Summary
Background and objective
The major cat allergen Fel d 1 represents one of the most important respiratory allergens. Aim of this study was to engineer recombinant Fel d 1 derivatives with reduced IgE reactivity and preserved T cell epitopes for vaccination and tolerance induction.
Methods
Seven recombinant mosaic proteins were generated by reassembly of non-IgE-reactive peptides of Fel d 1 which contained the sequence elements for induction of allergen-specific blocking IgG antibodies and T cell epitopes. Mosaic proteins were expressed in Escherichia coli using codon-optimized synthetic genes and compared with Fel d 1 regarding structural fold by circular dichroism, IgE-binding capacity, activation of allergic patients’ basophils and ability to induce allergen-specific blocking IgG antibodies upon immunization.
Results
Although each of the mosaic proteins had lost the alpha-helical fold typical for Fel d 1, a strong reduction in IgE reactivity as well as allergenic activity in basophil activation assays was only obtained for three constructs, two reassembled fragments (Fel d 1 MB, Fel d 1 MC) and a fusion of the latter two (Fel d 1 MF) in which the cysteines of Fel d 1 MC were replaced by serines. Immunization of rabbits with Fel d 1 MB, MC and MF induced high levels of IgG antibodies that inhibited IgE reactivity of cat-allergic patients to Fel d 1 in a comparable manner as IgG induced with the wild-type allergen.
Conclusions
We report the development of hypoallergenic reassembled Fel d 1 proteins suitable for vaccination and tolerance induction in cat-allergic patients.
Keywords: allergen-specific immunotherapy, cat allergy, Fel d 1, hypoallergenic vaccine, recombinant allergen, tolerance induction, vaccination
Introduction
The domestic cat (Felis domesticus) is one of the most common sources of indoor allergens and is responsible for IgE-mediated allergic disease in about 20% of atopic patients [1–3]. Symptoms of cat allergy range from mild manifestations of rhinitis and conjunctivitis to severe forms of asthma. Fel d 1 is the major cat allergen which is recognized by more than 90% of cat-allergic patients [4] and accounts for 60–90% of the total allergenic activity in cat dander [5]. Crystal structure analysis revealed that Fel d 1 is an α-helical protein [6] which forms tetramers consisting of two 18-kDa non-covalently linked heterodimers [7, 8]. Each dimer is composed of two antiparallel polypeptides, chain 1 and chain 2. In nature, these polypeptides are encoded by two separate genes [9] and are subsequently covalently linked by three disulphide bonds [7]. Bond et al. [10] showed that each Fel d 1 chain displays IgE-binding activity and is able to induce histamine release, although at a lower level than the naturally occurring Fel d 1 heterodimer. Therefore, recombinant Fel d 1, a chain 2 to chain 1 fusion protein [rFel d 1 (2 + 1)], mimicking immunological properties of the natural allergen has been produced [11].
Allergen-specific immunotherapy (SIT) is the only disease-modifying treatment for allergy and has longlasting effects even after discontinuation [12, 13]. SIT has been shown to be clinically effective for cat-induced asthma in several studies. Patients’ benefits were reported to be associated with the induction of IgG antibodies specific for the major cat allergen Fel d 1 and reduced cutaneous and respiratory symptoms [14–18]. However, SIT with cat allergen extracts is limited by severe side effects. For example, Mellerup et al. [19] reported that 41% of patients that underwent specific immunotherapy with cat-allergen extracts experienced severe side effects.
Therefore, several molecular approaches were developed for reducing SIT-induced side effects. T cell epitope-containing peptides for the induction of tolerance have been tested in several clinical trials with the goal of developing a tolerogenic cat vaccine [20–23]. Another approach was the development of recombinant hypoallergens of Fel d 1 by disrupting the disulfide bonds and duplicating selected T cell epitopes [24, 25]. We recently described an approach based on recombinant fusion proteins consisting of non-allergenic Fel d 1 peptides fused to hepatitis B PreS protein [26]. Other approaches comprise Fel d 1 coupled to bacteriophage Qβ-derived virus-like particles, Fel d 1 linked to human IgG or vitamin D3 or Fel d 1 fusion proteins with a target signal for MHC II presentation [27–30].
In this study, we aimed to develop recombinant hypoallergenic Fel d 1 derivatives which may be useful both for vaccination and induction of blocking IgG antibodies as well as for tolerance induction. We report the rational design, structural, immunological and pre-clinical characterization of seven recombinant Fel d 1 derivatives containing either the whole Fel d 1 sequence or fragments containing approximately half of the Fel d 1 sequence. Based on IgE reactivity testing, assessment of allergenic activity in basophils of allergic patients and immunization experiments searching for the derivatives which induce robust allergen-specific IgG responses blocking allergic patients IgE binding to the wild-type allergen, three derivatives were identified as possible candidates for vaccination and tolerance induction.
Materials and methods
Allergic patients, synthetic peptides
IgE reactivity testing and assessment of allergenic activity by basophil activation were performed with blood samples from twenty-one cat-allergic patients. Patients reported clinical symptoms of conjunctivitis, rhinitis and/or asthma upon exposure to cats (Table S1). They exhibited IgE antibodies to cat dander extract (e1) as measured by ImmunoCAP (Phadia, Uppsala, Sweden) and IgE antibodies to rFel d 1 as determined by ELISA and/or in a microarray assay (ISAC, Phadia, Uppsala, Sweden). Blood samples from three non-allergic subjects were used as controls. Blood and serum samples were analysed in an anonymous manner after informed written consent was obtained from patients with approval of the local ethics committee, Medical University of Vienna, Austria (EK number 565/2007).
Fel d 1-derived peptides (Table S2) were synthesized using an Applied Biosystems peptide synthesizer Model 433A (Foster City, CA, USA) and purified by HPLC [31].
Expression and purification of the recombinant hypoallergenic Fel d 1 derivatives
rFel d 1 was purified as previously described [11]. Genes (codon-optimized for Escherichia coli expression) coding for the hypoallergenic Fel d 1 derivatives were synthesized (ATG: biosynthetics, Merzhausen, Germany and GenScript, Piscataway, USA) and inserted into the NcoI/EcoRI sites of pET-28b (Fel d 1 M, Fel d 1 MA, Fel d 1 MB) or into NdeI/EcoRI sites of pET-27b (Fel d 1 MC, Fel d 1 ME and Fel d 1 MF; Novagen, Darmstadt, Germany). All Fel d 1 derivatives also contained sequences coding for a hexahistidine tag at the C-terminus. The DNA sequences were confirmed by means of restriction enzyme analysis with corresponding restriction enzymes (Roche, Mannheim, Germany) and by automated sequencing of both DNA strands. E. coli BL 21 DE3 (Stratagene, La Jolla, California) transformed with pET-28b-M, MA, MB or pET-27b-MC, ME, MF were grown at 37°C in a GFL 3033 incubator (GFL, Burgwedel, Germany) in LB medium containing kanamycin (30 μg/mL) for approximately 8 h at 37°C until a cell density (OD600 nm) of 0.3–0.6 was reached. Protein expression was induced by adding 0.5 mm isopropyl-ß-thiogalactopyranoside (Calbiochem, Merck, Darmstadt, Germany). Cells were harvested by centrifugation at 10 408 g for 10 min and lysed with an Ultra-Turrax (Janke & Kunkel-IKA Labortechnik, Staufen, Germany) in 100 mm NaH2PO4, 10 mm Tris-HCl, 8 m Urea, pH 8. Recombinant proteins were purified by Ni2+ metal ion affinity chromatography (Qiagen, Hilden, Germany) and refolded by extensive dialysis against distilled water. The purity of the recombinant proteins was analysed by SDS–PAGE, and the molecular mass was determined by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Bruker, Billerica, MA, USA). The final protein concentrations were determined using a Micro-BCA Protein Assay Kit (Pierce, Rockford, IL, USA). The stability of MF was studied after 28 months of storage at −20°C by SDS–PAGE performed under reducing and non-reducing conditions and by size-exclusion chromatography (SEC) as described [32].
Circular dichroism (CD) analysis
The CD spectra of the purified recombinant proteins were measured on a JASCO (Tokyo, Japan) J-810 spectropolarimeter. Measurements were carried out at protein concentrations of 0.1 mg/mL in a rectangular quartz cuvette with a path length of 0.2 cm. Spectra were recorded from 190 to 260 nm with a resolution of 0.5 nm at a scan speed of 50 nm/min and were the result from three scans. Final spectra were corrected by subtracting the baseline spectra obtained with the buffers alone. Results are expressed as the mean residue ellipticities (Θ) at given wavelengths. The secondary structure contents of the proteins were calculated with the secondary structure estimation program CDSSTR [33].
IgE reactivity, T cell reactivity and allergenic activity of Fel d 1 and hypoallergenic Fel d 1 derivatives
Serum IgE reactivity of hypoallergenic Fel d 1 derivatives and synthetic peptides was determined by means of direct ELISA. For this purpose, 3 μg/mL of Fel d 1, Fel d 1 derivatives or peptides were coated onto the ELISA plates. Sera from 21 cat-allergic patients and three non-allergic individuals were then added to the ELISA plates in a dilution of 1 : 10 in TBS containing 0.5% v/v Tween 20 (TBS-T), and bound human IgE was detected with HRP-coupled goat anti-human IgE antibodies diluted 1 : 2500 (KPL, Gaithersburg, MD). The OD values corresponding to bound antibodies were measured at 405 and 490 nm. All determinations were conducted as duplicates, and results were expressed as mean values with a variation coefficient of less than 5%. For liquid-phase ELISA competition experiments, sera from cat-allergic patients (n = 12) were diluted 1 : 10 in TBST and were pre-incubated with rFel d 1, M, MA, MB, MC and MF (10 μg/mL), respectively, and, for control purposes, with buffer alone or with the unrelated grass pollen allergen rPhl p 1 overnight at 4°C. Sera were then added to ELISA plates coated with rFel d 1, and ELISA was developed as described above. The percentage of inhibition of IgE binding was calculated as follows: 100−(ODA × 100)/ODB where ODA and ODB represent the OD values after pre-incubation with the antigen or the buffer (TBS-T), respectively. To calculate the amount of Fel d 1 needed for 50% of inhibition, sera of five patients were pre-incubated with raising amounts of rFel d 1 overnight at 4°C, then added to ELISA plates coated with Fel d 1 and developed as described above.
Peripheral blood mononuclear cells (PBMCs) were obtained from cat-allergic patients and cultured as described [26]. Cells were stimulated with different concentrations of rFel d 1 (2 + 1), rFel d 1 MB, MC, MF (5 and 1.25 μg per well) or with equimolar amounts of rFel d 1, MB, MC, MF and peptides 1–8 (0.132 nmol). For control, 4 U Interleukin-2 per well (Boehringer Mannheim, Germany) or medium alone was tested. Mean cpm values were calculated from the triplicates, and stimulation indices (SI) were calculated as the quotient of the cpm obtained for the antigen-stimulated cultures and medium control [26].
In vitro basophil activation tests were performed with heparinized peripheral blood from nine patients with cat allergy. Blood samples (100 μL) were incubated with serial dilutions of rFel d 1 (2 + 1), M, MA, MB, MC, MB+C, MF (0.01–0.00001 mg/mL), a monoclonal anti-IgE antibody (1 μg/mL; Immunotech, Marseille, France) or with PBS for 15 min at 37°C. CD203c expression on basophils was measured by flow cytometry with the phycoerythrin-conjugated mAb 97A6 (CD203c). Allergen-induced upregulation of CD203c on basophils was expressed as the stimulation index, as described previously [34].
To compare IgE reactivity with allergenic activity for each of the 21 patients studied, rat basophil leukemia cells (RBL) expressing human high-affinity IgE receptor FcεRI [35] (1 × 105/well) were loaded overnight with sera from the 21 cat-allergic patients and, for control purposes, with serum from one non-allergic individual at a dilution 1 : 30. Cells were washed three times with Tyrode’s buffer (Sigma, Vienna, Austria) and exposed to serial dilutions of allergen (0.0001, 0.001, 0.01 and 0.1 μg/mL of rFel d 1 (2 + 1), MB + MC and MF, respectively) for 1 h. Supernatants were analysed for β-hexosaminidase activity as described previously [36]. Experiments were carried out in triplicates, and results are presented as mean percentages of total β-hexosaminidase released after addition of 10% Triton X-100 ± SE of the mean (SEM).
Immunization of rabbits, determination of IgG antibody titres and epitope maping
Rabbits were immunized with 200 μg of rFel d 1 (2 + 1), M, MB, MC, MF and MB + C in equimolar amounts using Freund’s complete adjuvant, followed by two booster injections with 200 μg of the immunogens using Freund’s incomplete adjuvant (first booster injection after 4 weeks and a second booster injection after 7 weeks; Charles River Breeding Laboratories, Kisslegg, Germany) [26]. For immunization with aluminium hydroxide Al(OH)3, two rabbits were immunized with 200 μg of rFel d 1 and two with 200 μg of Fel d 1 MF (booster injections after 4, 7, 9 and 12 weeks). Pre-immune sera were obtained from the rabbits before immunization.
Rabbit immune responses were analysed by ELISA titrations. For the measurement of specific rabbit IgG antibodies, ELISA plates were coated overnight with 5 μg/mL rFel d 1. After blocking, the plates were incubated overnight with serial dilutions of the corresponding rabbit antisera, or the corresponding pre-immune sera (1 : 1000, 1 : 5000, 1 : 10 000, 1 : 20 000 and 1 : 50 000). Bound rabbit IgG antibodies were detected with a 1 : 1000 diluted horseradish peroxidase–labelled donkey anti-rabbit IgG antiserum (Amersham Biosciences, Little Chalfont, UK).
Epitope mapping was performed by direct ELISA. Synthetic peptides P1–P8 were coated on the ELISA plates at a concentration of 2 μg/mL, and after washing and blocking, 1 : 1000 diluted antisera were added, and bound rabbit IgG was detected with HRP-labelled donkey anti-rabbit IgG.
ELISA competition assay for analysing the inhibition of human IgE binding to rFel d 1 by IgG specific for the hypoallergenic Fel d 1 derivative
ELISA plates (Nunc, Roskilde, Denmark) coated overnight with 1 μg/mL rFel d 1 were pre-incubated for 24 h with each of the anti-hypoallergenic Fel d 1 derivatives antisera, anti-rFel d 1 antiserum, or, for control purposes, with the corresponding pre-immune sera (dilutions: 1 : 50 for CFA-immunized rabbits or 1 : 5 with Al(OH)3-immunized rabbits, respectively). After overnight incubation with sera from cat-allergic patients (diluted 1 : 10), bound IgE antibodies were detected with the horseradish peroxidase–labelled goat anti-human IgE antibodies (KPL). The percentage reduction in IgE binding achieved by means of pre-incubation with rabbit antisera was calculated as follows: 100−(ODI/ODP) × 100). ODI and ODP represent optical density values after pre-incubation with the rabbit immune serum or pre-immune serum, respectively.
Statistical analysis
For statistical analyses, the statistical program spss (2008; version 16.0 SPSS Inc, Chicago, USA) was used. P-values < 0.05 were considered significant. Differences between the groups are compared using Mann–Whitney U-test.
Results
Design of hypoallergenic rFel d 1 derivatives
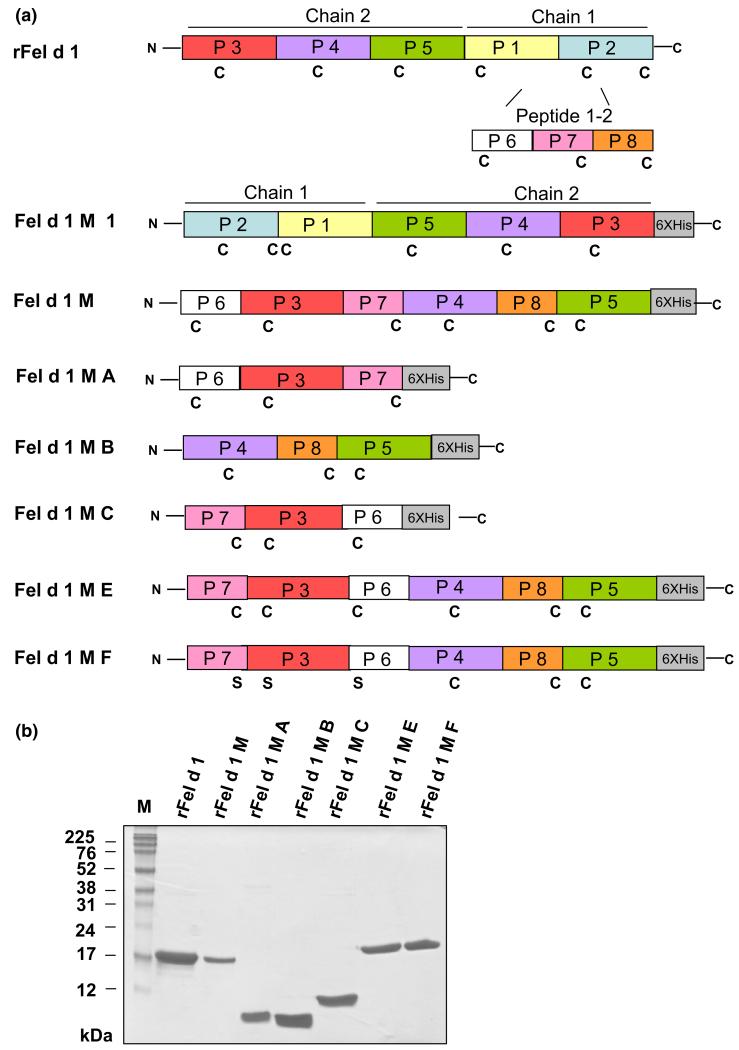
Fel d 1 peptides (Table S2: P1–P8; Fig. 1a) were shown to exhibit reduced IgE reactivity (Fig. 2, Table S3). To obtain hypoallergenic Fel d 1 molecules preserving Fel d 1-specific T cell epitopes, P1–P5 were recombined as mosaic derivatives of Fel d 1. As a fully IgE- and T cell-reactive reference molecule, we used rFel d 1 (2 + 1) in which chain 1 and chain 2 have been fused [11]. The first Fel d 1 derivative, Fel d 1 M1, was produced by engineering a fusion protein consisting of an N-terminal chain 1 in which the positions of P1 and P2 were exchanged and of an altered C-terminal chain 2 in which P3 and P5 had been exchanged (Fig. 1a). However, this derivative showed IgE reactivity comparable to rFel d 1 (2 + 1) and precipitated in biological buffers and therefore was not studied further (data not shown). We assumed that the reassembly of P1 and P2 might have restored IgE reactivity and therefore we divided chain 1 into three shorter peptides (peptides 6, 7 and 8; Table S2; Fig. 1a). The derivative Fel d 1 M was then obtained by reassembling P6-P3-P7-P4-P8-P5 from the N- to the C-terminus. As M exhibited residual IgE reactivity (Fig 2, Table S3) and allergenic activity, we thought to disrupt it by preparing the derivatives MA and MB consisting of P6-P3-P7 and P4-P8-P5 of M, respectively, as well as MC consisting of P7-P3-P6 (Fig. 1a). Interestingly, we observed that the residual allergenic activity resided in MA whereas MC showed lower allergenic activity although it consisted of the same peptides as MA, yet in another order. Finally, we recombined MC and MB to obtain a single hypoallergenic molecule containing all primary sequence elements of Fel d 1. For this purpose, ME consisting of a N-terminal MC and a C-terminal MB was generated, and MF which was identical to ME except that the three cysteines in MC were exchanged to serines (Fig. 1a).
Fig. 1.
Hypoallergenic Fel d 1 derivatives. (a) Representation of the recombinant Fel d 1 allergen and its mosaic derivatives. Peptides (P1–P8) used for construction of hypoallergenic Fel d 1 derivatives (Fel d 1 M1, M, MA, MB, MC, ME, MF) are indicated in rFel d 1 consisting of an N-terminal chain 2 and a C-terminal chain 1 in different colours. Cysteines (C), serines (S), N- and C-termini of proteins as well as C-terminal hexa-histidine tags (6XHis) are indicated. (b) Coomassie-stained SDS–PAGE of purified rFel d 1 and rFel d 1 derivatives. M, molecular weight marker (kDa).
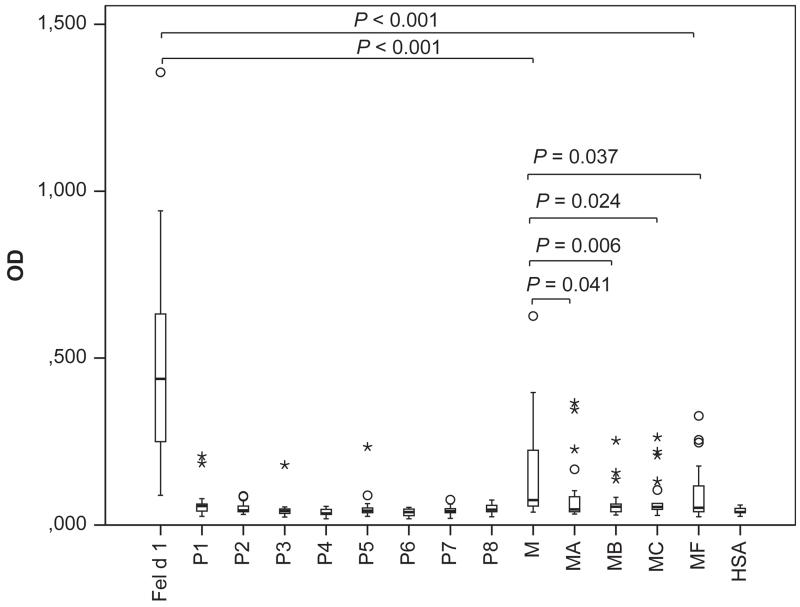
Fig. 2.
IgE reactivity of cat-allergic patients to Fel d 1, Fel d 1 peptides and derivatives as tested by ELISA in 21 patients. OD values correspond to bound IgE and are represented in box plots, where boxes mark the interquartile range containing 50% of the data and lines across the boxes indicate the median. ○ represent outliers and * are extreme values. P values are shown.
Expression, purification and structural characterization of recombinant Fel d 1 hypoallergens
The Fel d 1 mosaic derivatives (Fig. 1a) were expressed in E. coli under laboratory scale conditions (2–6 mg/L culture) and could be purified by affinity chromatography to more than 90% purity (Fig. 1b). The calculated mass for the Fel d 1 derivatives corresponded with the mass determined by matrix-assisted laser desorption/ionization time-of-flight analysis (i.e. M: 18 921.69 Da; MA: 10 384.06 Da; MB: 9501.92 Da; MC: 10 218.98 Da; ME: 18 755.62 Da; MF: 18 748.72 Da). CD analyses showed the altered fold of the mosaic derivatives when compared to rFel d 1 (Figure S1).
The analysis of a preparation of MF which had been stored for 28 months at −20°C showed no evidence for degradation of the protein when it was run on SDS–PAGE under reducing conditions but the protein occurred in the form of a dimer and higher oligomeric forms in non-reducing SDS–PAGE and in solution appeared as a tetramer and higher molecular weight aggregates as demonstrated by SEC (Figure S2).
rFel d 1 derivatives show reduced IgE reactivity and allergenic activity but retained T cell reactivity in comparison with Fel d 1
Serum IgE reactivity of the purified proteins was determined by means of direct ELISA (Fig. 2, Table S3). Each of the 21 patients showed IgE reactivity to Feld 1 while no reactivity to HSA was observed. Fel d 1 derivatives showed either complete loss of IgE reactivity in some patients or strong reduction in others. Fel d 1 M showed a significantly reduced IgE reactivity as compared to Fel d 1 (Fig. 2, P < 0.001). Each of the other mutants (i.e. MA, MB, MC and MF) showed an even more reduced IgE reactivity which was significantly reduced as compared to Fel d 1 M (Fig. 2). There was a consistent reduction in IgE reactivity for each of the tested patients (Table S3). Similar results were found for fluid-phase ELISA competition experiments (Table S4) where rFel d 1 almost completely inhibited IgE reactivity in sera of cat-allergic patients (n = 12) to rFel d 1 (i.e. 89% mean inhibition), whereas pre-incubation of sera with the hypoallergenic derivatives caused less than 16% mean inhibition of IgE reactivity to Fel d 1 (Mean inhibitions, M: 15%; MA: 12%; MB: 13%; MC: 16%; MF: 12%).
The fluid-phase inhibition was performed in antigen excess because a 50% inhibition of IgE reactivity to Fel d 1 was obtained with concentrations of rFel d 1 ranging from 0.0008 to 0.1 μg/mL (data not shown), and the inhibitions were performed with a concentration of 10 μg/mL of inhibitors.
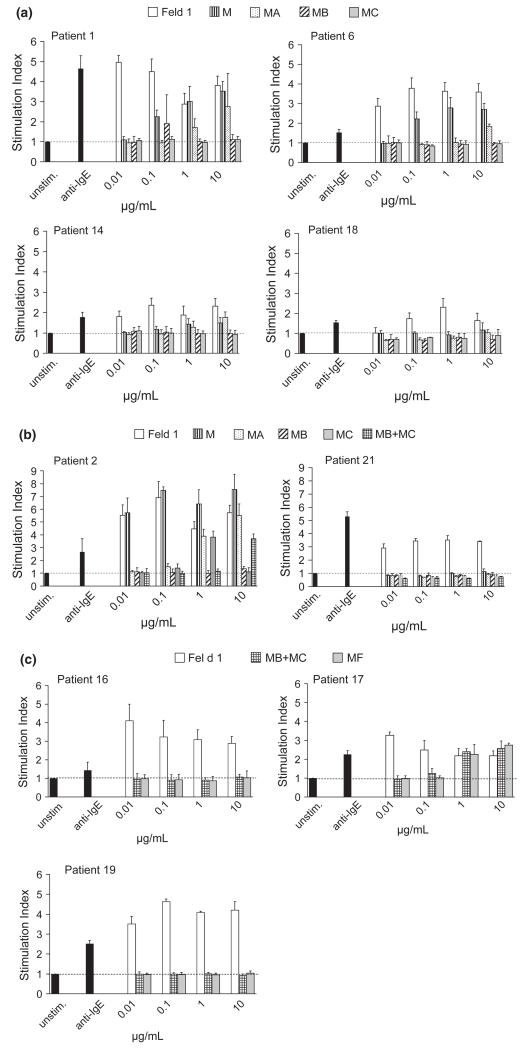
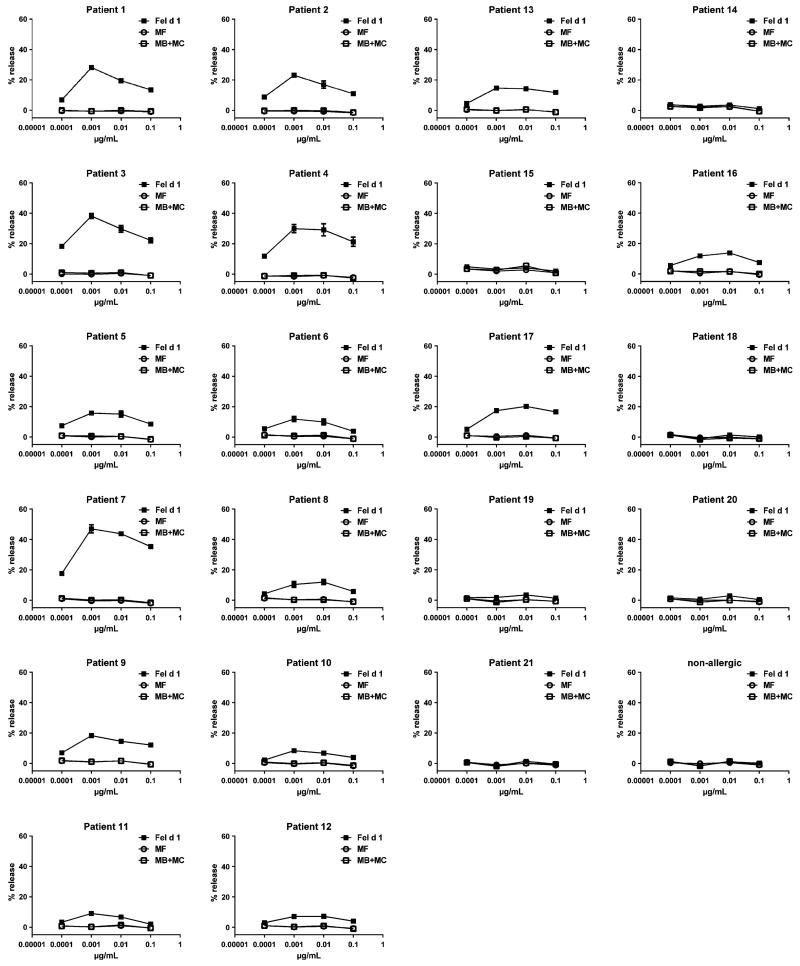
Next we investigated the allergenic activity of the Fel d 1 derivatives in basophil activation experiments (Fig. 3a). Fel d 1 MB and Fel d 1 MC did not cause a relevant upregulation of the CD203c expression up to the highest applied antigen concentration (i.e. 10 μg/mL) in the tested patients, whereas Fel d 1 started to induce upregulation at a concentration of 0.01 μg/mL in three out of four patients. In two patients, Fel d 1 M started to induce basophil activation at 0.1 μg/mL and Fel d 1 MA at 1 μg/mL. In a second set of experiments (Fig. 3b), we sought to additionally test equimolar mixes of fragments Fel d 1 MB and Fel d 1 MC and found a 1000-fold reduction in allergenic activity in comparison with rFel d 1. We then compared Fel d 1 MF with rFel d 1 and an equimolar mix of Fel d 1 MB and MC. The MB + MC mix as well as Fel d 1 MF showed an at least 100-fold reduced allergenic activity in comparison with rFel d 1 (Fig. 3c). The variants Fel d 1 M B, Fel d 1 MC and Fel d 1 MF which had shown the strongest reduction in allergenic activity were subjected to a detailed comparison with Fel d 1 using sera from all 21 cat-allergic patients by using rat basophil cells which had been transfected with the α-chain of the human Fcε receptor (Fig. 4). MB + MC and MF did not induce any relevant basophil activation, whereas rFel d 1 wild type induced a dose-dependent activation in 15 of the 21 patients indicating a more than 1000-fold reduction in allergenic activity of the derivatives as compared to Fel d 1 (Fig. 4).
Fig. 3.
Allergenic activity of rFel d 1 and rFel d 1 derivatives as determined by basophil activation. Basophils from cat-allergic patients were exposed to increasing concentrations (0.01–10 μg/mL) of rFel d 1 and equimolar concentrations of rFel d 1 derivatives (a: rFel d 1 M, MA, MB and MC). In (b), two patients were also tested with an equimolar mixture of MB plus MC, and in (c), the latter mix and MF was tested in three patients. Anti-IgE served as a positive control and unstimulated control were basophils stimulated with PBS only. The stimulation indices (SI) representing the upregulation of CD203c expression are shown on the y-axes. SI was calculated as a ratio of mean fluorescence intensities of stimulated and unstimulated basophils and is displayed as means ± standard deviation (SD) of triplicates.
Fig. 4.
Allergenic activity of rFel d 1 and rFel d 1 derivatives as determined using rat basophil leukemia cells (RBL) transfected with human FcεRI. RBL cells were loaded with sera of cat-allergic patients (1–21) or with serum from a non-allergic person and were then exposed to increasing concentrations (0.0001, 0.001, 0.01 and 0.1 μg/mL) of rFel d 1, or equimolar concentrations of rFel d 1 derivatives (rFel d 1 MB + MC, Fel d 1 MF). β-hexosaminidase releases are displayed as percentages of total β-hexosaminidase on the y-axes ± standard error mean (SEM) of triplicates.
Since Fel d 1 MB, MC and MF showed the greatest reduction in allergenic activity, we tested these molecules regarding the presence of T cell epitopes. When PBMC from two cat-allergic patients 21 and 16 (Table S1) were exposed to two concentrations of each of the molecules, we found that the hypoallergenic derivatives Fel d 1 MB, MC and MF induced comparable (patient 21: SI 0.25 μg/mL: Fel d 1: 2.6; Fel d 1 MB: 2.9; Fel d 1 MC: 2.7; Fel d 1 MF: 3.7; SI 1 μg/mL: Fel d 1: 3.1; Fel d 1 MB: 2.8; Fel d 1 MC: 3.0; Fel d 1 MF: 3.6) or even stronger (patient 16: SI 0.25 μg/mL: Fel d 1: 3.3; Fel d 1 MB: 5.3; Fel d 1 MC: 7.7; Fel d 1 MF: 7.0; SI 1 μg/mL: Fel d 1: 2.8; Fel d 1 MB: 8.9; Fel d 1 MC: 8.6; Fel d 1 MF: 7.4) T cell proliferation compared to Fel d 1 wild type indicating that Fel d 1-specific T cell epitopes were retained. To further compare the derivatives with the rFel d 1 wild type for T cell activation and to study T cell-reactive peptides, PBMC from three additional patients were exposed to the equimolar concentrations of Fel d 1 derivatives and peptides. Table S5 shows that peptides 1, 2, 3, 4, 6, 7 and 8 induced T cell proliferation (SI = or > 1.5) in PBMCs from patients with cat allergy. MF induced T cell proliferation in each of the tested patients.
Immunization with Fel d 1 derivatives induces IgG antibodies that recognize rFel d 1 and inhibit patients’ IgE binding to rFel d 1
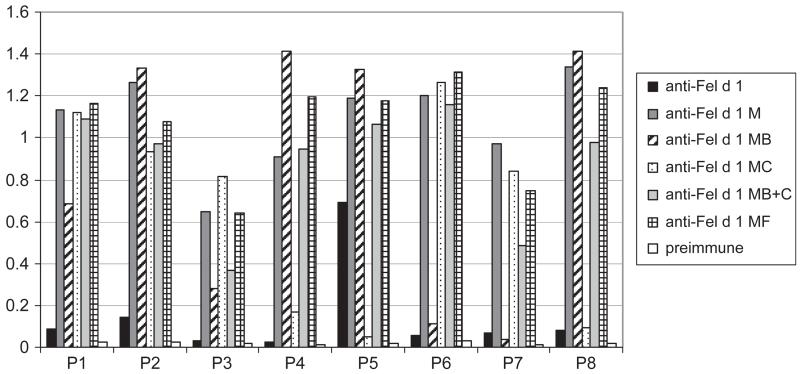
As shown in Figure S3, the immunization with Fel d 1 derivatives (i.e. Fel d 1 M, Fel d 1 MF, Fel d 1 MB and MC, mix of Fel d 1 MB and MC) induced in rabbits Fel d 1-specific IgG antibody responses. In the individual rabbits, antibody responses were comparable to those obtained with rFel d 1 except for the two rabbits which were immunized with Fel d 1 MB and MC, respectively. In these rabbits, antibody responses were lower than the ones induced with rFel d 1 (Figure S3). Interestingly, rabbit antibodies induced with the Fel d 1 derivatives recognized more synthetic Fel d 1 peptides and also with greater intensity than rabbit antibodies induced with rFel d 1 wild type (Fig. 5).
Fig. 5.
Reactivity of rabbit anti-Fel d 1 and anti-Fel d 1 variant antibodies with Fel d 1-derived peptides. Shown are the OD values corresponding to bound IgG antibodies (y-axis) specific for synthetic Fel d 1 peptides (P1–P8) for the individual antisera.
We then investigated whether Fel d 1 mosaic derivative–induced rabbit IgG antibodies can inhibit the binding of cat-allergic patients’ serum IgE to the wild-type rFel d 1 in an ELISA competition assay. Antibodies induced by immunization with rFel d 1 derivatives strongly inhibited patients’ IgE reactivity to Fel d 1 (Table S6). Anti–rFel d 1 M antibodies inhibited the binding of patients IgE to rFel d 1 between 36.9% and 90.3% (67.8% mean inhibition) which was close to the inhibition obtained with anti-rFel d 1 antibodies (56.6% and 88.1%, mean inhibition 74.9%; Table S6). The anti–rFel d 1 MB-antiserum showed inhibition rates between 36.9% and 77.7% (56.6% mean inhibition), and the anti-rFel d 1 MC-antiserum inhibited between 56.8% and 86.7% (73.7% mean inhibition). Interestingly, the rabbit antibodies obtained by immunization with an equimolar mix of fragments MB and MC (anti-rFel d 1 MB + MC) showed comparable inhibition rates (72.3% mean inhibition) with antibodies against fragment C alone (anti-Fel d 1 MC, 73.7%). Anti-Fel d 1 MF antibodies inhibited IgE binding to Fel d 1 between 61.6% and 89.9% (mean 70.9%) which was comparable to the inhibition achieved with anti-rFel d 1 antibodies. As CFA cannot be used in patients, two rabbits were immunized with Fel d 1 and two rabbits with Fel d 1 MF using alum, an adjuvant which is frequently used for SIT. We found that anti-Fel d 1 MF antibodies inhibited the binding of the patients’ sera similar to antibodies raised with Fel d 1 (Table 1).
Table 1.
Inhibition of allergic patients’ IgE reactivity to Fel d 1 by IgG antibodies raised with Al(OH)3-adsorbed proteins
| Anti-Fel d 1 |
Anti-Fel d 1MF |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rabbit 1 |
Rabbit 2 |
Rabbit 1 |
Rabbit 2 |
|||||||||
| Patient | Pre | Anti-Fel d 1 | % inh | Pre | Anti-Fel d 1 | % inh | Pre | Anti-Fel d 1MF | % inh | Pre | Anti-Fel d 1MF | % inh |
| 1 | 0.580 | 0.036 | 94 | 0.595 | 0.044 | 93 | 0.600 | 0.055 | 91 | 0.648 | 0.111 | 83 |
| 2 | 0.587 | 0.052 | 91 | 0.607 | 0.050 | 92 | 0.650 | 0.076 | 88 | 0.671 | 0.086 | 87 |
| 3 | 0.914 | 0.068 | 92 | 0.939 | 0.071 | 92 | 0.970 | 0.125 | 87 | 1.041 | 0.171 | 84 |
| 4 | 0.931 | 0.055 | 94 | 0.902 | 0.069 | 92 | 0.950 | 0.080 | 92 | 0.984 | 0.157 | 84 |
| 5 | 0.726 | 0.047 | 93 | 0.690 | 0.046 | 93 | 0.739 | 0.066 | 91 | 0.778 | 0.096 | 88 |
| 6 | 0.832 | 0.042 | 95 | 0.810 | 0.050 | 94 | 0.890 | 0.092 | 90 | 0.897 | 0.136 | 85 |
| 7 | 0.818 | 0.069 | 92 | 0.814 | 0.067 | 92 | 0.847 | 0.100 | 88 | 0.868 | 0.161 | 81 |
| 8 | 0.528 | 0.034 | 93 | 0.518 | 0.028 | 95 | 0.581 | 0.043 | 93 | 0.654 | 0.069 | 89 |
| 9 | 0.518 | 0.030 | 94 | 0.546 | 0.028 | 95 | 0.560 | 0.039 | 93 | 0.600 | 0.068 | 89 |
| 10 | 0.506 | 0.039 | 92 | 0.492 | 0.037 | 92 | 0.517 | 0.049 | 91 | 0.510 | 0.067 | 87 |
| 11 | 0.321 | 0.038 | 88 | 0.314 | 0.036 | 89 | 0.337 | 0.045 | 87 | 0.331 | 0.052 | 84 |
| 12 | 0.387 | 0.033 | 91 | 0.391 | 0.034 | 91 | 0.406 | 0.053 | 87 | 0.419 | 0.086 | 79 |
| 13 | 1.065 | 0.107 | 90 | 1.075 | 0.112 | 90 | 1.125 | 0.135 | 88 | 1.118 | 0.199 | 82 |
| 14 | 0.070 | 0.039 | 44 | 0.069 | 0.033 | 52 | 0.077 | 0.038 | 51 | 0.073 | 0.037 | 49 |
| 15 | 0.110 | 0.035 | 68 | 0.097 | 0.035 | 64 | 0.111 | 0.035 | 68 | 0.123 | 0.045 | 63 |
| 16 | 0.692 | 0.037 | 95 | 0.673 | 0.041 | 94 | 0.708 | 0.049 | 93 | 0.718 | 0.074 | 90 |
| 17 | 0.588 | 0.041 | 93 | 0.571 | 0.039 | 93 | 0.625 | 0.050 | 92 | 0.631 | 0.081 | 87 |
| 18 | 0.820 | 0.053 | 94 | 0.803 | 0.057 | 93 | 0.874 | 0.077 | 91 | 0.872 | 0.105 | 88 |
| 19 | 0.322 | 0.037 | 89 | 0.319 | 0.033 | 90 | 0.327 | 0.034 | 90 | 0.318 | 0.066 | 79 |
| 20 | 0.186 | 0.025 | 87 | 0.175 | 0.024 | 86 | 0.201 | 0.025 | 88 | 0.197 | 0.036 | 82 |
| 21 | 0.292 | 0.035 | 88 | 0.283 | 0.035 | 88 | 0.309 | 0.039 | 87 | 0.315 | 0.067 | 79 |
| Mean | 0.562 | 0.045 | 88 | 0.556 | 0.046 | 89 | 0.591 | 0.062 | 87 | 0.608 | 0.094 | 82 |
rFel d 1 was pre-incubated with rabbit IgG antibodies raised against Al(OH)3-adsorbed rFel d 1 or rFel d 1 MF or the corresponding rabbit pre-immune sera (pre) and then exposed to sera from 21 allergic patients. Two rabbits per allergen were immunized. Shown are OD values corresponding to bound IgE antibodies and the percentages of inhibition of IgE binding.
Discussion
In this study, we have used a combination of two strategies to reduce IgE reactivity and allergenic activity of an important respiratory allergen, the major cat allergen, Fel d 1. In a first set of experiments, we used rational molecular re-assembly of hypoallergenic fragments of Fel d 1 to identify recombinant hypoallergenic derivatives by IgE testing and analysis for allergenic activity using patients basophils. To improve solubility of the hypoallergenic mosaic protein which contained the entire primary sequence of Fel d 1 (i.e. Fel d 1 ME), we exchanged cysteines in its N-terminal building block Fel d 1 MC with serine residues. The latter manipulation reduced the aggregation of the recombinant mosaic protein which most likely had occurred by intermolecular disulphide bond formation. The strategy of molecular reassembly was based on previous IgE epitope mapping data where five hypoallergenic Fel d 1 peptides were identified [26] and which served in the initial constructs in a re-assembled form. After the initial IgE reactivity tests, the peptides were modified (i.e. shortened/prolonged) to further reduce the allergenic activity of the constructs. It was previously demonstrated for important respiratory allergens from grass pollen Phl p 2 [37] and Phl p 12 [38] that simple head to tail restructuring of non-IgE reactive fragments leads to hypoallergenic molecules. In the case of Fel d 1, this approach was not applicable as derivative Fel d 1 M1 showed IgE reactivity almost comparable to rFel d 1. Therefore, a more thorough restructuring was required to obtain a suitable hypoallergenic derivative which was based on further fragmentation of chain 1 and a rearrangement of these new fragments. One of the reasons for the persistence of IgE reactivity of Fel d 1 M1 could be the fact that Fel d 1 contains in addition to conformational also some sequential IgE epitopes. In fact, IgE epitopes as short as 14 amino acids have been reported [39]. Natural Fel d 1 consists of two chains which are linked by three disulphide bonds. They are formed between Cys3 of chain 1 and Cys73 of chain 2, Cys44 of chain 1 and Cys48 of chain 2, and between Cys70 of chain 1 and Cys7 of chain 2 [10]. It was previously reported that exchange of cysteines to serines contributes to solubility and reduced IgE reactivity of some respiratory allergens [24, 40]. As derivative ME containing all six cysteins showed only limited solubility, three of its cysteins were exchanged with serines (Cys 3 and Cys 44 of chain 1 and Cys 7 of chain 2) taking care that only one cysteine residue from each pair constituting disulfide bonds was exchanged to minimize divergence from the original Fel d 1 amino acid sequence. The resulting derivative MF indeed showed enhanced solubility in comparison with ME and could be well expressed and purified. Even after a storage of 28 months at −20°C, Fel d 1 MF showed no signs of degradation. However, the protein occurred as tetramer and in the form of high molecular weight aggregates. The latter does not speak against the use of MF for SIT because also allergoids which are registered for SIT represent denatured allergen extracts occuring in the form of high molecular weight aggregates, and a recombinant trimer of Bet v 1 which forms aggregates has been used in clinical SIT trials [32, 41]. Quite on the contrary, aggregated allergen derivatives such as the trimeric Bet v 1 were highly immunogenic and induced high titres of allergen-specific blocking antibodies. However, it will be necessary to develop GMP conditions which allow monitoring and standardizing the aggregation behaviour and the immunogenic as well as allergenic properties of the protein.
In total, we produced seven rFel d 1 derivatives of which five showed reduced IgE reactivity in liquid phase in comparison with rFel d 1 (2 + 1) (i.e. M, MA, MB, MC and MF). Basophil activation assay experiments and immunization experiments finally enabled us to select three candidate molecules: MB, MC and MF showed higher reduction in allergenic activity compared to the constructs M and MA. We observed an at least 100-fold reduction in allergenic activity for MB, MC and MF compared with that of wild-type rFel d 1 indicating that allergic patients should theoretically tolerate an up to 100-fold higher dose of these hypoallergens compared with Fel d 1 in the course of SIT.
In addition to hypoallergenicity, the ability to induce high levels of allergen-specific blocking IgG antibodies seems to be important for the suitability of a molecule which is used for subcutaneous immunotherapy [42–44]. Interestingly, when we performed immunization experiments of rabbits with our candidate molecules, MC showed the highest capacity of inducing allergen-specific blocking IgG although it contained only approximately half of the Fel d 1 sequence. Yet, the advantage of MF over MC or a mix of MB and MC is that it combines the whole Fel d 1 sequence within one protein. This property may to some degree be dispensable for a vaccination approach aiming mainly at the induction of allergen-specific blocking IgG antibodies, but for the induction of tolerance, a complete representation of T cell epitopes may be desired.
In this context, it has been demonstrated that it is quite difficult to make peptide-based tolerogenic vaccines because they need to contain a broad variety of many peptides to cover the major T cell epitopes of an allergen as demonstrated recently [23]. The latter was also confirmed in our study because we found that T cells from cat-allergic patients recognize several different epitopes (Table S5). Most of these sequences are part of MF, and we could show that MF induces comparable T cell proliferation as Fel d 1 wild type. MF may therefore be used to develop a tolerogenic vaccine based on one single recombinant protein. Moreover, MF, MC or a combination of MC and MB may also be used for the development of vaccines inducing high levels of allergen-specific IgG antibodies. Fel d 1 hypoallergens have been reported in other studies [24, 27, 45] but the reduction in their IgE reactivity and allergenic activity was much lower than that of the hypoallergenic derivatives described here. For example, three Fel d 1 mutants rFel d 1 (DTE I), rFel d 1 (DTE II) and rFel d 1 (DTE III) [24] started to induce catallergic patient’s basophil activation already at the concentration of 0.01 μg/mL while our best molecules (MB, MC and MF) started to induce basophil activation at concentrations of 1 μg/mL indicating that they are at least 100-fold more hypoallergenic. Likewise, our recombinant mosaic proteins appear to be less allergenic than the MAT-Fel d 1 construct which exhibits a 100-fold reduced allergenic activity as compared to wild type Fel d 1 [30]. Another approach to produce hypoallergenic cat vaccines describes chemical coupling of intact rFel d 1 to virus-like particles [27]. Although this vaccine showed high immunogenicity and reduced allergenicity in mouse model, it induced activation of human basophils already at concentration of 0.001 μg/mL. Another form of immunotherapy based on a chimeric fusion protein comprised of human Fcγ and intact Fel d 1 has been proposed by Zhu et al. [28]. They could show that Fcγ-Fel d 1 inhibited degranulation of human basophils in vitro as well as in sensitized human mast cells in vivo in an allergen-specific manner. Nevertheless, repeated administration of this molecule might induce autoantibodies against human Fcγ.
Fel d 1-PreS [26] a previously developed peptide carrier fusion protein was efficient in inducing allergen-specific blocking IgG antibodies but it contained only ~40% of Fel d 1 sequence and therefore cannot be used for direct induction of tolerance in T cells via the T cell receptor. As animal models, in particular murine models for cat allergy are based on artificial sensitization protocols and IgE and T cell epitope recognition in such models is different from that in allergic patients, next steps in evaluating our vaccine will be skin test studies in allergic patients to assess in vivo the allergenic activity of the components followed by safety and dose finding SIT studies in patients.
In conclusion, we have engineered and characterized hypoallergenic Fel d 1 derivatives which should be useful for several therapeutic or preventive approaches for cat allergy. These approaches include therapeutic and prophylactic tolerance induction as well as vaccination concepts.
Supplementary Material
Figure S1. Comparison of rFel d 1 and hypoallergenic derivatives by circular dichroism. The mean residue molar ellipticities for rFel d 1 and rFel d 1 derivatives (y-axis: degree cm2 dmol−1) were recorded for a range of wavelengths (x-axis).
Figure S2. (a) SDS–PAGE containing MF under non-reducing and reducing conditions. Lanes M and MF represent the molecular weight marker and purified MF which had been stored for 28 months. Molecular weights are indicated on the left margins. (b) Size exclusion (SEC) profile of MF. The positions of the standards (i.e. dextran blue: 2000 kDa; BSA: 66 kDa; cytochrome c: 12.4 kDa) are indicated, elution volumes (mL) are on the x-axis and the y-axis shows the absorbances at 280 nm.
Figure S3. Titration of IgG antibodies specific for rFel d 1. Different dilutions (x-axis) of antisera obtained by immunization of rabbits with rFel d 1(▬) or Fel d 1 derivatives (Fel d 1 M: ■; MB: ▲: MC: −; mix of MB + MC: ×; MF: ●) were reacted with ELISA plate-bound rFel d 1. OD values corresponding to bound antibodies are shown on the y-axis. PI: Pre-immune serum.
Table S1. Demographic, clinical and serological characteristics of cat-allergic patients.
Table S2. Characteristics of Fel d 1-derived peptides which were used as building blocks for the derivatives.
Table S3. IgE reactivity of sera from patients with cat allergy to Fel d 1, Feld 1-derived peptides and mosaic derivatives.
Table S4. Percentage inhibition of IgE binding to rFel d 1 after pre-adsorption of patients’ sera with rFel d 1, hypoallergenic Fel d 1 derivatives or rPhl p 1.
Table S5. T cell proliferative responses in cat-allergic patients.
Table S6. Inhibition of allergic patients’ IgE reactivity to Fel d 1 by IgG antibodies.
Acknowledgements
We thank Prof. Oswald Wagner, Department of Laboratory Medicine, Medical University of Vienna for his support. This study was supported by the SFB program F46 of the Austrian Science Fund (FWF) grants F4605, F4611; by research grants from Biomay, Vienna, Austria, and the Christian Doppler Research Association, Austria; and by the Swedish Research Council and Swedish Asthma and Allergy Association’s Research Foundation.
Footnotes
Conflict of interest
Rudolf Valenta has acted as a paid consultant to Biomay, Vienna, Austria and Thermofisher, Uppsala, Sweden, and has received funding for research carried out in this study. Other authors declare no conflict of interest.
Additional Supporting Information may be found in the online version of this article:
References
- 1.Perzanowski MS, Ronmark E, Nold B, Lundback B, Platts-Mills TA. Relevance of allergens from cats and dogs to asthma in the northernmost province of Sweden: schools as a major site of exposure. J Allergy Clin Immunol. 1999;103:1018–24. doi: 10.1016/s0091-6749(99)70173-9. [DOI] [PubMed] [Google Scholar]
- 2.Burbach GJ, Heinzerling LM, Edenharter G, et al. GA(2)LEN skin test study II: clinical relevance of inhalant allergen sensitizations in Europe. Allergy. 2009;64:1507–15. doi: 10.1111/j.1398-9995.2009.02089.x. [DOI] [PubMed] [Google Scholar]
- 3.Grönlund H, Saarne T, Gafvelin G, van Hage M. The major cat allergen, Fel d 1, in diagnosis and therapy. Int Arch Allergy Immunol. 2010;151:265–74. doi: 10.1159/000250435. [DOI] [PubMed] [Google Scholar]
- 4.van Ree R, van Leeuwen WA, Bulder I, Bond J, Aalberse RC. Purified natural and recombinant Fel d 1 and cat albumin in in vitro diagnostics for cat allergy. J Allergy Clin Immunol. 1999;104:1223–30. doi: 10.1016/s0091-6749(99)70017-5. [DOI] [PubMed] [Google Scholar]
- 5.Kleine-Tebbe J, Kleine-Tebbe A, Jeep S, Schou C, Løwenstein H, Kunkel G. Role of the major allergen (Fel d I) in patients sensitized to cat allergens. Int Arch Allergy Immunol. 1993;100:256–62. doi: 10.1159/000236421. [DOI] [PubMed] [Google Scholar]
- 6.Kaiser L, Grönlund H, Sandalova T, et al. The crystal structure of the major cat allergen Fel d 1, a member of the secretoglobin family. J Biol Chem. 2003;278:37730–5. doi: 10.1074/jbc.M304740200. [DOI] [PubMed] [Google Scholar]
- 7.Kristensen AK, Schou C, Roepstorff P. Determination of isoforms, N-linked glycan structure and disulfide bond linkages of the major cat allergen Fel d 1 by a mass spectrometric approach. Biol Chem. 1997;378:899–908. doi: 10.1515/bchm.1997.378.8.899. [DOI] [PubMed] [Google Scholar]
- 8.Duffort OA, Carreira J, Nitti G, Polo F, Lombardero M. Studies on the biochemical structure of the major cat allergen Felis domesticus I. Mol Immunol. 1991;28:301–9. doi: 10.1016/0161-5890(91)90141-6. [DOI] [PubMed] [Google Scholar]
- 9.Morgenstern JP, Griffith IJ, Brauer AW, et al. Amino acid sequence of Fel d I, the major allergen of the domestic cat: protein sequence analysis and cDNA cloning. Proc Natl Acad Sci USA. 1991;88:9690–4. doi: 10.1073/pnas.88.21.9690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bond JF, Brauer AW, Segal DB, Nault AK, Rogers BL, Kuo MC. Native and recombinant Fel d 1 as probes into the relationship of allergen structure to human IgE immunoreactivity. Mol Immunol. 1993;30:1529–41. doi: 10.1016/0161-5890(93)90461-j. [DOI] [PubMed] [Google Scholar]
- 11.Grönlund H, Bergman T, Sandström K, et al. Formation of disulfide bonds and homodimers of the major cat allergen Fel d 1 equivalent to the natural allergen by expression in Escherichia coli. J Biol Chem. 2003;278:40144–51. doi: 10.1074/jbc.M301416200. [DOI] [PubMed] [Google Scholar]
- 12.Durham SR, Walker SM, Varga EM, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–75. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 13.Larche M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–71. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 14.Taylor WW, Ohman JL, Jr, Lowell FC. Immunotherapy in cat-induced asthma. Double-blind trial with evaluation of bronchial responses to cat allergen and histamine. J Allergy Clin Immunol. 1978;61:283–7. doi: 10.1016/0091-6749(78)90048-9. [DOI] [PubMed] [Google Scholar]
- 15.Van Metre TE, Jr, Marsh DG, Adkinson NF, Jr, et al. Immunotherapy decreases skin sensitivity to cat extract. J Allergy Clin Immunol. 1989;83:888–99. doi: 10.1016/0091-6749(89)90102-4. [DOI] [PubMed] [Google Scholar]
- 16.Hedlin G, Graff-Lonnevig V, Heilborn H, et al. Immunotherapy with cat- and dog-dander extracts. V. Effects of 3 years of treatment. J Allergy Clin Immunol. 1991;87:955–64. doi: 10.1016/0091-6749(91)90417-m. [DOI] [PubMed] [Google Scholar]
- 17.Varney VA, Edwards J, Tabbah K, Brewster H, Mavroleon G, Frew AJ. Clinical efficacy of specific immunotherapy to cat dander: a double-blind placebo-controlled trial. Clin Exp Allergy. 1997;27:860–7. [PubMed] [Google Scholar]
- 18.Nanda A, O’Connor M, Anand M, et al. Dose dependence and time course of the immunologic response to administration of standardized cat allergen extract. J Allergy Clin Immunol. 2004;114:1339–44. doi: 10.1016/j.jaci.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 19.Mellerup MT, Hahn GW, Poulsen LK, Malling H. Safety of allergen-specific immunotherapy. Relation between dosage regimen, allergen extract, disease and systemic side-effects during induction treatment. Clin Exp Allergy. 2000;30:1423–9. doi: 10.1046/j.1365-2222.2000.00910.x. [DOI] [PubMed] [Google Scholar]
- 20.Norman PS, Ohman JL, Jr, Long AA, et al. Treatment of cat allergy with T-cell reactive peptides. Am J Respir Crit Care Med. 1996;154:1623–8. doi: 10.1164/ajrccm.154.6.8970345. [DOI] [PubMed] [Google Scholar]
- 21.Simons FE, Imada M, Li Y, Watson WT, HayGlass KT. Fel d 1 peptides: effect on skin tests and cytokine synthesis in cat-allergic human subjects. Int Immunol. 1996;8:1937–45. doi: 10.1093/intimm/8.12.1937. [DOI] [PubMed] [Google Scholar]
- 22.Oldfield WL, Larche M, Kay AB. Effect of T-cell peptides derived from Fel d 1 on allergic reactions and cytokine production in patients sensitive to cats: a randomised controlled trial. Lancet. 2002;360:47–53. doi: 10.1016/s0140-6736(02)09332-7. [DOI] [PubMed] [Google Scholar]
- 23.Worm M, Lee HH, Kleine-Tebbe J, et al. Development and preliminary clinical evaluation of a peptide immunotherapy vaccine for cat allergy. J Allergy Clin Immunol. 2011;127:89–97. doi: 10.1016/j.jaci.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 24.Saarne T, Kaiser L, Grönlund H, Rasool O, Gafvelin G, van Hage-Hamsten M. Rational design of hypoallergens applied to the major cat allergen Fel d 1. Clin Exp Allergy. 2005;35:657–63. doi: 10.1111/j.1365-2222.2005.02234.x. [DOI] [PubMed] [Google Scholar]
- 25.Saarne T, Neimert-Andersson T, Grönlund H, Jutel M, Gafvelin G, van Hage M. Treatment with a Fel d 1 hypoallergen reduces allergic responses in a mouse model for cat allergy. Allergy. 2011;66:255–63. doi: 10.1111/j.1398-9995.2010.02468.x. [DOI] [PubMed] [Google Scholar]
- 26.Niespodziana K, Focke-Tejkl M, Linhart B, et al. A hypoallergenic cat vaccine based on Fel d 1-derived peptides fused to hepatitis B PreS. J Allergy Clin Immunol. 2011;127:1562–70. doi: 10.1016/j.jaci.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitz N, Dietmeier K, Bauer M, et al. Displaying Fel d 1 on virus-like particles prevents reactogenicity despite greatly enhanced immunogenicity: a novel therapy for cat allergy. J Exp Med. 2009;206:1941–55. doi: 10.1084/jem.20090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu D, Kepley CL, Zhang K, Terada T, Yamada T, Saxon A. A chimeric human-cat fusion protein blocks cat-induced allergy. Nat Med. 2005;11:446–9. doi: 10.1038/nm1219. [DOI] [PubMed] [Google Scholar]
- 29.Grundström J, Neimert-Andersson T, Kemi C, et al. Covalent coupling of vitamin D3 to the major cat allergen Fel d 1 improves the effects of allergen-specific immunotherapy in a mouse model for cat allergy. Int Arch Allergy Immunol. 2012;157:136–46. doi: 10.1159/000327546. [DOI] [PubMed] [Google Scholar]
- 30.Martínez-Gómez JM, Johansen P, Rose H, et al. Targeting the MHC class II pathway of antigen presentation enhances immunogenicity and safety of allergen immunotherapy. Allergy. 2009;64:172–8. doi: 10.1111/j.1398-9995.2008.01812.x. [DOI] [PubMed] [Google Scholar]
- 31.Focke M, Mahler V, Ball T, et al. Non-anaphylactic synthetic peptides derived from B cell epitopes of the major grass pollen allergen, Phl p 1, for allergy vaccination. FASEB J. 2001;15:2042–4. doi: 10.1096/fj.01-0016fje. [DOI] [PubMed] [Google Scholar]
- 32.Campana R, Vrtala S, Maderegger B, et al. Altered IgE epitope presentation: a model for hypoallergenic activity revealed for Bet v 1 trimer. Mol Immunol. 2011;48:431–41. doi: 10.1016/j.molimm.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitmore L, Wallace BA. DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 2004;32(Web Server issue):W668–73. doi: 10.1093/nar/gkh371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hauswirth AW, Natter S, Ghannadan M, et al. Recombinant allergens promote expression of CD203c on basophils in sensitized individuals. J Allergy Clin Immunol. 2002;110:102–9. doi: 10.1067/mai.2002.125257. [DOI] [PubMed] [Google Scholar]
- 35.Vogel L, Luttkopf D, Hatahet L, Haustein D, Vieths S. Development of a functional in vitro assay as a novel tool for the standardization of allergen extracts in the human system. Allergy. 2005;60:1021–8. doi: 10.1111/j.1398-9995.2005.00803.x. [DOI] [PubMed] [Google Scholar]
- 36.Hartl A, Hochreiter R, Stepanoska T, Ferreira F, Thalhamer J. Characterization of the protective and therapeutic efficiency of a DNA vaccine encoding the major birch pollen allergen Bet v 1a. Allergy. 2004;59:65–73. doi: 10.1046/j.1398-9995.2003.00335.x. [DOI] [PubMed] [Google Scholar]
- 37.Mothes-Luksch N, Stumvoll S, Linhart B, et al. Disruption of allergenic activity of the major grass pollen allergen Phl p 2 by reassembly as a mosaic protein. J Immunol. 2008;181:4864–73. doi: 10.4049/jimmunol.181.7.4864. [DOI] [PubMed] [Google Scholar]
- 38.Westritschnig K, Linhart B, Focke-Tejkl M, et al. A hypoallergenic vaccine obtained by tail-to-head restructuring of timothy grass pollen profilin, Phl p 12, for the treatment of cross-sensitization to profilin. J Immunol. 2007;179:7624–34. doi: 10.4049/jimmunol.179.11.7624. [DOI] [PubMed] [Google Scholar]
- 39.van Milligen FJ, van ‘t Hof W, van den Berg M, Aalberse RC. IgE epitopes on the cat (Felis domesticus) major allergen Fel d I: a study with overlapping synthetic peptides. J Allergy Clin Immunol. 1994;93:34–43. doi: 10.1016/0091-6749(94)90230-5. [DOI] [PubMed] [Google Scholar]
- 40.Smith AM, Chapman MD. Reduction in IgE binding to allergen variants generated by site-directed mutagenesis: contribution of disulfide bonds to the antigenic structure of the major house dust mite allergen Der p 2. Mol Immunol. 1996;33:399–405. doi: 10.1016/0161-5890(95)00150-6. [DOI] [PubMed] [Google Scholar]
- 41.Vrtala S, Hirtenlehner K, Susani M, et al. Genetic engineering of a hypoallergenic trimer of the major birch pollen allergen Bet v 1. FASEB J. 2001;15:2045–7. doi: 10.1096/fj.00-0767fje. [DOI] [PubMed] [Google Scholar]
- 42.Valenta R, Ferreira F, Focke-Tejkl M, et al. From allergen genes to allergy vaccines. Annu Rev Immunol. 2010;28:211–41. doi: 10.1146/annurev-immunol-030409-101218. [DOI] [PubMed] [Google Scholar]
- 43.Linhart B, Valenta R. Vaccines for allergy. Curr Opin Immunol. 2012;24:354–60. doi: 10.1016/j.coi.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eifan AO, Shamji MH, Durham SR. Long-term clinical and immunological effects of allergen immunotherapy. Curr Opin Allergy Clin Immunol. 2011;11:586–93. doi: 10.1097/ACI.0b013e32834cb994. [DOI] [PubMed] [Google Scholar]
- 45.Nilsson OB, Adedoyin J, Rhyner C, et al. In vitro evolution of allergy vaccine candidates, with maintained structure, but reduced B cell and T cell activation capacity. PLoS One. 2011;6:e24558. doi: 10.1371/journal.pone.0024558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Comparison of rFel d 1 and hypoallergenic derivatives by circular dichroism. The mean residue molar ellipticities for rFel d 1 and rFel d 1 derivatives (y-axis: degree cm2 dmol−1) were recorded for a range of wavelengths (x-axis).
Figure S2. (a) SDS–PAGE containing MF under non-reducing and reducing conditions. Lanes M and MF represent the molecular weight marker and purified MF which had been stored for 28 months. Molecular weights are indicated on the left margins. (b) Size exclusion (SEC) profile of MF. The positions of the standards (i.e. dextran blue: 2000 kDa; BSA: 66 kDa; cytochrome c: 12.4 kDa) are indicated, elution volumes (mL) are on the x-axis and the y-axis shows the absorbances at 280 nm.
Figure S3. Titration of IgG antibodies specific for rFel d 1. Different dilutions (x-axis) of antisera obtained by immunization of rabbits with rFel d 1(▬) or Fel d 1 derivatives (Fel d 1 M: ■; MB: ▲: MC: −; mix of MB + MC: ×; MF: ●) were reacted with ELISA plate-bound rFel d 1. OD values corresponding to bound antibodies are shown on the y-axis. PI: Pre-immune serum.
Table S1. Demographic, clinical and serological characteristics of cat-allergic patients.
Table S2. Characteristics of Fel d 1-derived peptides which were used as building blocks for the derivatives.
Table S3. IgE reactivity of sera from patients with cat allergy to Fel d 1, Feld 1-derived peptides and mosaic derivatives.
Table S4. Percentage inhibition of IgE binding to rFel d 1 after pre-adsorption of patients’ sera with rFel d 1, hypoallergenic Fel d 1 derivatives or rPhl p 1.
Table S5. T cell proliferative responses in cat-allergic patients.
Table S6. Inhibition of allergic patients’ IgE reactivity to Fel d 1 by IgG antibodies.