Abstract
Introduction
Surface osteosarcoma are rare variant of osteosarcoma that include parosteal osteosarcoma, periosteal osteosarcoma and high grade surface osteosarcoma. These lesions have different clinical presentation and biological behavior compared to conventional osteosarcoma, and hence need to be managed differently.
Goal
The aim of this study is to analyze the clinico-pathological features and outcome of a series of surface osteosarcoma in an attempt to define the adequate treatment of this rare entity.
Patient and method
It is a retrospective and bicentric study of 18 surface osteosarcoma that were seen at the KASSAB’s Institute and SAHLOUL Hospital from 2006 to 2013. The authors reviewed the clinical and radiologic features, histologic sections, treatments, and outcomes in this group of patients.
Results
Seven patients were male (38.9%) and 11 were female (61.1%) with mean age of 25 years (range from 16 to 55 years). Eleven lesions were in the femur and 7 in the tibia. We identified 11 parosteal osteosarcoma (six of them were dedifferentiated), 3 periosteal osteosarcoma and 4 high grade surface osteosarcoma. Six patients had neoadjuvant chemotherapy and all lesions had surgical resection. Margins were wide in 15 cases and intra lesional in 3 cases. Histological response to chemotherapy was poor in all cases. The mean follow up was 34.5 months. Six patients (33.3%) presented local recurrence and 8 patients (44.4%) presented lung metastases. Six patients (33.3%) died from the disease after a mean follow up of 12 months (6–30 months); all of them had high grade lesions.
Conclusion
Histological grade of malignancy is the main point to assess in surface osteosarcoma since it determines treatment and prognosis. Low grade lesions should be treated by wide resection, while high grade lesions need more aggressive surgical approach associated to post operative chemotherapy.
Keywords: Parosteal osteosarcoma, Periosteal osteosarcoma, High grade surface osteosarcoma, Chemotherapy-surgery
1. Level of evidence: IV
1.1. Introduction
Osteosarcoma are primary malignant bone tumors in which neoplastic cells produce osteoid. The spectrum of lesions is very wide, which can be distinguished either by their grade of malignancy, the differentiation of neoplastic cells or even according to their location related to the bone. Most of sarcoma arise intramedullarly, and only few of them develop on the outer surface of the cortex when they are called “surface osteosarcoma” [1]. They are classified into three subtypes: parosteal osteosarcoma (POS), periosteal osteosarcoma (PerOS) and high grade surface osteosarcoma (HGSO). They account for 5%, 1.5% and 0.5% of all osteosarcomas respectively [2]. Classic parosteal osteosarcoma (cPOS) originates from the outer fibrous layer of the periosteum. Its cells are fibroblasts. It is characterized by the low grade of anaplasia and slow indolent course. Occasionally, it presents a progression in malignancy and becomes dedifferentiated (DPOS). Periosteal osteosarcoma developes within the inner, germinative layer of the periosteum (the cambium layer) from cells differentiating in osteoblasts and chondroblasts. Its histological grade of malignancy is intermediate. HGSO arises from the surface of bone and it is entirely high grade, with a high mitotic activity identical to that of conventional OS. We report the clinical data and the oncological outcome of 18 cases of surface osteosarcoma and suggest the appropriate management.
2. Patients and methods
We reviewed retrospectively the clinical data of 18 patients treated between 2006 and 2013 for a surface osteosarcoma. Patients were managed in 2 centers: Kassab’s National Institute and Sahloul Hospital. Clinical data were available from patients’ charts. Imaging records and histological slides were reviewed and discussed respectively with a radiologist and a pathologist that are specialized in musculo-skeletal pathology. Inclusive criteria were bone sarcoma producing osteoid originated at the surface of the bone regardless the grade of malignancy and the degree of medullar extension. The mean follow-up was 34.5 months (range: 6–96).
We analyzed the survival, local recurrence, and distant metastasis outcomes using survivorship techniques. Duration of symptoms was analyzed with t-test of student. We used Kaplan–Meier survival analysis to determine the cumulative probability of survival, survivorship free of recurrence, and survivorship free of metastasis. Differences in survivorship curves were evaluated using log-rank tests and p values less than 0.05 were considered significant. Statistical summaries, analyses, and plotting of survival curves were performed using SPSS version 22.
3. Results
3.1. Clinical findings
Seven patients were male (38.9%) and 11 were female (61.1%). The mean age of patients was 25 years (range from 16 to 55 years). All lesions involved the appendicular skeleton. Seven of them (38.9%) were in the right side and 11 (61.1%) were in the left side. The most common location was the knee (9 cases: 50%), with the distal part of the femur involved in 8 cases. The duration of symptoms before diagnosis ranged from 3 to 48 months (mean 12.6 months). cPOS had the longest duration compared to high grade lesions (22.5 vs. 12.1 months). The most common symptom was swelling, present in all patients. It was painful in 16 patients (88.8%). Only 4 patients (22.2%) had joint stiffness, mainly knee flexum. Clinical data are summarized in Table 1.
Table 1.
Clinical data and outcome of patients.
| Patient | Sex/age | Location | Duration of symtoms (months) | Histological diagnosis | Differentiation/grade | Neo adjuvant chemotherapy: Yes or No/response | Surgical management | Margins | Local recurrence/treatment | Metastases | Survival | Follow up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/30 | DF | 24 | cPOS | C/1 | No | Cortical resection | IL | Yes/ amputation | No | NED | 30 |
| 2 | F/25 | FD | 6 | cPOS | F/2 | No | Intercalary resection | W | No | No | NED | 60 |
| 3 | M/19 | TD | 36 | cPOS | F/1 | No | Cortical resection | W | No | No | NED | 48 |
| 4 | F/20 | DF | 24 | cPOS | F/1 | No | Prosthesis | W | No | No | NED | 72 |
| 5 | M/23 | DF | 12 | cPOS | F/2 | No | Knee arthrodesis | W | No | No | NED | 12 |
| 6 | F/55 | DF | 5 | DPOS | O/4 | No | Amputation | W | No | No | NED | 20 |
| 7 | F/27 | DF | 3 | DPOS | O/4 | Yes/poor | Knee arthrodesis | W | No | Yes | DOD | 12 |
| 8 | M/16 | DF | 6 | DPOS | O/4 | Yes/poor | Prosthesis | W | Yes/amputation | Yes | DOD | 15 |
| 9 | M/52 | PT | 48 | DPOS | C/3 | No | Prosthesis | W | No | No | NED | 40 |
| 10 | M/37 | DF | 4 | DPOS | O/4 | Yes/poor | Prosthesis | W | Yes | Yes | DOD | 6 |
| 11 | M/27 | DF | 24 | DPOS | F/4 | Yes/poor | Prosthesis | W | No | Yes | AWD | 72 |
| 12 | F/17 | TD | 6 | PeOS | C/3 | No | Cortical resection | IL | Yes/amputation | No | NED | 24 |
| 13 | F/16 | FD | 6 | PeOS | C/2 | No | Intercalary reconstruction | W | No | No | NED | 72 |
| 14 | M/17 | TD | 3 | PeOS | O/2 | No | Intercalary reconstruction | W | No | No | NED | 18 |
| 15 | F/19 | FD | 4 | HGSO | C/4 | No | Intercalary reconstruction | IL | Yes | Yes | DOD | 6 |
| 16 | F/16 | FD | 3 | HGSO | C/4 | Yes/poor | Intercalary reconstruction | W | Yes/amputation | Yes | DOD | 30 |
| 17 | F/16 | TD | 12 | HGSO | C/4 | No | Intercalary reconstruction | W | No | Yes | DOD | 6 |
| 18 | F/17 | TD | 18 | HGSO | C/4 | Yes/poor | Intercalary reconstruction | W | No | Yes | AWD | 12 |
M: male; F: female; DF: distal femur; FD: femoral diaphysis; PT: proximal tibia; TD: tibial diaphysis; cPOS: classic parosteal osteosarcoma; DPOS: dedifferentiated parosteal osteosarcoma; PeOS: periosteal osteosarcoma; HGSO: high grade surface osteosarcoma; C: chondroblastic; F: fibroblastic; O: osteoblastic; IL: intralesional; W: wide; NED: no evidence of disease; DOD: dead of disease; AWD: alive with disease.
3.2. Imaging features
Roentgenographic findings were analyzed for all patients. Seventeen patients had been evaluated with magnetic resonance imaging and 10 patients had computed tomography.
Mainly, two patterns were observed. The first one corresponded to parosteal lesions and the second one to periosteal and high grade surface lesions.
-
•
Parosteal tumors were the most frequent ones with 11 cases (61.1%). They had a predilection for the posterior cortex of the distal metaphysis of the femur (8 cases: 72.7%). One case involved the proximal metaphysis of the tibia and 2 cases were diaphyseal: one in the femur (case 1) and one in the tibia.
Typically, the tumor had a melon-shaped, mineralized mass, pasted on the cortex (Figs. 1a and 2a). The tumor size ranged from 5 to 10 cm. The pattern of mineral was amorphous (50%) (Fig. 1a), lobulated (40%) (Fig. 2a) or striated (10%). There was a thin radiolucent zone between the tumor and the underlying bone (Figs. 1a and 2a) in 81.1% of cases. A speculated periosteal reaction was present in only one case (9%). The underlying cortex was eroded or destroyed in 4 cases (36.3%). In the larger, broad-based tumors, the cortex of the host bone was expanded by a new-bone formation in 60% of cases. Medullary involvement was clearly shown on plan radiographs in 5 cases (45.4%). It was focal in 2 cases (Fig. 2a) and diffuse in 3 cases.
Seven of these lesions were evaluated by CT scan wich was useful to study the attachment of the tumor to the host bone, medullary involvement and the presence of radiolucency within the mineralized component (Figs. 1b and 2b).
MRI was available in 10 patients. It best evaluated local extension of the tumor and especially medullary involvement. This was observed in 8 cases (72.7%). It was focal in 5 cases (Fig. 2c) and diffuse in 3 cases.
Medullary extension was suspected radiologically in two cases of the cPOS (50%) and in all cases (100%) of DPOS.
-
•
The second pattern was that of PerOS (3 cases) and HGSO (4 cases). All lesions were diaphyseal and periosteal reaction was constant. Typically, lesion had saucer shape appearance characterized by a cortical thickening at the periphery with Codman triangle and a non mineralized soft tissue mass at the center (Figs. 3a and 4a). The soft tissue mass was characterized by an aggressive periosteal reaction perpendicular to the osseous long axis and causes scalloping that affected only the thickened cortex (Fig. 3a). There was no abnormality in the adjacent medullary canal. Fine ring calcifications were seen in 3 cases (42.8%) and they were related to the chondroblastic differentiation of the lesion (Fig. 4a).
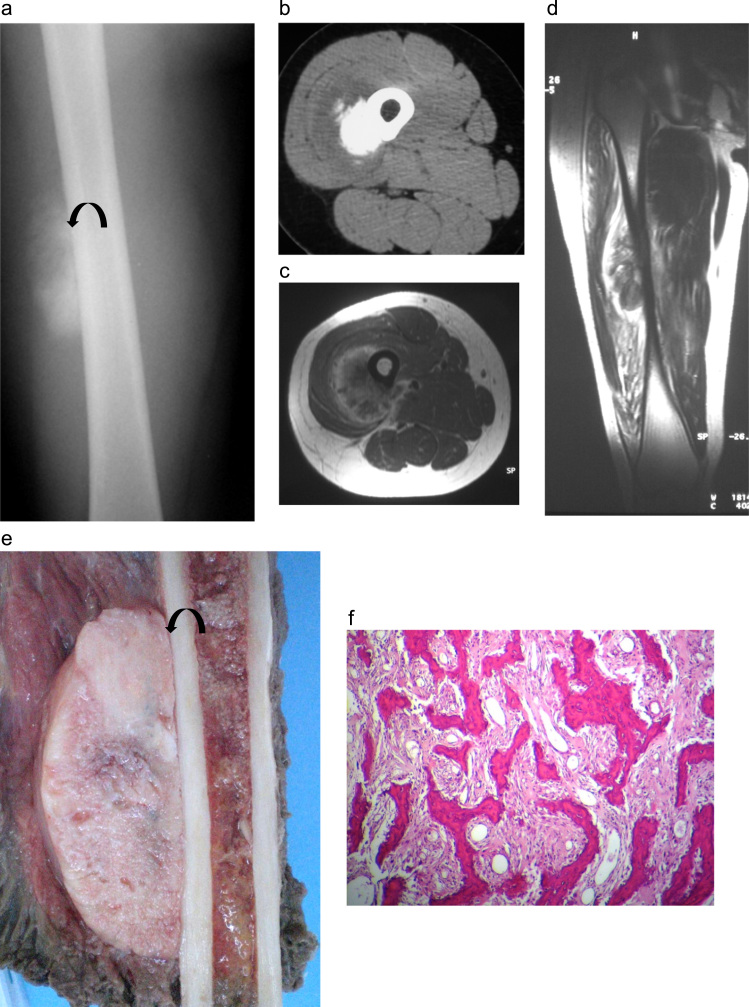
Fig. 1.
Case 1: parosteal osteosarcoma in a 24-year-old patient: (a) AP radiograph of the right femur shows an ossified exophytic tumor on the surface of the femur. A lucent cleavage plane (arrow) is seen between the tumor and the underlying cortex. (b) Transversal CT image in soft tissue algorithm: ossified lesion developed at the surface of bone cortex with hypodense peripheral rim. (c) Transversal T1 MR image after Gadolinium administration and (d) sagittal T2 image. The lesion shows low T1 and T2 intensity with peripheral enhancement but no adjacent bone signal abnormality. (e) Gross specimen showing a large, gritty white mass pasted on the underlying cortex with no medullary invasion. The cut surface displayed a homogeneous appearance with lucent areas. A thin lucent line ( arrow) is seen between the tumor and bone and corresponds to the periosteum. (f) Lower-power photomicrograph showing a well-formed bony trabeculae in a hypocellular spindle cell stroma. The tumor is low grade attested by the slight nuclear pleomorphism of the cellular component (hematoxylin and eosin 25×).
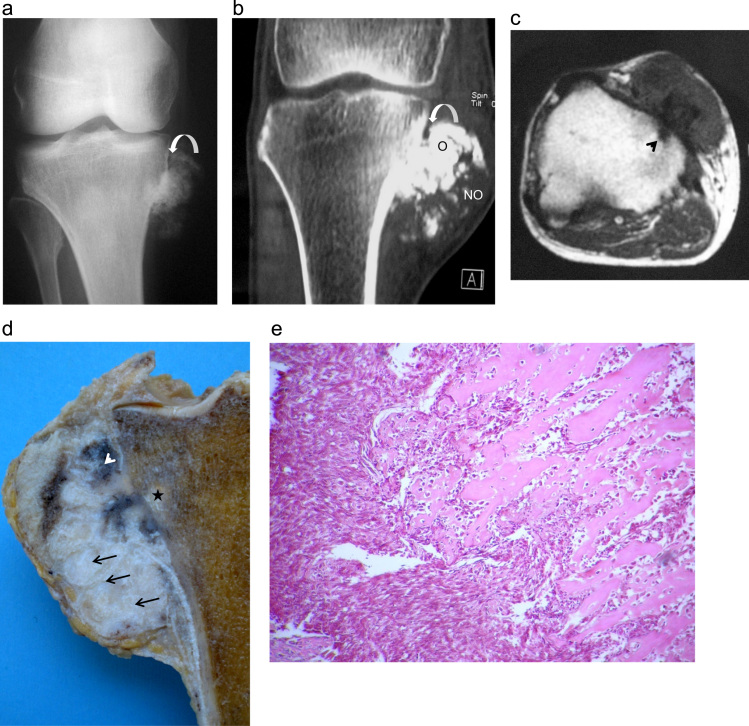
Fig. 2.
Case 2: DPOS in a 54-year-old male: (a) AP radiograph of the right knee shows an ossified tumor in medial proximal tibia with a lucent cleavage plane at its edge (arrow). (b) Coronal CT view of the right knee: surface bone lesion developed on the proximal tibia cortex. The lesion presents a proximal ossified (O) and distal hypodense (NO) appearance and is partially separated from bone by a hypodense thin band. (c) Transversal T1 MR image: the lesion presents a low T1 signal intensity and extends through the cortex to the bone marrow (arrowhead). (d) Gross specimen showing a nonhomogneous appearance of the DPOS. The tumor is composed of a mainly lytic (arrows), ivory areas intermixed with tan areas (arrow head) located close to the cortex and penetrate focally into the medullary canal (star). (e) Lower-power photomicrograph showing a conventional parosteal osteosarcoma to the right and area of dedifferentiation with pleomorphic appearing nuclei to the left (hematoxylin and eosin, 25×). Note the anastomosing arrangement of mature bony trabeculae with stromal cells in between. The dedifferentiation component corresponds to a high-grade spindle cell sarcoma. The transition between the two components is abrupt.
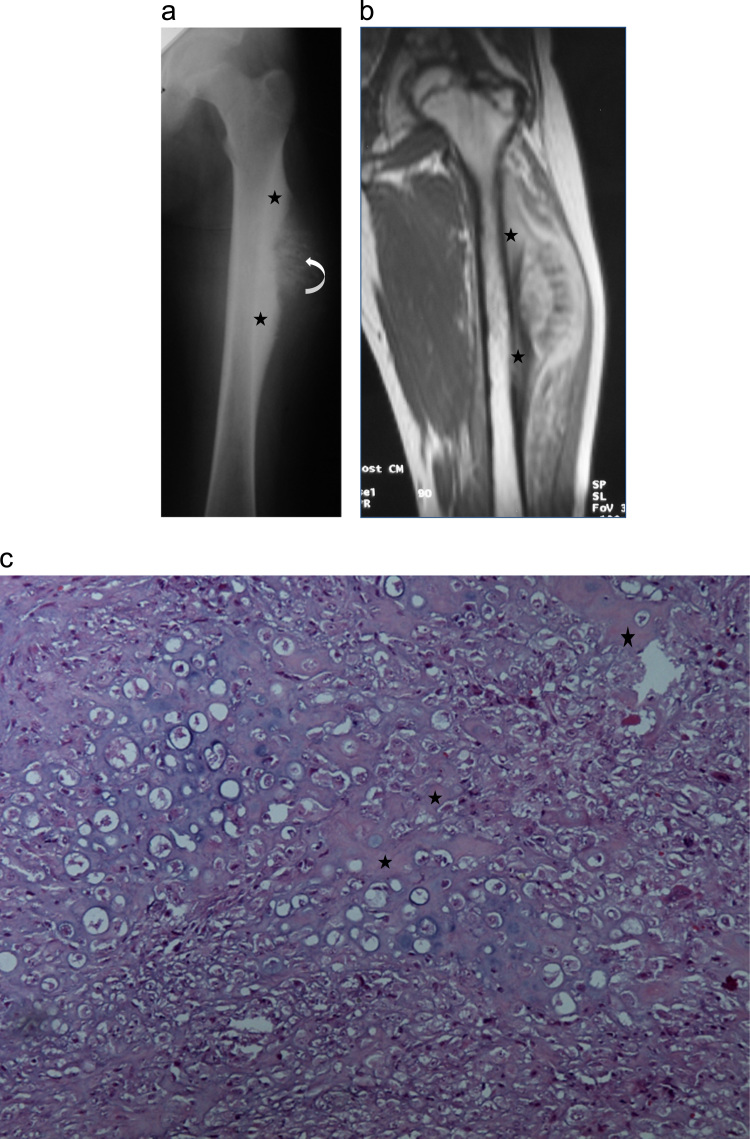
Fig. 3.
Case 3: periosteal osteosarcoma in a 16-year-old female: (a) AP radiograph of the left femur shows a surface bone tumor of the femoral diaphysis with cortical thickening and mostly at its edges (stars) and perpendicular peri-osteal spicules in the center (arrow) giving a tipical saucer-shape appearance. (b) Coronal T1 MR image after intravenous Gadolinium administration showing a surface bone lesion of the proximal femoral diaphysis with heterogenous MR signal, thickened cortex (stars) and no bone marrow abnormality. (c) Lower-power photomicrograph showing a periosteal osteosarcoma. The tumor is predominatly cartilaginous and the lace-like osteoid (stars) production is reduced (hematoxylin and eosin, 40×).
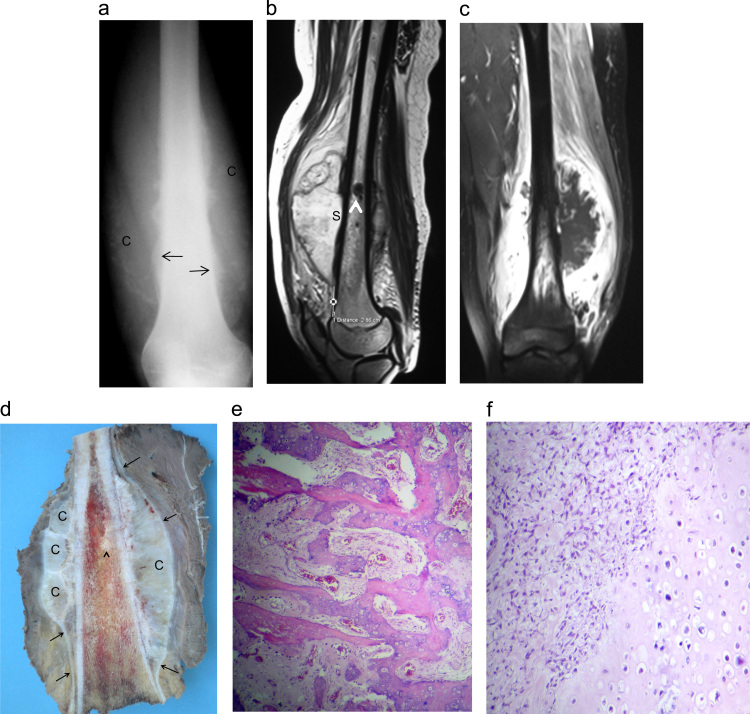
Fig. 4.
Case 4: High grade surface osteosarcoma in a 16-year-old female: (a) AP radiograph of the left femur: soft tissue swelling with chondroïd matrix (C) circonferential to the distal femur shaft. Note the periosteal bone formation with codman triangle (arrows). (b, c) MRI (sagittal T2 and coronal T1 image after fat suppression and intravenous Gadolinium administration) shows a low T1 and high T2 tumor mostly developed around the bone surface with cortical scalopping (S) and bone marrow signal abnormality (arrow head). (d) Gross specimen showing a huge circumferential white to grayish tumor. The tumor is mainly composed of lobulated chondroïd areas (C). The tumor develops in the deeper part of the periosteum and shows lifting of the periosteum (arrows). Bone marrows invasion (arrow head) High grade surface osteosarcoma wich shows prédominent chondroblastic differentiation: (e) photomicrograph showing large amounts of bone with spindle-cell stroma between the osseous trabeculae. (f) Photomicrograph showing the cartilaginous component with an irregular arrangement of chondrocytes.
HGSO were radiologically more aggressive. While PerOS affected only one part of the diaphyseal cylinder (Fig. 3a), HGSO were circumferential in 2 cases (Fig. 4a). Soft tissue mass was less mineralized in HGSO than in PerOS.
All patients were evaluated by MRI. Lesion margins were well defined with a pseudocapsule which corresponds to the periosteum (Figs. 3b and 4b). The marrow signal intensity was normal in all patients with PerOS (Fig. 3b) and abnormal in HGSO (Fig. 4b and c). In later cases, bone marrow invasion was focal in 3 cases (Fig. 4b) and diffuse in one case. MR images obtained after intravenous gadolinium administration showed a marked degree of enhancement in all patients. The enhancement pattern was the thick peripheral and septal enhancement with nodularity pattern in all cases (Figs. 3b and 4c).
3.3. Histological findings
Tumors were classified into the following subtypes:
-
●
Parosteal osteosarcoma (11 cases: 61.1%): Among parosteal tumors, 5 (45.4%) were low grade lesions (cPOS) with hypocellular spindle_cell stroma that contained well differentiated bone trabiculae (Fig. 1f). Spindle cells showed minimum cytological atypia and rare mitotic activity. Three lesions were graded 1 and two graded 2. Four were fibroblastic and one chondroblastic. In one case, the osseous trabeculea of the neoplasm had cement lines simulating the appearance of Paget disease and in one case they had fibrous dysplasia appearance.
Six of the parosteal osteosarcomas (54.6%) were considered to have undergone dedifferentiation (DPOS); that is, a high grade spindle cell sarcoma co-existed with a lower grade, more typical parosteal osteosarcoma (Fig. 2e). Four cases were recognized at the time of the initial diagnosis (synchronous lesions) and in two cases, dedifferentiation occurred at the time of recurrence (metachronous lesions). Cartilaginous component was identified in 4 cases (66%). In two cases, cartilage was predominant. Three cases were osteoblastic and one fibroblastic.
Medullary involvement was observed histologically in only one case in the group of cPOS, but was constant in the dedifferentiated lesions.
-
●
Periosteal osteosarcomas (3 cases: 16.6%): Tumors were diagnosed histologically as moderately differentiated (Grade 2 or 3). All cases were predominantly chondroblastic (Fig. 3c). Bone marrow was not invaded in any case.
-
●
High grade surface osteosarcomas (4 cases: 22.2%): Tumors were grade 4 on Broder’s scale (Fig. 4e). Three cases were chondroblastic (Fig. 4f) and one osteoblastic. Tumors were very permeative and involvement of bone marrow was observed in all cases (Fig. 4d).
3.4. Treatment
-
•
Surgical procedures: All patients underwent surgical resection. For diaphyseal tumors (9 cases), we performed cylindrical resection and intercalary reconstruction in 7 cases and partial resection of the cortex in two cases.
For metaphyseal and epiphyseal tumors (9 cases), we performed epiphyseal resection and reconstruction with endoprosthesis in 5 cases, knee arthrodesis in two cases, resection of the posterior cortex of the distal femur with bone grafting in one case and above the knee amputation in one case. Margins were considered wide in 15 cases and intralesional in 3 cases.
-
•
Chemotherapy: 10 patients with high grade lesions (DPOS or HGS) received modern protocols of chemotherapy incorporating doxorubicin and methotrexate. Six of them received pre and post operative chemotherapy, and 4 patients received only post operative chemotherapy. Response to chemotherapy in patients that received neoadjuvant was poor in all cases.
3.5. Local control
There were 6 cases of local recurrence (33.3%). Three cases were secondary to intralesional resections and three cases were observed after wide resections. In the latter cases, tumors were of a high grade (2 DPOS and 1 HGSO). Local recurrences were treated by amputation in 5 cases.
The mean survival period without local recurrence was 48.5±7.31 months. The survival rates without local recurrence at 2 and 5 years were respectively 72.4% and 54.3%. The survival rates without local recurrence at 5 years for low garde tumors (cPOS and PerOS) and in high grade tumors (DPOS and HGSO) were respectively 80% and 34.1%. The univariate analysis showed no significant difference (p=0.07).
3.6. Metastases
Seven patients (38.8%) developed lung metastases. All of them had high grade tumors (3 DPOS and 4 HGSO). Three patient had metastasectomy associated to chemotherapy and 4 patients had chemotherapy only.
The mean survival period without metastases was 47.42±7.71 months. The survival rates without metastases at 2 and 5 years were respectively 66.02% and 38.6%. The survival rates without metastases at 5 years for low garde tumors (cPOS and PerOS) and in high grade tumors (DPOS and HGSO) were respectively 100% and between 20% and 40%. The univariate analysis showed that histologic grade is a prognostic factor for metastases (p=0.004).
3.7. Survival
Twelve patients (66.7%) were still alive, and six (33.3%) died secondary to lung metastases after a mean time from surgery of 15.6 months (range: 6–30).
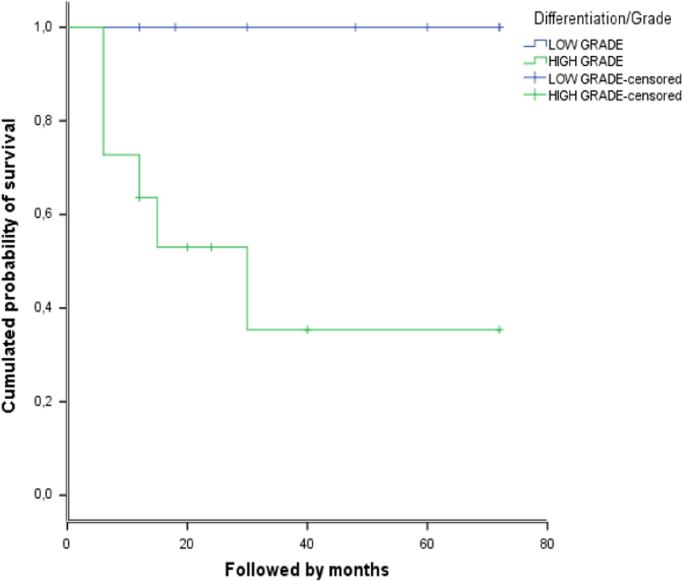
The overall survival period without death was 50±7 months. The survival rates at 2 and 5 years were respectively 71.3% and 62.4%. For low garde tumors (cPOS and PerOS) the survival rate without death was 100% while in high grade tumors (DPOS and HGSO) it was 35.4% (Fig. 5). The univariate analysis showed that malignancy grade was significantly related to prognosis (p=0.018).
Fig. 5.
Survival rate without death according the malignancy differentiation (low grade: cPOS and PerOS, high grade: DPOS and HGSO)).
Table 2 resumes statistical data.
Table 2.
Statistic data.
| Low and intermediate grade tumors | High grade tumors | p-value | |
|---|---|---|---|
| 5 years survival rate with local recurrence | 80% | 34.1% | 0.07 |
| 5 years survival rate with metastases | 100% | 20–40% | 0.004 |
| 5 years survival rate with death | 100% | 35.4% | 0.018 |
| Mean duration of symptoms | 16±4.66 months | 12±4.14 months | 0.56 |
4. Discussion
Our study is limited by the low number of cases reported. This is due to the rarity of surface osteosarcoma. These cases represented only 17.5% of all osteosarcoma treated in the same period in both centers. The most frequent lesion observed in our series was POS which represented 61% of surface osteosarcoma and 11% of all osteosarcoma. PerOS and HGSO represented each of them respectively 16.6% and 22.2% of surface osteosarcoma and 3.2% and 3.4 of all osteosarcoma.
In the literature, the proportion of surface osteosarcoma is much lower. They do not exceed 8% of all osteosarcoma. cPOS is the most frequent lesion with about 5% of all osteosarcoma [okada, campanacci]. PerOS and HGSO are much more unfrequent and represent respectively 1–2% and 0.5% of all osteosarcoma. The high rate of DPOS and HGSO reported in our series is probably related to the fact that we did not exclude cases with medullary extension which precludes the diagnosis of surface osteosarcoma for some authors [2], [3]. We think that the frequency of HGSO and DPOS are under estimated in the literature. Due to the permeative pattern and the rapid evolution of these lesions, a large number of them are misdiagnosed as convetional osteosarcoma.
Clinical history of patients with surface osteosarcoma depends on its grade of malignancy. In low grade lesions and especially in cPOS, symptoms are most often of prolonged duration [3]. However, in HGSO and DPOS—especially when the dedifferentiation is synchronous—clinical history is not distinguishable from that of conventional osteosarcoma. In our study, the mean duration of symptoms was longer in low and intermediate grade lesions than in high grade lesions (16±4.66 months vs. 12±4.14 months). However the difference was not statistically significant.
Radiologically, we observed two patterns corresponding to mainly 2 lesions: parosteal osteosarcoma in one hand and PerOS and HGSO in the other hand.
Distinctive features between cPOS and DPOS are debatable. As observed in our series (25% vs. 100%), invasion of the medullary canal is more frequent in cPOS then in DPOS [3], [4]. However, this cannot distinguish DPOS from cPOS according to Sheth et al. [5]. Other distinctive features were reported. Bertoni [6] studied the meaning of radioloucencies in parosteal osteosarcomas and concluded that the presence of deep intralesional radiolucencies within a parosteal osteosarcoma strongly suggests the possibility that the lesion is dedifferentiated.
Medullary involvement is also discussed in periosteal osteosarcomas and HGSO. Some authors think that this sign precludes the diagnosis of surface osteosarcoma [7] while others have described periosteal osteosarcoma with microscopic or gross medullary involvement [8], [9].
Histologically, diagnosis of cPOS can be overlopped with other lesions as fracture callus, fibrous dysplasia and Paget disease. Clinical history and radiological features are very helpful. Due to the chondroblastic differentiation, PerOS can sometimes be confused with chondrosarcoma and especially malignant transformation of an exostosis. Here also radiological appearance of the lesion is distinctive.
Currently, surgery is the main therapeutic procedure for surface osteosarcoma. Wide resection is recommended to achieve safe margins. Some authors advocated local excision for cPOS with marginal margins [10], [11], [12]. Indeed, resection of the cortical on which the cPOS is developed when the medullary canal is not invaded can be safe. Two cases had cortical resection with wide margins did not recur in our series. However, intralesional resection is absolutely prohibited because it is associated with high rate of local recurrence and high risk of dedifferentiation. In our experience, all cases in which resection was intralesional recurred. In one case, the tumor underwent dedifferentiation 4 years after first excision.
For high grade lesions (DPOS and HGSO), margins have to be as wide as possible in. One of the problems of these lesions is the assessment of the true extension of the tumor in the subperiosteal plane. According to Revell [9], even with axial MRI and CT, this can be difficult. These tumors are very permeative and spread further than can be assessed by MRI, and hence wider margins than might at first be considered are mandatory for safety. In the current study, three lesions recurred despite wide resections.
Chemotherapy has limited benefit in surface osteosarcoma. Rose [3] showed no benefit of chemotherapy in PerOS. Degree of necrosis that he observed in patients that received doxorobucine, ifosfamide and methotrexate was similar to the spontaneous necrosis seen in resected specimens from the other patients. Bertoni [4] reported poor response in all patients with DPOS that received neoadjuvant chemotherapy. Even for survival rate, the role of chemotherapy was not clear. Revel [5] reviews a series of 17 PerOS. Among 10 patients who received neoadjuvant chemotherapy, only 2 had greater than 90% necrosis. Although, both authors [4], [5] recommended by caution the use of neoadjuvant chemotherapy for DPOS and PerOS respectively.
To our opinion, chemotherapy should be used only in high grade lesions (DPOS and HGSO). Furthermore and due to the amount of poor response and especially the high risk of progression of the disease during chemotherapy, it would be safer and so recommendable to use chemotherapy post operatively only.
Prognosis of surface osteosarcomas is mainly related to histological grade. The probability of metastasis is significantly related to dedifferentiation. The second most important factor is surgical margins. Intralesional and marginal resections are associated to a high rate of recurrence and hence a progression of malignancy.
5. Conclusion
Surface osteosarcomas are rare but they cover a wide spectrum of lesions. We think that there are 2 kinds of lesions:
-
•
parosteal tumors that develop from the outer part of the périosteum and include cPOS and DPOS
-
•
and periosteal tumors that develop from the inner part of the periosteum and include PerOS and HGSO.
Histological grade of malignancy is the main point to assess since it determines treatment and prognosis. Low and intermediate grade lesions (cPOS and PerOS) should be treated by wide resection, however high grade lesions (DPOS and HGSO) need more aggressive surgical approach associated to post operative chemotherapy.
Conflict of interest
None.
References
- 1.Raymond A.K. Surface osteosarcoma. Clin. Orthop. Relat. Res. 1991;270:140–148. [PubMed] [Google Scholar]
- 2.Campanacci M. 2nd edition. Springer-Verlag; Wien, New York: 1999. Bone and Soft Tissue Tumors. [Google Scholar]
- 3.Okada K., Frassica F.J., Sim F.H., Beabout J.W., Bon J.R., Unni K.K. Parosteal osteosarcoma. A clinicopathological study. J. Bone Jt. Surg. Am. 1994;20:366–378. doi: 10.2106/00004623-199403000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Bertoni F., Bacchini P., Staals E.L., Davidovitz P. Dedifferientiated parosteal osteosarcoma: the experience of the Rizzoli Institute. Cancer. 2005;103:2373–2382. doi: 10.1002/cncr.21039. [DOI] [PubMed] [Google Scholar]
- 5.Sheth D.S., Yasko A.W., Raymond A.K. Conventional and dedifferentiated parosteal osteosarcoma. Diagnosis treatment and outcome. Cancer. 1996;78:2136–2145. [PubMed] [Google Scholar]
- 6.Bertoni F., Present D., Hudson T., Enneking W.F. The meaning of radiolucencies in parosteal osteosarcoma. J. Bone Jt. Surg. Am. 1985;67:901–910. [PubMed] [Google Scholar]
- 7.Rose P.S., Dickey I.D., Wenger D.E., Unni K.K., Sim F.H. Periosteal osteosarcoma. Clin. Orthop. Relat. Res. 2006;453:314–317. doi: 10.1097/01.blo.0000229341.18974.95. [DOI] [PubMed] [Google Scholar]
- 8.Hall R.B., Robinson L.H., Malawar M.M., Dunham W.K. Periosteal osteosarcoma. Cancer. 1985;55:165–171. doi: 10.1002/1097-0142(19850101)55:1<165::aid-cncr2820550126>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 9.Revell M.P., Deshmukh N., Grimer R.J., Carter S.R., Tillman R.M. Periostael osteosarcoma: a review of 17 cases with mean follow-up of 52 months. Sarcoma. 2002;6:123–130. doi: 10.1080/1357714021000066368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahuja S.C., Villacin A.B., Smith J., Bullough P.G., Huvos A.G., Marcove R.C. Juxtacortical (parosteal) osteogenic sarcoma. Histological grading and prognosis. J. Bone Jt. Surg. Am. 1977;59:632–647. [PubMed] [Google Scholar]
- 11.Enneking W.F., Springfield D., Gross M. The surgical treatment of parosteal osteosarcoma in long bones. J. Bone Jt. Surg. Am. 1985;67:125–135. [PubMed] [Google Scholar]
- 12.Kavanagh T.G., Cannon S.R., Pringle J., Stoker D.J., Kemp H.B.S. Parosteal osteosarcoma. Treatment by wide resection and prosthetic replacement. J. Bone Jt. Surg. Br. 1990;72:959–965. doi: 10.1302/0301-620X.72B6.2246298. [DOI] [PubMed] [Google Scholar]