Abstract
Background:
In the present scenario, we are made available with chemomechanical caries removal system containing a natural proteolytic enzyme for the ease in the excavation of infected dentine. The additive action for these agents is providing antimicrobial and anti-inflammatory properties.
Aim:
This study was undertaken for assessing the action of Carie Care™ and Papacarie Duo™ on Aggregatibacter actinomycetemcomitans.
Materials and Methods:
The samples were collected for cultivation of the periodontal pathogen from the clinical periodontal pockets using sterile paper points. The samples cultured under suitable conditions were analyzed with quantitative polymerase chain reaction targeting 16s r-DNA. The samples were divided into three groups namely, Group A: Control, Group B: With Papacarie Duo, Group C: With Carie Care. The pathogen inoculums plugs were inserted in the petri dishes containing chemically defined medium and the experimental gels at different concentrations and were incubated under optimal conditions. The inhibition of growth of the pathogen was studied visually.
Results:
There was visual inhibition of growth for Group B and C and also exhibited a dose-dependent effect also.
Conclusion:
Based on the results of the present study, Carie Care™ gel demonstrated better antimicrobial action against A. actinomycetemcomitans which is a major periodontal disease causing pathogen.
Keywords: Antimicrobial action, Carie Care, polymerase chain reaction
Introduction
Dental plaque is the etiological factor responsible for the gingival and periodontal disease which harbors a variety of pathogenic bacteria. The loss of equilibrium between microbial succession and reciprocal host–bacterial interaction will be capable of initiating the mechanism of destruction of the periodontal tissues.[1,2] Certain groups of Gram-negative bacteria such as Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, and Capnocytophaga species have been implicated in chronic or refractory periodontal diseases.[3,4] The periodontal pocket provides ideal conditions for the proliferation of these microorganisms.[5] Preventative or therapeutic strategies probably encounter a tendency of the ecosystem to return to the original equilibrium. However many a times, periodontal pathogens recolonize even after mechanical debridement, and if it continues to harbor bacteria associated with disease, a potential for further destructive phase exists. Antimicrobial therapy against periodontal pathogens can act as an adjunct for mechanical debridement of periodontal pocket followed by local drug delivery approach.[6] The papaya extract based gels with other natural ingredients (Carie Care and Papacarie Duo) with established antimicrobial activities are commercially available. There are no comparative studies available on the effect of these gels on the A. actinomycetemcomitans, a potential periodontal pathogen. Thus, the intent of this study was to evaluate the effects of papaya extract based gels on the growth of A. actinomycetemcomitans.
Materials and Methods
A series of microbial samples for the isolation of A. actinomycetemcomitans were collected from the subjects aged 35–55 years without nicotine or tobacco habit, affected with choric or localized periodontitis with periodontal pocket depth more than 4 mm. The relevant medical and dental history was recorded, and a signed informed consent document was obtained from the subjects. The examination and diagnosis were performed under standardized conditions. Periodontal status was assessed by determination of the probing pocket depth by community periodontal index, gingival state (gingival sulcus bleeding index according to Mühlemann and Son), and oral hygiene index (according to Silness and Löe).[7]
The sampling area was isolated with cotton rolls, carefully cleaned with sterile cotton pellets, and then air dried. From single site, sterile paper points (Hygenic, Coltene, and Whaledent, USA) were inserted to the bottom of the pocket for a 20-s period and then transferred into Luria Broth (LB) soft agar stabs (Hi-Media Pvt. Ltd., Mumbai, India) and incubated at 37°C for a week and then to 4°C until further use.[3]
DNA extraction and identification by DNA sequence analysis
Genomic DNA isolation
The clinical samples were processed for the isolation of A. actinomycetemcomitans, and an isolate was chosen for 16s r-DNA sequencing studies to ascertain their genetic background. A. actinomycetemcomitans genomic DNA was extracted using standard cetyltrimethylammonium bromide method. Well-grown cultures were taken in a microfuge tube and centrifuged at 10,000 rpm for 2 min at 4°C. The pellet obtained was resuspended in sterile water, washed, and centrifuged at 10,000 rpm for 2 min at 4°C. To the pellet, 675 μl of extraction buffer was added and incubated at 37°C for 30 min equal volume of chloroform: Isoamyl alcohol (24:1) was added. The sample was centrifuged at 10,000 rpm for 10 min at 4°C, and aqueous phase was taken in a sterile microfuge tube. Ice-cold isopropanol (0.6 volume) was added incubated at room temperature for 1 h. Samples were centrifuged at 10,000 rpm for 10 min, and then pellet is washed in 500 μl of 70% ice-cold ethanol and centrifuged at 10,000 rpm for 10 min at room temperature. The dried pellet was dissolved in sterile double distilled water and stored at −20°C. Quality of DNA was checked by running on a 1% agarose gel in ×1 tris acetate EDTA buffer and ethidium bromide (EtBr) staining. The DNA was quantified by measuring absorbance at 260 nm using a Shimadzu 1800 spectrophotometer.
16s r-DNA polymerase chain reaction amplification
The extracted DNA was then used as template for 16s r-DNA polymerase chain reaction (PCR) amplification with universal 16s primers–forward 5’ AGA GTT TGA TCC TGG CTC AG 3’ and reverse 5’ AAG GAG GTG ATC CAG CCG CA 3’. PCR sample volume was 25 µl per sample with the reaction mixture comprising 0.5 µl genomic DNA, ×1 PCR buffer (10 mM tris-HCl, 50 mM KCl, 1.5 mM MgCl2, pH 8.6), 0.3 µl Taq DNA polymerase (USB corporation, Cleveland, Ohio, USA), 2.5 µl deoxynucleotide triphosphates mix, and 2.5 µl reverse and forward primer each. PCR was performed on Master Cycler (Eppendorf, Germany) with initial denaturation for 2 min at 92°C. The first cycle had denaturation for 1 min at 92°C, primer annealing for 30 s at 48°C, and primer extension at 72°C for 2 min 10 s. The cycle was repeated for 34 times, and the process ends at the final polymerization step of 6 min 10 s at the temperature of 72°C. PCR product was resolved using electrophoresis in 1.2% agarose gel stained with EtBr, documented under ultraviolet light on UVI pro platinum gel documentation system.
16s r-DNA sequencing and identification of Aggregatibacter actinomycetemcomitans
16s PCR products were purified using AxyPrep™ DNA Gel Extraction Kit obtained from AXYGEN Biosciences. DNA sequencing was performed on ABI PRISM big dye terminator cycle sequence reading reaction kit at Chromous Biotech (Bengaluru, India), and the sequences obtained were compared and analyzed with databases at National Centre for Biotechnology Information (NCBI) using the BLAST program. The identified isolate of A. actinomycetemcomitans species was determined by percent sequence similarity (98.9%).
A. actinomycetemcomitans was grown to confluence in Petri dishes in anaerobic conditions at 37°C for 7 days to serve as source of pathogen plug to study its inhibition on the test samples.
Preparation of the culture plates
The samples were divided into three groups: Group A - control group, Group B - with three concentrations of Papacarie Duo™ (PC), and Group C - with three concentrations of Carie Care™ (CC). The chemically defined Socransky medium was developed to model the oral environment. Papacarie Duo™ (F&A Labartôrio Farmacéutico Ltda, São Paulo, Brazil) or Carie Care™ (Ecodentalworks India Pvt Ltd, Bengaluru, India) was mixed well into individual media plates, respectively just before plating. Control had nothing added to the media.
Growth of isolated pathogen on the culture plates
Each study groups were further divided into three subgroups (two petri dishes for each subgroup) depending on the dilution of the test samples at three different concentrations 10, 20, and 50 µl/ml of the gel (PC or CC) in media to study the dose-dependent effect.
Sub Group 1: Media plate with 10 µl/ml PC or CC
Sub Group 2: Media plate with 20 µl/ml PC or CC
Sub Group 3: Media plate with 50 µl/ml PC or CC.
In each subgroup, plug containing defined concentration of media with 1000 colony forming units of A. actinomycetemcomitans (logarithmic OD600 = 0.5) was inserted in the center of each plate (replacing the same size agarose in the media). All the culture plates were incubated at 370°C in an anaerobic chamber for 7 days and periodic visual observations were taken. Following incubation, the culture plates with test samples were analyzed for the bacterial growth inhibition.
Results
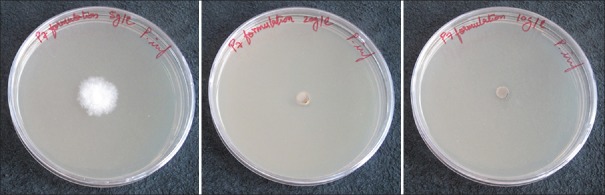
The inhibition of growth from the A. actinomycetemcomitans plug in the surrounding agar was indicative of the inhibitory role of the test samples. The petri dishes in the control group did not exhibit bacterial growth inhibition [Figure 1]. Both the study groups virtually represented the growth inhibition, thus indicative of antimicrobial effect [Figure 1b and c].
Figure 1.
Effect of Carie Care and Papacarie Duo on the growth of Aggregatibacter actinomycetemcomitans (from left to right)–control group, with Papacarie Duo, with Carie Care
In terms of growth of pathogen at different concentrations of test samples, Group B and C exhibited inhibition of growth from the plug in surrounding agar in a dose-dependent manner [Figures 2 and 3 respectively]. Group C was found to be significantly better than Group B in terms of in vitro inhibition of pathogen growth [Figure 3].
Figure 2.
Group B in concentration of (from left to right) - 10 μl, 20 μl, and 50 μl, respectively exhibiting growth inhibition
Figure 3.
Group C in concentration of (from left to right) - 10 μl, 20 μl, and 50 μl, respectively exhibiting growth inhibition
Discussion
Periodontal pocket provides ideal conditions for the proliferation of microorganisms mainly Gram-negative facultative species.[8] Advances in understanding of the etiology, epidemiology and microbiology of periodontal pocket flora have revolutionized the therapeutic strategies for management of periodontal disease progression. The conventional mechanical periodontal debridement is often accompanied by prolonged chemotherapeutic agent therapy aiming at eliminating the entire pathogenic bacterial flora.[9] In periodontal disease, there is an overproduction of collagenase causing destruction of healthy periodontal tissue.[10] The chemotherapeutic approach is intended not only kill the bacteria but also reduce the production of collagenase.
The papaya extract based gels in periodontal tissue could be significant, considering the likelihood of enhancing the postoperative period due to its anti-inflammatory and healing properties, as well as uniform tissue growth and the easy cleaning of necrotic tissues and secretions.[11] Hence, this study was undertaken to assess the antimicrobial action of the various commercially available papaya extract based gels with antimicrobial properties.
PCR techniques may be useful in investigating actual bacterial populations in individual patients, because of the high sensitivity of technique. However, PCR requires DNA probes for target DNA.[12,13] LB media, being a semi-solid, soft agar, offered the advantage of diluting the microorganisms while at the same time it is extensively used in recombinant DNA work and other molecular biology procedures. Being soft agar–it permitted some growth of anaerobic bacteria at the bottom of the slant where the conditions were more micro-anaerobic than at the top where aerobic bacteria could grow more easily.
A. actinomycetemcomitans is considered to be an opportunistic pathogen that produces a variety of potential virulence factors including an RTX (repeats in toxin) leukotoxin, and proinflammatory cytokines and chemokines, thus contributing to bacterial adhesion that alters host cells functions.[14] This opportunistic pathogen was considered for the present study as it is mainly responsible for aggressive periodontitis. In order to gain the clinical perspective, the causative pathogen was isolated from the periodontitis infected sites using PCR method with percent sequence similarity of more than 97%. It is interesting that at sequence homology values below about 97.5%, it is unlikely that two organisms have more than 60–70% DNA similarity and hence that they are related at the species level.[15]
The chemically defined culture medium is extremely useful during investigations of bacterial physiology to ensure in vitro experimental reproducibility and provide a point of reference.[16] Thus, Carie Care™ and Papacarie Duo™ were mixed separately with this chemically defined medium for obtaining a homogenous distribution as they are available in the gel form.
Papacarie Duo™ is a gel based on a combination of papain (a proteolytic enzyme with antibacterial and anti-inflammatory properties) and chloramine.[11] In case of Carie Care™, a particular fraction of papaya extract was isolated which had very gone do catalytic activity. This fraction was also found to have some antimicrobial activity as well along with proteolytic activity.[17]
Papain acts on the infected tissue (which lacks alpha 1 anti-trypsin, plasmatic antiprotease). It inhibited the protein digestion and allowed papain to break the partially degraded collagen molecules. It has bactericide and bacteriostatic properties, accelerates cicatricial process, and promotes debridement of necrotic tissue and secretions, thus facilitating wound healing.[18]
When assessed the antimicrobial action of the test samples, the Carie Care demonstrated significant antimicrobial action on the growth of A. actinomycetemcomitans when compared with Papacarie Duo. The results obtained in our study were in contrary to that obtained by Tanikawa-Vergillo et al. This may be attributed to the action potential of chloramine which is directly related to pH. Chloramine has less action potential in alkaline environment, Papacarie Duo™ which has chloramine as one of the main ingredients exhibited lesser antimicrobial activity.[11]
In the present study, dose-dependent effect of the Carie Care™ on the inhibition of A. actinomycetemcomitans appeared to be significantly better than Papacarie Duo™. This may be attributed to the presence of therapeutic essential oils as previously reported by Imai et al. 2001, Thapa et al. 2012, Nuñez and Aquino 2012.[19,20,21] The clove oil in itself is known to have both antimicrobial, analgesic, and antiseptic activities.[22]
There is very little scientific literature available on the antimicrobial effect of the various composition of papain gel on the periodontal disease causing pathogens. Due to the interest in the conservative management of the young individuals with compromised periodontal status, especially aggressive periodontitis, this study was undertaken. Thus, there is a need for the controlled trials to establish the here said hypothesis under clinical conditions.
Conclusion
Based on the findings, this study gives an initial lead in in vitro control of potential pathogens responsible for periodontal infection using therapeutic agents containing natural ingredients, thus providing an Insight to in-vivo control and therapeutic solutions for the defined diseases. The multiple therapeutic advances one can find using caries care is underlined in the present studies, which in turn could be an important milestone in minimal invasive dentistry.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2000. 2005;38:135–87. doi: 10.1111/j.1600-0757.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- 2.Szkaradkiewicz AK, Karpinski TM. Microbilogy of chronic periodontitis. J Biol Earth Sci. 2013;3:M14–20. [Google Scholar]
- 3.Salari MH, Kadkhoda Z. Rate of cultivable subgingival periodontopathogenic bacteria in chronic periodontitis. J Oral Sci. 2004;46:157–61. doi: 10.2334/josnusd.46.157. [DOI] [PubMed] [Google Scholar]
- 4.Dzink JL, Socransky SS, Haffajee AD. The predominant cultivable microbiota of active and inactive lesions of destructive periodontal diseases. J Clin Periodontol. 1988;15:316–23. doi: 10.1111/j.1600-051x.1988.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 5.Suresh PK, Dewangan MK. Development and in vitro characterization of metronidazole loaded chitosan microspheres for delivery to periodontal pocket. J Appl Pharm Sci. 2011;1:165–9. [Google Scholar]
- 6.Nair SC, Anoop KR. Intraperiodontal pocket: An ideal route for local antimicrobial drug delivery. J Adv Pharm Technol Res. 2012;3:9–15. doi: 10.4103/2231-4040.93558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniluk T, Tokajuk G, Cylwik-Rokicka D, Rozkiewicz D, Zaremba ML, Stokowska W. Aerobic and anaerobic bacteria in subgingival and supragingival plaques of adult patients with periodontal disease. Adv Med Sci. 2006;51(Suppl 1):81–5. [PubMed] [Google Scholar]
- 8.Schwach-Abdellaoui K, Vivien-Castioni N, Gurny R. Local delivery of antimicrobial agents for the treatment of periodontal diseases. Eur J Pharm Biopharm. 2000;50:83–99. doi: 10.1016/s0939-6411(00)00086-2. [DOI] [PubMed] [Google Scholar]
- 9.Haffajee AD, Dibart S, Kent RL, Jr, Socransky SS. Clinical and microbiological changes associated with the use of 4 adjunctive systemically administered agents in the treatment of periodontal infections. J Clin Periodontol. 1995;22:618–27. doi: 10.1111/j.1600-051x.1995.tb00815.x. [DOI] [PubMed] [Google Scholar]
- 10.Kaplish V, Walia MK, Hari Kumar SL. Local drug delivery systems in the treatment of periodontitis: A review. Pharmacophore. 2013;4:39–49. [Google Scholar]
- 11.Tanikawa-Vergillo KL, Cal S, Alfaya TA, Franca CM, Mesquita-Ferrari RA, Santos Fernandes KP, et al. Assessment of antimicrobial action of Papacarie™ against Aggregatibacter actinomycetemcomitans. Clin Exp Med Lett. 2012;53:63–6. [Google Scholar]
- 12.Boutaga K, van Winkelhoff AJ, Vandenbroucke-Grauls CM, Savelkoul PH. Periodontal pathogens: A quantitative comparison of anaerobic culture and real-time PCR. FEMS Immunol Med Microbiol. 2005;45:191–9. doi: 10.1016/j.femsim.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Lyons SR, Griffen AL, Leys EJ. Quantitative real-time PCR for Porphyromonas gingivalis and total bacteria. J Clin Microbiol. 2000;38:2362–5. doi: 10.1128/jcm.38.6.2362-2365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: The “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000. 2005;38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- 15.Stackebrandt E, Goebel BM. Taxonomic note: A place for DNA-DNA reassociation and 16s rRNA sequence analysis in the present species definition in bacteriology. Int J Syst Bacteriol. 1994;44:846–9. [Google Scholar]
- 16.Socransky SS, Dzink JL, Smith CM. Chemically defined medium for oral microorganisms. J Clin Microbiol. 1985;22:303–5. doi: 10.1128/jcm.22.2.303-305.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thakur R, Patil SS, Kush A. Chemomechanical caries removal technology – Dentistry at ease. Indian Dent Res Rev. 2014;9:22–7. [Google Scholar]
- 18.Bussadori SK, Castro LC, Galvão AC. Papain gel: A new chemo-mechanical caries removal agent. J Clin Pediatr Dent. 2005;30:115–9. doi: 10.17796/jcpd.30.2.xq641w720u101048. [DOI] [PubMed] [Google Scholar]
- 19.Imai H, Osawa K, Yasuda H, Hamashima H, Arai T, Sasatsu M. Inhibition by the essential oils of peppermint and spearmint of the growth of pathogenic bacteria. Microbios. 2001;106(Suppl 1):31–9. [PubMed] [Google Scholar]
- 20.Thapa D, Losa R, Zweifel B, Wallace RJ. Sensitivity of pathogenic and commensal bacteria from the human colon to essential oils. Microbiology. 2012;158(Pt 11):2870–7. doi: 10.1099/mic.0.061127-0. [DOI] [PubMed] [Google Scholar]
- 21.Nuñez L, Aquino MD. Microbicide activity of clove essential oil (Eugenia caryophyllata) Braz J Microbiol. 2012;43:1255–60. doi: 10.1590/S1517-83822012000400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alqareer A, Alyahya A, Andersson L. The effect of clove and benzocaine versus placebo as topical anesthetics. J Dent. 2006;34:747–50. doi: 10.1016/j.jdent.2006.01.009. [DOI] [PubMed] [Google Scholar]