---|---|---|---|
| Individual | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Sex | F | M | M | F | F | M | F | M |
| Ethnicity | Hutterite-D | Hutterite-D | Hutterite-S | Hutterite-S | Hutterite-S | Hutterite-S | Egyptian | Egyptian |
| Current age (years) | 18 | 23 | 10 | 6 | 6 | 9 | 9 | 3 |
| Age at last exam (years) | 13 | 17 | 8 | 5 | 5 | 9 | 8 | 2 |
| Growth | ||||||||
| Birth length | 50th–75th | 5th–10th | 5th–10th | <5th | <5th | ND | normala | normala |
| Birth weight | 50th | 50th | 25th–50th | <5th | <5th | 50th–75th | normala | normala |
| Birth OFC | 90th | 50th | normala | <5th | <5th | >95th | normala | normala |
| Height | −3 to −4 SD | −3 to −4 SD | −3 SD | 5th | 5th | <5th | 5th | 5th |
| Weight | −4 SD | −4 SD | −3 SD | 10th | 10th | 25th–50th | 25th | 5th |
| OFC | 50th | 50th | 50th | 25th–50th | 25th–50th | 95th | 50th | 50th |
| Development | ||||||||
| Head Control (years) | 2 | 4 | 2 | 2 | 2 | 2 | no | no |
| Sit (years) | 7 | with support | 3 | with support | with support | with support | no | no |
| Walk (years) | no | no | no | no | assisted | no | assisted | no |
| Intellectual disability | profound | profound | severe | profound | profound | profound | severe | severe |
| Clinical Features | ||||||||
| Hypotonia | profound | profound | severe | severe | severe | severe | severe | severe |
| Strabismus | yes | yes | yes | yes | yes | no | yes | yes |
| Seizures | no | no | no | no | no | yes | myoclonic | no |
| Other | recurrent infections, joint hypermobility | recurrent infections, joint hypermobility | constipation | recurrent infections | recurrent infections | recurrent infections | hyperreflexia | hyperreflexia |
| Imaging | ||||||||
| Skeletal | osteopenia | osteopenia, flat and broad long bone epiphyses | normal skeletal survey at 16 months | ND | ND | ND | ND | ND |
| MRI | severe atrophy of the cerebellar vermis and hemispheres | severe atrophy of the cerebellar vermis and hemispheres | severe atrophy of the cerebellar vermis and hemispheres | severe atrophy of the cerebellar vermis and hemispheres | ND | progressive atrophy of the cerebellar vermis and hemispheres | severe atrophy of the cerebellar vermis and hemispheres | mild atrophy of the cerebellar vermis and hemispheres; cerebral atrophy, particularly of the frontal lobes |
| MRS | ND | ND | ND | normal | ND | elevated lactate peak | ND | ND |
Abbreviations are as follows: Hutterite-D, Hutterite Dariusleut; Hutterite-S, Huterite-Schmiedeleut; ND, no data.
Reported as normal.
Figure 1.
MRI Findings in Individuals with Mutation in SLC39A8
MRI findings from individual 1 at 6 years of age (A), individual 2 at 12 years of age (B), individual 4 at 3 years of age (C), individual 7 at 8 years of age (D), and individual 6 at 9 years of age (E) demonstrate a small cerebellum with wide interfoliate sulci and major fissures demonstrating diffuse cerebellar vermian and hemispheric atrophy. The posterior fossa is normal in size. MRS over the left basal ganglia in individual 6 (E) demonstrates an elevated lactate peak.
Two additional children of Egyptian descent, an 8-year-old girl and her 2-year-old brother, presented with severe intellectual disability, developmental delay, hypotonia, and strabismus (Table 1). These individuals were ascertained as part of a large intellectual disability cohort, separate from the Hutterite children. In addition, the female sibling had early-onset generalized myoclonic seizures that are now well controlled. MRI of the brain showed diffuse cerebellar atrophy in both siblings that was much more severe in the older sister. The younger brother also showed striking cortical atrophy, particularly of the frontal lobes. All further examinations, including fundus examination and ultrasound of heart and abdomen, were unremarkable. Notably, there was a third sibling who passed away at 7 months of age with profound developmental delay and failure to thrive. The parents are consanguineous (first cousins) and healthy. Informed consent was obtained from the parents and study design approved by the Institutional Research Ethics boards at the University of Erlangen in Germany and the National Research Center in Cairo.
Given the history of the Hutterite community, we predicted that this rare disease would be caused by a shared homozygous ancestral mutation. Identity-by-descent mapping was performed with DNA from individuals 1, 2, and 3 and GeneChip Human Mapping 10 K (Xba 2.0) and 50 K (Xba 240) arrays (Affymetrix), as described elsewhere.12 This was followed by fine mapping with PCR amplification of microsatellite markers (True Allele PCR Premix, Applied Biosystems), which were then resolved on an ABI3130 automated sequencer (Applied Biosystems) and analyzed with GeneMapper Software (Applied Biosystems). We identified a single shared 11.7 Mb homozygous region at 4q22–25 and a maximal region of chr4: 94,964,050–106,621,864 (hg19) (Figure S1A). No genes within this region had previously been linked to a similar phenotype. To efficiently identify potential causative variant(s), we performed whole-exome sequencing using DNA from individual 2. The SureSelect Human All Exon Kit version 4 (Agilent) was used for target enrichment, and the library was sequenced with 100 base-pair paired-end reads on a HiSeq 2000 platform (Illumina), as described previously.13, 14 Bioinformatics analysis of exome sequencing data was carried out as described previously;13, 14 average coverage was 139× and 93% of exons in the 4q22–25 region were completely covered at >10×. After excluding common variants (≥1% minor allele frequency represented in the NHLBI exome variant server, in-house controls [435], or Exome Aggregation Consortium), a variant in SLC39A8 (GenBank: NM_022154.5; c.112G>C [p.Gly38Arg]) was identified. This highly conserved residue is located within a predicted cytoplasmic domain of the SLC39A8 transmembrane protein (UniProt) and predicted to be “probably damaging” by SIFT15 and PolyPhen-2.16 Sanger sequencing revealed all affected individuals to be homozygous for this variant, parents heterozygous, and healthy siblings were either heterozygous or carried the normal sequence (Figure S2). Finally, to determine the prevalence of this variant in the Hutterite population, we conducted genotyping in a Hutterite control cohort with a TaqMan SNP genotyping assay (Life Technologies). The SLC39A8 variant was present in heterozygous state at a low frequency, 1.7% in Lehrerleut (120 controls) and 3.8% in Dariusleut (92 controls), but was never observed in a homozygous state. The variant is present in the Exome Aggregation Consortium (ExAC) database as heterozygous in 2 out of 15,930 alleles that are reportedly from European individuals. It is unclear whether this is a founder mutation given the Hutterite population’s European roots, or separate mutation events.
Similarly, because of consanguinity of the Egyptian parents, a homozygous mutation was expected. Genome-wide genotyping of the two affected children and of their mother (since her parents were also consanguineous) using the HumanCoreExome BeadChips (Illumina) with 240K polymorphic variants followed by homozygosity mapping using the web-based application HomozygosityMapper17 revealed six candidate regions spanning a total length of 72 Mb (Figure S1B). DNA of the affected brother was enriched, using the SureSelect Human All Exon Kit version 5 (Agilent), and sequenced using 100-bp paired-end reads on a HiSeq2500 platform (Illumina). Analysis, base calling, and variant annotation were performed according to standard methods.18 An average coverage of 145× was achieved; 95% of the target sequence was covered at least 20×, and 96% was covered at least 5×. Only variants with coverage of 5× or more were analyzed. After excluding common variants (≥1% minor allele frequency represented in the NHLBI exome variant server, in-house controls [728], or ExAC database), one homozygous variant remained. We then repeated filtering steps based on different in silico parameters (conservation, pathogenicity prediction, mutation scoring) and also on combinations of different parameters (SIFT,15 PhyloP,19 PolyPhen-2,16 LRT,20 MutationTaster,21 MutationAssessor,22 GERP,23 and CADD24), and the same candidate variant remained that had a predicted pathogenic effect: c.112G>C (p.Gly38Arg) (GenBank: NM_022154.5) in SLC39A8. The variant segregated with the disease in the family (Figure S2). We conclude that the SLC39A8 c.112G>C (p.Gly38Arg) variant is responsible for the phenotype in the affected Hutterite and Egyptian individuals.
To investigate whether the c.112G>C (p.Gly38Arg) mutation homozygous in the affected individuals of Hutterite and Egyptian descent arose from a single event in a distant common ancestor, we assessed high-quality SNPs in the 4 Mb surrounding the mutation using WES data. This analysis demonstrated that these affected individuals did not share an extended common haplotype (Table S1). Given this finding and the origin and migration pattern of the Hutterite population,9 it is highly likely that this is a recurrent event and not derived from a single ancestral mutation.
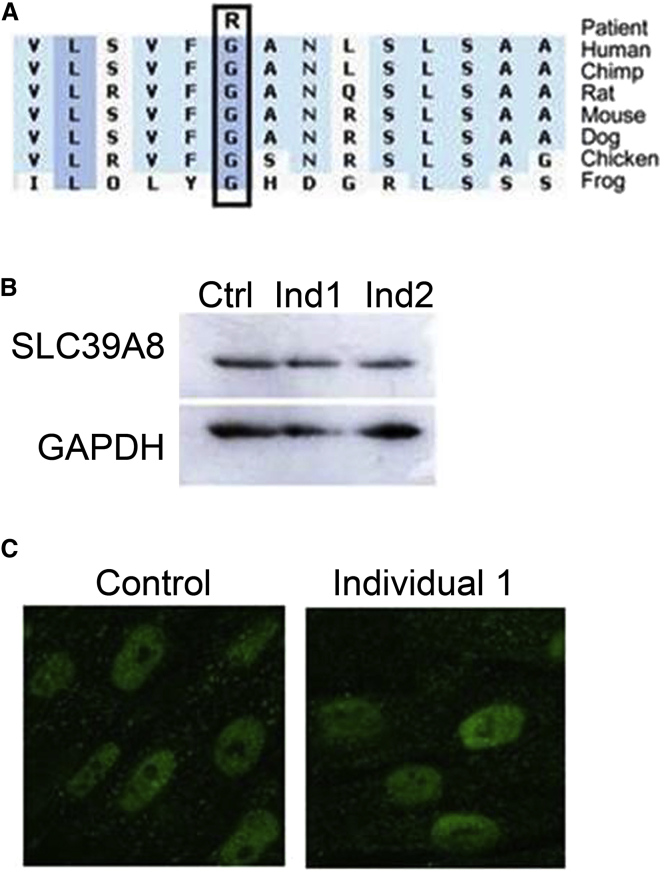
SLC39A8, also known as zinc- and iron-related protein 8 (ZIP8), is a member of the solute carrier gene (SLC) superfamily25, 26 and functions to transport Mn, Zn, Cd, and Fe across the plasma membrane.27, 28 Immunoblot analysis and immunofluorescence with confocal microscopy conducted according to standard protocols on control and affected individual lymphoblast and fibroblast cells (derived from individual 2) demonstrated that there was no overt effect on SLC39A8 protein abundance or localization (Figure 2), suggesting that the mutation probably impacts protein function. To further evaluate pathogenicity of the p.Gly38Arg variant, we measured the divalent cations of interest in affected individuals (Table 2). Mn and Zn levels in blood were either low or very low-normal in all tested individuals, whereas urine levels tended to be high, indicating renal wasting. Cd is a toxic metal that can have adverse health effects when inadvertent environmental exposure occurs; Cd levels were measured for two of the affected individuals but were not detectable (data not shown). These findings indicate the p.Gly38Arg SLC39A8 substitution probably impacts both Mn and Zn transport, resulting in elemental deficiency secondary to variable poor absorption and loss of these elements through the kidney and causes an intellectual disability with cerebellar atrophy syndrome.
Figure 2.
SLC39A8 Mutation Causes a Zinc and Manganese Deficiency Syndrome
(A) Evolutionary alignment of SLC39A8 amino acid sequence shows strict conservation of Gly38. Conservation plot was generated with Alamut software.
(B and C) Western blot (lymphoblasts) (B) and immunofluorescence (fibroblasts) (C) analyses demonstrate normal levels and localization of SLC39A8 in cells derived from individual 2 compared to controls. GAPDH was used as immunoblot loading control.
Table 2.
Zinc and Manganese Levels of Individuals with the SLC39A8 Homozygous Mutation c.112G>C (p.Gly38Arg)
|
Family |
Family A |
Family B |
Family C |
Family D |
Family E |
Family F |
||
|---|---|---|---|---|---|---|---|---|
| Individual | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Blood | ||||||||
| Mn | ND | erythrocyte: 20 nmol/l (NR 273–728) | blood levels: 20 nmol/l (NR 78–289) | blood levels: 14.2 nmol/l (NR 5.3–40.8); 0.78 μg/l (NR 0.29–2.24) | blood levels: 5.5 nmol/l (NR 5.3–40.8); 0.3 μg/l (NR 0.29–2.24) | blood levels: 18.4 nmol/l (NR 5.3–40.8); 1.01 μg/l (NR 0.29–2.24) | 1.1 μg/l (NR 5–12.4) | 1.1 μg/l (NR 5–12.4) |
| Zn | ND | serum value: 9.4 μmol/l (NR 8–20) | plasma values: 11.3 μmol/l (NR 11.8–16.4); 740 μg/l (NR 771–1,072) | plasma values: 10.7 μmol/l (NR 11.8–16.4); 702 μg/l (NR 771–1,072) | plasma values: 10.8 μmol/l (NR 11.8–16.4); 709 μg/l (NR 771–1,072) | serum value: 8.8 μmol/l (NR 10–20) | normal | 399 μg/dl (NR 408–787) |
| Urine | ||||||||
| Mn | ND | 7.7 μg/l (NR 0.07–0.5); 140.1 nmol/l (NR 1.3–9.1); 9.1 μmol/mol Cr (NR 0.2–1.2) | 0.22 μg/l (NR 0.07–0.5); 4.0 nmol/l (NR 1.3–9.1); 3.34 μmol/mol Cr (NR 0.17–1.2) | ND | 1.309 μg/l (NR 0.07–0.5); 25.5 nmol/l (NR 1.3–9.1); 10.61 μmol/mol Cr (NR 0.14–1.08) | ND | normal | normal |
| Zn | ND | 283 μg/l (NR 60–400); 4.3 μmol/l (NR 0.9–6.1); 0.28 nmol/mol Cr (NR 0.12–0.81) | 125 μg/l (NR 60–400); 1.9 μmol/l (NR 0.9–6.1); 1.59 nmol/mol Cr (NR 0.12–0.81) | ND | 252 μg/l (NR 60–400); 3.9 μmol/l (NR 0.9–6.1); 1,606 μmol/mol Cr (NR 104–729) | ND | normal | 144 μg/l (NR 150–1,200) |
Abbreviations are as follows: ND, no data; NR, normal range.
Notably, there is some variation among the affected individuals with regard to Mn and Zn levels. Variability in these levels can be attributed to a number of factors. First, there might be partial compensation by alternative transporters (>20 Zn transporters29 and ∼5 Mn transporters30 have been identified) and this response might differ between individuals. Of note, SLC39A8 is ubiquitously expressed, as are a number of the alternate SLC transporters with potential functional redundancy.31 Interestingly, human blood Mn levels have been correlated by genome-wide significance mapping with SNPs in both SLC39A8 and SLC30A10,32 supporting the important role of SLC39A8 in Mn homeostasis. Finally, it is likely that transient cycling of elemental levels between low-normal and severely low might exist as a result of dietary Mn and/or Zn intake.
Mn and Zn are essential elements that act as cofactors for a variety of proteins, including enzymes and transcription factors involved in energy metabolism, endocrine regulation, immune response, nervous system functions, and reproduction.1, 3, 29 Given the abundance and centrality of Mn- and Zn-dependent processes, it is not surprising that SLC39A8 transporter deficiency causes the severe multi-systemic developmental disorder reported here. Furthermore, Slc39a8 hypomorphic mice (expressing 15%–50% of wild-type levels depending on age and tissue) are observed at expected Mendelian ratios early in development, but die between embryonic day (E) 18.5 and 48 hr postnatally.33 These mice displayed stunted growth and pale appearance as early as E11.5, and by birth exhibited small size, poor weight gain, severe anemia, underdeveloped eyes, malformed craniums, and hypoplastic hind limbs and organs. These findings further support the essentiality of proper SLC39A8 function.
There are a number of lines of evidence from humans and animals that provide support for a link between the clinical presentation of these children and Mn deficiency. First, the pathogenic mechanism of a number of human conditions with features similar to the disease reported here has been attributed, at least in part, to a single Mn-dependent enzyme. For example, arginase deficiency causes an autosomal-recessive condition characterized by impaired growth and development, intellectual disability, and ataxia;34 glutamine synthase deficiency causes brain abnormalities, seizures, delayed development, and hypotonia.35 Second, a study in Mexico City found that diminished levels of blood Mn in childhood had a negative impact on neurological development.36 Third, dietary-induced Mn deficiency has severe developmental consequences in mice, rats, chickens, pigs, and guinea pigs; although there are some phenotypic differences, all species display impaired growth, skeletal abnormalities, and ataxic characteristics.1, 37 Finally, excess dietary and/or environmental Mn has been shown to have several neurotoxic effects in mice and rats,38 highlighting the vulnerability of the nervous system. Our findings demonstrate that many of the animal and human phenotypes caused by altered Mn homeostasis are paralleled in individuals with altered SLC39A8 function.
Current literature contains a number of reports of Zn deficiency in humans and model organisms with features akin to our affected individuals. First, spondylocheiro dysplastic Ehlers-Danlos syndrome is an autosomal-recessive condition caused by mutations in the SLC39A13 zinc transporter.6 Although this disease is clearly distinct from that presented here, the phenotype includes short stature, emphasizing a role for Zn in systemic growth. Second, a number of mice with genetic mutations in Zn transporters have been generated and characterized; for example, Zip13-null mice display delayed growth and connective tissue abnormalities and Zip14-null mice are growth deficient and have impaired skeletogenesis.2, 3, 29 Finally, studies in Drosophila and zebrafish have shown defective neuron migration and growth under conditions of Zn deficiency.2, 3, 29 Taken together, it is it clear that Zn is absolutely pivotal for human health; deficiency as a result of altered function of SLC39A8 undoubtedly contributes to this disorder.
Finally, mutations in SLC39A8 have been identified and reported in two additional individuals in an accompanying paper in this issue.39 One of these individuals shares the p.Gly38Arg substitution reported here in conjunction with a second alteration, p.Ile340Asn, and is more severely affected. Both of these subjects had an abnormal glycosylation pattern of serum transferrin and very recent studies of individuals 2, 4, and 5 in this report show similar, but less severe, abnormalities,39 identifying additional insight into the pathogenesis of this disease as well as a useful biomarker for future therapeutic studies.
In conclusion, we have identified a homozygous recurrent mutation in SLC39A8 that causes defective Mn and Zn transport, intellectual disability, developmental delay, hypotonia, strabismus, cerebellar atrophy, and variable short stature. It remains to be seen whether Mn and Zn supplementation will have a significant clinical impact for these affected individuals; this will be largely dependent on the residual ability of the mutant SLC39A8 to transport Mn and Zn, and/or whether alternative transporters are able to compensate. Importantly, our identification of this Mendelian form of Mn and Zn transporter deficiency provides, in addition to the identification of a rare disease, insights into the role and essentiality of these divalent cations in human health and development.
Acknowledgments
We thank the families for their enthusiastic participation in this study. We gratefully acknowledge Jackie Morris and Linda MacLaren for clinical support and Dr. Brian Lowry for the initial clinical investigation of individual 2. The authors wish to acknowledge the contribution of the high throughput sequencing platform of the McGill University and Génome Québec Innovation Centre and of FAU Erlangen-Nürnberg. This work was supported by the Care4Rare Canada Consortium (Enhanced Care for Rare Genetic Diseases in Canada) funded by Genome Canada, the Canadian Institutes of Health Research, the Ontario Genomics Institute, Ontario Research Fund, Genome Quebec, Children’s Hospital of Eastern Ontario Foundation, Alberta Children’s Hospital Research Institute, and Alberta Innovates Health Solutions. This project was also supported in part through funding of DFG (AB393/2-2 and AB393/4-1) to R.A.J. and A.R. and from the Alberta Children’s Hospital Foundation to J.S.P. D.R.M. was supported by the CIHR Training Program in Genetics, Child Development, and Health at the University of Calgary. Work from Cincinnati was supported by NIH grants R01 ES010416 (D.W.N.), T32 ES016646 (M.G.-P.), and P30 ES006096 (D.W.N.). The project was selected for analysis by the Care4Rare Consortium Gene Discovery Steering Committee, consisting of K.M.B. (lead; University of Ottawa), Alex MacKenzie (co-lead; University of Ottawa), J.M. (McGill University), Michael Brudno (University of Toronto), Dennis Bulman (University of Ottawa), and David Dyment (University of Ottawa).
Published: December 3, 2015
Footnotes
Supplemental Data include two figures and one table and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2015.11.002.
Contributor Information
Kym M. Boycott, Email: kboycott@cheo.on.ca.
Rami Abou Jamra, Email: rami.aboujamra@medizin.uni-leipzig.de.
Web Resources
The URLs for data presented herein are as follows:
ExAC Browser, http://exac.broadinstitute.org/
NHLBI Exome Sequencing Project (ESP) Exome Variant Server, http://evs.gs.washington.edu/EVS/
OMIM, http://www.omim.org/
UCSC Genome Browser, http://genome.ucsc.edu
UniProt, http://www.uniprot.org/
Supplemental Data
References
- 1.Avila D.S., Puntel R.L., Aschner M. Manganese in health and disease. Met. Ions Life Sci. 2013;13:199–227. doi: 10.1007/978-94-007-7500-8_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fukada T., Kambe T. Molecular and genetic features of zinc transporters in physiology and pathogenesis. Metallomics. 2011;3:662–674. doi: 10.1039/c1mt00011j. [DOI] [PubMed] [Google Scholar]
- 3.Kambe T., Hashimoto A., Fujimoto S. Current understanding of ZIP and ZnT zinc transporters in human health and diseases. Cell. Mol. Life Sci. 2014;71:3281–3295. doi: 10.1007/s00018-014-1617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pennington J.A., Schoen S.A. Total diet study: estimated dietary intakes of nutritional elements, 1982-1991. Int. J. Vitam. Nutr. Res. 1996;66:350–362. [PubMed] [Google Scholar]
- 5.Küry S., Dréno B., Bézieau S., Giraudet S., Kharfi M., Kamoun R., Moisan J.P. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat. Genet. 2002;31:239–240. doi: 10.1038/ng913. [DOI] [PubMed] [Google Scholar]
- 6.Giunta C., Elçioglu N.H., Albrecht B., Eich G., Chambaz C., Janecke A.R., Yeowell H., Weis M., Eyre D.R., Kraenzlin M., Steinmann B. Spondylocheiro dysplastic form of the Ehlers-Danlos syndrome--an autosomal-recessive entity caused by mutations in the zinc transporter gene SLC39A13. Am. J. Hum. Genet. 2008;82:1290–1305. doi: 10.1016/j.ajhg.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chowanadisai W., Lönnerdal B., Kelleher S.L. Identification of a mutation in SLC30A2 (ZnT-2) in women with low milk zinc concentration that results in transient neonatal zinc deficiency. J. Biol. Chem. 2006;281:39699–39707. doi: 10.1074/jbc.M605821200. [DOI] [PubMed] [Google Scholar]
- 8.Tuschl K., Clayton P.T., Gospe S.M., Jr., Gulab S., Ibrahim S., Singhi P., Aulakh R., Ribeiro R.T., Barsottini O.G., Zaki M.S. Syndrome of hepatic cirrhosis, dystonia, polycythemia, and hypermanganesemia caused by mutations in SLC30A10, a manganese transporter in man. Am. J. Hum. Genet. 2012;90:457–466. doi: 10.1016/j.ajhg.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hostetler J.A. History and relevance of the Hutterite population for genetic studies. Am. J. Med. Genet. 1985;22:453–462. doi: 10.1002/ajmg.1320220303. [DOI] [PubMed] [Google Scholar]
- 10.Nimgaonkar V.L., Fujiwara T.M., Dutta M., Wood J., Gentry K., Maendel S., Morgan K., Eaton J. Low prevalence of psychoses among the Hutterites, an isolated religious community. Am. J. Psychiatry. 2000;157:1065–1070. doi: 10.1176/appi.ajp.157.7.1065. [DOI] [PubMed] [Google Scholar]
- 11.Boycott K.M., Parboosingh J.S., Chodirker B.N., Lowry R.B., McLeod D.R., Morris J., Greenberg C.R., Chudley A.E., Bernier F.P., Midgley J. Clinical genetics and the Hutterite population: a review of Mendelian disorders. Am. J. Med. Genet. A. 2008;146A:1088–1098. doi: 10.1002/ajmg.a.32245. [DOI] [PubMed] [Google Scholar]
- 12.Puffenberger E.G., Hu-Lince D., Parod J.M., Craig D.W., Dobrin S.E., Conway A.R., Donarum E.A., Strauss K.A., Dunckley T., Cardenas J.F. Mapping of sudden infant death with dysgenesis of the testes syndrome (SIDDT) by a SNP genome scan and identification of TSPYL loss of function. Proc. Natl. Acad. Sci. USA. 2004;101:11689–11694. doi: 10.1073/pnas.0401194101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fahiminiya S., Majewski J., Mort J., Moffatt P., Glorieux F.H., Rauch F. Mutations in WNT1 are a cause of osteogenesis imperfecta. J. Med. Genet. 2013;50:345–348. doi: 10.1136/jmedgenet-2013-101567. [DOI] [PubMed] [Google Scholar]
- 14.McDonald-McGinn D.M., Fahiminiya S., Revil T., Nowakowska B.A., Suhl J., Bailey A., Mlynarski E., Lynch D.R., Yan A.C., Bilaniuk L.T. Hemizygous mutations in SNAP29 unmask autosomal recessive conditions and contribute to atypical findings in patients with 22q11.2DS. J. Med. Genet. 2013;50:80–90. doi: 10.1136/jmedgenet-2012-101320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar P., Henikoff S., Ng P.C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 2009;4:1073–1081. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 16.Adzhubei I.A., Schmidt S., Peshkin L., Ramensky V.E., Gerasimova A., Bork P., Kondrashov A.S., Sunyaev S.R. A method and server for predicting damaging missense mutations. Nat. Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seelow D., Schuelke M., Hildebrandt F., Nürnberg P. HomozygosityMapper--an interactive approach to homozygosity mapping. Nucleic Acids Res. 2009;37 doi: 10.1093/nar/gkp369. W593-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murakami Y., Tawamie H., Maeda Y., Büttner C., Buchert R., Radwan F., Schaffer S., Sticht H., Aigner M., Reis A. Null mutation in PGAP1 impairing Gpi-anchor maturation in patients with intellectual disability and encephalopathy. PLoS Genet. 2014;10:e1004320. doi: 10.1371/journal.pgen.1004320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollard K.S., Hubisz M.J., Rosenbloom K.R., Siepel A. Detection of nonneutral substitution rates on mammalian phylogenies. Genome Res. 2010;20:110–121. doi: 10.1101/gr.097857.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chun S., Fay J.C. Identification of deleterious mutations within three human genomes. Genome Res. 2009;19:1553–1561. doi: 10.1101/gr.092619.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwarz J.M., Cooper D.N., Schuelke M., Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat. Methods. 2014;11:361–362. doi: 10.1038/nmeth.2890. [DOI] [PubMed] [Google Scholar]
- 22.Reva B., Antipin Y., Sander C. Predicting the functional impact of protein mutations: application to cancer genomics. Nucleic Acids Res. 2011;39:e118. doi: 10.1093/nar/gkr407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper G.M., Stone E.A., Asimenos G., Green E.D., Batzoglou S., Sidow A., Sidow A., NISC Comparative Sequencing Program Distribution and intensity of constraint in mammalian genomic sequence. Genome Res. 2005;15:901–913. doi: 10.1101/gr.3577405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kircher M., Witten D.M., Jain P., O’Roak B.J., Cooper G.M., Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 2014;46:310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eide D.J. The SLC39 family of metal ion transporters. Pflugers Arch. 2004;447:796–800. doi: 10.1007/s00424-003-1074-3. [DOI] [PubMed] [Google Scholar]
- 26.He L., Vasiliou K., Nebert D.W. Analysis and update of the human solute carrier (SLC) gene superfamily. Hum. Genomics. 2009;3:195–206. doi: 10.1186/1479-7364-3-2-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He L., Girijashanker K., Dalton T.P., Reed J., Li H., Soleimani M., Nebert D.W. ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: characterization of transporter properties. Mol. Pharmacol. 2006;70:171–180. doi: 10.1124/mol.106.024521. [DOI] [PubMed] [Google Scholar]
- 28.Wang C.Y., Jenkitkasemwong S., Duarte S., Sparkman B.K., Shawki A., Mackenzie B., Knutson M.D. ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading. J. Biol. Chem. 2012;287:34032–34043. doi: 10.1074/jbc.M112.367284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liuzzi J.P., Cousins R.J. Mammalian zinc transporters. Annu. Rev. Nutr. 2004;24:151–172. doi: 10.1146/annurev.nutr.24.012003.132402. [DOI] [PubMed] [Google Scholar]
- 30.Avila D.S., Puntel R.L., Aschner M. Manganese in health and disease. In: Sigel A., Sigel H., Sigel R.K.O., editors. Interrelations between Essential Metal Ions and Human Diseases. Springer; 2013. pp. 199–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uhlén M., Fagerberg L., Hallström B.M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson Å., Kampf C., Sjöstedt E., Asplund A. Tissue-based map of the human proteome. Science. 2015;347:394–404. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 32.Ng E., Lind P.M., Lindgren C., Ingelsson E., Mahajan A., Morris A., Lind L. Genome-wide association study of toxic metals and trace elements reveals novel associations. Hum. Mol. Genet. 2015;24:4739–4745. doi: 10.1093/hmg/ddv190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gálvez-Peralta M., He L., Jorge-Nebert L.F., Wang B., Miller M.L., Eppert B.L., Afton S., Nebert D.W. ZIP8 zinc transporter: indispensable role for both multiple-organ organogenesis and hematopoiesis in utero. PLoS ONE. 2012;7:e36055. doi: 10.1371/journal.pone.0036055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris S.M., Jr. Arginases and arginine deficiency syndromes. Curr. Opin. Clin. Nutr. Metab. Care. 2012;15:64–70. doi: 10.1097/MCO.0b013e32834d1a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Häberle J., Görg B., Toutain A., Rutsch F., Benoist J.F., Gelot A., Suc A.L., Koch H.G., Schliess F., Häussinger D. Inborn error of amino acid synthesis: human glutamine synthetase deficiency. J. Inherit. Metab. Dis. 2006;29:352–358. doi: 10.1007/s10545-006-0256-5. [DOI] [PubMed] [Google Scholar]
- 36.Claus Henn B., Ettinger A.S., Schwartz J., Téllez-Rojo M.M., Lamadrid-Figueroa H., Hernández-Avila M., Schnaas L., Amarasiriwardena C., Bellinger D.C., Hu H., Wright R.O. Early postnatal blood manganese levels and children’s neurodevelopment. Epidemiology. 2010;21:433–439. doi: 10.1097/ede.0b013e3181df8e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erway L., Hurley L.S., Fraser A.S. Congenital ataxia and otolith defects due to manganese deficiency in mice. J. Nutr. 1970;100:643–654. doi: 10.1093/jn/100.6.643. [DOI] [PubMed] [Google Scholar]
- 38.Filipov N.M., Dodd C.A. Role of glial cells in manganese neurotoxicity. J. Appl. Toxicol. 2012;32:310–317. doi: 10.1002/jat.1762. [DOI] [PubMed] [Google Scholar]
- 39.Park J.H., Hogrebe M., Grüneberg M., DuChesne I., von der Heiden A.L., Reunert J., Schlingmann K.P., Boycott K.M., Beaulieu C.L., Mhanni A.A. SLC39A8 deficiency: A disorder of manganese transport and glycosylation. Am. J. Hum. Genet. 2015;97:894–903. doi: 10.1016/j.ajhg.2015.11.003. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.