Abstract
Objective
Dementia is a common clinical presentation among older adults with Down syndrome. The presentation of dementia in Down syndrome differs compared with typical Alzheimer’s disease. The performance of manualised dementia criteria in the International Classification of Diseases (ICD)-10 and Diagnostic and Statistical Manual of Mental Disorders-IV-Text Revision (DSM-IV-TR) is uncertain in this population.
We aimed to determine the concurrent validity and reliability of clinicians’ diagnoses of dementia against ICD-10 and DSM-IV-TR diagnoses. Validity of clinical diagnoses were also explored by establishing the stability of diagnoses over time.
Methods
We used clinical data from memory assessments of 85 people with Down syndrome, of whom 64 (75.3%) had a diagnosis of dementia. The cases of dementia were presented to expert raters who rated the case as dementia or no dementia using ICD-10 and DSM-IV-TR criteria and their own clinical judgement.
Results
We found that clinician’s judgement corresponded best with clinically diagnosed cases of dementia, identifying 84.4% cases of clinically diagnosed dementia at the time of diagnosis. ICD-10 criteria identified 70.3% cases, and DSM-IV-TR criteria identified 56.3% cases at the time of clinically diagnosed dementia. Over time, the proportion of cases meeting ICD-10 or DSM-IV-TR diagnoses increased, suggesting that experienced clinicians used their clinical knowledge of dementia presentation in Down syndrome to diagnose the disorder at an earlier stage than would have been possible had they relied on the classic description contained in the diagnostic systems.
Conclusions
Clinical diagnosis of dementia in Down syndrome is valid and reliable and can be used as the standard against which new criteria such as the DSM-5 are measured.
Keywords: dementia, Down syndrome, diagnosis, ICD-10, DSM-IV-TR, clinical judgement
Introduction
Down syndrome is the most common genetic cause of intellectual disability, and dementia is common in adults with Down syndrome. Post-mortem studies indicate that neuropathological changes typical of Alzheimer’s disease are almost universal in people with Down syndrome over the age of 40years (Wisniewski et al., 1985; Mann et al., 1986). Alongside this, an earlier mean age of onset of dementia has been shown to be between 50 and 55years in this group (Prasher and Krishnan, 1993), and data show that by the age of 60years between 30 and 40% of people with Down syndrome will warrant a diagnosis of dementia (Holland et al., 1998; Tyrrell et al., 2001; Coppus et al., 2006).
There is evidence to suggest that the presentation of dementia in people with Down syndrome is different from typical Alzheimer’s disease. In particular, it appears that symptoms related to frontal lobe deterioration such as changes in personality and behaviour are more notable early in the course of the disease (Ball et al., 2006; Deb et al., 2007a), although the reasons underlying this observation are not clear.
However, the prominent behavioural and emotional changes associated with Alzheimer’s disease in Down syndrome may also be an artefact representing the fact that people with Down syndrome are not diagnosed until later in the course of the disease. There are several possible reasons why a diagnosis of Alzheimer’s disease may be delayed in older adults with Down syndrome. Firstly, there are inherent difficulties in diagnosing cognitive decline in those with a pre-existing cognitive impairment. Standard screening tools such as the Mini-Mental State Examination (Folstein et al., 1975) and other direct neuropsychological tests are often not appropriate because of a ‘floor effect’ (Deb et al., 2007b). Subtle cognitive decline may be difficult to determine, particularly in people who have not attained many skills and in those living in a residential setting where environmental demands are low. Clinicians are therefore greatly dependent on informant history. In some cases, caregivers might not seek intervention until later in the course of the disease when behaviour becomes more difficult to manage. Because of these issues, there is debate about whether the current manualised criteria for diagnosing dementia, namely, the International Classification of Diseases (ICD)-10 (World Health Organisation, 1992) and Diagnostic and Statistical Manual of Mental Disorder-IV-Text Revision (DSM-IV-TR) (American Psychiatric Association, 2000), are applicable to the dementia syndrome associated with Alzheimer’s disease in Down syndrome, particularly as it has been shown that employing different systems of classification influences the reported prevalence of dementia in people with intellectual disability (Strydom et al., 2007).
As life expectancy increases for people with Down syndrome (Bittles et al., 2007), dementia will become a more common presentation. This has implications not only for individuals concerned but will also demand changes in service provision and impact in terms of increased caregiver stress and economic cost. Early diagnosis is important so that symptomatic medication such as acetylcholinesterase inhibitors can be offered at a suitable time and psycho-social interventions, including appropriate support for carers, can be implemented at a time that is most beneficial.
Given the complexities in diagnosing dementia in people with Down syndrome, many attempts have been made to standardise the assessment process. Instruments have been developed (or adapted) and can be based on either informant interview or direct cognitive assessment (Deb and Braganza, 1999), although there seems to be lack of consensus as to the most appropriate (Strydom and Hassiotis, 2003). Similarly, while the physician’s clinical judgement has regularly been regarded as the ‘gold standard’ against which other measures are tested, some authors have argued that this could result in a higher number of adults being diagnosed (Burt et al., 2005). It is important to evaluate the validity and reliability of clinical diagnoses in this population while new criteria are being introduced for the DSM-5 and ICD-11 as it could help to establish a standard against which new criteria can be measured.
We aimed to determine the concurrent validity and reliability of clinicians’ diagnoses of dementia against ICD-10 and DSM-IV-TR diagnoses. Validity of clinical diagnoses was also explored by establishing the stability of diagnoses over time, and we also aimed to establish the reliability of dementia diagnoses according to ICD-10, DSM-IV-TR and clinician’s judgement.
Materials and method
Identification of participants and collection of data
Anonymised clinical data from adults with Down syndrome or intellectual disability were eligible for inclusion in the Aging with Down Syndrome or Intellectual Disability (ADSID) database. This database consists of memory assessments conducted since 1996 by different clinicians across several sites in London and the surrounding area.
Each memory assessment included an examination of the patient’s functioning in several domains (cognitive ability, mood, daily living skills and social behaviours, neurological function and detailed mental state examination) and a comparison of how this may have changed from baseline levels, in accordance with published best-practice guidelines (Royal College of Psychiatrists and the British Psychological Society, 2009). Details of comorbid physical or mental health conditions were also reported. We excluded all data from adults who did not have Down syndrome and participants for whom insufficient data were available.
Data were collected retrospectively from participants’ medical records and entered into the ADSID database. Basic demographic information (age and gender) was obtained from the clinical records. The presence of Down syndrome was verified by review of the notes, although the method used for the original diagnosis varied. Information regarding cognitive, behavioural and emotional symptoms from each assessment was recorded. In addition, the results of screening tests (e.g. the Dementia questionnaire for people with Learning Disabilities (Evenhuis et al., 2006)), recent physical and mental health problems and physical investigations were also noted.
Ethics approval
The ADSID study was approved by the Newcastle and North Tyneside 1 Research Ethics Committee, and approval for collecting anonymised data from medical records was obtained from the National Information Governance Board. Where required, Caldicott Guardians of data providers gave approval for data transfer.
Concurrent validity ratings of clinical diagnoses of dementia
Clinical dementia diagnoses were defined as confirmed diagnoses of dementia recorded by clinicians following clinical assessment. All memory assessments were then summarised in anonymised case vignettes by the researchers, without making reference to clinical diagnosis. We also removed reference to treatment with acetylcholinesterase inhibitors and memantine. Case vignettes were also produced for assessments preceding and following the diagnosis of dementia, where available.
The case vignettes were then presented to two raters who worked independently to rate the case by applying diagnostic criteria delineated in ICD-10 and DSM-IV-TR; that is, the raters assigned the case to ‘no dementia’ or ‘dementia’ according to either ICD-10 or DSM-IV-TR criteria (Table 1). Use of the ICD-10 category of ‘tentative dementia’ was also included.
Table 1.
Summary of ICD-10 and DSM-IV-TR diagnostic criteria for Alzheimer’s dementia
| Criterion | ICD-10 | DSM-IV-TR |
|---|---|---|
| Memory impairment | X | X |
| Disturbance in higher cortical functiona | ||
| Executive functioning | X | X |
| Aphasia | X | |
| Apraxia | X | |
| Agnosia | X | |
| Decline in behavioural or emotional function | ||
| Emotional lability | X | |
| Irritability | X | |
| Apathy | X | |
| Coarsening of social behaviour | X | |
| Deficits cause significant impairment in functioning | X | |
| Gradual onset and progression | X | |
| Duration at least 6 months | X | |
| Exclusions | ||
| Delirium | X | X |
| Other CNS, systemic or substance-induced conditions | X | X |
| Other mental illness | X |
Only one of these symptoms need be present for DSM-IV-TR diagnosis
Raters were also asked to make a separate rating based on their clinical judgement and rate the cases as no dementia, ‘cognitive concern’, ‘possible dementia’ or ‘certain dementia’. Cognitive concern was defined as any evidence of deterioration in cognitive function but where this was of recent onset only and where the rater was unable to exclude a physical causation (such as hypothyroidism) or a mental illness causing changes in behaviour or memory (such as depression). Possible dementia was defined as likely dementia (i.e. significant decline over 6 months or more) but where the raters felt the vignette lacked evidence to be certain of a diagnosis and where they would have wanted further information or to offer another appointment to confirm results and symptoms.
Raters were clinicians (psychiatrists and psychologists) working in the fields of Psychiatry of Intellectual Disability or Psychiatry of Older People who were members of a ‘dementia in intellectual disabilities’ special interest group. Raters remained blinded to the patient’s true clinical diagnosis and any treatment received.
After rating each assessment independently, the raters compared outcomes for each assessment. Where there was disagreement between the raters’ outcomes, a discussion ensued, and a consensus was achieved and recorded. For a subset of assessments, the outcome of each individual rater was recorded prior to consensus allowing for determination of inter-rater reliability.
Stability of diagnoses over time
In order to explore the stability of clinical dementia diagnoses, we compared the outcomes of the rater’s assessments from the time of clinical diagnosis (t) and at the first (t+1) and second (t+2) assessments following a clinical diagnosis. The time between subsequent assessments (t, t+1 and t+2) was not less than 3 months and not more than 18 months.
Clinical assessments were available in 26 out of 64 (40.6%) of cases at all three of these time points.
Inter-rater reliability
In order to establish inter-rater reliability, we recorded the raters’ diagnoses before they agreed the diagnosis in a subset of 23 cases. This was conducted on data from participants’ first clinical assessments. In the ICD-10 analysis, the dementia group included ratings of tentative dementia and dementia. In the clinical judgement analysis, ratings of dementia and possible dementia were included in the dementia group, and the no dementia group included ratings of cognitive concern and no dementia.
Analysis
Statistical Package for the Social Sciences (SPSS) version 21 was used for data entry and analysis. Descriptive demographics and frequencies were calculated. Inter-rater reliability was calculated using Cohen’s kappa for inter-rater agreement.
Results
Demographic information
The final database for this analysis comprised 85 participants with Down syndrome. Three quarters (75.3%) of these had a clinical diagnosis of dementia (n = 64). Twenty one participants (24.7%) did not have a diagnosis of dementia recorded at clinical assessment and were included in order to help identify accuracy of diagnoses. The participants comprised 40 men (47.1%) and 45 women (52.9%).
Overall, 21 participants (24.7%) had mild (intelligence quotient (IQ) 70–50), 40 (47.1%) had moderate (IQ 35–50), and 12 (14.1%) had severe or profound (IQ < 35) intellectual disability. In 12 cases (14.1%), the level of intellectual disability was unknown.
The age range at which a clinical diagnosis of dementia was made was between 35.5 and 70.9 years (mean ± SD = 55.4 ± 6.6 years).
Concurrent validity ratings of clinical diagnoses of dementia
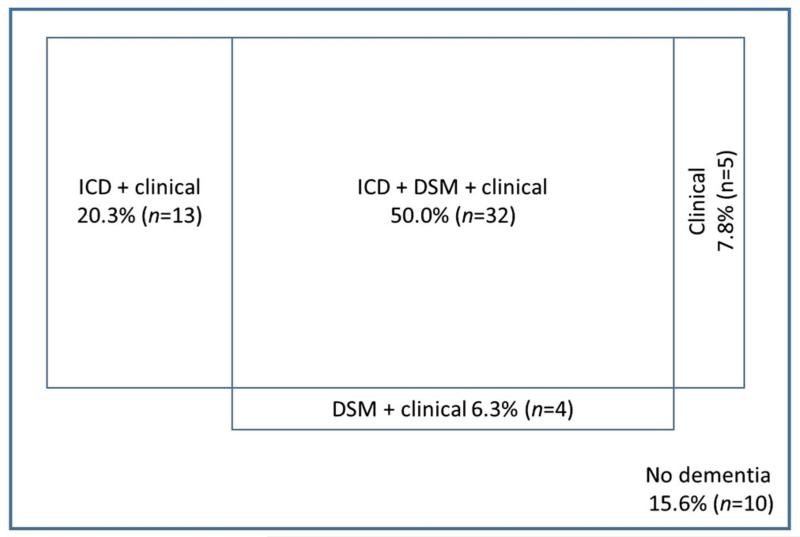
At the clinical assessment at which a diagnosis of dementia was made, the raters agreed with the clinical diagnosis in 84.4% (n =54) of all cases (n = 64) on the basis of ‘any diagnosis’ of dementia (i.e. using either ICD-10, DSM-IV-TR or clinical judgement, or a combination of these). In 10 (15.6%) cases where a clinical diagnosis of dementia had been made, the raters did not make a diagnosis of dementia.
The rater’s clinical judgement showed best agreement with original clinical diagnosis—the raters diagnosed dementia or possible dementia in 54 (84.4%) cases at the time point when a clinical diagnosis was first given, and in an additional 4 (6.2%) cases the raters chose cognitive concern to describe the case. In only 6 (9.4%) cases of clinically diagnosed dementia where the raters used their clinical judgement to inform their decision, the outcome was of no dementia. This was followed by ICD-10; 45 (70.3%) cases met ICD-10 criteria for dementia (28 cases, 43.8%) or tentative dementia (an additional 17 cases, 26.6%), while DSM-IV-TR criteria diagnosed 36 (56.3%) cases of clinician-diagnosed dementia.
Fifty per cent (n = 32) of those with a clinical diagnosis of dementia were rated as having dementia by all three measures, namely, ICD-10 and DSM-IV-TR criteria and using the rater’s clinical judgement.
See Figure 1 for more details.
Figure 1.
Diagram showing outcome of ratings at the time when the clinical diagnosis of dementia was made (not to scale).
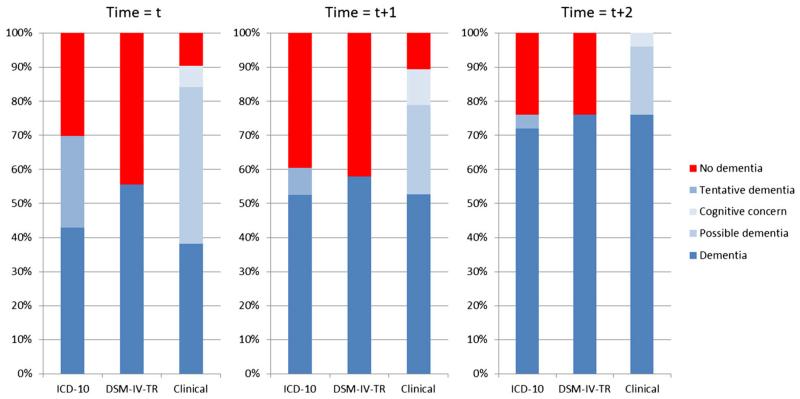
Stability of diagnoses over time
Figure 2 shows the trends in how rater’s diagnoses changed from the time of initial clinical diagnosis of dementia (t) and subsequent first (t+1) and second (t+2) assessment following this. This data are obtained from the subgroup of participants who had data for all three assessments (n =26).
Figure 2.
Stability of diagnoses over time showing ratings at time of clinician’s diagnosis of dementia (t) and first (t + 1) and second (t + 2) assessments following clinician’s diagnosis of dementia (n = 26).
Inter-rater reliability
In 23 of the initial assessments, the individual rater’s outcomes were recorded, in order to assess the inter-rater reliability of diagnoses using clinical judgement, and by application of standardised criteria (ICD-10 and DSM-IV-TR). This subset represented 27.1% of the overall sample. Cohen’s kappa was calculated as 0.911 for agreement between raters when ICD-10 was used, 0.704 for agreement when DSM-IV was applied and 0.826 for agreement between raters when clinical judgement was applied to the case. All were significant at p ≤ 0.001.
Discussion
Dementia diagnosis in people with Down syndrome
This study was conceived to investigate the validity and reliability of a clinical diagnosis of dementia in people with Down syndrome and to compare it with the performance of established criteria. We found that half of those with a clinical diagnosis of dementia were rated as having dementia by both ICD-10 and DSM-IV-TR criteria and rater’s clinical judgement, although the overall figure for diagnosis of dementia in participants made by any means was 84.4%. Rater’s clinical judgement had high levels of agreement with original diagnoses, but ICD-10 and DSM-IV-TR criteria fared worse, suggesting that experienced clinicians incorporated clinical features not included in ICD-10 and DSM-IV-TR criteria when making a diagnosis. Taken together with results demonstrating a high degree of inter-rater agreement, this suggests that interpretation of the criteria is consistent between clinicians. However, the results suggest that ICD-10 and DSM-IV-TR criteria are likely to underdiagnose dementia in this population, implying that the presentation of Alzheimer’s disease is sufficiently different in people with Down syndrome to affect performance of standardised criteria in this population. Furthermore, we have shown that clinical diagnoses have good validity, as the criteria diagnoses ‘caught up’ with clinical diagnoses at subsequent assessments.
We examined the characteristics of certain cases in more detail, that is, the cases where no diagnosis was made by the raters under any system (n=10) and the cases that were diagnosed by application of clinical judgement but neither ICD-10 nor DSM-IV-TR criteria (n=5). In the clinically diagnosed cases that were not deemed to have dementia by the raters, there were several people with comorbid mental and/or physical illness, suggesting that raters applied caution when other explanations for the decline could be relevant. In other cases, the decline was only recent, and in a small number of cases there was only limited information, which may have been insufficient for the raters to confidently diagnose.
In the cases that received a diagnosis when clinical judgement was applied but not when ICD-10 or DSM-IV-TR criteria were used, the decline tended to be only over a short duration, which may have precluded diagnosis under the stipulation that symptoms be present for at least 6 months (ICD-10).
As understanding and treatments advance, timely diagnosis of dementia is important to ensure access to interventions that improve functioning and quality of life. A diagnosis of dementia may be necessary for identifying and meeting changing needs in accommodation and nursing and social support. It is also important to facilitate adequate explanation of the diagnosis and prognosis for patients and relatives.
At the memory assessments following the diagnosis (t+1, and t+2), a firm diagnosis of dementia was made in progressively increasing proportions of cases. We therefore suggest that clinical judgement should be the preferred means of diagnosing dementia in people with Down syndrome following comprehensive assessment by clinicians who are experienced in recognising the symptoms of dementia in this population. Further work is required to define how standard criteria need to be adapted or interpreted in this population to enable valid and early diagnosis of dementia.
With regards to the performance of DSM-IV-TR criteria against ICD-10 criteria, we found DSM-IV-TR criteria to be the most exclusive, although if the ICD category of tentative dementia is excluded, the ICD criteria become the most exclusive and identify less than half (43.8%) of those clinically diagnosed cases. It has been suggested that ICD-10 criteria are specific and more demanding to apply as they rely on informant information to a greater extent (Henderson et al., 1994).
Inter-rater reliability
We found inter-rater agreement to be strong when both operationalised criteria were applied by raters. This is to be expected as the DSM-IV-TR and ICD-10 criteria for diagnosis are formed of clear statements that have been designed specifically to reduce ambiguity and allow a common language between professionals. However, agreement between raters when clinical judgement was applied to the cases was equally as strong. We had anticipated a greater degree of subjectivity in appraising and interpreting the details of the vignettes to be reflected in lower inter-rater agreement for clinical judgement, but this was not the case.
Because of the relatively small numbers in this subset, we were not able to compare outcomes of the ratings given by different professional groups (i.e. psychiatrists and psychologists).
Implications of the study
We have shown that clinical diagnoses of dementia by experienced clinicians appeared to be accurate and tended to identify dementia cases earlier than operationalised criteria in older adults with Down syndrome, although this may still be at a later stage than dementia diagnoses in an equivalent population without intellectual disability. We also identified a discrepancy in the diagnosis of dementia amongst a population with Down syndrome, depending on which criteria are used to identify the illness. Following the recent publication of DSM-5 and an updated ICD due in 2015, now is a timely juncture to explore the way in which clinicians diagnose dementia in people with Down syndrome. Changes to the defined criteria or the way it is interpreted in this population may be necessary in order to reflect the differing presentation of dementia in people with Down syndrome. Our results imply that clinical diagnoses should be used as the yardstick against which new criteria are measured. Use of the Diagnostic Criteria for psychiatric disorders for use with adults with Learning Disability (DC-LD) (Royal College of Psychiatrists, 2001) or Diagnostic Manual - Intellectual Disability (DM-ID) (Fletcher et al., 2007) as alternative diagnostic systems for people with intellectual disability is not widespread, and research evidence relating to these classifications is limited. They may be less suitable for adults with mild intellectual disability.
Our findings also have implications for research in dementia in Down syndrome, for example, to estimate the prevalence and estimates of dementia or to identify potential participants for clinical trials of treatments for dementia in this population.
Strengths and limitations of the study
The study has several strengths. It uses clinical data derived from memory assessments conducted over a wide time period from both urban and suburban locations. A range of people with Down syndrome between the ages of 35 and 70years with differing baseline levels of cognitive impairment and adaptive function were included. Our data are therefore fairly representative of clinical practice in the UK. Furthermore, in order to reduce bias, raters were blinded to the outcome of the clinical assessment and rated each case individually before reaching a consensus opinion.
Data from clinical assessments were transcribed onto anonymised data collection forms. Whilst every attempt to capture relevant information was made in both the design and completion of these forms, it is inevitable that some detail was lost and subtleties of clinical assessment that would be apparent to the treating clinician were not always available to our raters. Data were also dependent on the thoroughness and documentation of the clinical records. In addition, raters did not have the privilege of prior knowledge of the patient, as would often be the case in clinical practice. For these reasons, raters may have been less likely to make a diagnosis of dementia than their counterparts in the clinic.
In this study, we did not categorise the severity of dementia, although as the ratings were completed for the assessment at which a clinical diagnosis of dementia was made, it is most likely that the majority of new cases of dementia were in the early stage.
Conclusion
This study shows that when making a diagnosis of dementia in people with Down syndrome, clinicians who are experienced in assessing this population for cognitive decline are able to make accurate diagnoses at a relatively early stage of the disease. Clinical diagnoses were more inclusive than using ICD-10 or DSM-IV-TR criteria, suggesting that clinicians relied on dementia symptoms that are specific to this population. It remains to be seen whether new definitions of dementia in ICD-11 and DSM-5 will perform better in this population. We recommend that clinical diagnoses (following a comprehensive assessment including history, examination and consideration of investigations and formal cognitive assessments) are used as the standard against which new criteria can be judged. Further work may be required to establish how new criteria need to be adjusted in order to improve diagnosis in this population.
Key points.
We investigated the validity of dementia diagnoses in people with Down syndrome.
Application of DSM-IV-TR and ICD-10 criteria excluded some people with dementia.
Clinician’s judgement diagnosed dementia at an earlier stage.
Acknowledgements
We wish to thank Hannah Boardman, Sharon Jakobowitz and Camino Fernandez, Research Assistants, Khadija Rantell, Statistician. We would also like to express our thanks to all the raters who gave their time to assist with the ratings. Funding towards this study has been provided by The Baily Thomas Charitable Fund.
Footnotes
Conflicts of interest
None declared.
References
- American Psychiatric Association . Diagnostic and Statistical Manual-Text Revision (DSM-IV-TR) American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Ball SL, Holland AJ, Hon J, et al. Personality and behaviour changes mark the early stages of Alzheimer’s disease in adults with Down’s syndrome: findings from a prospective population-based study. Int J Geriatr Psychiatry. 2006;21(7):661–673. doi: 10.1002/gps.1545. [DOI] [PubMed] [Google Scholar]
- Bittles AH, Bower C, Hussain R, Glasson EJ. The four ages of Down syndrome. Eur J Public Health. 2007;17(2):221–225. doi: 10.1093/eurpub/ckl103. [DOI] [PubMed] [Google Scholar]
- Burt DB, Primeaux-Hart S, Loveland KA, et al. Comparing dementia diagnostic methods used with people with intellectual disabilities. J Policy Pract Intellect Disabil. 2005;2(2):94–115. [Google Scholar]
- Coppus A, Evenhuis H, Verberne G-J, et al. Dementia and mortality in persons with Down’s syndrome. J Intellect Disabil Res. 2006;50(10):768–777. doi: 10.1111/j.1365-2788.2006.00842.x. [DOI] [PubMed] [Google Scholar]
- Deb S, Braganza J. Comparison of rating scales for the diagnosis of dementia in adults with Down’s syndrome. J Intellect Disabil Res. 1999;43(5):400–407. doi: 10.1046/j.1365-2788.1999.043005400.x. [DOI] [PubMed] [Google Scholar]
- Deb S, Hare M, Prior L. Symptoms of dementia among adults with Down’s syndrome: a qualitative study. J Intellect Disabil Res. 2007;51(9):726–739. doi: 10.1111/j.1365-2788.2007.00956.x. [DOI] [PubMed] [Google Scholar]
- Deb S, Hare M, Prior L, Bhaumik S. Dementia screening questionnaire for individuals with intellectual disabilities. Br J Psychiatry. 2007;190(5):440–444. doi: 10.1192/bjp.bp.106.024984. [DOI] [PubMed] [Google Scholar]
- Evenhuis HM, Kengen MMF, Eurlings HAL. Dementia Questionnaire for People with Learning Disabilities (DLD) Harcourt Assessment; San Antonio: 2006. [Google Scholar]
- Fletcher R, Loschen E, Stavrakaki C, First M, editors. Diagnostic Manual—Intellectual Disability (DM-ID): A Textbook of Diagnosis of Mental Disorders in Persons with Intellectual Disability. NADD Press; Kingston, NY: 2007. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Henderson AS, Jorm AF, Mackinnon A, Christensen H, Scott LR, Korten AE, Doyle C. A survey of dementia in the Canberra population: experience with ICD-10 and DSM-III-R criteria. 1994. [DOI] [PubMed] [Google Scholar]
- Holland AJ, Hon J, Huppert FA, Stevens F, Watson P. Population-based study of the prevalence and presentation of dementia in adults with Down’s syndrome. Br J Psychiatry. 1998;172(6):493–498. doi: 10.1192/bjp.172.6.493. [DOI] [PubMed] [Google Scholar]
- Mann DMA, Yates PO, Marcyniuk B, Ravindra CR. The topography of plaques and tangles in Down’s syndrome patients of different ages. Neuropathol Appl Neurobiol. 1986;12(5):447–457. doi: 10.1111/j.1365-2990.1986.tb00053.x. [DOI] [PubMed] [Google Scholar]
- Prasher VP, Krishnan VHR. Age of onset and duration of dementia in people with down syndrome: integration of 98 reported cases in the literature. Int J Geriatr Psychiatry. 1993;8(11):915–922. [Google Scholar]
- Royal College of Psychiatrists . DC-LD: Diagnostic Criteria for Psychiatric Disorders for Use with Adults with Learning Disabilities/Mental Retardation. Gaskell Press; London, UK: 2001. [Google Scholar]
- Royal College of Psychiatrists and the British Psychological Society . CR155. Dementia and People with Learning Disabilities. British Psychological Society; Leicester: 2009. [Google Scholar]
- Strydom A, Hassiotis A. Diagnostic instruments for dementia in older people with intellectual disability in clinical practice. Aging Ment Health. 2003;7(6):431–437. doi: 10.1080/13607860310001594682. [DOI] [PubMed] [Google Scholar]
- Strydom A, Livingston G, King M, Hassiotis A. Prevalence of dementia in intellectual disability using different diagnostic criteria. Br J Psychiatry. 2007;191(2):150–157. doi: 10.1192/bjp.bp.106.028845. [DOI] [PubMed] [Google Scholar]
- Tyrrell J, Cosgrave M, McCarron M, et al. Dementia in people with Down’s syndrome. Int J Geriatr Psychiatry. 2001;16(12):1168–1174. doi: 10.1002/gps.502. [DOI] [PubMed] [Google Scholar]
- Wisniewski KE, Wisniewski HM, Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer’s disease in Down’s syndrome. Ann Neurol. 1985;17(3):278–282. doi: 10.1002/ana.410170310. [DOI] [PubMed] [Google Scholar]
- World Health Organisation . ICD-10: International Statistical Classification of Diseases and Related Health Problems. World Health Organisation; Geneva: 1992. [Google Scholar]