Abstract
Objective:
7-prenyloxycoumarins are a group of secondary metabolites that are found mainly in plants belonging to the Rutaceae and Umbelliferae families. This study was designed to evaluate and compare the cytotoxic and apoptotic activity of 7-prenyloxycoumarin compounds and herniarin on MCF-7, a breast carcinoma cell line.
Materials and Methods:
Cells were cultured in RPMI medium and incubated with different concentrations of auraptene, herniarin, umbelliferone, and umbelliprenin. Cell viability was quantified by MTT assay. Apoptotic cells were determined using propidium iodide staining of DNA fragmentation by flow cytometry (sub-G1peak). Bax protein expression was detected by western blot to investigate the underlying mechanism.
Results:
Doses which induced 50% cell growth inhibition (IC50) against MCF-7 cells with auraptene, herniarin, umbelliferone, and umbelliprenin were calculated (59.7, 207.6, 476.3, and 73.4 µM), respectively. Auraptene induced a sub-G1 peak in the flow cytometry histogram of treated cells compared to control cells, and DNA fragmentation suggested the induction of apoptosis. Western blot analysis showed that auraptene significantly up-regulated Bax expression in MCF-7 cells compared to untreated controls.
Conclusion:
Auraptene exerts cytotoxic and apoptotic effects in breast carcinoma cell line and can be considered for further mechanistic evaluations in human cancer cells. These results candidate auraptene for further studies to evaluate its biosafety and anti-cancer effects.
Key Words: Apoptosis, Bax, Cytotoxicity, MCF-7, 7-Prenyloxycoumarins
Introduction
Apoptosis is a sequence of regulated molecular events induced by many of the chemotherapeutic agents used in cancer treatment (Poncet and Kroemer, 2002 ▶; Mousavi et al., 2008 ▶). The induction of apoptosis in tumor cells is considered very useful in management and therapy as well as in the prevention of cancer. A wide variety of natural substances has been documented to have the ability to induce apoptosis in different tumor cells. In this regard, screening apoptotic inducers of plants, either in the form of crude extracts or as components isolated from them, is very important (Cassileth et al., 2008 ▶).
A natural product with interesting pharmacological properties is coumarin (benzopyran-2-one or chromen-2-one) ring. Many interesting coumarin derivatives have been synthesized including the furanocoumarins, pyranocoumarins, and coumarin sulfamates (COUMATES), which have been found to be useful in photo-chemotherapy, antitumor, and anti-HIV therapy, as a stimulant for the central nervous system, and as antibacterial, anti-inflammatory, and anti-coagulant drugs (Musa et al., 2008 ▶).
Various biological and pharmacological activities of 7-prenyloxycoumarins, a group of secondary metabolites found in families of Rutaceae and Umbelliferae, have gained the attention of researchers in the last two decades (Askari et al., 2009 ▶). Some of the most studied prenylated coumarins include auraptene, herniarin, umbelliferone, and umbelliprenin. Auraptene (7-geranyloxycoumarin) (Figure 1B) is the most abundant geranyloxycoumarin obtained from some species of the genus Citrus (Curini et al., 2004 ▶) with known cancer chemopreventive (Tanaka et al., 1998 ▶) and anti-tumor properties against many types of cancers (Sakata et al., 2004 ▶; Murakami et al., 2000 ▶). In addition, it possess anti-inflammatory (Curini et al., 2004 ▶; Murakami et al., 2000 ▶) and spasmolytic activities (Yamada et al., 1997 ▶) and can suppress the release of tumor necrosis factor alpha (TNF-α) (Tanaka et al., 1999 ▶), superoxide anion generation by inflammatory leukocytes (Murakami et al., 2000 ▶) and IкB (nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor) degradation (Murakami et al., 2003 ▶). Auraptene also has the ability to inhibit the platelet aggregation induced by arachidonic acid and platelet activated factor in vitro (Chen et al., 1995 ▶).
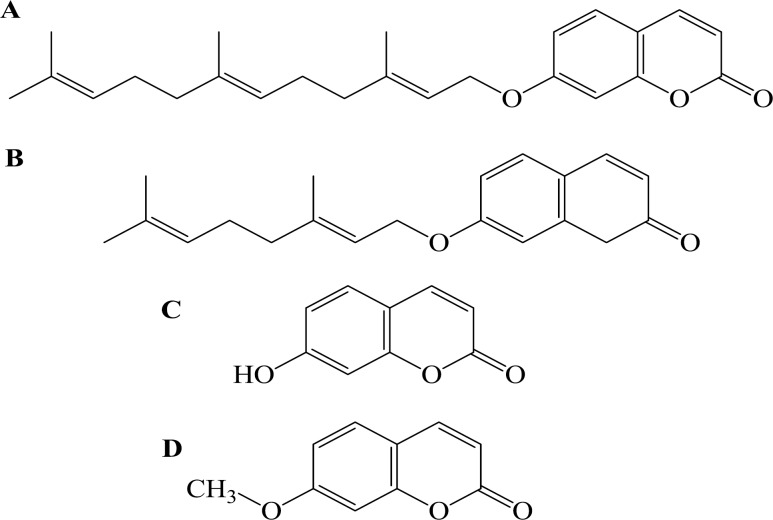
Figure 1.
Chemical structures of 7-prenyloxycoumarins: umbelliprenin (A), auraptene (B) umbelliferone (C), and herniarin (D).
Herniarin (Figure 1D) is a methoxy analogue of umbeliferone occuring naturally in some flowering plants (Ma et al., 2007 ▶). Herniarin was reported to have anti-dermatophytic activity (Mares et al., 1993 ▶).
Umbelliprenin is a prenylated coumarin (Figure 1A) and is found in various plant species such as Ferula (Umbellifera) species, Angelica archangelica (Linn.), Coriandrum sativum L., and Citrus limon (L.) Osbeck, which are consumed as food or used for food preparation. Previous researches have shown various pharmacological activities including inhibition of red pigment production in Serratia marcescens (Iranshahi et al., 2004 ▶), inhibition of squalene-hopene cyclase (SHC) (an enzyme taking part in sterol synthesis) (8), decreasing matrix metaloproteinase (MMP) activity (Baba et al., 2002 ▶), anti-leishmanial activity against promastigotes (Iranshahi et al., 2007 ▶), apoptosis induction in human M4Beu metastatic pigmented melanoma cells (Barthomeuf et al., 2008 ▶), and cancer chemopreventive activity (Iranshahi et al., 2008 ▶).
Umbelliferone (Figure 1C) is a synthesized chemical with the potential to be antimutagenic/ anticarcinogenic against mutations induced by benzo (a) pyrene, a potent mutagen/carcinogen, and hydrogen peroxide. It has also the ability to function as free radical scavengers.
Some coumarins and their active metabolite, 7-hydroxycoumarin analogs, have also shown inhibitory activity on breast cancer tumor cells (Musa et al., 2008 ▶).
Herein, we investigated the cytotoxic and proapoptotic effects of synthesized 7-prenyloxycoumarins and herniarin as bioactive natural coumarins in MCF-7 cells as a widely-used model system for the study of breast cancer (Simstein et al., 2003 ▶). We also explored the role of Bax protein in aurapten-induced apoptosis in MCF-7 cells.
Material and Methods
Preparation of 7-prenyloxycoumarins compounds and herniarin
Auraptene, herniarin, umbelliferone, and umbelliprenin were synthesized according to the previously described method (Askari et al., 2009 ▶). Bioactive 7-prenyloxycoumarins, namely, auraptene, umbelliprenin, and together with herniarin were synthesized from 7-hydroxycoumarin under alkaline conditions and then purified by column chromatography. The structures of the products were characterized by NMR spectroscopic method including 1H- and 13C-NMR experiments. For antiproliferative assay, auraptene, herniarin, umbelliferone, and umbelliprenin were diluted in DMSO. Immediately before use, they were diluted in the culture medium to obtain a final DMSO concentration of 0.5% (v/v).
Chemicals and reagents
RPMI-1640 medium and fetal bovine serum were purchased from Gibco (London, UK); 3-(4, 5-dimethylthiazol-2-yl)-2 and 5-diphenyl tetrazolium (MTT) from Promega (Madison, WI, USA); ethidium bromide, RNase A, and Proteinase K from Fermentas (Ontario, Canada); Bax antibody, β-actin antibody, FAS-L antibody, anti-rabbit IgG, and HRP linked antibody from CellSignaling technology (Boston, USA); ECL Western blotting detection reagent from Bio-RaD (USA).
Cell cultures and treatment agent
The human breast cancer cells (MCF-7) were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum at 37 ºC in a humidified atmosphere of 95% air and 5% CO2. The stock solution of compounds was prepared at 100 mg/ml in dimethylsulfoxide and kept at –20 °C. For MTT assay, cells were seeded at 104 cells per well onto 96-well culture plates. For assay of apoptosis, cells were seeded at 105 cell per well onto a 24-well plate.
For each concentration and time course study, there was a control sample that remained untreated which received the equal volume of medium.
Cell viability
Cell viability was determined using a modified MTT assay (Mosmann et al., 1983 ▶; Mousavi et al., 2010 ▶). Briefly, the cells were seeded (104 cells per well) onto flat-bottomed 96-well culture plates and allowed to grow 72 h after treatment with various concentration of auraptene, herniarin, umbelliferone, and umbelliprenin. After removing the medium, MTT solution (5 mg/ml in PBS) was added and incubated for 4 h and the resulting formazan was solubilized with DMSO (100 ml). The absorption was measured at 570 nm (620 nm as a reference) in an ELISA reader.
Cell morphology
The MCF-7 cells were plated in 96-well plates at a density of 104 cells/well and grown for 48 h in order to attach to the surface of the plates completely. Auraptene, herniarin, umbelliferone, and umbelliprenin were added in different concentrations (0, 25, 50, and 100 µM) to the cells and then the cells were grown at 37 °C in a humidified atmosphere with 5% CO2 for 48 h. For cell morphology experiments, the culture plates were examined and photographed by the inverted light microscope.
PI Staining
Apoptotic cells were detected using PI staining of treated cells followed by flow cytometry to detect the so-called sub-G1 peak (Tayarani-Najaran et al., 2010 ▶). It has been reported that DNA fragmentation creates small fragments of DNA that can be eluted following incubation in a hypotonic phosphate-citrate buffer. When stained with a quantitative DNA-binding dye such as PI, cells that have lost DNA will take up less stain and will appear to the left of the G1 peak. Briefly, 105 MCF-7 cells were seeded in each well of a 24-well plate and treated with IC50 concentrations: 70, 80, and 200 µM of auraptene, umbelliprenin, and herniarin, respectively for 48 h. MCF-7 cells were also treated with various concentrations of auraptene for 48 h. Floating and adherent cells were then harvested and incubated at 4 °C overnight in the dark with 750 μL of a hypotonic buffer (50 μg/mL PI in 0.1% sodium citrate plus 0.1% Triton X-100) before flow cytometric analysis using a FACScan flow cytometer (Becton Dickinson). 104 events were acquired with FACS.
DNA fragmentation
Isolation of apoptotic DNA fragments was performed based on the modified method reported by Gong et al. (Akins et al., 1992 ▶). In brief, the MCF-7 cells were incubated with 70, 80, and 200 µM of auraptene, umbelliprenin, and herniarin, respectively for 48 h. At the end of incubation, cells were centrifuged, washed twice with ice-cold PBS, and a volume equal to 4×106 cells of either type fixed overnight in 70° ethanol in -20 °C. The cells were centrifuged at 800 g for 5 min and the ethanol was removed.
The cell pellet was resuspended in 40 µl of phosphate-citrate buffer (PCB) at room temperature for 30 min. After centrifugation at 1000 g for 5 min, the supernatant was then incubated with 4 µl of 0.25% Nonidet P-40 followed by 3 µl of 1 mg/ml RNase A at 37 °C for 30 min. Three µl of 1 mg/ml proteinase K was added and the extract was incubated for an additional 30 min at 37 °C. An equal amount of this sample (20 ml) was electrophoresed through 1% agarose gel containing ethidium bromide in TBE buffer (45 mM Tris-borate, 1 mM EDTA) at 60 V for 2 h.
Western blotting analysis
MCF-7 cells were treated with 100 µM of auraptene, umbelliprenin, and herniarin for 48 h. The cells were harvested and rinsed with ice-cold PBS. The cell pellet was resuspended in a lysis buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% triton-X 100, 1 mM EDTA, 0.2% SDS, 1% protease inhibitor cocktail, 1% phosphatase inhibitor cocktail, and 1 mM phenylmethylsulfonyl fluoride, and left on ice for 30 min. After centrifugation at 10000 rpm for 20 min at 4 °C, the cell lysate was collected and protein concentration was determined according to the BCA detection kit (Gong et al., 1994). Equal amounts of proteins were subjected to 12.5% SDS–PAGE (w/v). The proteins were transferred to PVDF membrane and subjected to immune-blotting using Bax antibody and β-actin antibody, as primary antibodies, and anti-rabbit HRP linked IgG as secondary antibodies. Bax protein bands in MCF-7 cells were detected by enhanced chemiluminescence using the ECL Western blotting detection reagent. Scanned autorad images were quantified using Quantpro software (Molecular Dynamics).
Statistical analysis
One way analysis of variance (ANOVA) and Bonferroni’s post hoc were used for data analysis. All results were expressed as mean ± SEM and p-values below 0.05 were considered statistically significant.
Results
Inhibition of cell viability
Inhibition of cell viability caused by prenylated coumarins including auraptene, herniarin, umbelliferone, and umbelliprenin was examined using MTT assay.
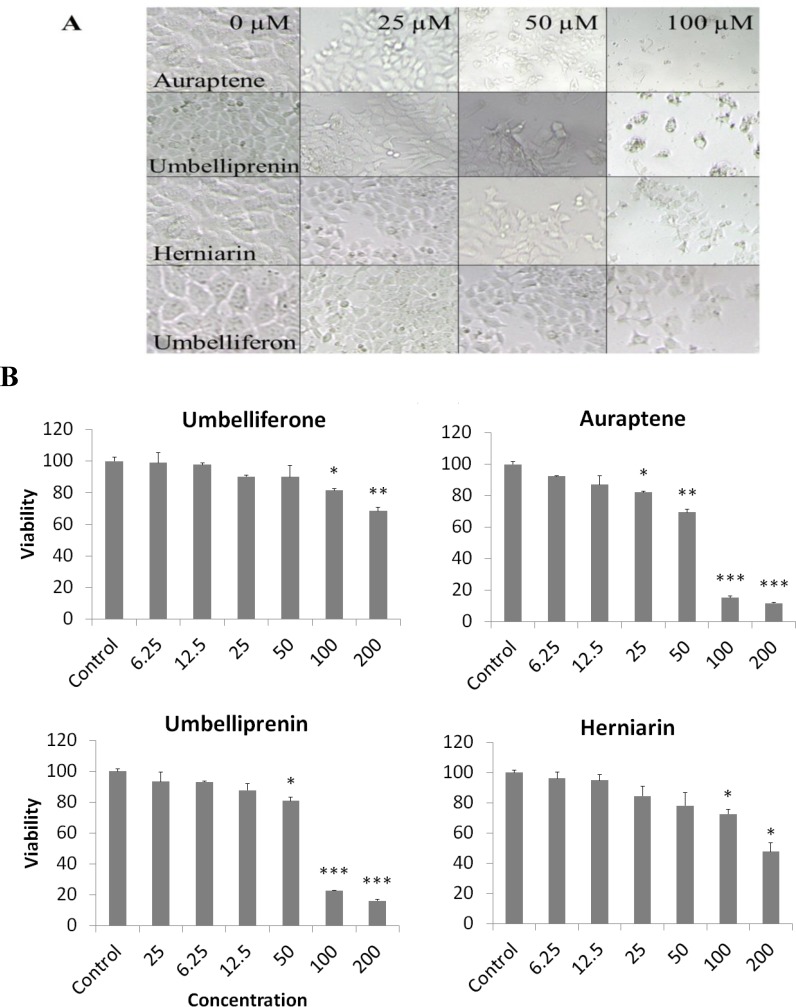
In order to compare the cytotoxicity of auraptene, herniarin, umbelliferone, and umbelliprenin, the MCF-7 cells were incubated with different concentrations of prenylated coumarins for 72 h. The results showed that these compounds decreased cell viability of cells in a concentration-dependent manner. The doses inducing 50% cell growth inhibition (IC50) against MCF-7 cells for auraptene, herniarin, umbelliferone, and umbelliprenin were calculated to be 59.7, 207.6, 476.3,and 73.4 µM, respectively (Figure 2). This toxicity was associated with morphological changes including reduction of cell volume and rounding of the cells. The substantial morphological changes observed in auraptene-treated MCF-7 cells were examined and photographed by the inverted light microscope. Damaged cells became round and shrunken, while the untreated cells retained the normal size and shape (Figure 2).
Figure 2.
(A). Morphological changes of MCF-7 cells after treatment with auraptene, herniarin, umbelliferone, and umbelliprenin for 48 h. Control cells remained untreated and received an equal volume of the solvent. (B). Cytotoxic effects of auraptene, herniarin, umbelliferone, and umbelliprenin on MCF-7 cells. Cells were treated for 72 h with different concentrations of prenylated coumarins. Cytotoxicity was determined by MTT assay. Results are the mean ± SEM of three independent experiments. ∗p <0.05, ∗∗p <0.01, and ∗∗∗p <0.001 compared to control
Apoptosis induction by auraptene, umbelliprenin, and herniarin in MCF-7 cells
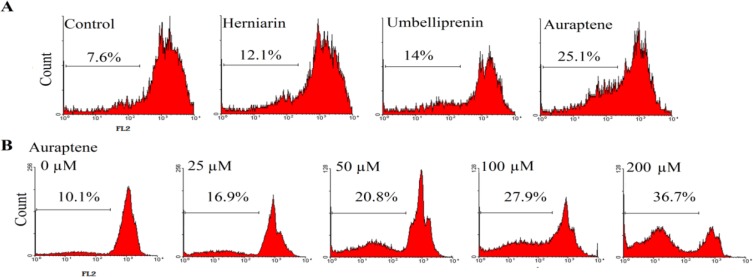
Apoptosis following treatment with auraptene, umbelliprenin, and herniarin was measured with PI staining and flow cytometry aiming to detect the sub-G1 peak resulting from DNA fragmentation. The MCF-7 cells treated with 100 µM auraptene, umbelliprenin, and herniarin for 48 h induced a sub-G1 peak in flow cytometry histogram compared to untreated control cells (Figure 3A).
Figure 3.
(A). Flow cytometry histograms of apoptosis assays by PI method in MCF-7 cells. Cells were treated with 70, 80, and 200 µM, auraptene, umbelliprenin, and herniarin, respectively for 48 h. Sub-G1 peak as an indicative of apoptotic cells, was induced in prenylated coumarins-treated but not in control cells. (B). Flow cytometry histograms of apoptosis assays by PI method in MCF-7 cells. Cells were treated with different concentrations of auraptene for 48 h. Sub-G1 peak as an indicative of apoptotic cells, was induced in auraptene-treated but not in control cells
According to the results, we treated the cell line with different concentrations of auraptene for 48 h. The results indicated the involvement of an apoptotic process in auraptene-induced cell death in a concentration-dependent manner (Figure 3B).
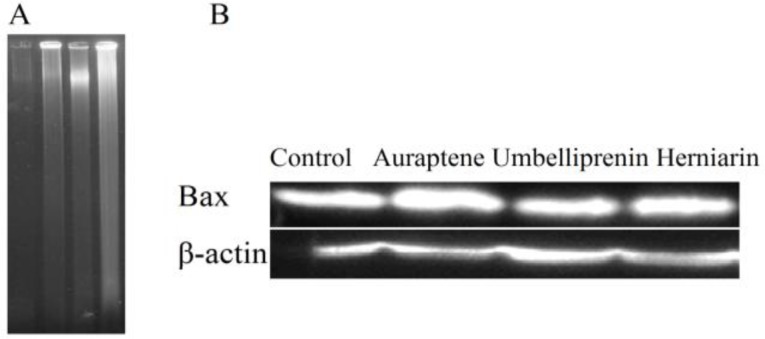
DNA fragmentation was also observed in MCF-7 cells, which confirmed apoptosis induction in this cell line (Figure 4A).
Figure 4.
A). Internucleosomal fragmentation of cells treated with auraptene, umbelliprenin, and herniarin for 48 h. After harvesting the cells, isolated DNA was analyzed by agarose gel elctrophoresis. Lane 1, untreated cells; lanes 2, 3, and 4 cells treated with 70, 80, and 200 µM, auraptene, umbelliprenin, and herniarin, respectively. (B). Western blot analysis of Bax protein expression in MCF-7 cells. Lane 1, untreated cells; lanes 2, 3, and 4 cells treated with 70, 80, and 200 µM, auraptene, umbelliprenin, and herniarin, respectively for 48 h. β-actin was used as a loading control
Expression of Bax proteins
Bax proteins play a pivotal role in controlling cytochrome c release and apoptosis initiation via the mitochondrial pathway (Gross et al., 1999 ▶). Auraptene in IC50 (60 µM) enhanced the expression of Bax protein in MCF-7 cells (Figure 4B).
Discussion
Breast cancer is the second leading cause of cancer-related death in women (Patil et al., 2010 ▶). The current treatment modalities of breast cancer causes leukemia, nausea, vomiting, blood clots endometrial cancer, and cardiotoxicity as side effects.
Hence, chemoprevention of cancer could be a better strategy. Pharmacological properties of bioactive compounds in cancer treatments and prevention have developed many natural-based cancer therapeutics (Scott et al., 2009 ▶).
7-prenyloxycoumarins are a group of compounds which have been shown to possess preventive and therapeutic effects on breast cancer but they have also cytotoxic effects on some other cancer cell lines.
We have demonstrated that treatment of MCF-7 cells with auraptene, herniarin, and umbelliprenin results in a significant decrease in cell viability by inducing apoptosis. In our research, assays for cell viability demonstrated that 59.74, 73.4, 476.3, and 207.6 μM of auraptene, umbelliprenin, umbelliferone, and herniarin were the IC50 values against MCF-7 cancer cells at 48 h, respectively.
Morphological characteristics of control cells, seemed to be normal and with increased concentration of these compounds, volume and number of the cells significantly decreased, chiefly with auraptene. The lowest IC50 and the most reduced volume in treated MCF-7 cells were related to auraptene, so it is the most potent inhibitor of MCF-7 growth compared to the other compounds. The cytotoxicity of auraptene started in concentration of 25 μM and at the concentration of 100 μM it could decrease the viability to below 20% (Figure 2B).
Literature has shown the cytotoxicity and chemo-preventive properties of umbelliprenin (Iranshahi et al., 2008 ▶; Iranshahi et al., 2009 ▶). Previous studies with umbelliprenin towards two human colon cancer cell lines, HT-29 and HCT 116, and breast cancer cells showed weak cytotoxic activities (Barthomeuf et al., 2008 ▶; Jabrane et al., 2010 ▶; Khorramizadeh et al., 2010 ▶).
It has been demonstrated that diet supplementation with auraptene in vivo has the potency to reduce the growth and number of metastatic lung tumors (Tanaka et al., 2000 ▶). Recently, an in vivo study also showed that dietary auraptene (500 ppm in the diet) decreased mammary carcinoma incidence and delayed median time to tumor in N-methylnitrosourea (MNU) treated rats (Krishnan et al., 2009 ▶). In two different studies, de Medina et al., and Krishnan et al., reported that auraptene induces concentration-dependent growth control through an arrest of the cell cycle in the G0-G1 phase in MCF-7, MDAMB-231, SW-620, and E151A cell lines, showing that auraptene inhibits both the proliferation of ER+ (MCF-7) and ER- (MDA-MB-231) human breast cancer cells (de Medina et al., 2010 ▶; and Krishnan et al., 2009 ▶). In addition, auraptene reduces cell survival in a clonogenic assay and also inhibits invasiveness. These data establish that auraptene reduces the proliferation, viability, and invasiveness of tumor cells of different origins (de Medina et al., 2010 ▶).
Barthomeuf et al., previously reported the proliferation of M4Beu cells is more potently inhibited by umbelliprenin (IC50 =12.3 μM) than by the citrus coumarin auraptene (7- geranyloxycoumarin, IC50 =17.1 μM) and they were capable of inhibiting the prevalence of lung metastasis in mice bearing B16BL6 murine melanoma (Barthomeuf et al., 2008 ▶). One study demonstrated that umbelliprenin has antitumor activity on both A549 and QU-DB cell lines with IC50 values of 59 and 47 μM for A549 and QU-DB cells, respectively (khaghanzadeh et al., 2012 ▶). Based on scientific reports, both auraptene and umbelliprenin have more toxicity on M4Beu cells in comparison with MCF7 cells and auraptene is more toxic on MCF7 cells in comparison with umbelliprenin.
Our results showed that the cytotoxicity of 7-prenyloxycoumarins is related to induction of apoptosis. Sub-G1 peak was observed in the flow cytometry histogram of breast cancer cells at the concentration of 70, 80, and 200 μM of auraptene, umbelliprenin, and herniarin, respectively at 48 h (Figure 4). The data from flow cytometry histogram of auraptene indicated the increase of sub-G1 peak in a dose-dependent manner. Induction of apoptosis in cancer cells by compounds is a key target for chemotherapeutic and chemopreventive applications. Mitochondria plays a pivotal role in apoptosis. The balance of pro-apoptotic proteins such as Bid, Bax, and Bak, and anti-apoptotic proteins including Bcl-2 and Bcl-xL, which are controlled by levels of cellular damage or stress distinguish the cell fate of apoptosis (Michael et al., 2000 ▶). Bax is a pore-forming pro-apoptotic protein that facilitates cytochrome c release and triggers caspase-mediated apoptotic cell death (Gross et al., 1999 ▶). Deficiency of Bax and Bak confers resistance to most conventional cancer therapies (Xu et al., 2015 ▶). Cells treated with auraptene increased levels of Bax, which is a key marker of mitochondrion involvement in auraptene-induced apoptosis.
In one study, umbelliprenin exhibited cytostatic effects and reduced the serum-induced proliferation of M4Beu cells through cell cycle blockade in G1 and induction of dose-dependent apoptosis with little cytotoxicity on primary fibroblasts (Shahverdi et al., 2006 ▶). khaghanzadeh et al., demonstrated that the pattern of cell death in both A549 and QU-DB cell lines was apoptosis (khaghanzadeh et al., 2012 ▶). Some researches showed other mechanisms for 7-prenyloxycoumarins. The proved toxicity of umbelliprenin for melanoma cancer cells and its ability to decrease MMP activity and expression in cancer cells (Shahverdi et al., 2006 ▶) support the hypothesis that foods or remedies containing umbelliprenin as well as coumarin might provide protection against the development and recurrence of malignant melanoma in humans with few side effects (Barthomeuf et al., 2008 ▶).
Auraptene reduces cyclin D1 protein which is overexpressed in human breast cancer (Krishnan et al., 2012 ▶). The effect of auraptene on cell cycle could persist for at least 24 h which could be the effect of parent compound or its active metabolites. The major metabolites of auraptene are umbelliferone and 7-ethoxycoumarin which also have been shown to possess chemopreventive effects (Murakami et al., 2000 ▶). Auraptene and umbelliprenin both have the same parent structure. The presence of an acyclic sesquiterpenene group in place of the geranyl group at C7-OH of the 1, 2- benzopyrone ring in umbelliprenin has been found to be related to the cytotoxicity of umbelliprenin (Barthomeuf et al., 2008 ▶). We conclude that the presence of a hydrophobic chain in place of the geranyl group at C7-OH plays an important role in cytotoxicity of auraptene via increasing lipophilicity and as a result, easier penetration to the cells. The absence of this group in umbelliferone, metabolite of auraptene, has caused a reduction in cell toxicity and our data confirmed this hypothesis. In comparison, herniarin with more hydrophobic group, is more cytotoxic than umbelliferone for cells. Li et al. reported the same result and the HIF-1 inhibitory activity of geranyloxycoumarins derivatives appears to be dependent upon the substitution pattern of both the coumarin backbone and the C-7 substituted geranyl side chain. Addition of certain fatty acyl chains to the basic system can increase the HIF-1 inhibitory activity. In acylated geranyloxycoumarins, membrane penetration is facilitated because of the lipophilic side chains. Auraptene, natural geranyloxycoumarin with the most lipophilic side chain, was among the most potent non-acylated HIF-1 inhibitors (Li et al., 2013 ▶).
Our results suggest that auraptene may be a potential chemotherapeutic or chemo-preventive agent based on its ability to induce apoptosis in cancer cells. While our investigation with MCF-7 cells as an in vitro model proposes strong evidence for auraptene-induced apoptosis, further studies with clinical trials need to be conducted to establish auraptene as a safe agent for cancer therapy.
Acknowledgements
This work was supported by grants from Research Affairs of Mashhad University of Medical Sciences, the Specialized Research Fund (No. 89299). We thank Dr. H. Nasirli for her kind assistance in flow cytometry. We are also grateful for the editorial assistance of Mrs. Z. Gonzalez.
Conflict of interest
The authors report no conflict of interest.
References
- Akins RE, Tuan RS. Measurement of protein in 20 seconds using a microwave BCA assay. BioTechniques. 1992;12:96–497. [PubMed] [Google Scholar]
- Askari M, Sahebkar AH, Iranshahi M. Synthesis and Purification of 7 Prenyloxycoumarins and Herniarin as Bioactive Natural Coumarins. Iran J Basic Med Sci. 2009;12:63–69. [Google Scholar]
- Baba M, Jin Y, Mizuno A, Suzuki H, Okada Y, et al. Studies on cancer chemoprevention by traditional folk medicines XXIV Inhibitory effect of a coumarin derivative 7-isopentenyloxycoumarin, against tumor-promotion. Biol Pharm Bull. 2002;25:244–246. doi: 10.1248/bpb.25.244. [DOI] [PubMed] [Google Scholar]
- Barthomeuf C, Lim S, Iranshahi M, Chollet P. Umbelliprenin from Ferula szowitsiana inhibits the growth of human M4Beu metastatic pigmented melanoma cells through cell-cycle arrest in G1 and induction of caspasedependent apoptosis. Phytomedicine. 2008;15:103–111. doi: 10.1016/j.phymed.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Cassileth B, Yeung KS, Gubili J. Herbs and Other Botanicals in Cancer Patient Care. Curr Treat Options, Oncol. 2008;9:109–16. doi: 10.1007/s11864-008-0061-5. [DOI] [PubMed] [Google Scholar]
- Chen IS, Lin YC, Tsai IL, Teng CM, Ko FN, et al. Coumarins and anti-platelet aggregation constituents from Zanthoxylum schinifolium. Phytochemistry. 1995;39:1091–1097. doi: 10.1016/0031-9422(95)00054-b. [DOI] [PubMed] [Google Scholar]
- Curini M, Epifano F, Maltese F, Marcotullioa MC, Tubarob A, et al. Synthesis and anti-inflammatory activity of natural and semisynthetic geranyloxycoumarins. Bioorg Med Chem Lett. 2004;14:2241–2243. doi: 10.1016/j.bmcl.2004.02.009. [DOI] [PubMed] [Google Scholar]
- de Medina P, Genovese S, Paillasse MR, Mazaheri M, Caze-Subra S, Bystricky K, Curini M, Silvente-Poirot S, Epifano F, Poirot M. Auraptene is an inhibitor of cholesterol esterification and a modulator of estrogen receptors. Mol Pharmacol. 2010;78:827–36. doi: 10.1124/mol.110.065250. [DOI] [PubMed] [Google Scholar]
- Gong J, Traganos F, Darzynkiewicz Z. A selective procedure for DNA extraction from apoptotic cells applicable for gel electrophoresis and flow cytometry. Anal Biochem. 1994;218:314–319. doi: 10.1006/abio.1994.1184. [DOI] [PubMed] [Google Scholar]
- Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- Iranshahi M, Arfa P, Ramezani M, Jaafari MR, Sadeghian H, et al. Sesquiterpene coumarins from Ferula szowitsiana and in vitro antileishmanial activity of 7-prenyloxycoumarins against promastigotes. Phytochemistry. 2007;68:554–561. doi: 10.1016/j.phytochem.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Iranshahi M, Kalategi F, Rezaee R, Shahverdi AR, Ito C, et al. Cancer chemopreventive activity of terpenoid coumarins from Ferula species. Planta Med. 2008;74:147–150. doi: 10.1055/s-2008-1034293. [DOI] [PubMed] [Google Scholar]
- Iranshahi M, Sahbkar A, Takasaki M, Konoshima T, Tokuda H. Cancer chemopreventive activity of the prenylated coumarin Umbelliprenin in vivo. Eur J Cancer Prev. 2009;18:412–415. doi: 10.1097/CEJ.0b013e32832c389e. [DOI] [PubMed] [Google Scholar]
- Iranshahi M, Shahverdi AR, Mirjani R, Amin G, Shafiee A. Umbelliprenin from Ferula persica roots inhibits the red pigment production in Serratia marcescens. Z Naturforsch. 2004;59:506–508. doi: 10.1515/znc-2004-7-809. [DOI] [PubMed] [Google Scholar]
- Jabrane A, Ben Jannet H, Mighri Z, Mirjolet JF, Duchamp O, Harzallah-Skhiri F, Lacaille-Dubois MA. Two new sesquiterpene derivatives from the Tunisian endemic Ferula tunetana Pom. Chem Biodivers. 2010;7:392–399. doi: 10.1002/cbdv.200900025. [DOI] [PubMed] [Google Scholar]
- Khaghanzadeh N, Mojtahedi Z, Ramezani M, Erfani N, Ghaderi A. Umbelliprenin is cytotoxic against QU-DB large cell lung cancer cell line but anti-proliferative against A549 adenocarcinoma cells. Daru. 2012;30:69. doi: 10.1186/2008-2231-20-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorramizadeh MR, Esmail-Nazari Z, Zarei-Ghaane Z, Shakibaieb M, Mollazadeh-Moghaddamd K, et al. Umbelliprenin-coated Fe3O4 magnetite nanoparticles. Antiproliferation evaluation on human Fibrosarcoma cell line (HT-1080) Fibrosarcoma cell line. Mater Sci Eng C Biomim Mater Sens Syst. 2010;30:1038–1042. [Google Scholar]
- Krishnan P, Yan K. J, Windler D, et al. Citrus auraptene suppresses cyclin D1 and significantly delays N-methyl nitrosourea induced mammary carcinogenesis in female Sprague-Dawley rats. BMC Cancer. 2009;9:259. doi: 10.1186/1471-2407-9-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Mahdi F, Du L, Jekabsons MB, Zhou YD, Nagle DG. Semisynthetic studies identify mitochondria poisons from botanical dietary supplements--geranyloxycoumarins from Aegle marmelos. Bioorg Med Chem. 2013;21:1795–803. doi: 10.1016/j.bmc.2013.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CM, Winsor L, Daneshtalab M. Quantification of spiroether isomers and Herniarin of different parts of Matricaria matricarioides and flowers of Chamaemelum nobile. Phytochem Anal. 2007;18:42–49. doi: 10.1002/pca.950. [DOI] [PubMed] [Google Scholar]
- Mares D, Romagnoli C, Bruni A. Antidermatophytic activity of Herniarin in preparations of Chamomilla recutita (L) Rauschert Plantes. Med Phytother. 1993;26:91–100. [Google Scholar]
- Michael O. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Mousavi SH, Tayarani NZ, Parsaee H. Protective Effect of Saffron Extract and Crocin on Reactive Oxygen Species-Mediated High Glucose-Induced Toxicity in PC12 Cells. Cell Mol Neurobiol. 2010;30:185–191. doi: 10.1007/s10571-009-9441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi SH, Tayarani-Najaran Z, Hersey P. Apoptosis. from signaling pathways to therapeutic tools. Iran J Basic Med Sci. 2008;11:121–142. [Google Scholar]
- Murakami A, Matsumoto K, Koshimizu K, Ohigashi H. Effects of selected food factors with chemopreventive properties on combined lipopolysaccharide- and interferon-g-induced IкB degradation in RAW2647 macrophages. Cancer Lett. 2003;195:17–25. doi: 10.1016/s0304-3835(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Murakami A, Nakamura Y, Ohto Y, Yano M, Koshiba T, et al. Suppressive effects of citrus fruits on free radical generation and nobiletin. an anti-inflammatory polymethoxyflavonoid. BioFactors. 2000;12:187–192. doi: 10.1002/biof.5520120130. [DOI] [PubMed] [Google Scholar]
- Murakami A, Nakamura Y, Tanaka T, Kawabata K, Takahashi D, et al. Suppression by citrus Auraptene of phorbol ester- and endotoxin-induced inflammatory responses. role of attenuation of leukocyte activation. Carcinogenesis. 2000;21:1843–1850. doi: 10.1093/carcin/21.10.1843. [DOI] [PubMed] [Google Scholar]
- Murakami A, Wada K, Ueda N, et al. In vitro absorption and metabolism of a citrus chemopreventive agent, auraptene, and its modifying effects on xenobiotic enzyme activities in mouse liver. Nutrition and Cancer. 2000;36:191–199. doi: 10.1207/S15327914NC3602_8. [DOI] [PubMed] [Google Scholar]
- Musa MA, Cooperwood JS, Khan MO. A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr Med Chem. 2008;15:2664–2679. doi: 10.2174/092986708786242877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil J, Kim J, ayaprakasha G.K. Berberine induces apoptosis in breast cancer cells (MCF-7) through mitochondrial–dependent pathway. Eur J Pharmacol. 2010;645:70–78. doi: 10.1016/j.ejphar.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Poncet KM, G Kroemer. Chemotherapy. targeting the mitochondrial cell death pathway. Oncogene. 2002;21:8786–8803. doi: 10.1038/sj.onc.1206039. [DOI] [PubMed] [Google Scholar]
- Sakata K, Hara A, Hirose Y, Yamada Y, Kuno T, et al. Dietary supplementation of the Citrus antioxidant Auraptene inhibits NN-Diethylnitrosamine-Induced rat hepatocarcinogenesis. Oncology. 2004;66:244–252. doi: 10.1159/000078001. [DOI] [PubMed] [Google Scholar]
- Scott EN, Gescher AJ, Steward WP, Brown K. Development of dietary phytochemical chemopreventive agents biomarkers and choice of dose for early clinical trials. Cancer Prev Res. 2009;6:525–530. doi: 10.1158/1940-6207.CAPR-08-0223. [DOI] [PubMed] [Google Scholar]
- Shahverdi AR, Saadat F, Khorramizadeh MR, Iranshahi M, Khoshayand MR. Two matrix metalloproteinases inhibitors from Ferula persica var persica. Phytomedicine. 2006;13:712–717. doi: 10.1016/j.phymed.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Simstein R, Burow M, Parker A, Weldon C, Beckman B. Apoptosis, chemoresistance and breast cancer. insights from the MCF-7 cell model system. Exp Biol Med. 2003;9:995–1003. doi: 10.1177/153537020322800903. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Kawabata K, Kakumoto M, Hara A, Murakami A, et al. Citrus Auraptene exerts dose-dependent chemopreventive activity in rat large bowel tumorigenesis the inhibition. correlates with suppression of cell proliferation and lipid peroxidation and with induction of phase II drug-metabolizing enzymes. Cancer Res. 1998;58:2550–2556. [PubMed] [Google Scholar]
- Tanaka T, Kohno H, Murakami M, Kagami S, El-Bayoumy K. Suppressing effects of dietary supplementation of the organoselenium 14-phenylenebis methylene. Cancer Res. 2000;60:3713–3716. [PubMed] [Google Scholar]
- Tanaka T, Sugiura H, Inaba R, Nishikawa A, Murakami A, et al. Immunomodulatory action of citrus Auraptene on macrophage functions and cytokine production of lymphocytes in female BALB/c mice. Carcinogenesis. 1999;20:1471–1476. doi: 10.1093/carcin/20.8.1471. [DOI] [PubMed] [Google Scholar]
- Tayarani-Najaran Z, Mousavi SH, Asili J, Emami SA. Growth-inhibitory effect of Scutellaria lindbergii in human cancer cell lines. Food Chem Toxicol. 2010;48:599–604. doi: 10.1016/j.fct.2009.11.038. [DOI] [PubMed] [Google Scholar]
- Xu W, Cheng M, Lao Y, Wang X, Wu J, Zhou L, Zhang Y, Xu H, Xu N. DNA damage and ER stress contribute to oblongifolin C-induced cell killing in Bax/Bak-deficient cells. Biochem Biophys Res Commun. 2015;13:300–306. doi: 10.1016/j.bbrc.2014.12.104. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Okamoto M, Kikuzaki H, Nakatani N. Spasmolytic activity of Auraptene analogs. Biosci Biotechnol. 1997;61:740–742. doi: 10.1271/bbb.61.740. [DOI] [PubMed] [Google Scholar]