Abstract
Introduction:
Malignancies constitute a wide variety of disorders having high mortality and morbidity rates. Current protocols for management include surgical intervention, chemotherapy, and radiation which possess numerous adverse effects. Many phytochemicals are available with anticancer properties similar to anticancer drugs. Major benefit of these compounds is apparent lack of toxicity to normal tissues. Graviola (botanical name: Annona Muricata) contain bioactive compound “annonaceous acetogenins” known for anticancer activity on cancer cell lines.
Aims:
To determine cytotoxicity of Graviola and percentage cell inhibition at G2M phase of cell cycle.
Settings and Design:
The cytotoxicity of aqueous extract of Graviola leaves on squamous cell carcinoma (SCC-25) cell lines at various concentrations evaluated using 3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The percentage of SCC-25 cell inhibition at G2M phase of cell cycle determined using flow cytometry.
Methods:
Graviola Leaves, American Type Culture Collection SCC-25 cell lines were procured from Skanda Laboratories, Bengaluru. The cytotoxicity of aqueous extract of Graviola on SCC-25 cells at various concentrations evaluated using MTT assay. The percentage of SCC-25 cell inhibition at G2M phase of cell cycle determined using flow cytometry.
Statistical Analysis:
Statistical analysis was done using one-way ANOVA.
Results:
MTT assay showed statistically significant (P < 0.001) dose-dependent inhibition of SCC-25 cell lines by Graviola with IC50 value of 12.42 μg/ml. Flow cytometry revealed that Graviola at 25 and 50 g/ml arrested 53.39% and 52.09% cells in G2M phase of cell cycle respectively, which was statistically significant.
Conclusion:
Graviola showed significant cytotoxic activity and percentage of cell inhibition at G2M phase cell cycle against SCC-25 cell lines.
Keywords: 3-(4,5-Dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide assay; flow cytometry; G2M phase; Graviola; squamous cell carcioma-25 cell lines
Introduction
Malignant neoplasms constitute a wide variety of disorders that have a high rate of mortality and morbidity. Cancer affects virtually every tissue type and region of the body with varying prognostic outcomes.
Head and neck cancers, arising from structures such as oral cavity, nasal cavity, sinus, and pharynx, often have a poor prognosis and are the most prevalent malignancies worldwide. Among all the head and neck cancers, Squamous cell carcinoma (SCC) ranks 6th in incidence and 8th in mortality rate. Current protocols for the management of cancer include surgical intervention, chemotherapy, and radiation therapy. The modalities possess numerous adverse effects, as is the case with much of present-day cancer intervention. These adverse effects arise from the nonspecific nature of chemotherapy and radiation, as normal nonneoplastic cells are equally affected by cytotoxic drugs.[1] Chemotherapeutic drugs can damage any part of the body, but most commonly affect the oral cavity, digestive tract, bone marrow, reproductive system, and hair follicles.
Parallel to the chemotherapeutic drugs, there are many studies which have shown that compounds obtained from natural substances are quite effective in managing cancer with minimal adverse effects as compared with conventional chemotherapeutic drugs.[2,3,4,5] Many natural products containing phytochemicals such as turmeric, grapes, Aloe vera, and flax seeds are currently employed in the treatment of cancer as they have anticancer properties similar to the routinely used anticancer drugs.
Graviola (Botanical name: Annona Muricata) is one such compound which has exhibited anticancer effect on various cell lines such as Ehrlich ascites carcinoma cells (EACC), breast cancer cell lines (MD Anderson [MDA] and SKBR3 [Breast adenocarcinoma cell line]), and pancreatic cancer cell lines (FG/COLO357 and CD18/HPAF).[6,7] However, the study on the effect of aqueous extract of Graviola on SCC cell (SCC-25) line is limited, and hence the study.
Aims and objectives
Aim
To determine the cytotoxic activity of aqueous extract of Graviola leaves and the percentage cell inhibition of SCC-25 lines in G2M phase of cell cycle using 3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide (MTT) assay and flow cytometry.
Objectives
To evaluate the cytotoxicity of the aqueous extract of Graviola leaves on SCC-25 cell lines using MTT assay
To evaluate the percentage of cells arrested in G2M phase of cell cycle in aqueous extract of Graviola leaves-treated SCC-25 cell lines using flow cytometry
To evaluate the amount of cells arrested at G2M phase of cell cycle in colchicine-treated SCC-25 cell lines using flow cytometry
Comparison of the amount of cells arrested at G2M phase of cell cycle in Graviola-treated and colchicine-treated SCC-25 cells.
Methods
Fresh leaves of Graviola, American Type Culture Collection SCC-25 cell lines and colchicine were procured from Skanda Laboratories, Bengaluru. The leaves of Graviola were shade dried, powdered, and aqueous extract was prepared by Soxhlet apparatus for 8 h using rota evaporator (PBV-7D).
3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide assay
The confluent cell lines of SCC-25 were procured and checked for viability. 50,000 cells/well of SCC-25 cells were seeded in a 96 well plate and incubated for 24 h at 37°C with 5% CO2. These cells were treated with aqueous extract of Graviola in 2 fold variation varying from 0 ug/ml to 320 ug/ml in Dulbecco's Modified Eagle Media (DMEM) media without fetal bovine serum and incubated for 24 h. Further after incubation, MTT reagent was added and incubated for 3–4 h. After incubation, the MTT reagent was pipetted out without disturbing cell, and 100 µl of dimethyl sulfoxide was added to the well so as to solubilize the formazan and the absorbance was measured at 590 nm using Tecan plate reader. The percentage of cells lysed was calculated using the under-mentioned formula.
Calculating inhibition
% Inhibition = (OD of control – OD of sample/OD of control) × 100
(OD = Optical density)
Cell cycle (G2M) phase analysis
Flow cytometry
SCC-25 cells were cultured as 1 × 106 cells in four 6 well plate containing DMEM and incubated for 24 h. The spent media was removed and washed once with 1 × phosphate buffered saline (PBS). Cells were starved with serum-free media for 24 h. After 24 h, cell plates were treated with 25 µg/ml and 50 µg/ml of aqueous extract of Graviola, 20 µM colchicine, and the fourth plate was left untreated and incubated for 24 h. After incubation, the media was removed, washed with ×1 PBS, and the cells were collected using Trypsin-ethylenediaminetetraacetic acid (EDTA) and centrifuged at 1500 rpm for 5 min at room temperature. The cell pellet was collected and gently resuspended in ×1 PBS and fixed overnight at 4°C in a fixing solution. The next day they were centrifuged at 4000 rpm for 10 min at room temperature discarding the supernatant. Again, the cell pellet was washed twice with ×1 PBS. Later, cells were incubated for 15 min at room temperature in 500 µl of Propidium iodide (PI) solution containing 0.05 mg/ml PI and 0.05 mg/ml RNase A in PBS and 0.1 m EDTA. The percentage of cells in G2M phase of cell cycle in compounds treated and untreated populations were determined using FACS Caliber (BD Biosciences, San Jose, CA, USA).
Results
3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide assay
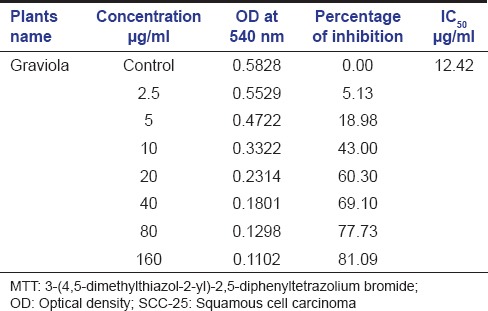
The aqueous Graviola extract showed significant dose-dependent inhibition of growth of SCC-25 cells at IC50 value of 12.42 µg/ml [Table 1 and Graph 1].
Table 1.
MTT assay performed on SCC-25 against ascending concentration of Graviola
Graph 1.
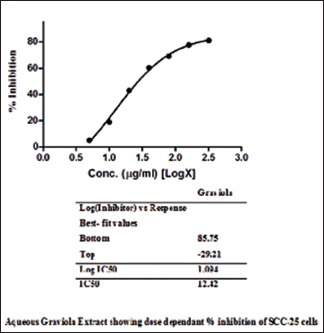
Aqueous extract of Graviola showed dose-dependent percentage inhibition of squamous cell carcinoma-25 cells
Flow cytometry
The aqueous extract of graviola at 25 µg/ml [Table 2 and Figure 1] exhibited 53.39% as compared to control [Table 3 and Figure 2] which exhibited 14.71% cell inhibition on SCC-25 cell, whereas colchicine [Table 4 and Figure 3] exhibited 47.77 % of cell inhibition at G2M phase of cell cycle in flowcytometry. Also aqueous extract of graviola at 50 µg/ml [Table 5 and Figure 4] exhibited 52.09% cell inhibition on SCC-25 cell.
Table 2.
Histogram statistics of flow cytometric analysis of Graviola at 25 μg/ml concentration on SCC-25 cells
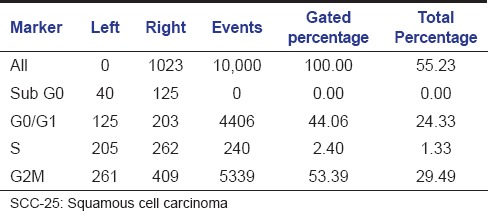
Figure 1.
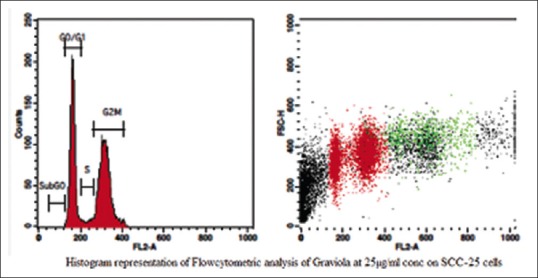
Histogram representation of flow cytometric analysis of the aqueous extract of Graviola at 25 μg/ml concentration on squamous cell carcinoma-25 cells
Table 3.
Histogram statistics of flow cytometric analysis of control (untreated cells)
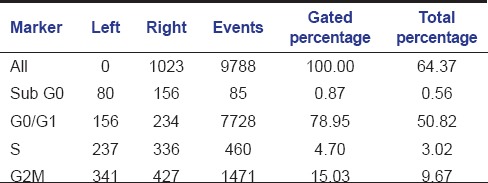
Figure 2.
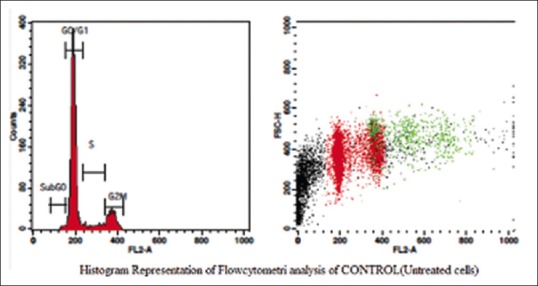
Histogram representation of flow cytometric analysis of control (untreated cells)
Table 4.
Histogram statistics of flow cytometric analysis of colchicine on SCC-25 cell lines
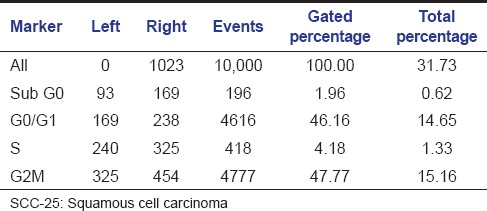
Figure 3.
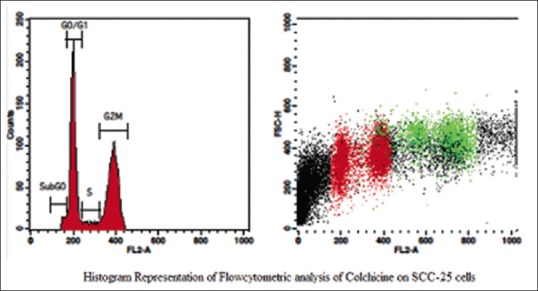
Histogram representation of flow cytometric analysis of colchicine on squamous cell carcinoma-25 cells
Table 5.
Histogram statistics of flow cytometric analysis of Graviola at 50 μg/ml concentration on SCC-25 cells
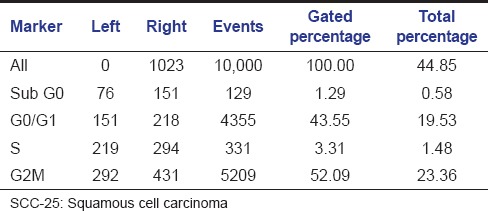
Figure 4.
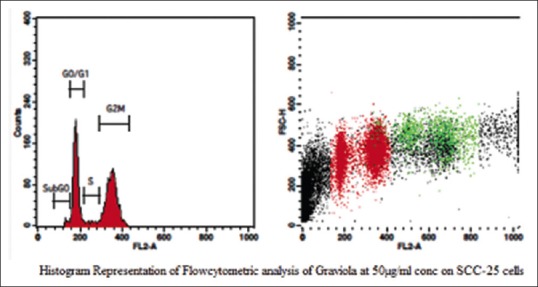
Histogram representation of flow cytometric analysis of the aqueous extract of Graviola at 50 μg/ml concentration on squamous cell carcinoma-25 cells
Discussion
A healthy diet rich in natural fruits and vegetables is associated with maintenance of health and reduced risk of diseases including cancers. The various phytochemicals such as phenols, phenolic acids, alkaloids, flavonoids, carotenoids, and vitamins play a major role in boosting the immunity.[4]
To combat SCC, these various anticancer drugs such as cisplatin, Doxorubicin, Fluorouracil, topotecan, and etoposide have been used. However, these drugs pose a serious of adverse effects. Hence, search for a compound with anticancer activity exhibiting minimal adverse effects has to be looked upon.
Annonaceous acetogenins is one such compound derived from Graviola tree, which is a low-branching bushy slender tree with leaves which are oblong, elliptic or narrow, and appear as smooth, glossy, dark green on the upper surface, and lighter beneath. It is commonly known as Soursop and the name Graviola is a Portuguese derivative.
Graviola is rich in secondary class metabolites such as saponins, alkaloids, terpenoids, flavonoids, coumarins, and tannins, which has proved to be a promising new antioxidant and anticancer drug.
Practitioners of herbal medicine use Graviola leaves, fruits, and barks to treat stomach ailments, fever, parasitic infections, and hypertension. Along with anticancer properties, Graviola also exhibits analgesic, anti-parasitic, antimicrobial, anti-inflammatory, and antirheumatic effects.
There are conflicting reports of neurotoxicity of Graviola bark and seeds which cause movement disorders and myeloneuropathy with symptoms mimicking Parkinson's disease.
Studies on Graviola have shown the anticancer and antiproliferative activity on various cell lines, such as EACC, breast cancer cell lines (MDA and SKBR3), and pancreatic cancer cell lines (FG/COLO357 and CE18/HPAF), wherein they have exhibited promising tumor cell toxicity, but the studies on anticancer effect of SCC-25 cell line is sparse.[7]
Probable Mechanism of action of Graviola.[8]
Depletion of ATP (Adenosine Triphosphate)
By inhibition of NADH (Nicotinamide Adenine Dinucleotide) Oxidase in plasma membrane along with the inhibition of Complex I (NADH: Ubiquinone oxidoreductase) in mitochondrial electron transport leading to the inhibition of oxidative phosphorylation, resulting in lowering the ATP's, thus inhibiting the cancer cell growth
-
Cancer cell usually develops multidrug resistance due to enhanced expression of a plasma membrane pump, P-glycoprotein, which consists of 2 intracellular ATP-binding sites through which the pump obtains the necessary energy and hence responsible for the elimination of anticancer drug. Graviola causes depletion of ATP, thus reducing the activity of shutting down the pump
- Cancer cells at the S-phase of their cell cycle are more vulnerable to the acetogenin annonacin. Annonacin arrests the cell cycle in the G1 phase and inhibits the S-phase progression. In addition, p53 and p21, cell cycle checkpoint proteins, were also enhanced by annonacin
- The acetogenin annonacin increases the expression of Bax and Bad and induces apoptosis
- Prevents the growth of blood vessels in or near the tumor
- Degrades or depletes the DNA and RNA building blocks necessary for cancer cell division.
Studies by Torres et al. (2012) showed that Graviola induces necrosis of pancreatic cells by inhibiting cellular metabolism. They stated that there is an increased expression of glucose transporters to enhance the uptake of glucose thus increasing ATP production which further leads to an enhanced tumor growth. Graviola extract inhibited multiple signaling pathways that regulate metabolism, cell cycle, survival, and metastatic properties in PC cells.[7]
Studies by Coothankandaswamy et al. (2010) showed that hypoxia inducible factor-1α (HIF) protein was inhibited by acetogenins. HIF-1 inhibition in combination with chemotherapy or radiation yielded enhanced treatment outcome in cancer patients. They concluded that the inhibition of HIF-1 activation and hypoxic tumor angiogenesis constitutes a novel mechanism of action for these anticancer alternative medicines.[9]
Studies by Gavamukulya et al. using ethanolic and aqueous extracts of Graviola leaves on EACC, MDA, and SKBR3 cell lines showed that aqueous extract had a higher free radical inhibition and a stronger antioxidant activity as compared to ethanolic extract. The ethanolic extracts had a very high selectivity for cancer spleen cells while sparing the normal spleen cells throughout their range of concentrations tested with 100% spleen cell viability.[10]
However, in their study, the aqueous extracts showed no effect throughout the range of tested concentrations, which is in contrast to our study wherein the aqueous extract of Graviola leaves exhibited SCC-25 cell cytotoxicity with IC50 of 12.42 µg/ml and had arrested 53.39% of SCC-25 cells at 25 µg/ml of Graviola extract at G2M phase of cell cycle.
Thus, the anticancer potential of aqueous extract of Graviola on SCC-25 cell is clearly documented and is evident in this study by MTT assay and flow cytometry.
Conclusion
Aqueous extract of Graviola leaf shows promising cytotoxic activity and cell inhibition in G2M phase of cell cycle on SCC-25 cell lines. The effect of Graviola on the normal cell lines is still debatable with the available literature showing conflicting reports.[5,10,11] Hence, further clinical research is required to elucidate the preparation, potential drug interactions, toxicity, mechanism of action, effective dose, so as to use this plant-derived extract as a potential drug for treating cancer patients.
Skanda Laboratories, Bengaluru.
Financial support and sponsorship
Skanda Laboratories, Bengaluru.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Colevas AD. Chemotherapy options for patients with metastatic or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol. 2006;24:2644–52. doi: 10.1200/JCO.2005.05.3348. [DOI] [PubMed] [Google Scholar]
- 2.Liu RH. Health-promoting components of fruits and vegetables in the diet. Adv Nutr. 2013;4:384S–92S. doi: 10.3945/an.112.003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodhead P. Cancer – Diet, Nutrition and Botanical Support. 2009. [Last cited on 2015 Sep 01]. Available from: http://www.wpb.radon.com/pdf/Brodhead%20Cancer%20recommendations .
- 4.Wang H, Khor TO, Shu L, Su ZY, Fuentes F, Lee JH, et al. Plants vs. cancer: A review on natural phytochemicals in preventing and treating cancers and their druggability. Anticancer Agents Med Chem. 2012;12:1281–305. doi: 10.2174/187152012803833026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Somkid K, Sincharoenpokai P, Ontong S, Niumsakul S, Chansuvanich N. Cytotoxicity Testing of Graviola (Annona muricata Linn.) Leaf Extracts In vitro. [Last accessed on 2015 Aug 26]. Available from: www.annualconference.ku.ac.th/cd53/06_020_P67.pdf .
- 6.Vijayameena C, Subhashini G, Loganayagi M, Ramesh M. Phytochemical screening and assessment of antibacterial activity for the bioactive compounds in Annona muricata. Int J Curr Microbiol Appl Sci. 2013;2:1–8. [Google Scholar]
- 7.Torres MP, Rachagani S, Purohit V, Pandey P, Joshi S, Moore ED, et al. Graviola: A novel promising natural-derived drug that inhibits tumorigenicity and metastasis of pancreatic cancer cells in vitro and in vivo through altering cell metabolism. Cancer Lett. 2012;323:29–40. doi: 10.1016/j.canlet.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor L. New York, United states: Sage Press; 2005. Technical Data Report for Graviola, The Healing Power of Rainforest Herbs. [Google Scholar]
- 9.Coothankandaswamy V, Liu Y, Mao SC, Morgan JB, Mahdi F, Jekabsons MB, et al. The alternative medicine pawpaw and its acetogenin constituents suppress tumor angiogenesis via the HIF-1/VEGF pathway. J Nat Prod. 2010;73:956–61. doi: 10.1021/np100228d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gavamukulya Y, Abou-Elella F, Wamunyokoli F, AEl-Shemy H. Phytochemical screening, anti-oxidant activity and in vitro anticancer potential of ethanolic and water leaves extracts of Annona muricata (Graviola) Asian Pac J Trop Med. 2014;7:S355–63. doi: 10.1016/S1995-7645(14)60258-3. [DOI] [PubMed] [Google Scholar]
- 11.George VC, Kumar DR, Rajkumar V, Suresh PK, Kumar RA. Quantitative assessment of the relative antineoplastic potential of the n-butanolic leaf extract of Annona muricata Linn. in normal and immortalized human cell lines. Asian Pac J Cancer Prev. 2012;13:699–704. doi: 10.7314/apjcp.2012.13.2.699. [DOI] [PubMed] [Google Scholar]


