Abstract
Aim:
This study was conducted to estimate and compare inorganic salivary calcium, phosphate, magnesium, salivary flow rate, and pH of unstimulated saliva and oral hygiene status of healthy subjects, subjects with periodontitis and dental caries, and to correlate salivary calcium level with number of intact teeth.
Materials and Methods:
The study population consisted of 48 systemically healthy subjects in the age group of 18-55 years, which was further divided into three groups: healthy, periodontitis, and dental caries. Oral hygiene index-simplified, probing pocket depth, clinical attachment level, the number of intact teeth, and active carious lesions were recorded. Estimation of inorganic salivary calcium, phosphate, and magnesium was performed spectrophotometrically using Vitros 5.1 FS. Statistical analysis was performed using the one-way analysis of variance test at 5% significance level.
Results:
There was a statistically significant increase in inorganic salivary calcium, phosphate, pH, flow rate, and poor oral hygiene status in periodontitis group compared to dental caries and healthy group.
Conclusion:
Subjects with increased inorganic salivary calcium, phosphate, pH, flow rate, and poor oral hygiene are at a higher risk of developing periodontitis. Since there is increased remineralization potential, these subjects have more number of intact teeth compared to the dental caries group.
Keywords: Flow rate, magnesium, pH, phosphate, salivary calcium
Introduction
Dental caries and periodontitis are the most commonly occurring diseases affecting the humankind.[1] Saliva acts as a major determinant of oral environment and serves as an easily available diagnostic and monitoring method. Saliva is a bodily fluid secreted by major and many minor salivary glands. It plays an important role not only in plaque formation, but also has a lubricating effect, thereby maintaining the mucosal integrity of oral and upper gastrointestinal surfaces.[2] The salivary flow rate may be a contributing factor in the incidence of caries, and reduction in salivary output may result in rapid deterioration in oral health.[3] Changes in salivary composition and the flow rates may compromise the integrity of both the soft and hard tissues in the oral cavity. Salivary flow rate, buffering capacity, pH, calcium, phosphate, and fluoride ion concentrations are essential factors in the determination of periodontal diseases and dental caries.[4] Acidic pH promotes the demineralization of enamel whereas alkaline pH promotes plaque mineralization to form calculus.[5] Salivary flow rate and composition influence the formation of calculus and periodontal disease.[6]
Studies have shown that periodontitis is associated with increased level of calcium content in saliva and it has been suggested that higher calcium concentration of plaque is associated with low caries incidence.[7] It was found by researchers that periodontitis-affected subjects had more intact teeth compared to the subjects who are free of disease. In a cross-sectional epidemiologic study, an inverse relationship between periodontitis and dental caries was found.[8] Periodontitis affected subjects have increased intraoral mineralization capacity as their saliva may contain many factors, which may favor mineralization. Magnesium may also play an important role in preventing periodontal disease as it has the unique ability to reduce inflammation caused by bacterial toxins.[9] Thus, reduced magnesium concentrations are associated with enhanced inflammatory response to bacterial challenge.[10]
The aim of the present study was to estimate and compare the inorganic salivary calcium, phosphate, magnesium, salivary flow rate, and salivary pH of unstimulated saliva and oral hygiene status of healthy subjects, subjects with periodontitis and dental caries, and to correlate salivary calcium level and the number of intact teeth.
Materials and Methods
The study included subjects visiting the out-patient section of Department of Periodontology of the Institution. Ethical clearance for the study was obtained from the Institutional Ethical Committee, Yenepoya University. All the participants were provided with the verbal explanation of the nature of the study, and informed consent was obtained. The study was carried out from 01 October, 2014, to 25 November, 2014. The sample size was calculated at 80% of power and 5% significance level. In this cross-sectional study, the study population consisted of 48 systemically healthy subjects in the age group of 18-55 years that was further categorized into three groups comprising 16 in each group. Group A (healthy): Healthy subjects without chronic periodontitis with less than two active carious lesions. Group B (periodontitis): Subjects with chronic periodontitis having more than one tooth with probing pocket depth (PPD) ≥ 4 mm and with an attachment loss of 1.5 mm or more. Group C (dental caries): Subjects without chronic periodontitis and more than two active carious lesions on single/multiple teeth.
Subjects who had received any periodontal treatment during the past 6 months, subjects with <20 natural teeth, with any systemic disease, using medications affecting salivary secretions and calcium level, pregnant, lactating women, and smokers were excluded from the study.
A complete medical and dental history was obtained. Periodontal examination included oral hygiene index-simplified (OHI-S),[11] PPD, and clinical attachment loss, which were measured at four sites for all teeth except third molar using William's graduated periodontal probe. A PPD ≥ 4 mm and with an attachment loss of 1.5 mm or more was considered as periodontitis. The number of intact teeth and the number of carious lesions were noted. Assessment of active and inactive caries lesions was done based on visual and tactile criteria. It was carried out depending on the depth of penetration and severity of the lesions (intact surface, surface discontinuity in enamel or manifest cavity in dentin). Explorers were used to gently clean the tooth surface of any debris and to check for loss of tooth structure (cavitation) and surface texture (hard or rough/soft/leathery).[12]
Collection of the saliva, measurement of salivary flow rate, and pH
All participants were instructed not to eat or drink at least one hour prior to the collection of saliva. Saliva was collected between 9 and 12 AM. Prior to the collection of saliva, subjects were asked to rinse their mouth with water and then wait for 1-2 min for water clearance. Unstimulated whole saliva was then collected by making the subjects sit in the upright position with head slightly inclined. The passively drooled saliva that was collected in the floor of mouth was expectorated into graduated saliva collecting vials. Three milliliter of unstimulated whole saliva was collected and flow was expressed as ml/min. pH was measured using a pH meter which has a reading from 0 to 14 (acidic pH < 7 and basic pH > 7 and neutral = 7). A minor change in the pH value by one unit corresponds to ten folds change in hydrogen ion concentration of the solution. Saliva was centrifuged at 3000 rpm for 10 min and the clear supernatant was obtained and subjected to biochemical analysis with Vitros 5.l FS.
Estimation of inorganic salivary calcium, phosphate, and magnesium
Calcium was measured colorimetrically in Vitros 5.1 FS using arsenazo dye III. Calcium forms complex with arsenazo III dye, which is measured spectrophotometrically at a wavelength of 680 nm. The amount of the colored complex formed is proportional to the calcium concentration in the sample. Inorganic phosphate reacts with ammonium molybdate to form ammonium phosphomolybdate, which is measured spectrophotometrically at a wavelength of 670 nm. The magnesium reacts with the formazan dye derivative to form magnesium dye complex, which is measured by reflection density at a wavelength of 630 nm.
Statistical analysis
The mean salivary calcium, phosphate, magnesium, flow rate, and pH of unstimulated saliva, probing depth, OHI-S, the number of intact teeth, and the number of carious lesions of the three groups were statistically analyzed using one-way analysis of variance. Inter-group comparison was done using Tukey's honestly significance difference test. A P < 0.05 was considered statistically significant.
Results
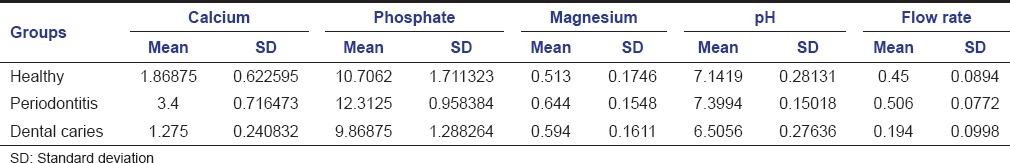
A statistically significant difference in the mean values of salivary calcium, phosphate, pH, and flow rate was noted between healthy, periodontitis, and dental caries group (P < 0.001). However, there was no statistically significant difference in mean salivary magnesium levels (P = 0.084) [Table 1].
Table 1.
Comparison of mean value of inorganic salivary calcium, phosphate, magnesium, pH, and flow rate among healthy group, periodontitis, and dental caries group
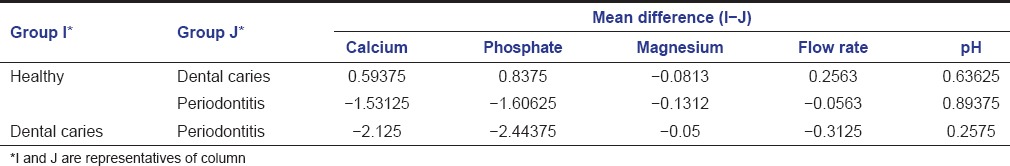
Multiple comparisons and mean differences for salivary calcium and pH among the three groups was statistically significant (P < 0.001). The mean difference for salivary phosphate between healthy and periodontitis group and dental caries and periodontitis group was statistically significant (P < 0.001). The mean difference for the flow rate was statistically significant for the healthy and dental caries group and dental caries and periodontitis group (P < 0.001) [Table 2].
Table 2.
Multiple comparison and mean difference of inorganic salivary calcium, phosphate, magnesium, flow rate, and pH among healthy group, periodontitis, and dental caries group
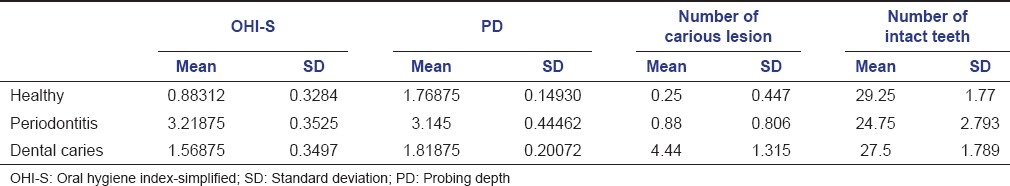
The mean of OHI-S, probing depth, the number of carious lesions, and the number of intact teeth among the three groups are summarized in Table 3. Comparison among the three groups showed statistically significant results (P < 0.001).
Table 3.
Comparison of mean value of OHI-S, PD, number of carious lesion, and number of intact teeth among healthy group, periodontitis and dental caries group
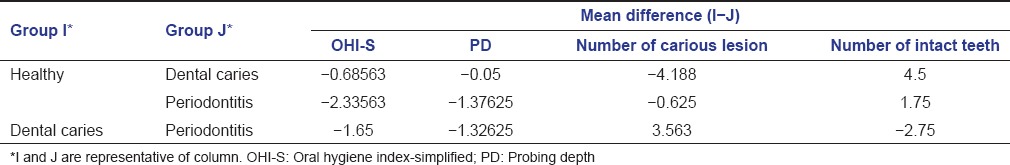
Multiple comparisons and the mean difference of OHI-S among the three groups was statistically significant (P < 0.001). The mean difference of probing depth was statistically significant for healthy and periodontitis group and dental caries and periodontitis group (P < 0.001). The mean difference of the number of carious lesions and the number of intact teeth was statistically significant between the healthy and dental caries group and dental caries and periodontitis group (P < 0.001) [Table 4].
Table 4.
Multiple comparison and mean difference of OHI-S, PD, number of carious lesion, and number of intact teeth among healthy group, periodontitis, and dental caries group
Discussion
Dental caries and periodontal disease are thought to share common contributory factors with each other. Epidemiological studies showing high periodontal index scores in caries-free populations support the concept that an inverse relationship exists between periodontal disease and dental caries.[13] However, some studies have reported a positive correlation between the two.[14,15] In the present study, when salivary inorganic calcium and phosphate levels were compared among three groups, there was statistically significant differences showing high levels of calcium and phosphate in periodontitis group. Similar observations have been made in earlier studies showing a positive correlation between high salivary calcium content and periodontitis.[7,16] Therefore, higher salivary calcium levels could be a risk factor for the development of periodontal disease.[17] Higher calcium concentration in the plaque is associated with low incidence of caries.[18] Salivary magnesium levels among the three groups were not statistically significant. However, few other studies found an association between periodontitis and reduced magnesium level.[9,19,20]
Periodontitis group showed higher pH levels when compared to other groups. An alkaline pH is associated with increased proteolytic activity of Porphyromonas gingivalis, the optimum pH required for the growth being 7.5.[21] Alkaline pH is favorable for deposition of calcium phosphate, thereby promoting plaque mineralization.[5]
Dental caries group showed lower pH values reflecting that acidic pH is favorable for enamel demineralization. The frequency of acidogenic episodes may be more important in caries progression than the degree of acidogenicity during any one episode.[22] The salivary flow rate was higher in periodontitis group due to the inflammatory effect of periodontal disease activity that could trigger the salivary innervations. This salivary protection effect itself can trigger body defense mechanism toward the inflammatory process.[23]
The oral hygiene status was poor in the periodontitis group and dental caries group. According to Axelsson and Lindhe,[24] the subjects who received only symptomatic treatment without any oral hygiene instructions suffered from gingivitis, loss of periodontal support, and recurrent carious lesion.
A higher number of the intact teeth were observed in the healthy group compared to periodontitis group. The higher number of the intact teeth observed in periodontitis group may be attributed to a better remineralization capacity associated with periodontitis. It is suggested that calculus, a well-known predisposing factor for periodontitis, is a consequence of a high mineralization potential in the mouth.[8]
Findings from the present study suggest that subjects who had increased inorganic salivary calcium, phosphate, high salivary pH, increased salivary flow rate, and poor oral hygiene are at a higher risk of developing periodontitis. These subjects had less dental caries with a high number of intact teeth probably due to the increased remineralization potential of saliva. As the salivary calcium increases, calcium levels in the plaque also increases thereby providing calcium for remineralization.[25] However, subjects with decreased inorganic salivary calcium, phosphate, low salivary pH, and reduced salivary flow rate are at increased risk of developing dental caries as the plaque is more acidogenic, which may result in demineralization of enamel.
Conclusion
From the present study, it can be concluded that subjects with increased inorganic salivary calcium, phosphate, and poor oral hygiene are at a higher risk of developing periodontitis. The groups with a higher risk of periodontitis showed increased pH and salivary flow rate. Because of increased remineralization potential, these subjects have a high number of intact teeth when compared to dental caries group. These findings reemphasize physicochemical factors of saliva can be important markers to assess the risk of periodontitis and caries activity. However, further cross-sectional studies with larger samples are required to extrapolate the findings.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
I would like to thank Dr. Sharif M.H (Professor, Department of Pathology) for his guidance and help in biochemical analysis.
References
- 1.Schoen MH, Freed JR. Prevention of dental disease: Caries and periodontal disease. Annu Rev Public Health. 1981;2:71–92. doi: 10.1146/annurev.pu.02.050181.000443. [DOI] [PubMed] [Google Scholar]
- 2.Fabian TK, Fejerdy P, Csermely P. Chemical biology of saliva in health and disease. In: Begley TP, editor. Wiley Encyclopedia of Chemical Biology. Wiley Encyclopedia of Chemical Biology New York, USA: John Wiley and Sons; 2008. pp. 1–9. [Google Scholar]
- 3.Shannon IL, Kilgore WI, Terry JM. Relation of parotid flow rate, sodium, potassium, and chloride concentrations to caries experience. J Oral Med. 1969;24:3–5. [PubMed] [Google Scholar]
- 4.Animireddy D, Reddy Bekkem VT, Vallala P, Kotha SB, Ankireddy S, Mohammad N. Evaluation of pH, buffering capacity, viscosity and flow rate levels of saliva in caries-free, minimal caries and nursing caries children: An in vivo study. Contemp Clin Dent. 2014;5:324–8. doi: 10.4103/0976-237X.137931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong L, Sissons CH, Pearce EI, Cutress TW. Calcium phosphate deposition in human dental plaque microcosm biofilms induced by a ureolytic pH-rise procedure. Arch Oral Biol. 2002;47:779–90. doi: 10.1016/s0003-9969(02)00114-0. [DOI] [PubMed] [Google Scholar]
- 6.Miller CS, King CP, Jr, Langub MC, Kryscio RJ, Thomas MV. Salivary biomarkers of existing periodontal disease: A cross-sectional study. J Am Dent Assoc. 2006;137:322–9. doi: 10.14219/jada.archive.2006.0181. [DOI] [PubMed] [Google Scholar]
- 7.Sewón L, Söderling E, Karjalainen S. Comparative study on mineralization-related intraoral parameters in periodontitis-affected and periodontitis-free adults. Scand J Dent Res. 1990;98:305–12. doi: 10.1111/j.1600-0722.1990.tb00977.x. [DOI] [PubMed] [Google Scholar]
- 8.Sewón LA, Parvinen TH, Sinisalo TV, Larmas MA, Alanen PJ. Dental status of adults with and without periodontitis. J Periodontol. 1988;59:595–8. doi: 10.1902/jop.1988.59.9.595. [DOI] [PubMed] [Google Scholar]
- 9.Aun WA. Inorganic ions level in saliva of patients with chronic periodontitis and healthy subjects. J Bagh Coll Dent. 2012;24:93–7. [Google Scholar]
- 10.Malpuech-Brugère C, Nowacki W, Daveau M, Gueux E, Linard C, Rock E, et al. Inflammatory response following acute magnesium deficiency in the rat. Biochim Biophys Acta. 2000;1501:91–8. doi: 10.1016/s0925-4439(00)00018-1. [DOI] [PubMed] [Google Scholar]
- 11.Greene JC, Vermillion JR. The simplified oral Hygiene index. J Am Dent Assoc. 1964;68:7–13. doi: 10.14219/jada.archive.1964.0034. [DOI] [PubMed] [Google Scholar]
- 12.Nyvad B, Machiulskiene V, Baelum V. Reliability of a new caries diagnostic system differentiating between active and inactive caries lesions. Caries Res. 1999;33:252–60. doi: 10.1159/000016526. [DOI] [PubMed] [Google Scholar]
- 13.Iwano Y, Sugano N, Matsumoto K, Nishihara R, Iizuka T, Yoshinuma N, et al. Salivary microbial levels in relation to periodontal status and caries development. J Periodontal Res. 2010;45:165–9. doi: 10.1111/j.1600-0765.2009.01213.x. [DOI] [PubMed] [Google Scholar]
- 14.Lang NP, Kiel RA, Anderhalden K. Clinical and microbiological effects of subgingival restorations with overhanging or clinically perfect margins. J Clin Periodontol. 1983;10:563–78. doi: 10.1111/j.1600-051x.1983.tb01295.x. [DOI] [PubMed] [Google Scholar]
- 15.Albandar JM, Buischi YA, Axelsson P. Caries lesions and dental restorations as predisposing factors in the progression of periodontal diseases in adolescents. A 3-year longitudinal study. J Periodontol. 1995;66:249–54. doi: 10.1902/jop.1995.66.4.249. [DOI] [PubMed] [Google Scholar]
- 16.Fiyaz M, Ramesh A, Ramalingam K, Thomas B, Shetty S, Prakash P. Association of salivary calcium, phosphate, pH and flow rate on oral health: A study on 90 subjects. J Indian Soc Periodontol. 2013;17:454–60. doi: 10.4103/0972-124X.118316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Acharya A, Kharadi MD, Dhavale R, Deshmukh VL, Sontakke AN. High salivary calcium level associated with periodontal disease in Indian subjects – A pilot study. Oral Health Prev Dent. 2011;9:195–200. [PubMed] [Google Scholar]
- 18.Sewón LA, Karjalainen SM, Sainio M, Seppä O. Calcium and other salivary factors in periodontitis-affected subjects prior to treatment. J Clin Periodontol. 1995;22:267–70. doi: 10.1111/j.1600-051x.1995.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhang MF, Huang YJ, Zhang HF, Tang W, Zhou J. Oxidative stress and susceptibility of periodontal disease. Shanghai Kou Qiang Yi Xue. 2013;22:571–6. [PubMed] [Google Scholar]
- 20.Meisel P, Schwahn C, Luedemann J, John U, Kroemer HK, Kocher T. Magnesium deficiency is associated with periodontal disease. J Dent Res. 2005;84:937–41. doi: 10.1177/154405910508401012. [DOI] [PubMed] [Google Scholar]
- 21.Zilm PS, Mira A, Bagley CJ, Rogers AH. Effect of alkaline growth pH on the expression of cell envelope proteins in Fusobacterium nucleatum. Microbiology. 2010;156(Pt 6):1783–94. doi: 10.1099/mic.0.035881-0. [DOI] [PubMed] [Google Scholar]
- 22.Dong YM, Pearce EI, Yue L, Larsen MJ, Gao XJ, Wang JD. Plaque pH and associated parameters in relation to caries. Caries Res. 1999;33:428–36. doi: 10.1159/000016547. [DOI] [PubMed] [Google Scholar]
- 23.Sinor Z, Azirrawani A. Association between salivary parameters and periodontal disease. Int Med J. 2013;20:1–5. [Google Scholar]
- 24.Axelsson P, Lindhe J. Effect of controlled oral hygiene procedures on caries and periodontal disease in adults. J Clin Periodontol. 1978;5:133–51. doi: 10.1111/j.1600-051x.1978.tb01914.x. [DOI] [PubMed] [Google Scholar]
- 25.Sewón LA, Karjalainen SM, Söderling E, Lapinleimu H, Simell O. Associations between salivary calcium and oral health. J Clin Periodontol. 1998;25(11 Pt 1):915–9. doi: 10.1111/j.1600-051x.1998.tb02390.x. [DOI] [PubMed] [Google Scholar]


