Abstract
TGFβ is over expressed in advanced human cancers. It correlates with metastasis and poor prognosis. However, TGFβ functions as both a tumor suppressor and a tumor promoter. Here we report for the first time that genetic deletion of Tgfbr2 specifically in myeloid cells (Tgfbr2MyeKO) significantly inhibited tumor metastasis. Reconstitution of tumor-bearing mice with Tgfbr2MyeKO bone marrow recapitulates the inhibited metastasis phenotype. This effect is mediated through decreased production of type 2 cytokines, TGFβ1, arginase 1 and iNOS, which promoted IFN-γ production and improved systemic immunity. Depletion of CD8 T cells diminished metastasis defect in the Tgfbr2MyeKO mice. Consistent with animal studies, myeloid cells from advanced stage cancer patients demonstrated an increased TGFβ receptor II expression. Our studies demonstrate that myeloid-specific TGFβ signaling is an essential component of the metastasis-promoting puzzle of TGFβ. This is in contrast to the previously reported tumor-suppressing phenotypes in fibroblasts, epithelial or T cells.
Keywords: Tumor metastasis, TGFβ signaling, myeloid cells, immune response, cytokines, tumor microenvironment, breast cancer
INTRODUCTION
TGFβ signaling plays an important role in tumor initiation and progression. Paradoxically TGFβ can function as both a tumor suppressor and promoter (1, 2). The mechanisms underlying the dual role of TGFβ are very intricate and are poorly understood. In the past, most work dissecting the molecular mechanisms was largely focused on differential regulation of signaling pathways by tumor autonomous TGFβ signaling and cross talk with other signaling network (2–4). It was thought that changes in signal intensity and connectivity of SMAD-dependent and independent pathways including PI3K, MAPK, as well as small GTPases may explain the complex transition of TGFβ from a tumor suppressor to a tumor promoter (5).
Interestingly, disruption of TGFβ signaling in a number of epithelial cells results in a more malignant tumor phenotype in breast, intestinal, pancreatic, colon, and head-and-neck squamous cell carcinoma (6). Deletion of Tgfbr2 in FSP1+ fibroblasts induces the development of invasive squamous cell carcinoma in the fore-stomach, and intraepithelial neoplasia in the prostate (7, 8). Additionally, deletion of Smad4, an important down-stream mediator of TGFβ signaling in T cells, induces development of gastrointestinal cancers (9). These studies suggest that TGFβ signaling in epithelial cells, fibroblasts, and T cells play a tumor suppressive function. Recent work from our group and others showed that down regulation of TGFβ signaling, a frequent event observed in many tumor types, leads to increased CXCL1-CXCL5/CXCR2 and SDF-1/CXCR4 chemokine/chemokine receptor signaling, and subsequent recruitment of host derived immature myeloid Gr-1+CD11b+ cells or myeloid derived suppressor cells (MDSCs) and macrophages (10, 11) into tumors. These infiltrating myeloid cells produce large quantities of TGFβ1 and matrix metalloproteinases (MMPs) that suppress the host immune system and concurrently promote tumor invasion (10).
Myeloid cells play an important role in tumor progression. They suppress host immune surveillance (12–15) and influence the tumor microenvironment (10, 13, 14, 16). These cells are also present in the lungs prior to tumor cell arrival and contribute to pre-metastatic niche formation (17) and alteration of the local lung environment (18). These cells include tumor-associated macrophages (TAM, Mac-1+ or F4/80+ cells)(14), Gr-1+CD11b+ myeloid derived suppressor cells (MDSCs) (12), and tumor associated neutrophils (TAN, CD11b+Ly6G+) (15). One of the most important properties of these cells is increased TGFβ production (10, 19). In fact, depletion of Gr-1+CD11b+ cells diminished the antitumor effect of TGFβ neutralization, suggesting that immature Gr-1+CD11b+ cells are responsible for tumor promoting effect of TGFβ in breast cancer progression (20). However, it is not known how TGFβ signaling in myeloid cells affects tumor phenotype. Delineation of TGFβ pathways in myeloid cells may unravel the paradoxical role of TGFβ in cancer. In this report, we demonstrate that TGFβ signaling in myeloid cells of tumor host is fundamentally important for tumor metastasis. Genetic deletion of Tgfbr2 specifically in myeloid cells dramatically decreases tumor metastasis. Our data implicate myeloid TGFβ signaling as a potential novel therapeutic target.
RESULTS
Increased Expression of TβRII in Myeloid Cells under Tumor Conditions, and LysM-Cre Mediated Myeloid-specific Tgfbr2 Deletion
To assess the role of TGFβ signaling in tumor associated myeloid cells, we used Gr-1+CD11b+ cells as samples for myeloid cells as they constitute the majority of tumor-associated myeloid cells and produce high levels of TGFβ1. We used murine 4T1 mammary tumor and Lewis lung carcinoma (LLC) mouse models that are in Balb/c and C57Bl/6 backgrounds respectively. For both models, we found that splenic Gr-1+CD11b+ cells from tumor-bearing mice express significantly higher levels of TβRII compared with their non-tumor-bearing counterparts (Fig. 1A, and B, data not shown for LLC model). The impact of elevated TβRII expression is likely amplified since the frequencies of these myeloid cells are also increased in the bone marrow, spleen, and peripheral blood of tumor-bearing mice (Supplementary Fig. S1A).
Figure 1.

Increased expression of TβRII in myeloid cells under tumor conditions and mouse models for myeloid specific deletion of Tgfbr2 A and B, Western Blotting and Immunofluorescence of TβRII. Sorted splenic Gr-1+CD11b+ cells were used for protein extraction or cytospin slides. Cells from mice bearing 4T1 tumors were compared with non-tumor bearing normal mice. Scale bar, 20 µm. C, TβRII Western of sorted human blood myeloid cells enriched using CD33+ or CD34+ or CD15+ markers, from both normal individuals and later stage lung cancer patients. Right panels: MACS sorting of the myeloid cells, before (left) and after (right) sorting. D, Heat map (upper panels) and dot plots (lower panels) of Tgfb1 and Tgfbr2 mRNA in human peripheral blood mononuclear cells in cohorts of breast (GSE27567) and lung cancers (GSE20189). The datasets were analyzed using Genespring GX 10.0 software. Red and green colors indicate increased and decreased expression, respectively. *p<0.05. E, Southern hybridization showing specific deletion of Tgfbr2 in sorted myeloid cells from 4T1 tumor bearing Balb/c mice, but not in T or B cells. F, IF of TβRII (red color) in splenic Gr-1+CD11b+ cells from Tgfbr2flox/flox and Tgfbr2MyeKO mice bearing LLC (left panels) and 4T1 tumors (right panels). G, Western blotting of TβRII, p-Smad2, and Smad2 of sorted splenic Gr-1+CD11b+ cells from Tgfbr2MyeKO and Tgfbr2flox/flox mice in Balb/c background bearing 4T1 tumors.
The overproduction of immature myeloid cells has also been reported in patients with a variety of cancers (16, 21), in which they are identified as CD33+, CD34+, or CD15+ cells (16, 22, 23). We used these markers to enrich the myeloid cells from peripheral blood of 16 patients with metastatic non-small cell lung cancer. These myeloid cells, which include granulocytes, monocytes and their precursors, were accounted for approximately 82% of the total leukocytes, compared to 72% from the healthy individuals (n=11, p<0.05) (Supplementary Fig. S1B). Sorted myeloid cells from the patients showed increased TβRII expression compared to healthy individuals (Fig. 1C). Further examination of the human breast cancer and lung cancer datasets using Genespring GX 10.0 software showed a significant increase in TβRII and TGFβ1 mRNA in human peripheral blood mononuclear cells of breast cancer patients compared to normal individuals (Fig. 1D) (GSE27567) (24). In lung cancer, TGFβ1 was increased at stages IIIA and IV compared to the stage I and II, and correlates well with modest increased TβRII mRNA level (p>0.05 but with a trend of increase) in the lung cancer patient cohort (GSE20189) (25) (Fig. 1D). Together, these data suggest that increased TβRII expression correlates with cancer progression in a clinical setting.
The over-expression of TβRII in both human and mouse myeloid cells from cancer hosts raised the possibility that TβRII signaling in myeloid cells affects tumor progression and metastasis. Although TGFβR3 has been recently implicated in tumor progression (26, 27), the TGFβ-induced and TGFβR3-mediated responses have not been shown to be independent of TβRII. On the other hand, overwhelming data from the field supporting a central role of the TβRII in TGFβ signaling. Thus in order to test our hypothesis, mice with a targeted deletion of Tgfbr2 in myeloid cells (Tgfbr2MyeKO) were generated through the cross-breeding of floxed Tgfbr2 (Tgfbr2flox/flox) mice with LysM-Cre transgenic mice. LysM-Cre transgenic mice have been well characterized and used in many studies to delete genes specifically in myeloid cells (28, 29). Indeed, sorted Gr-1+CD11b+ cells, but not B cells (B220+) or T cells (CD3+) (over 95% purity, Supplementary Fig. S1C) from Tgfbr2MyeKO mice showed Tgfbr2 recombination (Fig. 1E) and a clearly decreased TβRII expression and phosphorylation of Smad2 (Fig. 1F, and G). These data support that Tgfbr2 deletion was efficient and specific in myeloid cells.
Deletion of Tgfbr2 in Myeloid Cells Significantly Inhibited Tumor Metastasis
The Tgfbr2MyeKO mice appeared phenotypically normal with no alteration in the number or percentage of T (CD4 & CD8), B, NK, or Gr-1+CD11b+ and F4/80+ cells in bone marrow, spleen, thymus and lymph node (data not shown). However, Tgfbr2MyeKO mice showed a decreased ability to develop tumor metastasis following injection of 4T1 mammary tumor cells into the #2 mammary fat pads (MFP) (Fig. 2A), with modest effect on 4T1 primary tumor growth (p>0.05) and later stage of LLC growth (p<0.05) (Supplementary Fig. S2A and B). The 4T1 mammary tumor model shares many characteristics with human breast cancer, particularly its ability to spontaneously metastasize to the lungs. In an experimental metastasis design in which 2 × 105 4T1 cells were injected into the tail vein, there was also a significant reduction in metastasis in Tgfbr2MyeKO mice (Fig. 2B). This result was recapitulated in the B16 melanoma orthotopic and LLC experimental metastasis models in Tgfbr2MyeKO mice in a C57BL/6 background (Fig. 2C and 2D). Similar to 4T1 tumor model, the primary tumor growth of B16 was not different between Tgfbr2MyeKO and control mice (data not shown). Together these data suggest that the major effect of myeloid Tgfbr2 deletion is on tumor metastasis. This is supported by two additional experimental metastasis tumor models: MC26 colon cancer and B16 melanoma, which showed significantly decreased number of lung metastases in Tgfbr2MyeKO mice compared to controls after tail vein injection of the tumor cells (Supplementary Fig. S2C and D).
Figure 2.

Deletion of Tgfbr2 in myeloid cells significantly inhibited tumor metastasis. A, Lung metastasis in Tgfbr2MyeKO and Tgfbr2flox/flox control mice 28 days after 4T1 injection (5 × 104) in #2 MFP (n=6 for each group). Shown is one of the four experiments performed. * indicate p<0.05. B, H&E staining of representative butterfly sections of the lungs showing dramatic reduction of 4T1 lung metastasis in Tgfbr2MyeKO mice (n=12) compared to Tgfbr2flox/flox mice (n=5) received tail vein injection of 4T1 cells (2 × 105) for 25 days. Quantitative data is on the right. **p<0.01. C, Decreased metastasis in Tgfbr2MyeKO mice subcutaneous injection of B16 melanoma cells (1 × 106) (n=7). Tumors were resected on day 16, lungs were harvest on day 37. *p<0.05. D, A dramatic reduction of lung metastasis in Tgfbr2MyeKO mice on a C57BL/6 background that received tail vein injection of LLC cells (2.5 × 105)(n=5–16 mice). Quantitative data are on the right. One of the two experiments is shown. **p<0.01. E, Schematic experimental design for adoptive transfer of Tgfbr2MyeKO bone marrow to wild type 4T1 tumor bearing mice. F, Kaplan-Meier survival curve (left panel) and metastasis counts (right panel) in mice received BM transplant from Tgfbr2MyeKO and floxed control mice. Shown is one of the two experiments performed. *p<0.05. All data are represented as mean ± SEM.
To further confirm the inhibitory effect of myeloid-specific Tgfbr2 deletion on tumor metastasis, we transplanted bone marrow (B.M.) from Tgfbr2MyeKO mice into wild type mice bearing 4T1 tumors. To better model clinical metastatic disease, the primary tumor was surgically removed on day 15 and metastasis was allowed to continue until day 34, when the animals were irradiated and subjected to B.M. transplantation (Fig. 2E). In these therapeutic experiments, 4T1 tumors were injected into the #4 MFP to allow surgical removal of tumors. We observed 100% survival of the mice that received B.M. from the Tgfbr2MyeKO mice, whereas approximately 55% of the mice that received B.M. from Tgfbr2flox/flox control mice showed decreased survival (Fig. 2F). In addition, a significant reduction in the number of lung metastases was observed in mice that received Tgfbr2MyeKO B.M. relative to those that received control B.M. (Fig. 2F). These data suggest that myeloid-specific TGFβ signaling constitutes an essential part of the metastasis-promoting role of TGFβ.
Deletion of Tgfbr2 in Myeloid Cells Decreased Type 2 Cytokine Expression, and Increased IFN-γ Production and Host Anti-tumor Immunity
TGFβ signaling is a critical mediator of immune cell polarization (19). It is not clear whether it has similar function in the myeloid cells. We examined type 1 and type 2 cytokine expression in sorted Gr-1+CD11b+ cells. Interestingly, the expression of type 2 cytokines, including IL-10 and IL-4, was reduced in myeloid cells with the Tgfbr2 deletion compared to controls, with no difference in type 1 cytokine production (e.g., IL-12 and TNF-α) (Fig. 3A). There was also reduced expression in arginase I and iNOS (Fig. 3A), the critical mediators implicated in the immune suppression effects of Gr-1+CD11b+ cells. The decreased expression of type 2 cytokines was further confirmed in a cytokine protein array (Fig. 3B, upper panels, with semi-quantitative data of dot density on the lower panel). Deletion of myeloid Tgfbr2 also decreased TGFβ1 production in sorted Gr-1+CD11b+ cells (Fig. 3C), suggesting an autocrine effect of TGFβ signaling. The decreased expression of iNOS and arginase I is consistent with decreased nitric oxide (NO) production and arginase 1 activity (Fig. 3D).
Figure 3.

Myeloid specific deletion of Tgfbr2 reduced the expression of type 2 cytokines, TGFβ1, arginase and iNOS in myeloid cells. A, Q-PCR of type 1 and type 2 cytokines, arginase, and iNOS expression in Gr-1+ myeloid cells sorted from spleen of Tgfbr2MyeKO and control mice bearing 4T1 tumors. Shown is the mean ± SEM of three samples per group. *p<0.05; **p<0.01; ***p<0.001. B, Cytokine protein array analysis of sorted Gr-1+ myeloid cells (left panel), with semi-quantitative data of dot density on the right. Shown is one of the two experiments performed. ***p<0.001. C, TGFβ1 ELISA of Gr-1+ myeloid cells sorted from the spleen of 4T1 tumor bearing mice. Samples from 3 mice were collected and triplicates per sample were analyzed. D, The decreased production of NO and arginase 1 function. Samples were from 4T1 tumor bearing Tgfbr2MyeKO and control mice.
Tumor-associated myeloid cells inhibit multiple immune cell functions in tumor hosts (12). We therefore examined whether deletion of myeloid-specific Tgfbr2 resulted in improved immune function of CD4+, CD8+, B, NK or macrophage cells. We observed an increased percentage of IFN-γ-positive CD8+ T cells in the spleen of tumor-bearing Tgfbr2MyeKO mice compared to Tgfbr2flox/flox mice (Fig. 4A). No difference was found in other cytokines or cell types (data not shown). This was consistent with the increased number of IFN-γ producing cells detected by ELISPOT in the spleens of Tgfbr2MyeKO mice (Supplementary Fig. S3A). To examine whether the enhanced T cell immunity is antigen specific, we co-cultured Gr-1+ myeloid cells sorted from Tgfbr2MyeKO mice with T cells from OT1 transgenic mice, and observed an increased IFN-γ production when pulsed the T cells with a specific ovalbumin (ova) peptide (Fig. 4B). Importantly, systemic neutralization of IFN-γ diminished the inhibitory effect of myeloid Tgfbr2 deletion on metastasis (Fig. 4C), with no significant effect on primary tumor size (Supplementary Fig. S3B).
Figure 4.
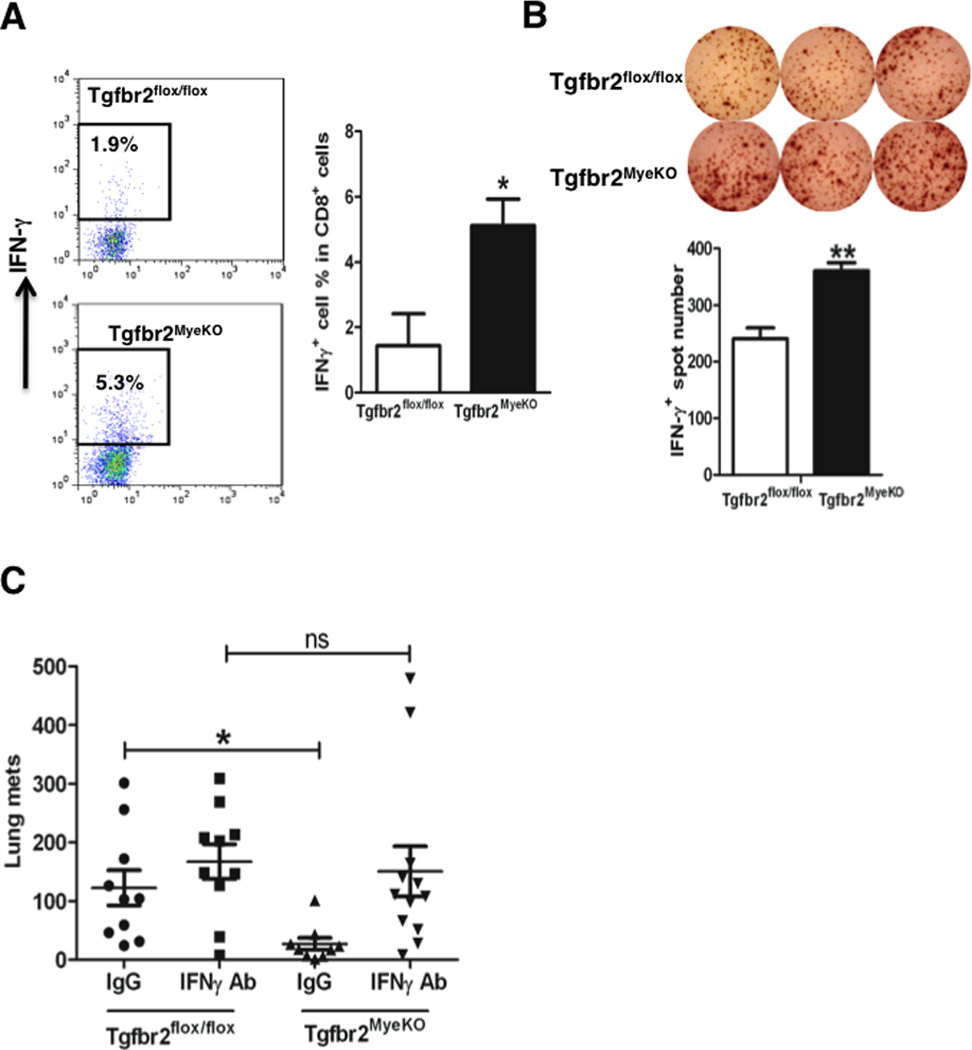
Deletion of Tgfbr2 in myeloid cells elevated IFN-γ production in CD8+ T cells, and systemic IFN-γ neutralization diminished metastasis inhibition in Tgfbr2MyeKO mice. A, Flow cytometry of IFN-γ intracellular staining of CD8+ T cells from spleens of Tgfbr2MyeKO and Tgfbr2flox/flox mice bearing 4T1 tumors (left panels). Quantitative data is on the right. Shown is one of the two experiments performed. * p<0.05. B, IFN-γ ELISPOT of T cells from OT1 transgenic mice when co-cultured with Gr-1+ myeloid cells sorted from the speens of Tgfbr2MyeKO mice bearing 4T1 tumors. Quantitative data is on the lower panel. Two experiment were performed, with each sample in triplicates. **p<0.01. C, Lung metastasis of 4T1 tumor bearing Tgfbr2MyeKO and Tgfbr2flox/flox mice received IFN-γ neutralization antibody or IgG control injection. *p<0.05.
We next over-expressed IL-4, IL-10, and TGFβ1, as well as arginase I and iNOS in sorted myeloid cells with Tgfbr2 deletion (Supplementary Fig. S4A and B) to see whether this would result a reduction of the IFN-γ producing T cells. We found expression of all IL-4, IL-10, and TGFβ1, or both arginase I and iNOS, but not either one alone in sorted Gr-1+CD11b+ cells with Tgfbr2 deletion decreased the number of IFN-γ+ T cells in the co-culture by ELISPOT assay (Fig. 5A and B), which was not the case in Gr-1+CD11b+ cells sorted from floxed control mice (data not shown). These data, together with the results showing that myeloid cells with Tgfbr2 deletion have decreased IL-4, IL-10, TGFβ1, as well as arginase I and iNOS (Fig. 3A) suggest that IL-4, IL-10, TGFβ1, arginase I and iNOS mediated mechanisms play a major role in enhanced IFN-γ producing CD8+ T cells as a result of inhibition of TGFβ signaling in Gr1+CD11b+ cells.
Figure 5.

Mechanisms of myeloid TGFβ mediated immunosuppressive function. A and B, Quantitative results of IFN-γ ELISPOT of T cells when co-cultured with sorted Gr-1+ cells (from Tgfbr2MyeKO mice) with over-expression of IL-4, IL-10, TGFβ1 (A) or arginase 1 and iNOS (B). Gr-1+ cells were sorted from spleens of 4T1 tumor bearing mice in a Balb/c background. T cells were isolated from the spleens of CL4 transgenic mice. Shown is one of the two experiments performed. *p<0.05, **p<0.01, ***p<0.001. C, Flow cytometry of IFN-γ intracellular staining of CD11b+Ly6C+ cells in the lungs of Tgfbr2MyeKO mice, with gating on the left panel and quantitative data in the middle panel. They are mostly F4/80+ macrophages (65%) and some are Ly6G+ neutrophils (32%) (right panel). Three mice each for Tgfbr2flox/flox and Tgfbr2MyeKO were examined. *p<0.05. All data are represented as mean ± SEM.
Local Innate Immunity in Premetastatic Lung Microenvironment of Tgfbr2MyeKO Mice
Tgfbr2MyeKO mice showed a decreased ability to develop tumor metastasis following tail vein injection of a number types of tumor cells including 4T1 (Fig. 2B), LLC (Fig. 2D), MC26 and B16 (Supplementary Fig. S2C and S2D). We suspect there is an altered lung microenvironment in Tgfbr2MyeKO mice. When we examined the percentage and the number of B, CD4 and CD8 T cells, Treg cells, as well as different myeloid subsets including CD11b+Ly6G+ neutrophils, CD11b+Ly6C+ monocytes, CD11b+F4/80+ macrophages, and CD11b+CD11c+ dendritic cells, no difference was found between Tgfbr2MyeKO and Tgfbr2flox/flox mice (Supplementary Fig. S5). This is true for both normal (Supplementary Fig. S5A) or tumor conditions (Supplementary Fig. S5B). In addition, the production of IFNγ is similar in lung-residing CD8+ T cells in tumor-bearing Tgfbr2MyeKO and Tgfbr2flox/flox mice (Supplementary Fig. S5C). Interestingly, Tgfbr2 deletion in myeloid cells increased IFN-γ production in one subset of CD11b+Ly6C+ (Fig. 5C, left and middle panels) but not other subsets (Supplementary Fig. S6A). These CD11b+Ly6C+ cells express F4/80 and Ly6G thus are likely macrophages and neutrophils (Fig. 5C, right panel). The production of the IFN-γ in these myeloid cells is likely mediated by IL-12 and IL-18 as neutralization of both IL-12 and 18 decreased IFN-γ level in these myeloid cells stimulated with lipopolysaccharide (LPS) (Supplementary Fig. S6B). This is consistent with previous reports (30, 31). In addition, CD11b+CD11c+ cells in the lungs of Tgfbr2MyeKO mice showed increased expression of the co-stimulatory molecule CD86 (Supplementary Fig. S7), which was not observed in tumor tissues, spleen, or draining lymph nodes of tumor-bearing mice (Supplementary Fig. S7). This suggests an increased functional maturation of CD11b+CD11c+ myeloid cells in Tgfbr2MyeKO mice compared to the control littermates. Therefore, our data suggest that an improved innate immunity in the lung environment of Tgfbr2MyeKO mice may have a critical role in the decreased metastasis phenotype in these mice.
In contrast to metastasis, myeloid Tgfbr2 deletion had little effect on primary tumor growth. We carefully examined the number and percentage of infiltrating immune cells including B, CD4 and CD8 T cells, Treg cells, as well as different myeloid subsets including CD11b+Ly6G+ neutrophils, CD11b+Ly6C+ monocytes, CD11b+F4/80+ macrophages, CD11b+CD11c+ dendritic cells. Again, no difference was found between Tgfbr2MyeKO and Tgfbr2flox/flox mice (Supplementary Fig. S8A). Additionally, there was no difference in IFN-γ production in CD8 T cells (Supplementary Fig. S8B left panel), or in the myeloid cells (Supplementary Fig. S8B right panel), the latter is different from our observations of the lung. Thus, despite a systemic increase in IFN-γ+CD8+ T cells and a local increase in IFN-γ+CD11b+Ly6G+ or IFN-γ+CD11b+F4/80+ cells in the lung, the tumor microenvironment remained a sanctuary site to protect tumor cells from host anti-tumor immunity.
Myeloid Cell Subsets Responsible for the Decreased Lung Metastasis in Tgfbr2MyeKO Mice
Different myeloid cell subsets have been implicated in tumor progression. To investigate which subset of the myeloid cells might be responsible for the decreased metastasis as results of Tgfbr2 deletion, we sorted CD11b+Ly6G+, CD11b+Ly6C+, and CD11b+F4/80+ cells(Supplementary Fig. S9A and B). We first examined TβRII expression. We found that the CD11b+Ly6G+ subset expressed higher level of TβRII compared to the CD11b+Ly6C+ and CD11b+F4/80+ subsets (Supplementary Fig. S9C). Consistent with this, CD11b+Ly6G+ cells with Tgfbr2 deletion also showed a significantly decreased production of TGFβ1 and IL-10, as well as modest decreased IL-4 (Fig. 6A), which is not observed in CD11b+Ly6C+ monocytes or CD11b+F4/80+ macrophages (Fig. 6A). The expression of iNOS and arginase 1 was decreased in both CD11b+Ly6G+ neutrophils and CD11b+Ly6C+ monocytes (Fig. 6B). Additionally, both CD11b+Ly6G+ and CD11b+Ly6C+ myeloid cells showed inhibited NO production when co-cultured with T cells (Fig. 6C). These data suggest that TGFβ signaling affects the properties and function of both CD11b+Ly6G+ and CD11b+Ly6C+ myeloid subsets. However, one should be noted that the percentage of CD11b+Ly6G+ cells in Gr1+ cells is significantly higher than the CD11b+Ly6C+ in the 4T1 tumor model (32). Therefore the effect from the CD11b+Ly6G+ cells is likely major.
Figure 6.

Reconstitution of wild type myeloid cell subset CD11b+Ly6G+ cells in Tgfbr2MyeKO mice reversed the diminished metastasis phenotype. A, Decreased TGFβ1 (ELISA), IL-10 and IL-4 (Q-PCR) was found in CD11b+Ly6G+ cells from Tgfbr2MyeKO mice. B, Q-PCR show decreased iNOS and Arginase 1 in CD11b+Ly6G+ and CD11b+Ly6C myeloid cell subsets. For both A and B, myeloid subsets were sorted by FACS. RNA was extracted and subjected to Q-PCR analysis. Supernatant from cultured subsets were used for ELISA. Myeloid cells were isolated from spleens of 4T1 tumor bearing mice. C, NO production of myeloid cell subsets sorted from Tgfbr2MyeKO mice (C57BL/6 background), when cocultured with OVA peptide stimulated splenocytes from OT-I mice (C57BL/6 background). For A, B, and C, three mice each for Tgfbr2flox/flox and Tgfbr2MyeKO were examined. D, Reconstitution of CD11b+Ly6G+ or Gr-1+CD11b+ cells, but not CD11b+Ly6C+ or CD11b+F4/80+ cells diminished the decreased 4T1 tumor lung metastasis in Tgfbr2MyeKO mice (n=7–10). All data are represented as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001.
To further confirm the effect of these myeloid cell subsets in vivo, we injected sorted wild type myeloid cell subsets into 4T1 tumor bearing Tgfbr2MyeKO mice through tail vein. We first tested the homing efficiency of tail vein injected cells using luciferase imaging. Approximately over 65–75% of the luciferase expressing 4T1 cells injected through tail vein homed to the lungs 24 hours after injection (Supplementary Fig. S10A). We found that the injection of CD11b+Ly6G+ cells reversed the metastasis defect in Tgfbr2MyeKO mice (Fig. 6D). These data suggest that the CD11b+Ly6G+ myeloid subset is responsible for the inhibition of metastasis that results from the myeloid-specific deletion of Tgfbr2. Our conclusion may more broadly apply to mouse models in which the CD11b+Ly6G+ myeloid cells constitute the majority of myeloid cells in at least 8 tumor models studied including MC26 colon cancer, LLC Lewis lung carcinoma, and B16 melanoma (32).
Cellular Mechanisms underlying Improved Host Anti-tumor Immunity and Decreased Metastasis in the Tgfbr2MyeKO Mice
Our data clearly demonstrate an IFN-γ dependent mechanism in decreased metastasis in Tgfbr2MyeKO mice. We next ask the question what cell type (CD8 T or Ly6G neutrophils) is critical this process. Tgfbr2MyeKO mice were inoculated with 4T1 tumor cells in the mammary fat pad. The mice were also injected with CD8 neutralization antibody or IgG control every 2–3 days to deplete the CD8 T cells (Fig. 7A). The depletion of CD8 T cells diminished the metastasis inhibition in the Tgfbr2MyeKO mice compared to the Tgfbr2MyeKO mice received IgG control treatment (Fig. 7B). In addition, to examine the direct contribution of the myeloid derived IFN-γ in the inhibition of the lung metastasis in the lung environment, we sorted IFN-γ deficient or wild type CD11b+Ly-6G+ cells, and then coinjected them with the tumor cells through tail vein. No significant effect on tumor metastasis was found (data not shown). However, this observation may be limited by the small number of IFNγ-producing myeloid cells within the Ly6G+ subset. Our data suggest that IFN-γ producing CD8 T cells are critical for the metastasis defect in the Tgfbr2MyeKO mice.
Figure 7.

CD8 depletion and IFN-γ deficient myeloid cells and lung metastasis inhibition in Tgfbr2MyeKO mice. A, flow cytometry show significantly decreased CD8 T cells in Tgfbr2MyeKO mice after received injection of CD8 neutralization antibody. B, CD8 neutralization antibody significantly decreased lung metastasis in Tgfbr2MyeKO mice. All data are represented as mean ± SEM. ***p<0.001. C, Schematic hypotheses for mechanisms underlying decreased metastasis in the Tgfbr2MyeKO mice. TGFβ1 production is enhanced in myeloid cells through autocrine (TGFβ1 produced in CD11b+Ly6G+ cells) and/or paracrine mechanisms (TGFβ1 from tumor cells or others such as CD4 Treg cells or stromal fibroblast). Deletion of Tgfbr2 in myeloid cells decreased the production of type 2 cytokines, TGFβ1, as well as arginase and iNOS, which in turn increased IFN-γ expression in CD8+ T cells. This likely improves systemic immune surveillance and result decreased lung metastasis in the Tgfbr2MyeKO mice. In the metastatic lung, deletion of Tgfbr2 also enhances IFN-γ production in a subset of macrophages and neutrophils, and functional maturation of dendritic cells, which may improve local innate immunity.
DISCUSSION
We report here for the first time that deletion of Tgfbr2 specifically in myeloid cells significantly inhibited tumor metastasis, suggesting that myeloid-specific TGFβ signaling is an essential component of the metastasis-promoting puzzle of TGFβ. This is in contrast to the tumor suppressing effect of TGFβ in epithelial cells, fibroblasts, and T cells (6, 7, 9, 33). Our study provides new mechanistic insight into TGFβ regulation of tumor metastases. Our data are reminiscent of those observed following blockade of TGFβ signaling in T cells using CD4dnTGFβ-RII mice, which confers resistance to an EL-4 lymphoma or a B16-F10 melanoma tumor challenge (34). However, those mice developed an autoimmune pathology that is not seen in our mouse model. The lack of pathology in our mouse model is likely due to the fact that myeloid cells are significantly expanded under tumor conditions (Supplementary Fig. S1A). Therefore, the specific deletion of myeloid Tgfbr2 produces a pronounced antitumor effect with very few adverse effects. A previous report demonstrated CD8+ CTL mediated suppression of melanoma and prostate metastasis upon silencing of TGFβ signaling in total bone marrow cells by retroviral-mediated transfection of a dominant negative TβRII (35). Our findings indicate that the myeloid cell associated TGFβ signaling may be the key player driving this effect.
Our studies demonstrate that TGFβ signaling is a critical mediator of the tumor-promoting effect of myeloid cells, supported by a recent report of anti-tumor properties of myeloid Tgfbr2 deletion on primary tumor growth (36). Myeloid cells have been shown to promote tumor progression through modulation of host immune surveillance (12–15), and alteration of the tumor microenvironment (10, 13, 14, 16). Recent evidence also suggests that myeloid cells have a substantial impact on the premetastatic lung (17, 18, 37, 38). A number of studies have tried to identify the molecular mechanisms for these myeloid cells in tumor metastasis. One of the better-studied molecules involves the paracrine loop of colony-stimulating factor 1 (CSF-1) and epidermal growth factor (EGF) between tumor cells and TAMs (13, 39). Apparently, CSF-1 is produced by tumor cells, and acts on the CSF-1R in the TAM (40, 41). Interestingly, tumor cells also express EGFR that mediate the signaling of EGF produced by the TAMs (39, 41–43). These two molecule pathways are critical for the effects of TAM upon tumor metastasis (13, 39). Hypoxia-inducible factor 1α (HIF-1α), in myeloid cells was also shown to control the inflammatory response through the regulation of the metabolic switch to glycolysis (44), and contributing to T cell suppression in the tumor microenvironment (45). Myeloid-specific HIF-2α promoted tumor cell proliferation and progression in murine hepatocellular and colitis associated colon carcinoma models (46). In addition, the transcription factor Ets2 in myeloid cells is important in metastasis as its deletion decreased the frequency and size of lung metastases in three different mouse models of breast cancer metastasis, which is mediated through inhibition of a gene program for angiogenesis (47). Recently, mice with myeloid specific deletion of HuR, a RNA-binding protein that regulates mRNAs transcription and translation, displayed enhanced sensitivity to endotoxemia, rapid progression of chemical-induced colitis, and increased severe susceptibility to colitis-associated cancer through increased inflammatory cytokines and enhanced CCR2-mediated macrophage chemotaxis (48). However, most of these reports did not focus on tumor metastasis. Of interest, the deletion of myeloid specific vascular endothelial growth factor (VEGF-A), resulted in an accelerated tumor progression despite the fact that myeloid-derived VEGF-A is essential for the tumorigenic alteration of vasculature and signaling to VEGFR2, and rather these changes act to retard, not promote, tumor progression (49).
Our results provide insight into the molecular mechanisms for TGFβ regulation of myeloid cell tumor-promoting function (Fig. 7C). First, TGFβ is produced in high levels by tumor-associated myeloid cells including Gr-1+CD11b+ cells (10, 50, 51). In fact, depletion of Gr-1+CD11b+ cells diminished the antitumor effect of TGFβ neutralization (20). Deletion of Tgfbr2 significantly decreased TGFβ1 production, which is not the case for TGFβ2 or TGFβ3 (Supplementary Fig. S10B and C). In addition, CD11b+Ly6G+ myeloid cell subset that we found play a critical role in the decreased metastasis in the Tgfbr2MyeKO mice, showed significantly higher expression of the TβR2 compared to the CD11b+Ly6C+ and CD11b+F4/80+ cells (Supplementary Fig. S9C). Thus our data suggest that there is likely an autocrine and/or paracrine loop that enhances TGFβ1 production and signaling in myeloid cells through the TβR2 in CD11b+Ly6G+ myeloid cell subset (Fig. 7C). Deletion or down regulation of TGFβ signaling in myeloid cells would disrupts TGFβ1 production. However, it is not clear whether TGFβ1 production directly converts the myeloid cells from a type I to type II phenotype or is the result of type II myeloid cell polarization. Second, myeloid TGFβ signaling is critical in tumor associated immune suppression. The critical mediators down stream of TGFβ signaling include type 2 cytokines, arginase and iNOS, which have a significant impact on both systemic adaptive immunity and innate immunity in the lung (Fig. 7C). It is very clear in our studies that TGFβ signaling is critical in shaping the type 1/type 2 phenotype of myeloid cells.
Interestingly, in the lung, there was an increase in IFN-γ+CD11b+Ly6G+ or IFN-γ+CD11b+F4/80+ cells, as well as CD11b+CD11c+CD86+ dendritic cells, which were not present in the tumor microenvironment. Apparently, deletion of Tgfbr2 decreased iNOS production in the splenic Gr-1+CD11b+ cells and improved systemic immunity, whereas deletion of Tgfbr2 also increased IFN-γ production in the lung residing macrophages and neutrophils. This appears to be different from the observations that IFN-γ level correlates with iNOS in macrophages (52). We want to point out that these two effects are in fact on very different population of cells (myeloid immune suppressor cells vs lung residing macrophages/neutrophils) and in two very different organ environments (spleen vs lung). A number of studies supports that regulation of iNOS in vivo may depend on the relative tempo with which the inflammatory and immune responses evolve. We believe this increased innate immunity in the lung may be important in the diminished tumor metastasis in Tgfbr2MyeKO mice. A recent study reported that type I interferon (IFN-α/β) selectively within the innate immune compartment is essential for tumor-specific T cell priming and tumor elimination (53). Tumor-associated macrophages cross talk with adaptive immune components (54). We found that the critical mediators in the improved IFN-γ producing CD8+ T cells in the Tgfbr2MyeKO mice involve IL-4, IL-10, TGFβ as well as arginase 1 and iNOS. Overexpression of these factors in sorted Gr-1+CD11b+ cells with Tgfbr2 deletion decreased the number of IFN-γ+ T cells (Fig. 5 A and B). Indeed, these type 2 cytokines as well as arginase I and iNOS are known for cancer associated immune suppression by Gr1+CD11b+ cells (12). Our data suggest an improvement in both systemic adaptive immunity and local innate immunity in the lung is important for the reduced metastasis observed in Tgfbr2MyeKO mice. However, our data support that CD8 T cells play a prominent role in adaptive immunity that is critical in metastasis inhibition in Tgfbr2MyeKO mice.
Consistent with the observations in tumor-bearing mice, myeloid cells from the peripheral blood of advanced stage lung cancer patients also demonstrated increased TβRII expression, suggesting a clinical relevance of our studies. Importantly, transplant of Tgfbr2MyeKO bone marrow into wild type mice bearing 4T1 tumors significantly increased survival and decreased lung metastasis. It is quite feasible with the current technology to manipulate TGFβ signaling in bone marrow-derived myeloid cells through viral vector induced recombination and bone marrow transplantation. This novel approach, by targeting myeloid TGFβ signaling, may overcome some of the problems of systemic application of small molecular inhibitors of TβRII kinase or neutralizing antibodies that often have adverse effects in normal healthy tissues. This is particularly exciting in two aspects: 1) clinical inhibitors have not shown high rates of tumor response and deletion of Tgfbr2 in bone marrow-derived cells may provide a novel option that has not been described before; 2) the finding that myeloid specific TGFβ signaling is a significant part of the tumor-promoting effects of TGFβ allows specific and effective targeting. This finding is particularly important as TGFβ functions as both a tumor suppressor and promoter. The reduced tumor metastasis that we have observed suggests that myeloid-specific Tgfbr2 deletion is likely to be effective in countering the tumor-promoting role of TGFβ. Taken together, our studies demonstrate that myeloid-specific TGFβ signaling is a significant part of the tumor-promoting effects of TGFβ. This may provide therapeutic opportunities for new approaches to cancer therapy.
METHODS
Cell Lines and Generation of Tgfbr2MyeKO Mice
Murine 4T1, MC26, B16 and LLC cell lines were obtained from ATCC, and kept in the liquid nitrogen when not in use. Cells were thawed, cultured, and passaged less than 6 month for experiments. These mouse cell lines have not been authenticated by the authors. The Tgfbr2flox/flox mouse line was established as described (55, 56). LysM-Cre mice (C57BL/6 and 129 background) were obtained from Jackson Laboratory (Bar Harbor, ME). Tgfbr2flox/flox mice were bred with LysM-Cre mice to generate the Tgfbr2 deletion in myeloid cells (Tgfbr2MyeKO). Mice heterozygous for LysM-Cre and heterozygous for the floxed Tgfbr2 allele were further bred with wild type Balb/c or C57BL/6 for 10 generations to generate a Balb/c or C57BL/6 background. The genotyping of Tgfbr2flox/flox and Tgfbr2MyeKO mice, as well as Southern blotting on sorted T and B lymphocytes, and Gr-1+CD11b+ cells were done as described previously (55). The OT-1 and CL4 transgenic mice specific to OVA 257–264 peptide (SIINFEKL) and HA 518–526 peptide (IYSTVASSL) were obtained from Taconic and Jackson lab respectively. IFN-γ ko mice in C57BL/6 background were provided by Cancer Inflammation Program in NCI. All animal studies are approved by the National Cancer Institute Animal Care and Use Committee.
Flow Cytometry and Cell Sorting
Single cell suspensions were made from peripheral blood, lymph node, spleen, thymus and bone marrow from normal and tumor-bearing mice (16), as well as tumor and lung tissues (57). Gr-1+CD11b+ cells, CD3+ T cells, B220+ B cells, CD11b+Ly6G+ cells, CD11b+Ly6C+ cells, and CD11b+F4/80+ cells were sorted from splenocytes by FACSAria flow cytometer (BD) for various assays. For intracellular staining, cells were stimulated with PMA (50ng/ml), ionomycin (1 µg/ml), and monensin (2µM) for 4 hours, fixed, permeabilized using a BD Fixation/Permeabilization kit and stained for cytokines. The cells were analyzed on a FACS Calibur flow cytometer (BD, San Jose, CA). For human immature myeloid cells, peripheral blood from patients with metastatic non-small cell lung cancer was collected at the National Cancer Institute, Bethesda, MD, on an IRB-approved protocol. The cells were labeled with anti-CD33-PE, anti-CD15-PE, and anti-CD34-PE (BD Pharmingen) and sorted by MACS according to manufacturer’s protocol (Miltenyi Biotec).
Immunofluorescence (IF) Staining
The sorted myeloid cells were centrifuged on cytospin slides, fixed with 4% paraformaldehyde and incubated with a rat polyclonal anti-TβRII antibody (Santa Cruz CA,) followed by Alexa fluor 488 goat anti-rat or 594 goat anti-rabbit (Invitrogen, CA) antibodies.
TGFβ1 ELISA
Conditioned media was collected from cultured Gr-1+CD11b+ cells, CD11b+Ly6G+ cells, CD11b+Ly6C+ cells, and CD11b+F4/80+ cells (2% FBS RPMI1640, overnight). The samples were analyzed for TGFβ1 expression by using an ELISA kit (R&D Systems, MN).
Cytokine Antibody Array
Gr-1+CD11b+ cells were sorted from Tgfbr2MyeKO and Tgfbr2flox/flox mice bearing 4T1 tumors. The proteins were extracted and cytokine antibody array was performed per manufacturer protocol (Raybiotech, GA). The relative quantification was determined by dot density using Image J software.
iNOs and Arginase Functional Assays
FACS sorted myeloid cells were co-cultured with splenocytes from OT-1 mice with the stimulation of OVA peptide (Youn et al., 2008). Cell culture supernatant was collected and subjected to Nitrite assay using Nitric Oxide Quantitation Kit (Active Motif). For Arginase functional assay, sorted myeloid cells (1 × 106) cells from spleens of 4T1 tumor bearing Tgfbr2flox/flox and Tgfbr2MyeKO mice were lysed in Tris-HCl (pH 7.4) containing protease inhibitor cocktails and 1% Triton X-100. After centrifugation, cell lysate supernatant was analyzed for arginase activity with QuantiChrom Arginase Assay Kit (DARG-200).
Electroporation
MACS sorted Gr1+ myeloid cells (2×106) from spleens of Tgfbr2flox/flox and Tgfbr2MyeKO mice were electroporated with over expression plasmids of TGFβ1 (gift from Dr. Lalage Wakefield), IL-4 (pORF-mIL-4, InvivoGen) and IL-10 (pORF-mIL-10, InvivoGen) using Amaxa mouse macrophage nuclefector kit according to the manufacturer’s instructions.
IFN-γ ELISPOT
Single cell suspensions from the spleens of Tgfbr2MyeKO and Tgfbr2flox/flox mice bearing 4T1 tumors were prepared. Splenocytes (2 × 105) were stimulated with CD3 (0.5 ug/ml, eBioscience, CA), cultured overnight per manufacturer’s protocol (BD). The ELISPOT plate was scanned in ImmunoSpot (Cellular Technology Ltd. OH) and quantification was assessed using the CTL Scanning and CTL counting 4.0. For antigen specific T cell response regulated by myeloid cells, Gr-1+ myeloid cells were sorted from Tgfbr2flox/flox and Tgfbr2MyeKO mice, and co-cultured with splenocytes (2 × 105) from OT1 or CL4 transgenic mice at 6:1 ratios (splenocytes: myeloid cells). Over expression of arginase 1 (Origene, MD) and iNOS (gift from Victor Laubach, University of Virgina) in myeloid cells isolated from Tgfbr2MyeKO was done using mouse macrophage nucleofector kit (Lonza, Germany). Irradiated splenocytes (2000 rad, 5 × 105) were added as antigen presenting cells. OVA257–264 peptide or HA 518–526 peptide (1µg/ml) was added as stimulator. After 24-hour culture, IFN-γ ELISPOT assay were preformed and spot numbers were counted as described above.
Western Blotting
Gr-1+CD11b+ cells sorted from the spleens or lung tissues of normal or 4T1 tumor-bearing mice were lysed and analyzed by Western. Primary antibodies include TβRII, pSmad2, Smad2: Cell Signaling, MA; and beta-actin: Sigma.
Quantitative RT-PCR
Total RNA was extracted from sorted Gr-1+CD11b+ cells or subsets using an RNeasy Mini Kit (Qiagen, CA) and cDNA was synthesized using Invitrogen superscripttm First-strand synthesis system. Relative gene expression was determined using a BioRad iCycler-iQ SYBR Green PCR kit. Primer sequences are available upon request.
Spontaneous and Experimental Metastasis
For orthotopic metastasis, mammary tumor 4T1 cells (5 × 104) were injected into the #2 MFP. The numeration referred to: the neck to the groin, the neck (#1, left and right), the arm (#2, left and right), the thoracic (#3, left and right) and abdominal (#4 and #5, left and right). Mice were sacrificed 28 days later. For B16 orthotopic model, 1 × 106 B16 cells were injected subcutaneously, tumors were removed at day 16, mice were euthanized at day 37. For experimental metastasis, mice received tail vein injection (TVI) of 4T1 cells (2 × 105), LLC cells (2.5 × 105), MC26 cells (2 × 105), or B16 cells (2 × 105). Tumor size was measured at 2–3 day intervals using calipers as: Volume = length × (width)2 × 0.5. The number of lung metastasis was evaluated as described (58) or by H&E staining of lung section when mice died or became moribund, or when the primary tumors reached a size of 2.0 cm in diameter.
IFN-γ Neutralization
4T1 cells (5 × 104) were injected into #2 MFP of Tgfbr2flox/flox and Tgfbr2MyeKO mice. The mice were treated with IFN-γ neutralizing antibody XMG-6 or IgG control by intraperitoneal (IP) injection. Dosage: 1 mg antibody or IgG per mouse on D1, 3, and 6; 0.5 mg on D9, 12, 15, 18, 21, 24, and 27. Mice were sacrificed on D28 after tumor injection. Lung metastases were evaluated as described above and tumors were weighed.
CD8 T cell depletion
For in vivo depletion of CD8 T cells, CD8α neutralizing antibody (2.43 clone) and IgG2b (100 ug per mouse) were intraperitoneally injected every two days starting from the day 0 of 4T1 injection until the mice were sacrificed and evaluated for lung metastasis.
Myeloid Cell Reconstitution
The Tgfbr2flox/flox and Tgfbr2MyeKO mice were injected with 2 × 105 4T1 cells through tail vein (day 0). Tgfbr2MyeKO mice were then injected with different myeloid subsets: CD11b+Ly6G+; CD11b+Ly6C+; CD11b+F4/80+ cells; Gr-1+CD11b+ cells on day −1, 1, 3, 6, 8, 10, 13, 15, 17 via tail vein. The mice received 3 × 105 myeloid cells for the first 6 injections, then 1 × 106 for CD11b+Ly6G+ and Gr-1+CD11b+ cells for the last 3 injections. This is based on an increase of those myeloid cells in the peripheral blood over the time after tumor injection. Mice were euthanized on day 20. Lung metastasis was evaluated.
Ex vivo Pulmonary Metastasis Assay (PuMA)
B16BL6-GFP cells (5 × 105) were co- injected with sorted myeloid cells (106) through tail vein. Mice were euthanized 5 minutes after injection, and the lungs were infused with agarose as described (59). Lung sections were sliced (1–2mm thick) and placed on Gelfoam (Pfizer-Pharmacia & Upjohn Co.) for culture. LEICA-DM IRB fluorescent inverted microscope (Leica) and Retiga-EXi Fast 1394 Mono Cooled CCD camera (QImaging) were used to capture GFP positive cells at ×10 magnification. The area was quantified by OpenLab software (Improvision)(59). For the effect of IFN-γ deficient myeloid cells on metastasis, the Ly-6G cells were sorted from IFN-γ ko mice bearing B16 melanoma (C57/Bl6 background). The cells were then injected with B16 cells through tail vein. The lung section culture was performed as described above. The metastasis was evaluated after 2–3 weeks.
Bone Marrow Transplant
4T1 cells (5 × 105) were injected into the #4 & 5 MFP of wild type Balb/c mice (recipient). Fifteen days later, the primary tumors were surgically removed and weighed. The mice were left to recover until day 34 after tumor injection, which allow them to develop invasive tumor and metastasis. On day 34, these mice were irradiated (900 cGy). Bone marrow cells (5 × 105) from Tgfbr2MyeKO mice or Tgfbr2flox/flox control mice (donor mice) were injected into the tail vein of recipient mice in 100 µl PBS. Acidified water (pH 1.3 to 2.0), autoclaved food, and autoclaved cages were used for the recipient mice for two weeks after irradiation. Lung metastases were evaluated starting day 63.
Statistical Analysis
Graphpad Prism v5.04 was used for the graphs and for statistics. All data other than indicated were analyzed using the Student’s t-test, and was expressed as mean ± SE. Differences were considered statistically significant when the p-value was < 0.05.
Supplementary Material
SIGNIFICANCE.
Our study identifies myeloid-specific TGFβ signaling as a critical mediator in tumor metastasis, distinct from the tumor suppression effect of TGFβ signaling in epithelial cells, fibroblasts, and T cells. We further provide mechanistic insight into host anti-tumor immunity, and suggest a cell type specific cancer-targeting strategy.
ACKNOWLEDGMENTS
We thank Drs. Glenn Merlino and Stuart Yuspa for their critical reading of the manuscript. We thank Ana Chytil, Vanderbilt Cancer Center, Nashville, TN, for technical assistance to Hannah Yan on the Southern hybridization. We are grateful for the technical assistance from FACS Core of the Center for Cancer Research, National Cancer Institute.
This work was supported by NCI intramural funding to Li Yang
Abbreviation list
- CTL
cytotoxic T Lymphocyte
- ELISPOT
Enzyme Linked Immunosorbent Spot
- FACS
Fluorescence Activated Cell Sorting
- iNOS
Inducible Nitric Oxide Synthase
- MACS
Magnetic Activated Cell Sorting
- MDSC
Myeloid Derived Suppressor Cells or Gr-1+CD11b+immature myeloid cells
- MFP
Mammary Fat Pad
- NO
Nitric Oxide
- PuMA
Pulmonary Metastasis Assay
- TAM
Tumor Associated Macrophages
- TAN
Tumor Associated Neutrophil
- TβR
TGFβ receptor
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interest.
REFERENCES
- 1.Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 2.Massague J. TGFbeta in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adorno M, Cordenonsi M, Montagner M, Dupont S, Wong C, Hann B, et al. A Mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell. 2009;137:87–98. doi: 10.1016/j.cell.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 4.Liu IM, Schilling SH, Knouse KA, Choy L, Derynck R, Wang XF. TGFbeta-stimulated Smad1/5 phosphorylation requires the ALK5 L45 loop and mediates the pro-migratory TGFbeta switch. EMBO J. 2009;28:88–98. doi: 10.1038/emboj.2008.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 6.Yang L, Moses HL. Transforming growth factor beta: tumor suppressor or promoter? Are host immune cells the answer? Cancer Res. 2008;68:9107–9111. doi: 10.1158/0008-5472.CAN-08-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 8.Achyut BR, Bader DA, Robles AI, Wangsa D, Harris CC, Ried T, et al. Inflammation-Mediated Genetic and Epigenetic Alterations Drive Cancer Development in the Neighboring Epithelium upon Stromal Abrogation of TGF-beta Signaling. PLoS Genet. 2013;9:e1003251. doi: 10.1371/journal.pgen.1003251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim BG, Li C, Qiao W, Mamura M, Kasprzak B, Anver M, et al. Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature. 2006;441:1015–1019. doi: 10.1038/nature04846. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, et al. Abrogation of TGFbeta Signaling in Mammary Carcinomas Recruits Gr-1+CD11b+ Myeloid Cells that Promote Metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bierie B, Stover DG, Abel TW, Chytil A, Gorska AE, Aakre M, et al. Transforming growth factor-beta regulates mammary carcinoma cell survival and interaction with the adjacent microenvironment. Cancer Res. 2008;68:1809–1819. doi: 10.1158/0008-5472.CAN-07-5597. [DOI] [PubMed] [Google Scholar]
- 12.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mantovani A. Cancer: Inflaming metastasis. Nature. 2009;457:36–37. doi: 10.1038/457036b. [DOI] [PubMed] [Google Scholar]
- 15.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang L, Debusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan HH, Pickup M, Pang Y, Gorska AE, Li Z, Chytil A, et al. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 2010;70:6139–6149. doi: 10.1158/0008-5472.CAN-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10:554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Z, Pang Y, Gara SK, Achyut BR, Heger C, Goldsmith PK, et al. Gr-1+CD11b+ cells are responsible for tumor promoting effect of TGF-beta in breast cancer progression. Int J Cancer. 2012;131:2584–2595. doi: 10.1002/ijc.27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 22.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 23.Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120:457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaBreche HG, Nevins JR, Huang E. Integrating factor analysis and a transgenic mouse model to reveal a peripheral blood predictor of breast tumors. BMC Med Genomics. 2011;4:61. doi: 10.1186/1755-8794-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rotunno M, Hu N, Su H, Wang C, Goldstein AM, Bergen AW, et al. A gene expression signature from peripheral whole blood for stage I lung adenocarcinoma. Cancer Prev Res (Phila) 2011;4:1599–1608. doi: 10.1158/1940-6207.CAPR-10-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill CR, Sanchez NS, Love JD, Arrieta JA, Hong CC, Brown CB, et al. BMP2 signals loss of epithelial character in epicardial cells but requires the Type III TGFbeta receptor to promote invasion. Cellular signalling. 2012;24:1012–1022. doi: 10.1016/j.cellsig.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker L, Millena AC, Strong N, Khan SA. Expression of TGFbeta3 and its effects on migratory and invasive behavior of prostate cancer cells: involvement of PI3-kinase/AKT signaling pathway. Clin Exp Metastasis. 2013;30:13–23. doi: 10.1007/s10585-012-9494-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun CX, Downey GP, Zhu F, Koh AL, Thang H, Glogauer M. Rac1 is the small GTPase responsible for regulating the neutrophil chemotaxis compass. Blood. 2004;104:3758–3765. doi: 10.1182/blood-2004-03-0781. [DOI] [PubMed] [Google Scholar]
- 29.Hazenbos WL, Clausen BE, Takeda J, Kinoshita T. GPI-anchor deficiency in myeloid cells causes impaired FcgammaR effector functions. Blood. 2004;104:2825–2831. doi: 10.1182/blood-2004-02-0671. [DOI] [PubMed] [Google Scholar]
- 30.Munder M, Mallo M, Eichmann K, Modolell M. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: A novel pathway of autocrine macrophage activation. J Exp Med. 1998;187:2103–2108. doi: 10.1084/jem.187.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schindler H, Lutz MB, Rollinghoff M, Bogdan C. The production of IFN-gamma by IL-12/IL-18-activated macrophages requires STAT4 signaling and is inhibited by IL-4. J Immunol. 2001;166:3075–3082. doi: 10.4049/jimmunol.166.5.3075. [DOI] [PubMed] [Google Scholar]
- 32.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31:220–227. doi: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med. 2001;7:1118–1122. doi: 10.1038/nm1001-1118. [DOI] [PubMed] [Google Scholar]
- 35.Shah AH, Tabayoyong WB, Kundu SD, Kim SJ, Van Parijs L, Liu VC, et al. Suppression of tumor metastasis by blockade of transforming growth factor beta signaling in bone marrow cells through a retroviral-mediated gene therapy in mice. Cancer Res. 2002;62:7135–7138. [PubMed] [Google Scholar]
- 36.Novitskiy SV, Pickup MW, Chytil A, Polosukhina D, Owens P, Moses HL. Deletion of TGF-beta signaling in myeloid cells enhances their anti-tumorigenic properties. J Leukoc Biol. 2012;92:641–651. doi: 10.1189/jlb.1211639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 38.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 40.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 42.Goswami S, Sahai E, Wyckoff JB, Cammer M, Cox D, Pixley FJ, et al. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65:5278–5283. doi: 10.1158/0008-5472.CAN-04-1853. [DOI] [PubMed] [Google Scholar]
- 43.Hernandez L, Smirnova T, Kedrin D, Wyckoff J, Zhu L, Stanley ER, et al. The EGF/CSF-1 paracrine invasion loop can be triggered by heregulin beta1 and CXCL12. Cancer Res. 2009;69:3221–3227. doi: 10.1158/0008-5472.CAN-08-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, DeNardo DG, et al. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010;70:7465–7475. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, Yuan LJ, et al. Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest. 2010;120:2699–2714. doi: 10.1172/JCI39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zabuawala T, Taffany DA, Sharma SM, Merchant A, Adair B, Srinivasan R, et al. An ets2-driven transcriptional program in tumor-associated macrophages promotes tumor metastasis. Cancer Res. 2010;70:1323–1333. doi: 10.1158/0008-5472.CAN-09-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yiakouvaki A, Dimitriou M, Karakasiliotis I, Eftychi C, Theocharis S, Kontoyiannis DL. Myeloid cell expression of the RNA-binding protein HuR protects mice from pathologic inflammation and colorectal carcinogenesis. J Clin Invest. 2012;122:48–61. doi: 10.1172/JCI45021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stockmann C, Doedens A, Weidemann A, Zhang N, Takeda N, Greenberg JI, et al. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature. 2008;456:814–818. doi: 10.1038/nature07445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, et al. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sugiyama Y, Kakoi K, Kimura A, Takada I, Kashiwagi I, Wakabayashi Y, et al. Smad2 and Smad3 are redundantly essential for the suppression of iNOS synthesis in macrophages by regulating IRF3 and STAT1 pathways. Int Immunol. 2012;24:253–265. doi: 10.1093/intimm/dxr126. [DOI] [PubMed] [Google Scholar]
- 53.Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 55.Chytil A, Magnuson MA, Wright CV, Moses HL. Conditional inactivation of the TGF-beta type II receptor using Cre:Lox. Genesis. 2002;32:73–75. doi: 10.1002/gene.10046. [DOI] [PubMed] [Google Scholar]
- 56.Forrester E, Chytil A, Bierie B, Aakre M, Gorska AE, Sharif-Afshar AR, et al. Effect of conditional knockout of the type II TGF-beta receptor gene in mammary epithelia on mammary gland development and polyomavirus middle T antigen induced tumor formation and metastasis. Cancer Res. 2005;65:2296–2302. doi: 10.1158/0008-5472.CAN-04-3272. [DOI] [PubMed] [Google Scholar]
- 57.Ljung BM, Mayall B, Lottich C, Boyer C, Sylvester SS, Leight GS, et al. Cell dissociation techniques in human breast cancer--variations in tumor cell viability and DNA ploidy. Breast Cancer Res Treat. 1989;13:153–159. doi: 10.1007/BF01806527. [DOI] [PubMed] [Google Scholar]
- 58.Jessen KA, Liu SY, Tepper CG, Karrim J, McGoldrick ET, Rosner A, et al. Molecular analysis of metastasis in a polyomavirus middle T mouse model: the role of osteopontin. Breast Cancer Res. 2004;6:R157–R169. doi: 10.1186/bcr768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mendoza A, Hong SH, Osborne T, Khan M, Campbell K, Briggs J, et al. Modeling metastasis biology and therapy in real time in the mouse lung. J Clin Invest. 2010;120:2979–2988. doi: 10.1172/JCI40252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


