Abstract
Objective
We sought to determine the validity of cancer antigen 125 (CA-125) and the risk of malignancy index (RMI) in the diagnosis of ovarian cancer in women presenting with adnexal lesions of various histopathology types.
Methods
This retrospective cross- sectional study included all women with adnexal lesions who were evaluated at the Royal Hospital, Oman, between January 2012 and December 2014. The inclusion criteria included women who underwent surgical intervention and who had preoperative CA-125 testing and pelvic ultrasound in the work-up plan of their management. The surgical intervention was usually followed by a histopathological diagnosis of the nature of the lesion, which was used as the gold standard for the evaluation of both CA-125 and RMI.
Results
The cohort included 361 women who had serum CA-125 and pelvic ultrasound prior to the surgical intervention of the adnexal lesion. Of these women, 61 (17%) had malignant ovarian lesions. Using the proposed cut-off 35 U/ml for CA-125 and 200 for RMI, the CA-125 test was more sensitive for detecting the majority of malignant ovarian tumors compared to the RMI (69% vs. 57%). Both tests were more sensitive in detecting epithelial ovarian cancer compared to other ovarian cancers. However, RMI was more specific in excluding benign ovarian lesions compared to CA-125 (81% vs. 68%). Additionally, RMI had a better area under the curve compared to CA-125 (0.771 vs. 0.745; p<0.005). Lowering the RMI cut-off to 150 resulted in a better sensitivity (62% vs. 57%) and had an acceptable specificity (78% vs. 81%) compared to a cut-off of 200.
Conclusion
Both CA-125 and RMI have good validity in the diagnosis of ovarian tumors. CA-125 has higher sensitivity; however, RMI has higher specificity. In combination, CA-125 might be more valid for the diagnosis of malignant ovarian cancer while RMI is more valid for excluding the diagnosis of these tumors. Differential use of these two tools will improve the triage of women with suspected ovarian tumors since both are measured in their work-up. We recommended the use of both tools in primary care to reduce referral to gynecology or oncology units.
Keywords: Adnexal Mass, CA-125 Antigen, Ovarian Cancer, Risk of Malignancy Index, Validity of Results
Introduction
Carbohydrate antigen 125 or cancer antigen 125 (CA-125), also known as mucin 16 (MUC16), is a member of mucin glycoproteins, which contains 22,000 amino acids. It is significantly expressed by most ovarian epithelial tumors but also by the normal epithelium of the female reproductive system, gastrointestinal mucosal cells, and the luminal surface of mesothelium lining the peritoneum, pleura, and pericardium.1,2 CA-125 has been the focus of most clinicians in the initial evaluation and investigation of females who presented with unexplained abdominal symptoms or an adnexal mass. It was first described in 1983 as a biomarker for epithelial ovarian cancer (EOC). Since, it is increasingly used alone or in combination with other markers with or without pelvic ultrasound for the diagnosis of EOC.3 It has been approved by the US Food and Drug Association (FDA) and was recommended by the National Institute for Health and Care Excellence (NICE) for the diagnosis and monitoring response to therapy in women with established EOC.4
Despite its wide use, CA-125 has a known limitation in terms of its diagnostic performance particularly for early-stage disease. It has been reported to be elevated only in 47% of women with early stage ovarian cancer but is elevated in 80–90% of patients with advanced stage disease.5 However, it can also be elevated in some benign conditions of the ovary including ovarian endometrioma.5 These findings were illustrated further by others who also reported a poor sensitivity and specificity when the test was used alone.6
To improve the validity of CA-125, the risk of malignancy index (RMI) was developed by Jacobs et al7 in 1990. The RMI uses a multimodality approach that combines the CA-125 result with ultrasound findings and menopausal state to calculate an index score that helps to predict the risk of ovarian cancer in women presenting with an adnexal mass. The RMI is widely used in the UK and has been recommended by NICE guidelines as a tool for screening high-risk women in the primary care setting.8
The RMI was modified by Tingulstad et al,9 in 1996 to the RMI 2. There are minor differences between the two indices in the evaluation of ultrasound and menopausal scoring. Both RMIs were assessed in a few studies with some favor towards the modified version.10-12 The studies recommended the use of RMI in the primary care setting as it is a simple and cheap approach that can facilitate referral to specialized gynecological centers. A systematic review supported the usefulness of RMI in clinical practice as the test of choice in the preoperative evaluation of women with adnexal masses.13
The aim of this study was to highlight the usefulness of RMI as a diagnostic tool for evaluating women with suspected ovarian tumors attending the gynecology department (outpatient clinic and wards) at the Royal Hospital, Oman, during a three-year period. The study aimed to evaluate the validity of CA-125 alone as a currently used tumor marker and compare its performance with RMI. Although this study is not new in this field, it is the first in Oman where the use of CA-125 with or without RMI is not common practice by the primary care clinicians or gynecologists assessing women presenting with a suspected ovarian lesion or tumor.
Methods
This retrospective cross-sectional study looked at all operated cases of ovarian diseases performed at the Royal Hospital between January 2012 and December 2014. The study included all women who were referred to the gynecology department for the evaluation and management of an ovarian mass or disorder for which an ovarian operation/biopsy was done and laboratory work-up, including measurement of serum CA-125 and pelvic ultrasound, were performed prior to surgery. All clinical and laboratory data were collected using the hospital information system (Al-Shifa 3 Plus).
The CA-125 assay was performed by a two-step immunoassay chemiluminescent microparticle immunoassay technology (Architect 2000i, Abbott, US). The assays followed the manufacturers recommendations for quality assessment (internal and external). The between run precisions as reflected by the coefficient of variation were 2.8%, 3.2%, and 2.2% for the three levels of internal quality control materials (low, middle, and high concentration of CA-125), respectively.
From the variables collected, the RMI was calculated based on the equation recommended by Tingulstad et al,9 as follows:
RMI 2 = U × M × serum CA-125
Where U is the total ultrasound score, M is the menopausal status and the CA-125 value in U/ml. In this formula, a score was assigned for the ultrasonography characteristics looking for the presence of multilocular cystic lesions, solid areas, bilateral lesions, ascites, and intra-abdominal metastasis. A score of one was given if present. The total ultrasound score was calculated for each patient, where a total ultrasound score of zero or one was given a value of one and a score greater than two was given a U-score of four. The postmenopausal status was defined as one year or more of amenorrhea or aged 50 years or more if the woman had undergone a hysterectomy. The menopausal status score was one for premenopausal women and four for postmenopausal women. Finally, the value of serum CA-125 concentration was substituted directly into the formula.
RMI 2 scoring was implemented and calculated for all women included in this study hence when RMI it is mentioned we are referring to RMI 2. The performance of the RMI scoring system will be presented in this cohort and its validity will be compared to CA-125 values based on the histopathology report of the ovarian lesion, which is considered as the gold standard in this validation study. The cut-off value for CA-125 of 35 U/ml was used as recommended by the manufacturer and internationally. A cut-off value of 200 for the RMI was followed as recommended by Jacobs et al,7 to achieve relatively high levels of sensitivity and specificity.
SPSS Statistics (SPSS Inc., Chicago, US) was used to calculate the mean and standard deviation (SD). The non-parametric t-test, Kruskal-Wallis test, and Mann-Whitney U test were used to compare the differences in the means of each parameter (measured or calculated) between the different groups. Statistical significance was assigned to p<0.050.
The classification of the results for each parameter as normal or abnormal was based on the result of histopathology report versus the cut-offs used for CA-125 and RMI. Quality assurance for CA-125 was followed using both Internal QC (BIORAD, USA) and the External QA scheme (RIQAS, UK). The validity indicators (sensitivity, specificity, negative and positive predictive values, and efficiency) for CA-125 and MRI compared to the histopathology examination were calculated and compared at the recommended cut-offs. Receiver operator characteristic (ROC) and the area under the curve (AUC) for CA-125 and RMI were constructed to identify the preferred cut-off for both tools in our cohort.
Results
The total number of ovarian biopsies/surgeries in the study period was 441. However, only 361 cases had complete data that included both CA-125 and ultrasound report prior to the intervention and only these were included in this evaluation study. Table 1 summarizes the demographic characteristics of the studied population, and the histological results of the examined ovarian specimens are given in Table 2. The histopathology classifications of ovarian tumors included surface epithelial-stromal, sex cord-stromal, and germ cell tumors. Lesions that did not fit into these three groups were grouped as others.
Table 1. Demographic characteristics of the study patients.
| Characteristic | Benign | Malignant | p-value |
|---|---|---|---|
| Total patients | 376 (85.3%) | 65 (14.7%) | |
| Age (years)* Median (range) |
33±12 30 (10–80) |
43±17 43 (15–83) |
0.001 |
| BMI (kg/m2)* Median (range) |
27±7 27 (15–50) |
26±6 26 (15–48) |
0.081 |
| Premenopausal** Postmenopausal** |
339 (90.4%) 36 (9.6%) |
42 (64.6%) 23 (35.4%) |
0.000 |
| Parity** | |||
| Null | 186 (50.1%) | 23 (39.0%) | 0.004 |
| Primi | 47 (12.7%) | 2 (3.4%) | |
| Multi | 138 (37.2%) | 34 (57.6%) |
*mean±SD.**number (%). BMI: body mass index.
Table 2. Histological findings of the ovarian lesions in the study population. Classification includes surface epithelial-stromal (epithelial), sex cord-stromal (sex cord), and germ cell tumors (germ cell). Lesions that did not fit into any category were group "others". Secondaries include metastasis from the gastrointestinal tract and peritoneal cancers.
| Classification | Benign (n=376) | n | Malignant (n=65) | n |
|---|---|---|---|---|
| Epithelial n=207 (46.9%) |
Serous cystadenoma | 53 | Serous adenocarcinoma | 13 |
| Mucinous cystadenoma | 11 | Mucinous adenocarcinoma | 3 | |
| Endometrial cysts | 107 | Endometrial adenocarcinoma | 5 | |
| Seromucinous | 4 | Borderline epithelial tumors | 11 | |
| Total | 175 (46.5%) | Total | 32 (49.2%) | |
| Sex cord n=20 (4.5%) |
Fibroma | 8 | Granulosa | 7 |
| Thecoma | 2 | Steroid tumor | 2 | |
| Sclerosing stromal tumor | 1 | |||
| Total | 11 (2.9%) | Total | 9 (13.8%) | |
| Germ cell n=138 (31.3%) |
Teratoma | 123 | Dysgerminoma | 2 |
| Struma ovarii | 3 | Yolk sac cancer | 2 | |
| Immature teratoma | 6 | |||
| Struma ovarii with carcinoid | 2 | |||
| Total | 126 (33.5%) | Total | 12 (18.5%) | |
| Others n=76 (17.2%) |
Simple cyst | 8 | Secondaries | 11 |
| Functional cyst | 41 | Lymphoma | 1 | |
| Abscess | 2 | |||
| Paraovarian cyst | 9 | |||
| Normal pathology | 4 | |||
| Total | 64 (17.0%) | Total | 12 (18.5%) |
Of the 441 ovarian specimens examined, 376 (85.3%) were benign, and 65 (14.7%) were malignant. The malignant tumors included epithelial tumors (49.2%), in which serous cystadenocarcinoma (20.0%) was predominant, followed by borderline epithelial tumors (16.9%). Germ cell tumor (18.5%) was the second common malignant type followed by sex cord tumor (13.8%). In contrast, the most common benign lesions were epithelial types (46.5%) followed by germ cell types (33.5%). Among these, the most common benign lesions were teratoma (32.7%) followed by endometriotic cysts (28.4%) and serous cystadenoma (14.1%).
The descriptive analysis for CA-125 and RMI in the benign and malignant ovarian tumor groups with the various histological lesions are shown in Table 3. Both CA-125 and RMI were not normally distributed; hence non-parametric t-tests (Kruskal-Wallis test and Mann-Whitney U test) were applied.
Table 3. CA-125 (U/ml) and RMI in the tumor groups.
| Test | Tumor group |
Tumor type | Mean±SD | Median (range) | p-value |
|---|---|---|---|---|---|
| CA-125 | Epithelial | Benign | 80±165 | 33 (4–1578) | <0.001 |
| Malignant | 510±671 | 139 (9–2034) | |||
| Benign (excluding endometriosis | 18±11 | 17 (4–50) | NA | ||
| Malignant (excluding borderline) | 680±718 | 322 (18–2034) | NA | ||
| Endometriosis | 122±205 | 61 (9–1578) | NA | ||
| Borderline lesions | 185±433 | 44 (9–1483) | |||
| Sex cord | Benign | 63±120 | 22 (9–401) | 0.093 | |
| Malignant | 13±5 | 13 (8–22) | |||
| Germ cell | Benign | 24±35 | 16 (6–324) | <0.001 | |
| Malignant | 100±130 | 49 (13–411) | |||
| Others | Benign | 40±67 | 15 (5–265) | <0.001 | |
| Malignant | 319±340 | 218 (10–1081) | |||
| All | Benign | 56±126 | 21 (4–1578) | <0.001 | |
| Malignant | 346±541 | 81 (9–2034) | |||
| Total | 105±272 | 23 (4–2034) | NA | ||
| RMI | Epithelial | Benign | 230±463 | 68 (4–3044) | <0.001 |
| Malignant | 6130±9626 | 1300 (9–31792) | |||
| Benign (excluding endometriosis) | 50±105 | 21 (4–768) | NA | ||
| Malignant (excluding borderline) | 9190±10721 | 3664 (18–31792) | NA | ||
| Endometriosis | 354±565 | 144 (9–3044) | NA | ||
| Borderline lesions | 287±426 | 137 (9–1483) | NA | ||
| Sex cord | Benign | 294±476 | 124 (9–1604) | 0.093 | |
| Malignant | 41±29 | 40 (10–88) | |||
| Germ cell | Benign | 73±167 | 27 (6–1296) | <0.001 | |
| Malignant | 289±446 | 126 (19–1644) | |||
| Others | Benign | 109±214 | 28 (9–1024) | <0.001 | |
| Malignant | 2567±3815 | 872 (40–12160) | |||
| All | Benign | 164±368 | 44 (4–3044) | <0.001 | |
| Malignant | 3739±7575 | 304 (9–31792) | |||
| Total | 768±3388 | 56 (4–31792) | NA |
CA-125: cancer antigen 125; RMI: risk of malignancy index; SD: standard deviation; NA: non-applicable
The validity indicators for CA-125 and RMI for diagnosing all malignant ovarian tumors and epithelial ovarian tumors, with and without borderline tumors, are shown in Table 4. A high rate of false negative CA-125 and RMI results in patients with borderline epithelial lesions (5/11 and 7/11, respectively) and sex cord ovarian lesions (6/6 for both) was observed. Also, in patients with benign ovarian lesions, a different rate of true negative and false positive results were observed. A high proportion of false-positive CA-125 results (66/89) was noted in patients with endometriosis, with a median CA-125 of 144 U/ml (range 9–3044). In contrast, RMI showed a better performance in endometriosis with only 38/89 false positive results. Also, there were two patients with stage five chronic kidney disease (CKD) with eGFR <15 ml/min/1.73 m2, one had a hemorrhagic functional cyst (CA-125 was 104 U/ml) and the other had normal ovarian pathology but presented with ascites (CA-125 was 224 U/ml).
Table 4. Validity indicators of CA-125 and RMI in patients with malignant ovarian tumors and EOC, with and without borderline tumors (cut-off: CA-125 35 U/ml; RMI 200).
| Cases (n) | Test | Sensitivity (%) | Specificity (%) | NPV | PPV | Efficiency |
|---|---|---|---|---|---|---|
| All cases (n= 61) | CA-125 | 69 | 68 | 92 | 31 | 69 |
| RMI | 57 | 81 | 90 | 38 | 69 | |
| All EOC (n=32) | CA-125 | 78 | 68 | 97 | 21 | 73 |
| RMI | 69 | 81 | 96 | 28 | 75 | |
| EOC without borderline tumors (n=21) | CA-125 | 90 | 68 | 99 | 17 | 79 |
| RMI | 86 | 81 | 99 | 24 | 84 |
CA-125: cancer antigen 125; RMI: risk of malignancy index; EOC: epithelial ovarian cancer; NPV: negative predictive value; PPV: positive predictive value.
The ROC curves for both CA-125 and RMI are shown in Figure 1. The AUC for CA-125 and RMI were 0.745 and 0.771, respectively, (p<0.001). The validity indicator for both CA-125 and RMI was checked at different cut-off points [Table 5].
Figure 1.
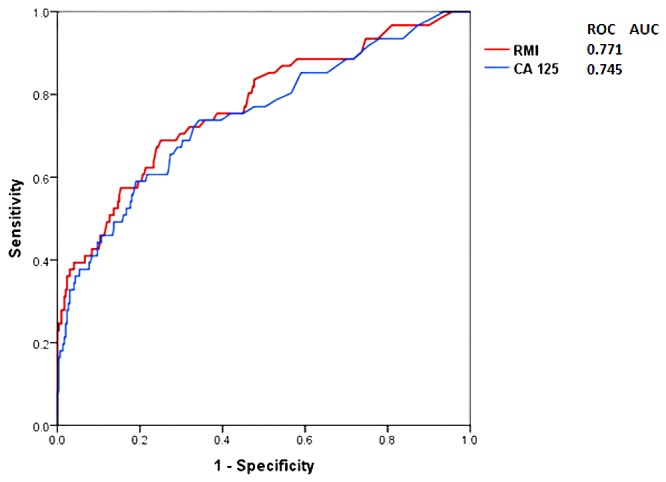
The receiver operating characteristic (ROC) curve and area under the curve (AUC) for CA-125 and RMI in the studied patients.
Table 5. Validity indicators for CA-125 and RMI at different cut-offs for all patients with malignant ovarian tumors (n= 61).
| Test | Cut-off | Sensitivity (%) | Specificity (%) | NPV | PPV | Efficiency |
|---|---|---|---|---|---|---|
| CA-125 U/ml | 35 | 69 | 68 | 92 | 31 | 69 |
| 50 | 61 | 75 | 90 | 33 | 68 | |
| 70 | 54 | 82 | 90 | 38 | 68 | |
| RMI | 150 | 62 | 78 | 91 | 37 | 70 |
| 200 | 57 | 81 | 90 | 38 | 69 | |
| 250 | 54 | 85 | 90 | 42 | 70 |
CA-125: cancer antigen 125; RMI: risk of malignancy index; NPV: negative predictive value; PPV: positive predictive value
Discussion
Ovarian cancer is a common gynecological cancer in women and has a poor prognosis with a five-year survival rate less than 35%. International health organizations including NICE,4 the European Society for Medical Oncology (ESMO),14,15 the US Preventive Services Task Force (USPSTF),16 and other bodies have set clinical guidelines on the recognition and management of women with ovarian cancer. These guidelines have increased the awareness towards initiating early investigations of women with suggestive symptoms and signs who present to primary care physicians.8 Despite the high mortality of these tumors, screening asymptomatic women is still questionable and is not recommended unless the patients have known genetic mutations that increase their risk for ovarian cancer (e.g. BRCA mutations) or have suggestive clinical scenarios.4,14-16
In Oman, the Ministry of Health (MOH) Oman Cancer Incidence Registry (2011) showed a crude incidence ovarian cancer rate of 1.8 and an age-standardized incidence rate of 3.1 per 100,000. This took into consideration the estimated mid-year Omani population in 2011 of 2,137,807 with a sex ratio of 983 females per 1000 males.17 No studies are available in Oman related to the incidence and whether ovarian cancer is being underdiagnosed. An audit conducted at the Royal Hospital revealed an awareness in using tumor markers effectively in monitoring cancer cases, including ovarian cancer, but not for diagnostic purposes.18 Our cohort revealed a prevalence rate of malignant ovarian cancer of 15% among all surgically proven ovarian lesions for women referred to the Royal Hospital. Looking at both CA-125 and RMI as indicators for ovarian cancer [Table 3], a significant difference (p<0.005) was noticed in both tools between benign and malignant groups in all sample populations as well as among the various histological types (except for sex cord lesions; p=0.094). CA-125 was raised (>35 U/ml) in 69% of women with malignant ovarian cancers and 32% of women with benign ovarian cancers. In contrast, RMI was raised (>200) in 57% of women with malignant ovarian cancers and 18% of women with benign ovarian cancers. The validity indicators of both tests [Table 4] showed that CA-125 has better sensitivity (69% vs. 57%) than RMI, but also has a lower specificity (68% vs. 81%). In addition, the ROC curve showed better AUC for RMI compared to CA-125 (0.771 vs. 0.745; p<0.005). The sensitivity of CA-125 and RMI was much higher when used to detect EOC (excluding the borderline cases) with values of 90% for CA-125 and 86% for RMI.
Among the malignant ovarian cancer group, all women with sex cord ovarian cancer and the majority of women with borderline epithelial cancer had false negative CA-125 and RMI results. The CA-125 results also showed a high proportion of false positive results among women with endometriosis where the median CA-125 was 144 U/ml, and in one case CA-125 was raised 45 folds in a 30 years old patient who had bilateral ovarian endometriosis lesions with peritoneum involvement. However, the RMI test in this setting appears to be better than CA-125 and could identify the benign nature of ovarian endometriosis cysts in women with high CA-125 levels. Considering different cut-offs of the RMI [Table 5], decreasing the index to 150 can increase its sensitivity to 62% keeping a specificity of 78%. Data obtained by different studies revealed a comparable outcome for CA-125 and RMI.19-21 Liao et al,22 reported in their meta-analysis of 19 studies a sensitivity and specificity of CA-125 of 75% and 80%, respectively.
Currently, CA-125 is still the most widely used tumor biomarker for the detection of ovarian cancer. However, its poor specificity is considered to be the main drawback that has limited its use alone, and its incorporation in the RMI has improved its specificity. It is known that CA-125 levels can be elevated in benign gynecological conditions particularly endometriosis and fibroids, and other medical disorders such as congestive heart failure and cirrhosis.23,24 This limitation has facilitated researchers to look for more specific biomarkers or tools for use in this setting. The RMI can easily be adopted when assessing women with adnexal mass by utilizing both CA-125 and ultrasound, which are part of the work-up investigations in these patients. In addition to CA-125 and RMI, other diagnostic tests for EOC have been recommended and evaluated.21 These include the human epididymis protein (HE4) and the Risk of Malignancy Algorithm (ROMA) using dual CA-125 and HE4 markers. Karlsen et al,21 reported equal performance of RMI and ROMA, despite the ROMA having a lower specificity.21 However, Moore et al,25 reported a higher sensitivity for ROMA than RMI for distinguishing benign status from EOC (94.3% vs. 84.6%) at a specificity of 75%. In patients with stage I and II disease, ROMA achieved a sensitivity of 85.3% compared to 64.7% for RMI.
The OVA1 test is another In Vitro Diagnostic Multivariate Index Assay (IVDMIA) that has been studied in the triage of women with an adnexal mass. The assay utilizes five proteomic biomarkers: CA-125, prealbumin, apolipoprotein A1, beta-2 microglobulin, and transferrin.26 However, in addition to the inclusion of different tests that are not routinely used in laboratory practice, this multi-marker showed comparable performance with RMI and ROMA.27
Conclusion
Both CA-125 and RMI have good validity in the diagnosis of ovarian tumors. CA-125 has a higher sensitivity; however, RMI has a higher specificity. In combination, CA-125 might be valid for the diagnosis of malignant ovarian cancer while RMI is valid for excluding the diagnosis of these tumors. Differential use of these two tools will improve the triage of women with suspected ovarian tumors since both are measured in their work-up. We recommended the use of both tools in primary care to reduce unnecessary referral of women with benign ovarian masses to gynecology or oncology units.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
References
- 1.Yin BW, Dnistrian A, Lloyd KO. Ovarian cancer antigen CA125 is encoded by the MUC16 mucin gene. Int J Cancer 2002. Apr;98(5):737-740. 10.1002/ijc.10250 [DOI] [PubMed] [Google Scholar]
- 2.Gniewek P, Kolinski A. Coarse-grained modeling of mucus barrier properties. Biophys J 2012. Jan;102(2):195-200. 10.1016/j.bpj.2011.11.4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bast RC, Jr, Klug TL, St John E, Jenison E, Niloff JM, Lazarus H, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med 1983. Oct;309(15):883-887. 10.1056/NEJM198310133091503 [DOI] [PubMed] [Google Scholar]
- 4.Frederick R, Ueland FR, Li AJ. Serum biomarkers for evaluation of adnexal mass for epithelial carcinoma of the ovary, fallopian tube, or peritoneum. UpToDate. Available at http://www.accessdata.fda.gov/cdrh_docs/reviews/K042731.pdf. Accessed Mar 2015.
- 5.Skates SJ, Mai P, Horick NK, Piedmonte M, Drescher CW, Isaacs C, et al. Large prospective study of ovarian cancer screening in high-risk women: CA125 cut-point defined by menopausal status. Cancer Prev Res (Phila) 2011. Sep;4(9):1401-1408. 10.1158/1940-6207.CAPR-10-0402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dodge JE, Covens AL, Lacchetti C, Elit LM, Le T, Devries-Aboud M, et al. Gynecology Cancer Disease Site Group . Preoperative identification of a suspicious adnexal mass: a systematic review and meta-analysis. Gynecol Oncol 2012. Jul;126(1):157-166. 10.1016/j.ygyno.2012.03.048 [DOI] [PubMed] [Google Scholar]
- 7.Jacobs I, Oram D, Fairbanks J, Turner J, Frost C, Grudzinskas JG. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. Br J Obstet Gynaecol 1990. Oct;97(10):922-929. 10.1111/j.1471-0528.1990.tb02448.x [DOI] [PubMed] [Google Scholar]
- 8.NICE Clinical Guideline 122. The recognition and initial management of ovarian cancer. 2011. Available at http://guidance.nice.org.uk/CG/Wave17/22. Accessed Mar 2015.
- 9.Tingulstad S, Hagen B, Skjeldestad FE, Onsrud M, Kiserud T, Halvorsen T, et al. Evaluation of a risk of malignancy index based on serum CA125, ultrasound findings and menopausal status in the pre-operative diagnosis of pelvic masses. Br J Obstet Gynaecol 1996. Aug;103(8):826-831. 10.1111/j.1471-0528.1996.tb09882.x [DOI] [PubMed] [Google Scholar]
- 10.Davies AP, Jacobs I, Woolas R, Fish A, Oram D. The adnexal mass: benign or malignant? Evaluation of a risk of malignancy index. Br J Obstet Gynaecol 1993. Oct;100(10):927-931. 10.1111/j.1471-0528.1993.tb15109.x [DOI] [PubMed] [Google Scholar]
- 11.Morgante G, la Marca A, Ditto A, De Leo V. Comparison of two malignancy risk indices based on serum CA125, ultrasound score and menopausal status in the diagnosis of ovarian masses. Br J Obstet Gynaecol 1999. Jun;106(6):524-527. 10.1111/j.1471-0528.1999.tb08318.x [DOI] [PubMed] [Google Scholar]
- 12.Kader Ali Mohan GR, Jaaback K, Proietto A, Robertson R, Angstetra D. Risk Malignancy Index (RMI) in patients with abnormal pelvic mass: Comparing RMI 1, 2 and 3 in an Australian population. Aust N Z J Obstet Gynaecol 2010. Feb;50(1):77-80. 10.1111/j.1479-828X.2009.01105.x [DOI] [PubMed] [Google Scholar]
- 13.Geomini P, Kruitwagen R, Bremer GL, Cnossen J, Mol BW. The accuracy of risk scores in predicting ovarian malignancy: a systematic review. Obstet Gynecol 2009. Feb;113(2 Pt 1):384-394. 10.1097/AOG.0b013e318195ad17 [DOI] [PubMed] [Google Scholar]
- 14.Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C, ESMO Guidelines Working Group . Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013. Oct;24(Suppl 6):vi24-vi32. 10.1093/annonc/mdt333 [DOI] [PubMed] [Google Scholar]
- 15.Colombo N, Peiretti M, Garbi A, Carinelli S, Marini C, Sessa C, ESMO Guidelines Working Group . Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012. Oct;23(Suppl 7):vii20-vii26. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Preventive Services Task Force (USPSTF). Screening for ovarian cancer. Clinical summary of USPSTF Recommendation. Available at http://www.uspreventiveservicestaskforce.org/. Accessed Mar 2015.
- 17.Cancer incidence in Oman 2011. Dept of non-communicable disease surveillance and control, Directorate General of Health Affairs, Ministry of Health, Oman. Avaialble at http///www.moh.gov.om. Accessed Mar 2015.
- 18.Al Rafay SM, Mula-Abed WS. Biochemical tumor markers requesting in hospital practice: an audit study. Oman Med J 2007;22(1):8-15. [Google Scholar]
- 19.Kader Ali Mohan GR, Jaaback K, Proietto A, Robertson R, Angstetra D. Risk Malignancy Index (RMI) in patients with abnormal pelvic mass: Comparing RMI 1, 2 and 3 in an Australian population. Aust N Z J Obstet Gynaecol 2010. Feb;50(1):77-80. 10.1111/j.1479-828X.2009.01105.x [DOI] [PubMed] [Google Scholar]
- 20.Anton C, Carvalho FM, Oliveira EI, Maciel GA, Baracat EC, Carvalho JP. A comparison of CA125, HE4, risk ovarian malignancy algorithm (ROMA), and risk malignancy index (RMI) for the classification of ovarian masses. Clinics (Sao Paulo) 2012;67(5):437-441. 10.6061/clinics/2012(05)06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlsen MA, Sandhu N, Høgdall C, Christensen IJ, Nedergaard L, Lundvall L, et al. Evaluation of HE4, CA125, risk of ovarian malignancy algorithm (ROMA) and risk of malignancy index (RMI) as diagnostic tools of epithelial ovarian cancer in patients with a pelvic mass. Gynecol Oncol 2012. Nov;127(2):379-383. 10.1016/j.ygyno.2012.07.106 [DOI] [PubMed] [Google Scholar]
- 22.Liao XY, Huang GJ, Gao C, Wang GH. A meta-analysis of serum cancer antigen 125 array for diagnosis of ovarian cancer in Chinese. J Cancer Res Ther 2014. Nov;10(Suppl):C222-C224. 10.4103/0973-1482.145884 [DOI] [PubMed] [Google Scholar]
- 23.Molina R, Filella X, Bruix J, Mengual P, Bosch J, Calvet X, et al. Cancer antigen 125 in serum and ascitic fluid of patients with liver diseases. Clin Chem 1991. Aug;37(8):1379-1383. [PubMed] [Google Scholar]
- 24.Park Y, Lee JH, Hong DJ, Lee EY, Kim HS. Diagnostic performances of HE4 and CA125 for the detection of ovarian cancer from patients with various gynecologic and non-gynecologic diseases. Clin Biochem 2011. Jul;44(10-11):884-888. 10.1016/j.clinbiochem.2011.04.011 [DOI] [PubMed] [Google Scholar]
- 25.Moore RG, Jabre-Raughley M, Brown AK, Robison KM, Miller MC, Allard WJ, et al. Comparison of a novel multiple marker assay vs the Risk of Malignancy Index for the prediction of epithelial ovarian cancer in patients with a pelvic mass. Am J Obstet Gynecol 2010. Sep;203(3):228.e1-228.e6. 10.1016/j.ajog.2010.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fung ET. A recipe for proteomics diagnostic test development: the OVA1 test, from biomarker discovery to FDA clearance. Clin Chem 2010. Feb;56(2):327-329. 10.1373/clinchem.2009.140855 [DOI] [PubMed] [Google Scholar]
- 27.Grenache DG, Heichman KA, Werner TL, Vucetic Z. Clinical performance of two multi-marker blood tests for predicting malignancy in women with an adnexal mass. Clin Chim Acta 2015. Jan;438:358-363. 10.1016/j.cca.2014.09.028 [DOI] [PubMed] [Google Scholar]


