Abstract
Aim:
Oral lichen planus (OLP) is a chronic mucocutaneous disease with an immunological etiology. This study was conducted to evaluate the effect of selenium combined with Vitamins A, C & E (Selenium-ACE) in the treatment of erosive-ulcerative OLP as an adjunctive to topical corticosteroids plus antifungal agent.
Subjects and Methods:
Thirty patients with a confirmed clinical and histopathologic diagnosis of OLP participated in this clinical trial. Patients were randomly allocated into one of three groups and treated as follows: (I) Topical corticosteroids, (II) topical corticosteroids plus antifungal, and (III) SE-ACE combined with topical corticosteroids plus antifungal. The patients were followed for 6 weeks. The pain and severity of the lesions were recorded at the initial and follow-up visits. All recorded data were analyzed using paired t-test and ANOVA test. A P ≤ 0.05 was considered significant.
Results:
The experimental groups showed a marked reduction in pain sensation and size of lesions, particularly in the final follow-up period, but there was no significant difference between the first two Groups I and II. However, healing of lesions and improvement of pain sensation was effective in Group III since a significant difference was found favoring Group III over both Groups I and II.
Conclusion:
No significant difference was found in treating erosive-ulcerative lesions of OLP by topical corticosteroids alone or combined with antifungal. However, when using SE-ACE in combination with topical corticosteroids plus antifungal, this approach may be effective in managing ulcerative lesions of OLP; but more research with a larger sample size and a longer evaluation period may be recommended.
Keywords: Antioxidant, clinical trial, Egypt, erosive, oral lichen planus, Selenium-ACE, treatment, ulcerative
Introduction
Oral lichen planus (OLP) is a chronic inflammatory mucocutaneous autoimmune disorder affects the stratum basal of the epithelium. It involves a type IV hypersensitivity reaction in which cell-mediated immunological dysfunction is found, mostly mediated by T-lymphocyte.[1,2,3] OLP has a prevalence rate of 0.1–4% in the general population,[4] it usually affects middle-aged and elderly people[5] with a female:male ratio 2:1.[6] Intraorally, buccal mucosa (usually occurs bilaterally and symmetrically), tongue, and gingiva are most commonly involved whereas other areas such as the palatal mucosa and floor of the mouth are rarely affected.[2] The histopathological characteristics of OLP are dense subepithelial lymphocytic infiltration, epithelial invasion, and hydropic degeneration of the basal keratinocytes.[6] The World Health Organization classified OLP as a possible premalignant lesion[7] indicating its potentiality to change into squamous cell carcinoma.[6] Therefore, every patient diagnosed with OLP should be regularly monitored, since malignant transformation can occur in all forms of OLP[8] with a variable frequency rate from 0% to 12.5%.[3]
The clinical presentation ranges from asymptomatic white keratotic lesions to painful erosions and ulcerations[9] with six clinical forms: Keratotic reticular, papular, plaque-like white patches, erosive, atrophic, and bullous (ulcerative).[6] The most common are reticular and erosive form.[2] The first three types are often present without any complaints that is, painless,[10] with no need for any intervention or treatment, but patients should be checked on regularly with a recommended follow-up visits every 4–6 months or sooner if any symptoms occur.[4,8] On the other hand, the erosive, atrophic, and ulcerative lesions, that are surrounded by keratotic forms suggest a damaged epithelium, a painful and/or a burning sensation, and thus interfere with eating, speaking, and swallowing. Oral pain resulting from OLP may be ranged from a little bothersome to annoying pain that can inhibit patients from their daily function.[11] It can persist in some patients for a long time, but a spontaneous resolution of the atrophic lesions is sometimes observed.[10]
Numerous different topical and general treatments have been suggested for relieving pain and reducing or eliminating exacerbations of the symptomatic lesions of erosive, atrophic, and ulcerative forms. However, most of the current available treatments are palliative rather than curative and recurrences may be encountered with high frequency. Topically, the following have been tried:
Corticosteroids, immunosuppressants such as cyclosporin, tacrolimus, and retinoids.[8] Corticosteroids are the most commonly used drugs, but other drugs such as calcineurin inhibitors, azathioprine, mycophenolate mofetil, retinoids, dapsone, and hydroxychloroquine can be used in recalcitrant cases.[12] Thus, there have been wide comparisons observed between different modalities in previous literature, but the most favorable curing treatment modality has not been yet established for the symptomatic OLP.
The exact etiology of OLP is still unknown, but it is mostly considered as a multifactorial process with different triggers such as: Genetic susceptibility, immunological illnesses, malnutrition, psychological as well as infectious factors.[3,13] In addition, the level of reactive oxygen species (ROS) and lipid peroxidation may be related to OLP.[14] Any certain condition which leads to increased level of ROS (either by over production or impaired removal) or reduced function of antioxidant is called oxidative stress. ROS may be toxic to cells via inactive enzymes, denaturizing proteins, DNA destruction, and lipid peroxidation. These events lead to damaged cell membrane, increased reactive aldehydic materials, and impaired cell function.[15] Different types of scavengers for free radicals have been suggested, such as different enzymes, minerals as well as vitamins. Vitamin A and E inhibits the lipid peroxidation of cell membrane, whereas Vitamin C plays as a cofactor for many enzymes which stabilize collagen structure and also help Vitamin E reproduction.[16] It is assumed that markers of oxidative stress are associated with different local oral conditions. The level of antioxidant is a potential determinant of susceptibility to be affected by OLP. This suggest that oxidative stress is a major trigger for OLP.[17,18,19]
Antioxidants have long been advocated for the treatment and prevention of a wide range of serious diseases such as stroke, cancer, diabetes, cataracts, Parkinson's disease, Alzheimer's disease, and arthritis, but still have some debate.[20] It, therefore, seems timely to assess the clinical evidence supporting the use of antioxidants specifically in lichen planus. Thus, if shown to be effective, antioxidants may act as a safe alternative to long-term use of nonsteroidal anti-inflammatory drugs or treatment by other drugs that are associated with adverse effects.
Selenium (Se) is an essential trace mineral in the soil which enters the food chain through plants and plays an important role in many functions of the body, especially when taken in combination with other antioxidants such as Vitamins A, C, and E. It plays a crucial role in the immune system. These trace elements act as cofactors for antioxidant enzymes involved in the destruction of toxic free radicals produced in the body. The serum levels of antioxidants vary in many diseases with the occurrence of some alterations which are part of the defense strategies of the organism and are induced by different cytokines.[21,22,23] In this study, we investigated the efficacy of using a Selenium-ACE in combination with topical corticosteroids plus antifungal agent in the management of symptomatic erosive-ulcerative lesions of OLP with a moderate degree of severity.
Subjects and Methods
Patient inclusion criteria
The patients have a symptomatic lesions of OLP (erosive-ulcerative areas) with moderate severity regarding lesion size and pain or burning sensation
Willing to participate and continue in the study.
Patient exclusion criteria
Presence of other systemic diseases
Receiving any systemic treatment such as systemic steroids, immunosuppressive medications or nonsteroidal anti-inflammatory drugs, or any supplementary vitamins, for at least 2 months prior to starting the present study
Receiving any topical therapy, for at least 1-month prior to initiating the present study
Smoking
Pregnancy and lactation for female patients.
Preparation of study design
Thirty patients (21 female and 9 male) were enrolled in this study from the Department of Oral Medicine, Periodontology, Oral Diagnosis, and Radiology. All the patients were affected with symptomatic lesions of OLP (erosive-ulcerative areas) with moderate severity. They were diagnosed clinically and histopathologically. The lesions were located bilaterally on the oral buccal mucosa. The research protocol has been approved and registered by the University Ethical Committee and it conforms to the provisions of the Declaration of Helsinki (as revised in Tokyo 2004). All patients gave an informed consent form. A questionnaire on the name, sex, date of onset and diagnosis, number, and location of the lesions were fulfilled. Regarding the date of onset and diagnosis, 20 cases were diagnosed for 6 months whereas the remaining 10 cases were diagnosed for 1-year. The lesions were encountered on the cheek mucosa and the number was located in the moderate degree of severity.
Study groups
The subjects were randomly and equally divided into three groups (10 per group) and managed as follows:
Group I: Ten patients received topical corticosteroids therapy alone (Orazone syrup, each 100 ml contains 10 mg dexamethasone, that is, each 10 ml has 1 mg dexamethasone; manufactured by the Arab Drug Company for Pharmaceutical and Chemical Industries “ADCO,” Cairo, ARE)
Group II: Ten patients received the same topical corticosteroids but plus an antifungal agent (Itrapex capsules, each capsule has 100 mg Itraconazole; manufactured by Multi-Apex Pharma SAE, Badr City-Cairo, Egypt)
Group III: Ten patients received SE-ACE (SE-ACE, 100% natural tablets; manufactured by Sigma, for Interpharma, under license of Wassen International Ltd., UK) in addition to the same topical corticosteroids plus the same antifungal agent.
The topical steroid therapy was prescribed as one and half teaspoonful (0.75 mg) four times daily, every 6 h, as only mouthwash for a period of 3–5 min without swallowing. Itraconazole was prescribed as an antifungal agent in the form of one capsule once daily, swallowed immediately after lunch meal. SE-ACE was prescribed in Group III once daily, on an empty stomach every morning.
Each tablet of SE-ACE contains: Vitamin A (Beta-Carotene and Retinol, 1500 i.u.); Vitamin E (natural source, 30 mg); Vitamin C, 90 mg; and SE 100 µg. The treatments were applied for a total period of 6 weeks, except itraconazole that is used for a period of 4 weeks, to avoid any possible side effects such as hypokalemia that may occur in rare cases if Itraconazole treatment is used for more than 1 month.
Evaluation of lesion size
Grading of size was defined as: 0 = Normal mucosa, 1 = lesion size is >0 up to 1.5 cm, 2 = lesion size is >1.5 cm and ≤3 cm, and 3 = lesion size is >3 cm.
Evaluation of pain or burning sensation
Pain or burning sensation was assessed and marked by the patients as points from 0 (no pain) to 10 (extreme pain), representing their pain perception as follows: 0 = no pain, 1 = mild pain (>0 and ≤3.5), 2 = moderate pain (>3.5 and ≤7), and 3 = severe pain (>7 up to 10).
Evaluation of the clinical response (improvement of lesions and symptoms)
The clinical improvement and patient satisfaction were assessed every 2 weeks as follows:
No resolution of lesions, that is, no change. This is scored as “0”
The partial resolution, with mild degrees of improvement in signs and symptoms and also in patient satisfaction. Patients still have Grade 2 of lesion size and score 2 of pain sensation but minimized to the lower half of these grades. This is scored as “1”
Moderate resolution, with reasonable and/or notable degrees of improvement in signs and symptoms and a moderate patient satisfaction. Thus, patients are changed into Grade 1 of lesion size and score 1 of pain sensation, but in the upper half of these grades. This is scored as “2”
The marked resolution, with evident degrees of improvement in signs and symptoms and a remarkable patient satisfaction. Patients still located in Grade 1 of lesion size and score 1 of pain sensation, but improved and become in the lower half of these grades that is, more nearer to complete healing and normal mucosa. This is scored as “3”
The complete resolution, with frank improvement in signs and symptoms and a complete patient satisfaction. Patients become in Grade 0 of lesion size and pain sensation, that is, almost reached to the normal mucosa without lesion and no pain. This is scored as “4”.
Statistical analysis
The statistical analyses were performed using mean and standard deviation values. The sample size was determined using PASS sample size software (NCSS statistical software, Kaysville, UT, USA). A Kolmogorov–Smirnov test of normality was done. The variables were compared among the study groups by ANOVA test using Scheffe test. The comparison between different follow-up periods (2 vs. 4 and 6 weeks/4 vs. 6 weeks) was done within each group by paired t-test [Tables 1 and 2]. Statistical differences were considered significant at (P ≤ 0.05). All of the statistical analyses were performed using SPSS ver. 21; (SPSS Inc., Chicago, IL, USA).
Table 1.
Results of improvement of OLP lesion size among studied groups at different follow-up periods, using mean±SD values along with significance level of both ANOVA test and paired t-test
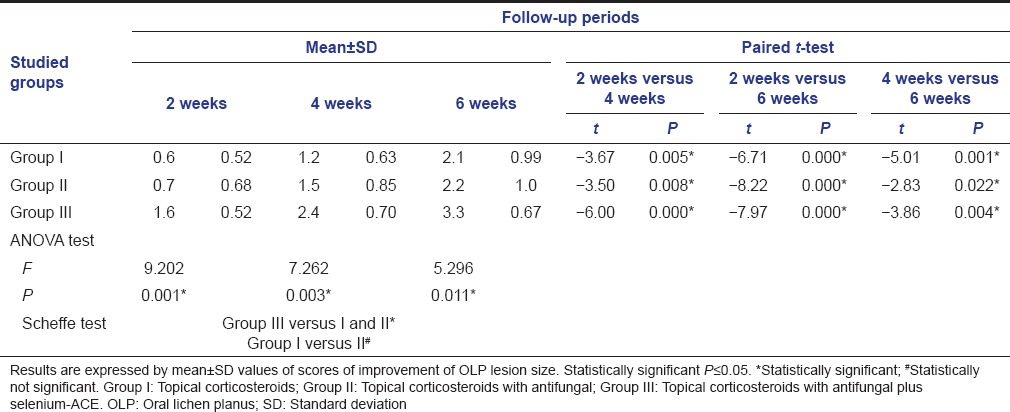
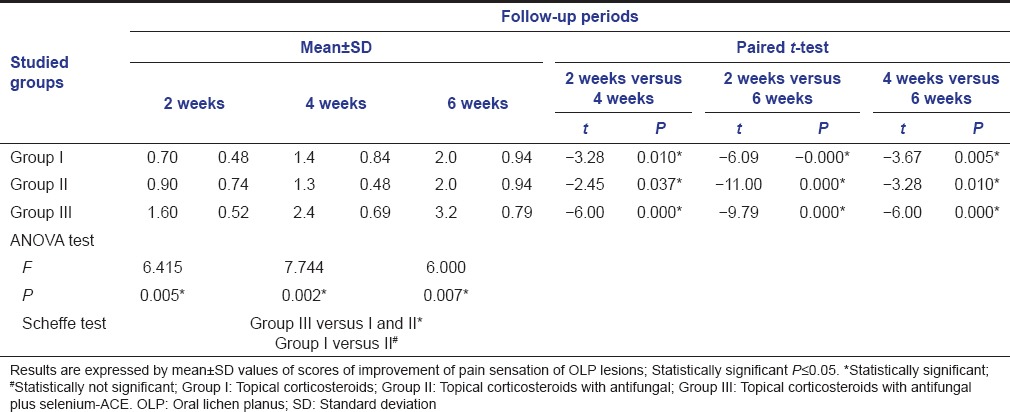
Table 2.
Results of improvement of pain sensation of OLP lesions among studied groups at different follow-up periods, using mean±SD values along with significance level of both ANOVA test and paired t-test
Results
There were no differences between the experimental groups in lesion size, pain sensation and severity of lesions, and previous treatment for OLP at the start of treatment. The lesions were located in the moderate severity in both lesion size and pain or burning sensation that is, Grade 2 of lesion size and score 2 of pain sensation. Data from ten patients per each group were analyzed.
Evaluation of improvement of clinical response (pain sensation and size of oral lichen planus lesions)
In general, pain sensation and lesion size were significantly reduced in the experimental groups upon using the intended treatment therapies with favored significant differences for Group III over Groups I and II.
The paired t-test was used to analyze and compare the results of the different follow-up periods (2 weeks vs. both 4 and 6 weeks as well as 4 vs. 6 weeks) within each group [Tables 1 and 2]. Statistically significant differences were noticed between the different follow-up intervals within the experimental groups. This means an evident reduction of OLP lesion size and alleviation of pain sensation throughout the whole experimental study of all groups.
Furthermore, the intergroup differences of pain sensation and lesion size were analyzed by ANOVA test using Scheffe test. Statistically significant differences were found between Group III versus both Groups I and II, whereas no significant difference observed between Group I versus Group II [Tables 1 and 2, Figures 1 and 2].
Figure 1.
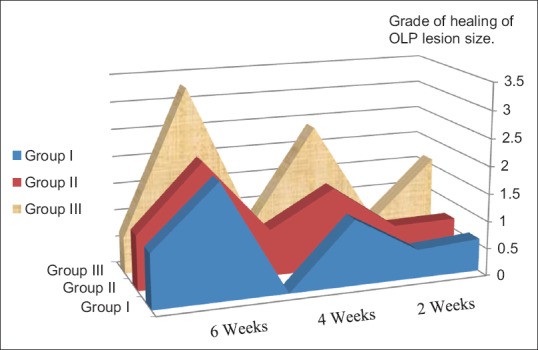
Area graph of the different experimental groups showing improvement of healing of Oral lichen planus lesion size at the different follow-up periods
Figure 2.

Area graph of the different experimental groups showing improvement of pain sensation in oral lichen planus lesion at the different follow-up periods
Meanwhile, the lesions of OLP that were not fully recovered during treatment therapies of the present study, are remained under observation until complete resolution of lesions.
Discussion
The aim of the present study was to test the efficacy of using SE-ACE combined with topical corticosteroids plus antifungal agent in the management of symptomatic erosive-ulcerative lesions of OLP with a moderate degree of severity. The nutritional performance of each element in SE-ACE reaches its peak effectiveness when used in combination. Taking SE-ACE in a regular course, once daily every morning on an empty stomach, as a food supplement is an ideal way of ensuring an adequate intake of these essential nutrients and providing an ideal natural cell protection for the entire body. SE is included in the present study because although it is not itself an antioxidant, it is considered an essential component of the endogenous antioxidant enzyme that is called glutathione peroxidase (GSH-Px).[24]
The increased oxidative stress or deficient antioxidant status has been shown to be important in the pathogenesis of several diseases.[25,26,27] Some previous studies suggested that there is a relation between high oxidative stress and low antioxidant activity in those who have OLP.[28] ROSs that are produced during macrophage activity, such as superoxide anion radicals, hydrogen peroxide and hydroxyl radicals are generated as a host defense mechanism.[29] The occurrence of severe oxidative stress may lead to increased accumulation of ROSs that can damage the cells.[30] ROSs may damage the extracellular matrix and inhibit collagen and proteoglycan synthesis.[26] SE acts as a cofactor of GSH-Px enzyme to protect the body from ROSs. The GSH-Px activity was found with lower levels in patients having diseases compared to the control subjects.[31] The decreased activity of GSH-Px reflects an inefficient removal of hydrogen peroxide from the cellular milieu.[32] In accordance, some observational and epidemiological studies[25,33,34] suggested that diets deficient in antioxidants may be associated with an increased incidence of rheumatoid arthritis and osteoarthritis or faster disease progression. Furthermore, animal studies have demonstrated an anti-inflammatory role for some antioxidants including superoxide dismutase and Vitamin E in experimentally induced arthritis.[20,25]
Steroids in the many cases of erosive forms of OLP are the first line of drugs in controlling symptoms and inducing clinical improvement. However, different formulations, dosages, time of use have lead to diversified effects during the treatment, with different responses as a result of individual susceptibility. There is no evidence that one steroid is more or less effective. Systemic steroids are mainly considered in exacerbations or widespread lesions as well as in managing recalcitrant lesions. The most suitable corticosteroid therapy in the management of OLP is the topical therapy, since this may cause significant reductions in the surface erythema and ulceration without exposing the patient to systemic side effect.[35] Thus, topical corticosteroids have been widely used in the treatment of symptomatic lesions of the oral mucosa including vesiculo-erosive lesions of OLP.
In general, the results of the present study showed a significant intergroup improvement of pain sensation and reduction of lesion size favoring Group III over Groups I and II, but no significant differences found between the two later Groups I and II [Tables 1 and 2, Figures 1 and 2]. In this context, the use of fluocinonide (either in 0.05% or 0.025% concentration) is found to be safe and effective in reducing the sings and complaints of OLP lesions without observing any adverse effects during the follow-up period.[36,37] In addition, mometasone furoate microemulsion when used topically, 3 times daily, over 30 days, showed a significant reduction in the erythema and ulceration of OLP lesions without severe side effects.[38]
In further studies, the effectiveness of topical use of betamethasone,[39] betamethasone sodium phosphate mouth rinse, fluticasone propionate spray,[40] and clobetasol propionate in three forms: Ointment (0.05%); adhesive denture paste; and oral analgesic base (orabase-B) has been described since the lesion areas were significantly reduced and a significant remission was occurred in the treatment of ulcerative lesions.[41] Gonzalez-Moles et al.[42] also found clobetasol 0.05% mouthwash as a safe and effective treatment of severe erosive OLP lesions, with a total recovery of 93.3%, but in a long period of 48-week. The present study used a follow-up period of 2, 4, and 6 week and found a significant improvement in signs and complaints within all the groups when compared different follow-up periods [Tables 1 and 2]. Campisi et al.[43] evaluated the efficacy of new lipid microspheres loaded with 0.025% of clobetasol propionate and suggested that it may enhance the remission of atrophic/erosive OLP lesions.
Furthermore, the efficacy of dexamethasone in the treatment of erosive lichen planus was assessed by detecting the salivary levels of proinflammatory cytokines. The topical 0.1% dexamethasone has been used for 6 weeks on only thirteen patients. The levels of all investigated cytokines were significantly decreased and the subjects’ symptoms were decreased in a significant way.[44] Thongprasom et al.[45] investigated the effect of fluocinolone acetonide in orabase 0.1% in OLP patients and found that its topical use had an effect on the reduction of tumor necrosis factor-alpha expression.
On the other hand, other agents have been used and evaluated in the treatment of OLP and showed significant degrees of effectiveness. The efficacy of Aloe vera was evaluated and showed a significant improvement with no adverse effects.[46] Another study[47] showed a significant reduction in pain and burning sensation, score, and size of lesions with similar degrees of healing when compared A. vera with triamcinolone acetonide 0.1% mouthwash. The authors finally concluded that A. vera can act as an effective alternative for triamcinolone treatment. Another safe agent such as hyaluronic acid 0.2% gel, when used topically, may form a protective coat and enhances hydration of oral mucosa and accelerates healing. The evaluated data indicated a significant reduction in the size of the erosive/ulcerated lesions.[48] On the contrary, topical retinoids[49] are less effective compared to corticosteroids and are rarely used currently since they are not the treatment agents of choice.
In addition, the findings of the present study showed that the addition of antifungal agent did not has a significant effect in the management of erosive-ulcerative lesions of OLP, since no significant differences found between Groups I and II throughout the whole follow-up periods [Tables 1 and 2, Figures 1 and 2]. The antifungal is mainly employed with corticosteroids to prevent or treat candidal infection and/or overgrowth which may occur as a side effect, especially with prolonged usage of steroid therapy. However, the present study showed some signs of candidias are only in the steroid group (Group I), that is, in 20% of the affected subjects (two patients of 10). In this context, Logi et al.[50] compared clobetasol gel with and without miconazole gel in OLP treatments and indicated no clinical signs of candidosis in the patients taking miconazole, but 30% affected in the steroid group. Other rare adverse effects were also observed, such as: Bad taste and smell, dry mouth, swollen mouth, and nausea.[40] However, the results of the present study did not show such adverse effects throughout the study period. As a general hygienic rule, proper oral hygiene control was found to be a very important factor enhancing the healing of the lesions. Thus, we considered the importance of maintaining good oral hygiene as an essential cofactor in the present study.
However, in a final conclusion, it should be remembered that the most of the available treatment modalities are considered only as palliative therapies aimed at relieving pain, reducing symptoms, and improving quality of patient's life through the healing of erosive and/or ulcerative lesions. Recurrences of OLP may also be faced at a different time periods due to the chronic autoimmune nature of the disease as a result of exposure to unknown antigens within the oral epithelium. Therefore, further studies may be recommended using a larger sample size and a longer evaluation period, especially after the discontinuation of the medication.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The author would like to thank all the kind contributors to the preparation and revision of this manuscript, especially, Ms. Afaf El-Ewaidy. The author is also grateful to the kind efforts and help of Prof. Abdulaziz Yassin, Prof. Public Health and Community Medicine, Faculty of Medicine, Tanta University, Egypt; Community Medicine Consultant, MOH, Saudi Arabia, in preparing and revising the statistical analysis of this manuscript.
References
- 1.Roopashree MR, Gondhalekar RV, Shashikanth MC, George J, Thippeswamy SH, Shukla A. Pathogenesis of oral lichen planus – A review. J Oral Pathol Med. 2010;39:729–34. doi: 10.1111/j.1600-0714.2010.00946.x. [DOI] [PubMed] [Google Scholar]
- 2.Oliveira Alves MG, Almeida JD, Balducci I, Guimarães Cabral LA. Oral lichen planus: A retrospective study of 110 Brazilian patients. BMC Res Notes. 2010;3:157. doi: 10.1186/1756-0500-3-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torrente-Castells E, Figueiredo R, Berini-Aytés L, Gay-Escoda C. Clinical features of oral lichen planus. A retrospective study of 65 cases. Med Oral Patol Oral Cir Bucal. 2010;15:e685–90. doi: 10.4317/medoral.15.e685. [DOI] [PubMed] [Google Scholar]
- 4.Sugerman PB, Savage NW, Walsh LJ, Zhao ZZ, Zhou XJ, Khan A, et al. The pathogenesis of oral lichen planus. Crit Rev Oral Biol Med. 2002;13:350–65. doi: 10.1177/154411130201300405. [DOI] [PubMed] [Google Scholar]
- 5.Ingafou M, Leao JC, Porter SR, Scully C. Oral lichen planus: A retrospective study of 690 British patients. Oral Dis. 2006;12:463–8. doi: 10.1111/j.1601-0825.2005.01221.x. [DOI] [PubMed] [Google Scholar]
- 6.Shen ZY, Liu W, Zhu LK, Feng JQ, Tang GY, Zhou ZT. A retrospective clinicopathological study on oral lichen planus and malignant transformation: Analysis of 518 cases. Med Oral Patol Oral Cir Bucal. 2012;17:e943–7. doi: 10.4317/medoral.17778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van der Meij EH, van der Waal I. Lack of clinicopathologic correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. J Oral Pathol Med. 2003;32:507–12. doi: 10.1034/j.1600-0714.2003.00125.x. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan I, Ventura-Sharabi Y, Gal G, Calderon S, Anavi Y. The dynamics of oral lichen planus: A retrospective clinicopathological study. Head Neck Pathol. 2012;6:178–83. doi: 10.1007/s12105-011-0318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pakfetrat A, Javadzadeh-Bolouri A, Basir-Shabestari S, Falaki F. Oral lichen planus: A retrospective study of 420 Iranian patients. Med Oral Patol Oral Cir Bucal. 2009;14:E315–8. [PubMed] [Google Scholar]
- 10.Radwan-Oczko M, Kozłowski Z. Oral lichen planus lesion assessment in relation to general health and oral symptoms. Adv Clin Exp Med. 2011;20:295–501. [Google Scholar]
- 11.Thongprasom K, Chaimusig M, Korkij W, Sererat T, Luangjarmekorn L, Rojwattanasirivej S. A randomized-controlled trial to compare topical cyclosporin with triamcinolone acetonide for the treatment of oral lichen planus. J Oral Pathol Med. 2007;36:142–6. doi: 10.1111/j.1600-0714.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 12.Lavanya N, Jayanthi P, Rao UK, Ranganathan K. Oral lichen planus: An update on pathogenesis and treatment. J Oral Maxillofac Pathol. 2011;15:127–32. doi: 10.4103/0973-029X.84474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: Reference interval and effects of life-style factors. Clin Chem. 1997;43:1209–14. [PubMed] [Google Scholar]
- 14.Nagao T, Warnakulasuriya S, Ikeda N, Fukano H, Yamamoto S, Yano M, et al. Serum antioxidant micronutrient levels in oral lichen planus. J Oral Pathol Med. 2001;30:264–7. doi: 10.1034/j.1600-0714.2001.300502.x. [DOI] [PubMed] [Google Scholar]
- 15.Alfadda AA, Sallam RM. Reactive oxygen species in health and disease. J Biomed Biotechnol 2012. 2012:936486. doi: 10.1155/2012/936486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller KL, Fenske NA. Uses of Vitamins A, C, and E and related compounds in dermatology: A review. J Am Acad Dermatol. 1998;39:611–25. doi: 10.1016/s0190-9622(98)70011-8. [DOI] [PubMed] [Google Scholar]
- 17.Yang LL, Liu XQ, Liu W, Cheng B, Li MT. Comparative analysis of whole saliva proteomes for the screening of biomarkers for oral lichen planus. Inflamm Res. 2006;55:405–7. doi: 10.1007/s00011-006-5145-8. [DOI] [PubMed] [Google Scholar]
- 18.Sezer E, Ozugurlu F, Ozyurt H, Sahin S, Etikan I. Lipid peroxidation and antioxidant status in lichen planus. Clin Exp Dermatol. 2007;32:430–4. doi: 10.1111/j.1365-2230.2007.02436.x. [DOI] [PubMed] [Google Scholar]
- 19.Azizi A, Farshchi F. Comparison of salivary and plasma antioxidant levels in lichen planus patients and healthy subjects. J Oral Pathol Med. 2012;41:524–6. doi: 10.1111/j.1600-0714.2012.01138.x. [DOI] [PubMed] [Google Scholar]
- 20.Melton L. The antioxidant myth: A medical fairy tale. New Sci. 2006 [Google Scholar]
- 21.Faryadi M, Mohebali M. Alterations of serum zinc, copper and iron concentrations in patients with acute and chronic cutaneous leishmaniasis. Iran J Public Health. 2003;32:53–8. [Google Scholar]
- 22.Barber EF, Cousins RJ. Interleukin-1 – stimulated induction of ceruloplasmin synthesis in normal and copper-deficient rats. J Nutr. 1988;118:375–81. doi: 10.1093/jn/118.3.375. [DOI] [PubMed] [Google Scholar]
- 23.Klasing KC. Nutritional aspects of leukocytic cytokines. J Nutr. 1988;118:1436–46. doi: 10.1093/jn/118.12.1436. [DOI] [PubMed] [Google Scholar]
- 24.Canter PH, Wider B, Ernst E. The antioxidant Vitamins A, C, E and selenium in the treatment of arthritis: A systematic review of randomized clinical trials. Rheumatology (Oxford) 2007;46:1223–33. doi: 10.1093/rheumatology/kem116. [DOI] [PubMed] [Google Scholar]
- 25.Mahajan A, Tandon VR. Antioxidants and rheumatoid arthritis. J Indian Rheumatol Assoc. 2004;12:139–42. [Google Scholar]
- 26.Hitchon CA, El-Gabalawy HS. Oxidation in rheumatoid arthritis. Arthritis Res Ther. 2004;6:265–78. doi: 10.1186/ar1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagfors L, Leanderson P, Sköldstam L, Andersson J, Johansson G. Antioxidant intake, plasma antioxidants and oxidative stress in a randomized, controlled, parallel, Mediterranean dietary intervention study on patients with rheumatoid arthritis. Nutr J. 2003;2:5. doi: 10.1186/1475-2891-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scully C, Beyli M, Ferreiro MC, Ficarra G, Gill Y, Griffiths M, et al. Update on oral lichen planus: Etiopathogenesis and management. Crit Rev Oral Biol Med. 1998;9:86–122. doi: 10.1177/10454411980090010501. [DOI] [PubMed] [Google Scholar]
- 29.Chaturvedi UC, Shrivastava R, Upreti RK. Viral infections and trace elements: Complex interaction. Curr Sci. 2004;87:1536–54. [Google Scholar]
- 30.Neupane DP, Majhi S, Chandra L, Rijal S, Baral N. Erythrocyte glutathione status in human visceral leishmaniasis. Indian J Clin Biochem. 2008;23:95–7. doi: 10.1007/s12291-008-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bahrami S, Hatam GR, Razavi M, Nazifi S. In vitro cultivation of axenic amastigotes and the comparison of antioxidant enzymes at different stages of Leishmania tropica. Trop Biomed. 2011;28:411–7. [PubMed] [Google Scholar]
- 32.Sodhi CP, Katyal R, Rana SV, Attri S, Singh V. Study of oxidative-stress in rotavirus infected infant mice. Indian J Med Res. 1996;104:245–9. [PubMed] [Google Scholar]
- 33.McAlindon TE, Jacques P, Zhang Y, Hannan MT, Aliabadi P, Weissman B, et al. Do antioxidant micronutrients protect against the development and progression of knee osteoarthritis? Arthritis Rheum. 1996;39:648–56. doi: 10.1002/art.1780390417. [DOI] [PubMed] [Google Scholar]
- 34.Kowsari B, Finnie SK, Carter RL, Love J, Katz P, Longley S, et al. Assessment of the diet of patients with rheumatoid arthritis and osteoarthritis. J Am Diet Assoc. 1983;82:657–9. [PubMed] [Google Scholar]
- 35.Radwan-Oczko M. Topical application of drugs used in treatment of oral lichen planus lesions. Adv Clin Exp Med. 2013;22:893–8. [PubMed] [Google Scholar]
- 36.Lozada F, Silverman S., Jr Topically applied fluocinonide in an adhesive base in the treatment of oral vesiculoerosive diseases. Arch Dermatol. 1980;116:898–901. [PubMed] [Google Scholar]
- 37.Voûte AB, Schulten EA, Langendijk PN, Kostense PJ, van der Waal I. Fluocinonide in an adhesive base for treatment of oral lichen planus. A double-blind, placebo-controlled clinical study. Oral Surg Oral Med Oral Pathol. 1993;75:181–5. doi: 10.1016/0030-4220(93)90091-h. [DOI] [PubMed] [Google Scholar]
- 38.Aguirre JM, Bagán JV, Rodriguez C, Jimenez Y, Martínez-Conde R, Díaz de Rojas F, et al. Efficacy of mometasone furoate microemulsion in the treatment of erosive-ulcerative oral lichen planus: Pilot study. J Oral Pathol Med. 2004;33:381–5. doi: 10.1111/j.1600-0714.2004.00213.x. [DOI] [PubMed] [Google Scholar]
- 39.McGrath C, Hegarty AM, Hodgson TA, Porter SR. Patient-centred outcome measures for oral mucosal disease are sensitive to treatment. Int J Oral Maxillofac Surg. 2003;32:334–6. doi: 10.1054/ijom.2002.0377. [DOI] [PubMed] [Google Scholar]
- 40.Hegarty AM, Hodgson TA, Lewsey JD, Porter SR. Fluticasone propionate spray and betamethasone sodium phosphate mouthrinse: A randomized crossover study for the treatment of symptomatic oral lichen planus. J Am Acad Dermatol. 2002;47:271–9. doi: 10.1067/mjd.2002.120922. [DOI] [PubMed] [Google Scholar]
- 41.Lo Muzio L, della Valle A, Mignogna MD, Pannone G, Bucci P, Bucci E, et al. The treatment of oral aphthous ulceration or erosive lichen planus with topical clobetasol propionate in three preparations: A clinical and pilot study on 54 patients. J Oral Pathol Med. 2001;30:611–7. doi: 10.1034/j.1600-0714.2001.301006.x. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez-Moles MA, Morales P, Rodriguez-Archilla A, Isabel IR, Gonzalez-Moles S. Treatment of severe chronic oral erosive lesions with clobetasol propionate in aqueous solution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:264–70. doi: 10.1067/moe.2002.120522. [DOI] [PubMed] [Google Scholar]
- 43.Campisi G, Giandalia G, De Caro V, Di Liberto C, Aricò P, Giannola LI. A new delivery system of clobetasol-17-propionate (lipid-loaded microspheres 0.025%) compared with a conventional formulation (lipophilic ointment in a hydrophilic phase 0.025%) in topical treatment of atrophic/erosive oral lichen planus. A Phase IV, randomized, observer-blinded, parallel group clinical trial. Br J Dermatol. 2004;150:984–90. doi: 10.1111/j.1365-2133.2004.05943.x. [DOI] [PubMed] [Google Scholar]
- 44.Rhodus NL, Cheng B, Bowles W, Myers S, Miller L, Ondrey F. Proinflammatory cytokine levels in saliva before and after treatment of (erosive) oral lichen planus with dexamethasone. Oral Dis. 2006;12:112–6. doi: 10.1111/j.1601-0825.2005.01165.x. [DOI] [PubMed] [Google Scholar]
- 45.Thongprasom K, Dhanuthai K, Sarideechaigul W, Chaiyarit P, Chaimusig M. Expression of TNF-alpha in oral lichen planus treated with fluocinolone acetonide 0.1% J Oral Pathol Med. 2006;35:161–6. doi: 10.1111/j.1600-0714.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- 46.Salazar-Sánchez N, López-Jornet P, Camacho-Alonso F, Sánchez-Siles M. Efficacy of topical Aloe vera in patients with oral lichen planus: A randomized double-blind study. J Oral Pathol Med. 2010;39:735–40. doi: 10.1111/j.1600-0714.2010.00947.x. [DOI] [PubMed] [Google Scholar]
- 47.Mansourian A, Momen-Heravi F, Saheb-Jamee M, Esfehani M, Khalilzadeh O, Momen-Beitollahi J. Comparison of Aloe vera mouthwash with triamcinolone acetonide 0.1% on oral lichen planus: A randomized double-blinded clinical trial. Am J Med Sci. 2011;342:447–51. doi: 10.1097/MAJ.0b013e3182171164. [DOI] [PubMed] [Google Scholar]
- 48.Nolan A, Badminton J, Maguire J, Seymour RA. The efficacy of topical hyaluronic acid in the management of oral lichen planus. J Oral Pathol Med. 2009;38:299–303. doi: 10.1111/j.1600-0714.2008.00739.x. [DOI] [PubMed] [Google Scholar]
- 49.Scardina GA, Messina P, Carini F, Maresi E. A randomized trial assessing the effectiveness of different concentrations of isotretinoin in the management of lichen planus. Int J Oral Maxillofac Surg. 2006;35:67–71. doi: 10.1016/j.ijom.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 50.Lodi G, Tarozzi M, Sardella A, Demarosi F, Canegallo L, Di Benedetto D, et al. Miconazole as adjuvant therapy for oral lichen planus: A double-blind randomized controlled trial. Br J Dermatol. 2007;156:1336–41. doi: 10.1111/j.1365-2133.2007.07883.x. [DOI] [PubMed] [Google Scholar]


