Abstract
Severe vitamin D deficiency is known to cause rickets, however epidemiological studies and RCTs did not reveal conclusive associations for other parameters of bone health. In our study, we aimed to investigate the association between serum levels of 25(OH) vitamin D and bone turnover markers in a population-based sample of children. 25(OH)D, calcium (Ca), osteocalcin (OC), and β-Crosslaps (β-CTx) were measured in 2798 ten-year-old children from the German birth cohorts GINIplus and LISAplus. Linear regression was used to determine the association between bone turnover markers and 25(OH)D levels. 25(OH)D, OC, and β-CTx showed a clear seasonal variation. A 10 nmol/l increase in 25(OH)D was significantly associated with a 10.5 ng/l decrease (p < 0.001) in β-CTx after adjustment for design, sex, fasting status, time of blood drawn, BMI, growth rate, and detectable testosterone/estradiol. For OC alone no significant association with 25(OH)D was observed, whereas the β-CTx-to-OC ratio was inversely associated with 25(OH)D (−1.7% change, p < 0.001). When stratifying the analyses by serum calcium levels, associations were stronger in children with Ca levels below the median. This study in school-aged children showed a seasonal variation of 25(OH)D and the bone turnover markers OC and β-CTx. Furthermore a negative association between 25(OH)D and the bone resorption marker β-CTx was observed.
Vitamin D can be obtained by diet, however most of the body’s vitamin D is formed from 7-dehydrocholesterol within the epidermal layer of skin after exposure to UVB radiation. Vitamin D is then transported to the liver and converted to 25-hydroxyvitamin D (25(OH)D) which is the major circulating and storage form of vitamin D with a half-life of about 2 weeks1 and thereby reflecting the current vitamin D status. The active form calcitriol (1,25(OH)2D), is mainly, but not exclusively produced by 1α-hydroxylation of 25(OH)D in the kidney. The main function of calcitriolis increasing calcium calcium absorption from the gut2. The perception of vitamin D’s importance for bone health is well established, as severe vitamin D deficiency or very low calcium intake cause rickets - a defective mineralization of bones- in the growing child. Besides its role in intestinal calcium absorption, calcitriol may also affect bone health directly, as its receptors are expressed by osteoblasts3,4. However the relative importance of these mechanisms and their role in the growing skeleton of healthy children is unclear.
Clinical practice guidelines emphasize the importance of vitamin D in bone health. However systematic reviews of randomized controlled trials in children and adolescents5 as well as adults6 did not show significant effects on bone mineral density after vitamin D supplementation, especially in individuals with normal vitamin D levels. Therefore, we aimed to investigate the association between serum levels of 25(OH)D and bone turnover markers in a large population-based sample of children, and the effects of serum calcium levels on this association.
Methods
The study population consists of 2,798 participants of two German birth cohorts. In both cohorts only healthy full-term neonates were recruited. The German Infant Study on the influence of Nutrition Intervention plus environmental and genetic influences on allergy (GINIplus) is a multicentre, two armed study consisting of 5,991 new-borns. One study arm is a prospective, double-blind, randomised intervention trial with hypoallergenic formulae, while the second arm is observational and does not include an intervention. The study design has been previously described in detail7. The Lifestyle-related factors on the Immune System and the development of Allergies in childhood (LISAplus) study consists of 3,097 healthy neonates, recruited at birth, who have a birth weight greater than 2500 g. LISAplus was designed as a population-based observational study and children have been followed up at the age of six, twelve and 18 months and two, four, six and ten years8. Parents of the study participants gave written informed consent. The study complied with the Ethical Principles of the World Medical Association Declaration of Helsinki and experimental protocols were approved by the regional ethics committees (Bavarian Board of Physicians, Ethics Commission of the Medical Faculty at the University of Leipzig, and Board of Physicians of North-Rhine-Westphalia).
The present analysis is restricted to 2,798 children who attended a clinical examination at age 10 years and had valid information on 25(OH)D and bone turnover markers.
Laboratory Analyses
Blood samples, either fasting or non-fasting, were drawn at age of 10 years between November 2006 and May 2009 during all seasons and months, centrifuged after collection, and stored frozen at –80° until assayed for bone turnover markers and 25 (OH)D. Total serum 25 (OH)D concentration was determined by Roche “vitamin D total” on the fully automated Modular system (E170, Roche Diagnostics, Mannheim, Germany). The specificity is reported by the manufacturer as 25(OH)D2 = 81%; 25(OH)D3 = 98%; 1,25(OH)2D2 = 6%; 1,25(OH)2D3 = 5%; 24,25(OH)2 = 121%, and the lower limit of detection as 7.5 nmol/L. The intra-assay coefficient of variation was 2.2-6.8% for sera with levels between 20.8–173.7 nmol/L, the inter-assay coefficient of variation as provided by the manufacturer was 3.4–13.1% for levels between 20.8–173.7 nmol/L. According to the manufacturer and further validations9,10 the “vitamin D total” immunoassay shows a good comparability with LC-MS/MS.
The automated Modular system (E170, Roche) was used to measure the two bone turnover marker osteocalcin (OC) and β-isomer of the C-terminal telopeptide (β-CTx) as well as calcium (Ca), 17beta-estradiol, and 5alpha-testosterone in the same lab. Intra- and inter-assay coefficients of variation varied between 3.2% and 2.9% for concentrations of 4.1 and 21.1 nmol/l osteocalcin, 4.7% and 4.8% for concentrations of 343 and 792 ng/l CTx, and between 2.1% and 1.5% for concentrations of 2.0 and 2.9 nmol/l calcium, respectively. The analytical sensitivity was 0.087 nmol/l for testosterone and 18.4 pmol/l for estradiol. Intra- and interassay coefficients of variation were below 4.06% and 2.83% for testosterone concentrations of 6.2 and 20.2 nmol/l, respectively. For estradiol concentrations of 378 and 1941 pmol/l, intra- and interassay coefficients of variation were lower than 5.29% and 3.56%.
Covariates
Equivalent net income was calculated by dividing the household income by the number of people in the household (the first adult weighted with factor 1, other household members at least 14 years old with factor 0.5, and all other household members with factor 0.3). Equivalent net income was then categorized into city-specific tertiles, with cutoffs 972 and 1310 Euro/month for Wesel, 1406 and 2104 Euro/month for Munich, 1086 and 1528 Euro/month for Leipzig, and 1071 and 1548 Euro/month for Bad Honnef. Maternal and paternal education level was categorized into less than 10 years, 10 years or more than 10 years of education. Height and weight were measured at age of 6 years by paediatricians during preventive health screenings and at age of 10 years during a clinical examination at the study center. Growth rate was calculated as change in age and sex standardized WHO z-score of height between age 6 and age 10 years. Physical activity was measured based on questionnaire data and categorized according to Janssen 200711 as moderate and vigorous physical activity for less than 7 h per week, moderate and vigorous physical activity for more than 7 h/week, or moderate and vigorous physical activity for at least 10.5 h/week and vigorous physical activity for at least 3.5 h/week.
Statistical analysis
All analyses were carried out using the statistical software R (version 3.1.0). Comparisons of categorical variables were performed using Fisher’s exact test in case of binary variables, and χ2 test for variables with more than two categories. For comparisons of normally distributed variables between groups, t-test was used.
To explore the seasonal variation of 25(OH)D, β-CTx, and OC generalized additive mixed models (gamm) as implemented in the R-packages “mgcv” were used. For month of measurement, thin plate regression splines were added to the model and the year of measurement was treated as random effect. Associations between 25(OH)D concentrations and bone turnover markers were assessed using linear regression. As adjustment variables, we tested study, city, fasting status, time of blood drawn, personal characteristics (age, sex, BMI, BMI2, growth rate, pubertal status, total physical activity level, time spent in front of pc/tv in summer/winter) and socioeconomic factors (parental education, single parent status and income). Only variables that showed a significant association (p < 0.05) with either OC or β-CTx in univariate models were selected for multivariable models. As the half-life time of 25(OH)D, β-CTx, and OC is very limited, we decided not to adjust for month of measurement. Regression models were additionally performed stratified by serum Ca levels and using 25(OH)D quartiles as sensitivity analyses.
Results
The numbers of blood samples taken per month were: January (161), February (220), March (216), April (283), May (212), June (235), July (304), August (241), September (247), October (264), November (224), and December (191). There was a clear seasonal dependency within the measurements β-CTx, OC, and 25(OH)D (Fig. 1). 25(OH)D ranged from 21.8 nmol/l to 142.4 nmol/l with a mean of 63.1 nmol/l in the winter months from November until April, while during the summer months values from 23.2 nmol/l to 224.6 nmol/l with a mean of 83.9 nmol/l were observed. For all three parameters, a significant (p < 0.001) non-linear effect of months of measurement was seen. The adjusted R2 was 32.4% for 25(OH)D, 34.2% for β-CTx and 10.1% for OC.
Figure 1. Seasonal mean estimated using generalized additive modeling adjusted for BMI, age, fasting status, time of blood drawn.
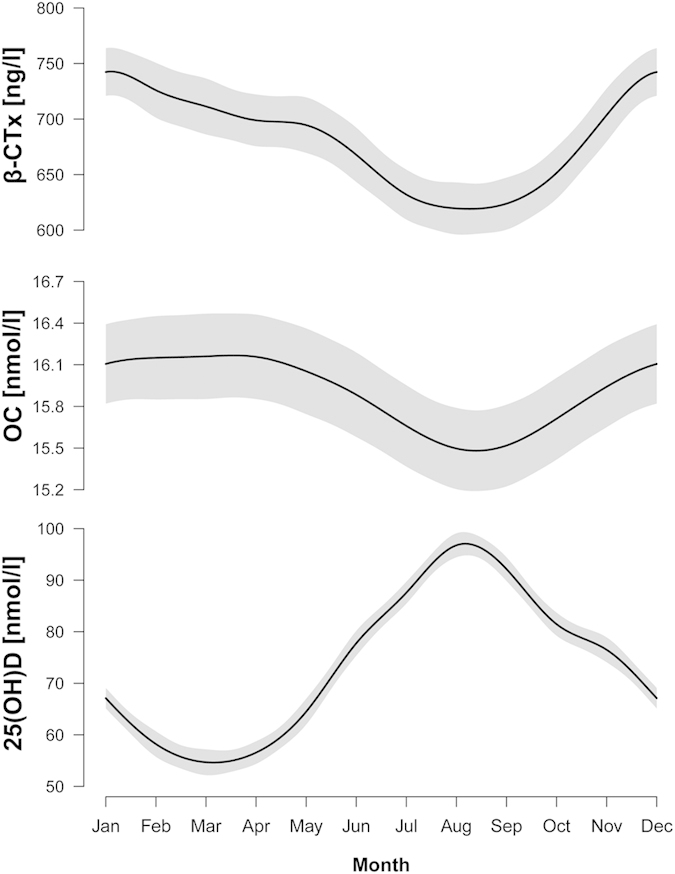
Point wise confidence intervals shaded in grey.
Children had a mean age of 10.2 and 51.2% were male (Table 1). Estradiol was detectable in 72.3% of girls, whereas testosterone was only detectable in 27.7% of the boys (p < 0.001). The two bone turnover markers showed a significant positive correlation (Pearson’s ρ = 0.60, p < 0.001).
Table 1. Study population characteristics.
| All participants | Girls | Boys | ||
|---|---|---|---|---|
| Study and centre,% | ||||
| GINI Munich | 36.7 | 1027/2798 | 37.6 | 35.8 |
| GINI Wesel | 25.7 | 718/2798 | 26.6 | 24.7 |
| LISA Munich | 18.8 | 526/2798 | 17.6 | 20.0 |
| LISA Wesel | 3.7 | 104/2798 | 3.5 | 3.9 |
| LISA Leipzig | 9.9 | 276/2798 | 9.4 | 10.3 |
| LISA Bad Honnef | 5.3 | 147/2798 | 5.2 | 5.3 |
| Age [years], mean (sd) | 10.2 (0.2) | 10.2 (0.2) | 10.2 (0.2) | |
| Sex | ||||
| male | 51.2 | 1432/2798 | 0.0 | 100.0 |
| BMI [kg/m2] | 17.4 (2.5) | 17.4 (2.5) | 17.4 (2.5) | |
| Growth ratea | 0.18 (0.50) | 0.10 (0.53) | 0.26 (0.45) | |
| Detectable testosterone/estradiolb, % | 50.0 | 1370/2741 | 74.0 | 27.1 |
| Fasting status at blood drawn, % | 17.4 | 487/2798 | 18.7 | 16.1 |
| Time of examination,% | ||||
| before 11am | 28.0 | 739/2641 | 28.8 | 27.2 |
| 11am–2pm | 12.0 | 318/2641 | 12.9 | 11.3 |
| after 2 pm | 60.0 | 1584/2641 | 58.4 | 61.5 |
| Physical activity,% | ||||
| moderate or vigorous physical activity <7h | 27.0 | 633/2346 | 32.4 | 22.0 |
| moderate or vigorous physical activity ≥7h | 37.6 | 883/2346 | 39.6 | 35.8 |
| moderate or vigorous physical activity ≥10.5h and vigorous physical activity ≥ 3.5 h | 35.4 | 830/2346 | 27.9 | 42.2 |
| Maternal education,% | ||||
| Low (<10 y) | 9.5 | 265/2784 | 8.8 | 10.2 |
| medium (10 y) | 38.3 | 1065/2784 | 39.1 | 37.5 |
| High (>10 y) | 52.2 | 1454/2784 | 52.2 | 52.3 |
| Paternal education, % | ||||
| Low (<10 y) | 18.4 | 505/2747 | 18.0 | 18.7 |
| medium (10 y) | 22.6 | 622/2747 | 22.4 | 22.9 |
| High (>10 y) | 59.0 | 1620/2747 | 59.6 | 58.4 |
| Center specific income tertiles,% | ||||
| 1st tertile | 32.6 | 836/2568 | 31.5 | 33.5 |
| 2nd tertile | 30.3 | 778/2568 | 31.3 | 29.3 |
| 3rd tertile | 37.1 | 954/2568 | 37.2 | 37.1 |
| 25 (OH) D [nmol/l], mean (sd) | 74.3 (25.2) | 73.0 (24.6) | 75.5 (25.7) | |
| OC [nmol/l], mean (sd) | 15.9 (5.3) | 16.9 (5.8) | 15.0 (4.7) | |
| β-CTx [ng/l], mean (sd) | 679.4 (333.4) | 696.3 (351.7) | 663.3 (314.9) | |
| β-CTx/OC [ng/nmol], geometric mean (sd) | 39.7 (1.5) | 38.4 (1.5) | 41.1 (1.5) | |
adefined as height z-score 10 years minus height z-score 6 years.
b0.09nmol/l for testosterone, 0.0184 nmol/l for estradiol.
Boys had a higher physical activity level (p < 0.001), higher 25(OH)D concentrations (p = 0.009), lower OC concentrations (p < 0.001) and lower β-CTx concentrations (p = 0.009), but there was no significant difference in BMI between girls and boys (p = 0.926). Children with detectable estradiol/testosterone showed lower 25(OH)D concentrations (p < 0.001) and an increase in bone turnover markers (p < 0.0001).
A 10 nmol/l increase in 25(OH)D was associated with a significant decrease of −10.5ng/l (95%-CI: −14.9,−6.2; p < 0.001) in the bone resorption marker β-CTx when controlling for city, study, sex, fasting status BMI, BMI2, growth rate, time of blood drawn, and detectable sex hormones (Table 2). For the bone formation marker OC a non-significant increase of 0.03 nmol/l (95%-CI: -0.06,0.09; p = 0.795) was observed resulting in a −1.7% (95%-CI: −2.3, −1.2, p < 0.001) decrease of the β-CTx to OC ratio. Additional adjustment for maternal and paternal education and physical activity only marginally changed the association results. Fasting status, growth rate and gender had the largest effect sizes among the covariates for OC, and fasting status, growth rate, study, and city for β-CTx, but crude models (results not shown) yielded the similar results. There was no effect modification of gender in any of the models (p for interaction between gender and 25(OH)D >0.05 in all models).
Table 2. Results of linear regression between bone metabolism marker and 25(OH) D in different sets of adjustment.
| All | low calcium (<median) | high calcium (≥median) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| effecta | (CI) | p | effecta | (CI) | p | effecta | (CI) | p | |
| Basic modelb | |||||||||
| OC | 0.01 | (−0.07, 0.08) | 0.896 | −0.02 | (−0.12, 0.10) | 0.765 | −0.06 | (−0.16, 0.04) | 0.268 |
| β-CTx | −10.2 | (−14.4, –6.1) | <0.001 | −14.4 | (−20.6, −8.1) | <0.001 | −9.0 | (−14.7, −3.4) | 0.002 |
| β-CTx to OC ratio | −1.7% | (−2.2, −1.1) | <0.001 | −2.2% | (−3.1, −1.4) | <0.001 | −1.0% | (−1.7, −0.4) | 0.003 |
| Extended modelc | |||||||||
| OC | 0.01 | (−0.07, 0.09) | 0.795 | −0.01 | (−0.13, 0.11) | 0.872 | −0.05 | (−0.15, 0.05) | 0.343 |
| β-CTx | −10.5 | (−14.9, –6.2) | <0.001 | −15.3 | (−21.8, –8.8) | <0.001 | −8.9 | (−14. 8, −3.0) | 0.003 |
| β-CTx to OC ratio | −1.7% | (−2.3, –1.2) | <0.001 | −2.3% | (−3.2, −1.4) | <0.001 | −1.1% | (−1.8, −0.4) | 0.002 |
| Full modeld | |||||||||
| OC | 0.03 | (−0.06, 0.11) | 0.504 | 0.01 | (−0.12, 0.14) | 0.899 | −0.03 | (−0.14, 0.09) | 0.636 |
| β-CTx | −9.6 | (−14.3, −45.0) | <0.001 | −14.7 | (−21.6, –7.7) | <0.001 | −7.8 | (−14.2, –1.4) | 0.018 |
| β-CTx to OC ratio | −2.4% | (−2.4, −1.2) | <0.001 | −2.4% | (−3.4, –1.5) | <0.001 | −1.2% | (−2.0, –0.4) | 0.003 |
aeffect: beta for OC and β-CTx, %change for β-CTx to OC ratio.
bBasic model: adjusted for city, study, sex, fasting status, time of blood drawn.
cExtended model: basic model adjustment plus BMI, BMI2, growth rate, detectable testosterone/estradiol.
dFull model: extended model adjustment plus maternal and paternal education and physical activity.
When stratifying the results for serum calcium levels (median or higher versus lower than median) the association between bone turnover markers and 25(OH)D was stronger in children with calcium concentrations below the median. The formal test for an interaction between serum calcium and bone turnover marker yielded a p-value of 0.03 for β-CTx and p = 0.001 for the β-CTx to OC ratio.
In sensitivity analyses, we restricted the study population to fasting subjects, and observed slightly increased effect estimates. Also further adjustment of the models for parental income, single parent status, age, and pubertal status obtained from questionnaires did not lead to substantial differences in effect estimates. When using quartiles of 25(OH)D levels in the analysis, we observed significantly decreased β-CTx concentrations for the 2nd–4th quartile (medium to high 25(OH)D) compared with the first quartile (low 25(OH)D) (2nd quartile: β = −43.4, p = 0.006, 3rd quartile: β = −69.2, p < 0.001, 4th quartile: β = −67.9, p < 0.001) and no effect on OC (2nd quartile: β = −0.08, p = 0.775, 3rd quartile: β = 0.03, p = 0.918, 4th quartile: β = 0.03, p = 0.917).
Discussion
Our study showed a clear seasonal variation of bone turnover markers and a negative association between serum 25(OH)D and β-CTx concentrations in a large population-based sample of school-aged children, with an effect modification of serum calcium levels.
OC is solely secreted by osteoblasts and a marker for bone formation. β-CTx is a marker that is specific for bone resorption and shows a circadian rhythm. Both markers are increased during periods of rapid bone turnover such as during growth in childhood and adolescence12 and typically show a positive association with growth rate13,14.
Most studies on bone health have focused mainly on the elderly or post-menopausal woman owing to their greater risk of osteoporosis15,16.
Smaller observational studies investigating the association between serum levels of 25(OH)D and bone turnover markers have been performed in healthy adolescents. Jones et al.17 showed a significant association between bone turnover markers in 136 Tasmanian boys and Viljakainen et al.18 in 196 Finnish girls, whereas in 172 10–17 years old students from schools in Beirut no association was seen in samples collected during November and December19. A study in 138 6–12 year old youth living in Pittsburgh, Pennsylvania) who were examined once during summer (June-September) and once during winter (December-March) showed higher 25(OH)D concentrations in summer accompanied by lower CTx levels20. However the study did not reveal any significant association neither between 25(OH)D and CTx nor with OC. In 196 Swiss adolescents aged 11–16 years, also no association between bone turnover and 25(OH)D was observed21. A further study was conducted in 301 Chinese adolescent girls and revealed significantly reduced BMC and higher concentrations for bone-specific alkaline phosphatase (BAP) in vitamin D deficient individuals, but no differences were found in OC22.
Baseline concentrations of the calcitriol were associated with an 0.51 ng/ml increase in OC one year later in a prospective study in 178 healthy female adolescents with a mean age of 11.7 (SD = 2.3) years23.
Cashman et al.24 investigated the association between 25(OH)D levels and BMD measured by dual-energy X-ray absorptiometry on the forearm and heel in boys and girls of two age groups (12 years and 15 years). A significantly higher forearm BMD was observed for girls in the highest 25(OH)D tertile compared to the lowest tertile, but neither for heel BMD nor for boys any association was observed. 25(OH)D concentrations in the highest tertile were additionally associated with lower OC concentrations compared to the lowest tertile in 12 and 15-year old boys24,25. There was no association between 25(OH)D and CTx in any sex or age group.
Bone turnover markers (serum BAP, OC, urine Ntx) were also not influenced by serum 25(OH)D levels in 302 pubertal healthy black and white children enrolled during winter (October-January), neither at baseline nor after 12 weeks of supplementation with vitamin D326. A longer supplementation of 12 months vitamin D3 did also not show any association with bone turnover markers (serum OC, urine Pyr, Dpyr) in 221 girls aged 11-12 years27.
Thus, results of previous observational studies in children and adolescents were inconclusive, with some showing effects of vitamin D concentrations on either bone formation or bone resorption17,18,22,23,24,25, while others did not19,20,21,26,27. Taken together with the notion of a lacking effect of vitamin D supplementation on bone mineral density in clinical trials5 there remains uncertainty about the presence and direction of an association between vitamin D status and markers of bone turnover in children and adolescents. There are several possible reasons why trials did not detect significant associations. The meta-analysis of Winzenberg et al.5 revealed a trend towards larger effects in studies of participants with low baseline 25(OH)D levels, therefore high baseline 25(OH)D concentrations might have prevented any additional effect of vitamin D supplementation. Also the dosage of supplementation in some of the trials (mostly 200 IU vitamin D) was likely too low to provoke a detectable effect. In adults, vitamin D is often supplemented together with calcium, which makes it difficult to interpret isolated effects of vitamin D.
Here, we observed lower concentrations of β-CTx in children with higher circulating vitamin D concentrations and a decreased β-CTx to OC ratio. The assumed pathophysiological basis of this association is that low 25(OH)D levels can be considered as a marker for low calcitriol levels which lead to a lower intestinal calcium absorption. To compensate for this, parathyroid hormone (PTH) is released to increase the circulating Ca levels by bone resorption and demineralization. In our study, the association of 25(OH)D and bone turnover marker was stronger in children with calcium levels below the median, also indicating that these mechanisms may be operative to prevent low calcium serum levels. In previous studies, Ca was not considered as a potential effect modifier. Some studies accounted only for dietary Ca intake18,19,20,21,23,24,25, others in which Ca was measured in serum treated it as outcome22,27 no study investigated the effects of vitamin D status on bone turnover marker in relation to serum Ca status.
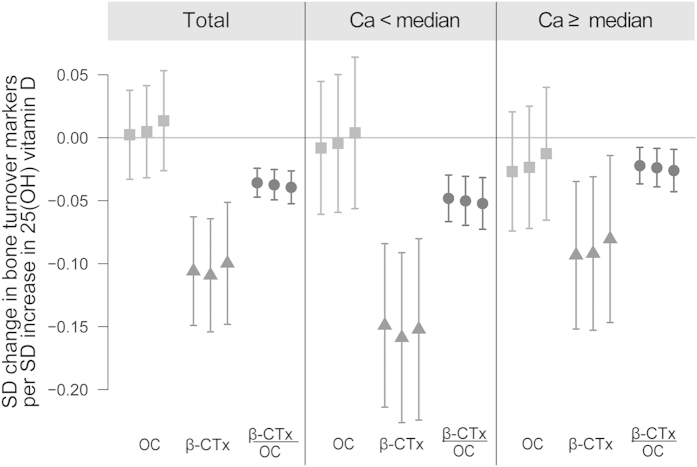
In general, the effect sizes in our study were low (about −0.10 SD decrease in β-CTx per SD increase in 25 (OH) D, and about −0.04 SD decrease in β-CTx to OC ratio, see Fig. 2. We hypothesize that the lack of power might be a plausible reason why smaller studies often did not find any statistically significant association between bone turnover markers and vitamin D status, especially in population without vitamin D deficiency and with sufficient Ca supply.
Figure 2. Results of linear regression on standardized values.
Description: Model 1: city, study, sex, fasting status, time of blood drawn; Model 2: Model 1 + BMI, BMI2, growth rate, detectable testosterone/estradiol; Model 3: Model 2 + maternal and paternal education, center specific income tertiles, single parent status) and physical activity.
Limitations and strength
The main strength of the present study is that it was conducted in a large population-based sample of children which allows detection of small effect sizes. Furthermore, detailed information on personal characteristics has been prospectively collected. Therefore adjustment for many covariates and potential confounders was possible.
It is challenging to measure vitamin D status as the obtained concentrations vary with the methods applied28. In GINIplus and LISAplus the measured 25(OH)D levels were higher than reported for this age group in the KIGGS study29 and NHANES30. Therefore, we used in our analyses 25(OH)D concentrations as continuous variable as well as in quartiles and avoided interpretation in terms of vitamin D sufficiency. To better investigate the pathophysiological basis for the observed association, an additional measurement of PTH and receptor activator of nuclear factor kappa-B ligand (RANKL) would be required. Therefore, the unavailability of these measures has to be considered as a major limitation of this manuscript. Especially as serum calcium concentration is a very poor marker for the determination of calcium status. Furthermore calcium was not albumin adjusted and thus might not reflect the true biologic effect. Furthermore, as the design of the study was observational and cross-sectional, it cannot establish a causal link between 25(OH)D levels and bone turnover.
Conclusion
This study showed associations between a marker of bone turnover and 25(OH)D which are dependent on serum calcium level in healthy school-aged children. Higher levels of 25(OH)D were associated with slightly decreased β-CTx, and a decreased β-CTx to OC ratio. Nevertheless, clinical relevance of the findings is limited due to small effect sizes in this population with comparably high 25(OH)D levels.
Additional Information
How to cite this article: Thiering, E. et al. Associations between serum 25-hydroxyvitamin D and bone turnover markers in a population based sample of German children. Sci. Rep. 5, 18138; doi: 10.1038/srep18138 (2015).
Acknowledgments
An abstract summarizing the results of the data when analyzed with a different approach was submitted to the Vitamin D and Human Health Conference 2014 and published in the conference report. The GINIplus study was mainly supported for the first 3 years of the Federal Ministry for Education, Science, Research and Technology (interventional arm) and Helmholtz Zentrum Munich (former GSF) (observational arm). The 4 year, 6 year, and 10 year follow-up examinations of the GINIplus study were covered from the respective budgets of the 5 study centres (Helmholtz Zentrum Munich (former GSF), Research Institute at Marien-Hospital Wesel, LMU Munich, TU Munich and from 6 years onwards also from IUF - Leibniz Research-Institute for Environmental Medicine at the University of Düsseldorf) and a grant from the Federal Ministry for Environment (IUF Düsseldorf, FKZ 20462296). The LISAplus study was mainly supported by grants from the Federal Ministry for Education, Science, Research and Technology and in addition from Helmholtz Zentrum Munich (former GSF), Helmholtz Centre for Environmental Research - UFZ, Leipzig, Research Institute at Marien-Hospital Wesel, Pediatric Practice, Bad Honnef for the first 2 years. The 4 year, 6 year, and 10 year follow-up examinations of the LISAplus study were covered from the respective budgets of the involved partners (Helmholtz Zentrum Munich (former GSF), Helmholtz Centre for Environmental Research - UFZ, Leipzig, Research Institute at Marien-Hospital Wesel, Pediatric Practice, Bad Honnef, IUF – Leibniz-Research Institute for Environmental Medicine at the University of Düsseldorf) and in addition by a grant from the Federal Ministry for Environment (IUF Düsseldorf, FKZ 20462296). We thank the families for their participation in the studies, the obstetric units for allowing recruitment, the GINI and LISA study teams for their excellent work. The GINIplus birth cohort study was designed and/or conducted by: J. Heinrich, H.E. Wichmann, S. Sausenthaler, M. Chen, M. Schnappinger, P. Rzehak (Helmholtz Zentrum München); D. Berdel, A. von Berg, C. Beckmann, I. Groß (Department of Pediatrics, Marien-Hospital, Wesel, Germany); S. Koletzko, D. Reinhard, S. Krauss-Etschmann (Department of Pediatrics, Ludwig Maximilians University, Munich, Germany); C.P. Bauer, I. Brockow, A. Grübl, U. Hoffmann (Department of Pediatrics, Technical University, Munich, Germany); U. Krämer, E. Link, C. Cramer (IUF - Institut für Umweltmedizinische Forschung at the Heinrich Heine University, Düsseldorf, Germany); H. Behrendt (Centre for Allergy and Environment, Technical University, Munich, Germany). The LISAplus birth cohort study was designed and/or conducted by: J. Heinrich, H.E. Wichmann, S. Sausenthaler, C.M. Chen, M. Schnappinger (Helmholtz Zentrum München - German Research Center for Environmental Health, Institute of Epidemiology,Munich, Germany); M. Borte, U. Diez (Department of Pediatrics, Municipal Hospital ‘St Georg’, Leipzig, Germany): A. von Berg, C. Beckmann, I. Groß (Marien-Hospital Wesel, Department of Pediatrics, Wesel, Germany); B. Schaaf (Pediatric Practice, Bad Honnef, Germany); I. Lehmann, M. Bauer, S. Röder (Helmholtz Centre for Environmental Research – UFZ, Department of Environmental Immunology/Core Facility Studies, Leipzig, Germany); O. Herbarth, C. Dick, J. Magnus (University of Leipzig, Institute of Hygiene and Environmental Medicine, Leipzig, Germany); U. Krämer, E. Link, C. Cramer (IUF - Institut für Umweltmedizinische Forschung, Düsseldorf, Germany); C.P. Bauer, U. Hoffmann (Technical University Munich, Department of Pediatrics, Munich, Germany); H. Behrendt (ZAUM - Center for Allergy and Environment, Technical University, Munich, Germany).
Footnotes
Author Contributions E.T. analyzed the data, is responsible for the integrity of the data analysis and drafted the manuscript. J.K. performed the measurement of all biomarkers. D.B., A.v.B., I.L., B.H., S.K. and J.H. were responsible for study conception and the acquisition of all additional data. I.L. and L.C.H. were involved in the analysis and interpretation of the data. All authors critically revised the manuscript for important intellectual content, and approved the final version of the submitted manuscript.
References
- Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr 88, 582S–586S (2008). [DOI] [PubMed] [Google Scholar]
- Haussler M. R. et al. Molecular mechanisms of vitamin D action. Calcif Tissue Int 92, 77–98 (2013). [DOI] [PubMed] [Google Scholar]
- Atkins G. J. et al. Metabolism of vitamin D3 in human osteoblasts: evidence for autocrine and paracrine activities of 1 alpha,25-dihydroxyvitamin D3. Bone 40, 1517–28 (2007). [DOI] [PubMed] [Google Scholar]
- van Driel M. & van Leeuwen. J. P. Vitamin D endocrine system and osteoblasts. Bonekey Rep 3, 493 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzenberg T., Powell S., Shaw K. A. & Jones G. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ 342, c7254 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid I. R., Bolland M. J. & Grey A. Effects of vitamin D supplements on bone mineral density: a systematic review and meta-analysis. Lancet 383, 146–55 (2014). [DOI] [PubMed] [Google Scholar]
- Berg A. et al. Impact of early feeding on childhood eczema: development after nutritional intervention compared with the natural course - the GINIplus study up to the age of 6 years. Clin Exp Allergy 40, 627–36 (2010). [DOI] [PubMed] [Google Scholar]
- Zutavern A. et al. Timing of solid food introduction in relation to atopic dermatitis and atopic sensitization: results from a prospective birth cohort study. Pediatrics 117, 401–11 (2006). [DOI] [PubMed] [Google Scholar]
- Knudsen C. S., Nexo E., Højskov C. S. & Heickendorff L. Analytical validation of the Roche 25-OH Vitamin D Total assay. Clin Chem Lab Med 50(11), 1965–8 (2012). [DOI] [PubMed] [Google Scholar]
- Emmen J. M., Wielders J. P., Boer A. K., van den Ouweland J. M. & Vader H. L. The new Roche Vitamin D Total assay: fit for its purpose? Clin Chem Lab Med 8, 0(0):1–4 (2012). [DOI] [PubMed] [Google Scholar]
- Janssen I. Physical activity guidelines for children and youth. Can J Public Health 98 Suppl 2, S109–21 (2007). [PubMed] [Google Scholar]
- Gracia-Marco L. et al. Bone mass and bone metabolism markers during adolescence: The HELENA Study. Horm Res Paediatr 74, 339–50 (2010). [DOI] [PubMed] [Google Scholar]
- Kanbur N. O., Derman O., Sen T. A. & Kinik E. Osteocalcin. A biochemical marker of bone turnover during puberty. Int J Adolesc Med Health 14(3), 235–44.. [DOI] [PubMed] [Google Scholar]
- Matsukura T. et al. The characteristics of bone turnover in the second decade in relation to age and puberty development in healthy Japanese male and female subjects–Japanese Population-based Osteoporosis Study. Ann Hum Biol 30(1), 13–25. [DOI] [PubMed] [Google Scholar]
- Kuchuk N. O. et al. Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons J Clin Endocrinol Metab 94, 1244–50 (2009). [DOI] [PubMed] [Google Scholar]
- Ardawi M. S., Qari M. H., Rouzi A. A., Maimani A. A. & Raddadi R. M. Vitamin D status in relation to obesity, bone mineral density, bone turnover markers and vitamin D receptor genotypes in healthy Saudi pre- and postmenopausal women. Osteoporos Int 22, 463–75 (2011). [DOI] [PubMed] [Google Scholar]
- Jones G., Dwyer T., Hynes K. L., Parameswaran V. & Greenaway T. M. Vitamin D insufficiency in adolescent males in Southern Tasmania: prevalence, determinants, and relationship to bone turnover markers. Osteoporos Int 16, 636–41 (2005). [DOI] [PubMed] [Google Scholar]
- Viljakainen H. T. et al. A seasonal variation of calcitropic hormones, bone turnover and bone mineral density in early and mid-puberty girls - a cross-sectional study. Br J Nutr 96, 124–30 (2006). [DOI] [PubMed] [Google Scholar]
- Fares J. E. et al. Effect of gender, puberty, and vitamin D status on biochemical markers of bone remodedeling. Bone 33, 242–7 (2003). [DOI] [PubMed] [Google Scholar]
- Rajakumar K. et al. Impact of seasonal flux on 25-hydroxyvitamin D and bone turnover in pre- and early pubertal youth. Pediatr Int 56, 35–42 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginty F. et al. Effects of usual nutrient intake and vitamin D status on markers of bone turnover in Swiss adolescents. Eur J Clin Nutr 58, 1257–65 (2004). [DOI] [PubMed] [Google Scholar]
- Foo L. H. et al. Low vitamin D status has an adverse influence on bone mass, bone turnover, and muscle strength in Chinese adolescent girls. J Nutr 139, 1002–7 (2009). [DOI] [PubMed] [Google Scholar]
- Ilich J. Z. et al. Calcitriol and bone mass accumulation in females during puberty. Calcif Tissue Int 61, 104–9 (1997). [DOI] [PubMed] [Google Scholar]
- Cashman K. D. et al. Low vitamin D status adversely affects bone health parameters in adolescents. Am J Clin Nutr 87, 1039–44 (2008). [DOI] [PubMed] [Google Scholar]
- Hill T. R. et al. Vitamin D status and parathyroid hormone relationship in adolescents and its association with bone health parameters: analysis of the Northern Ireland Young Heart’s Project. Osteoporos Int 21, 695–700 (2010). [DOI] [PubMed] [Google Scholar]
- Hill K. M. et al. Bone turnover is not influenced by serum 25-hydroxyvitamin D in pubertal healthy black and white children. Bone 51, 795–9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mølgaard C. et al. Does vitamin D supplementation of healthy Danish Caucasian girls affect bone turnover and bone mineralization? Bone 46, 432–9 (2010). [DOI] [PubMed] [Google Scholar]
- Enko D. et al. 25-hydroxy-Vitamin D status: limitations in comparison and clinical interpretation of serum-levels across different assay methods. Clin Lab 60(9), 1541–50 (2014). [DOI] [PubMed] [Google Scholar]
- Lämmle L., Bergmann K., Bös K. & Koletzko B. Predictors of differences in vitamin D levels in children and adolescents and their relation to endurance performance. Ann Nutr Metab 62(1), 55–62 (2013). [DOI] [PubMed] [Google Scholar]
- Looker A. C. et al. Vitamin D status: United States, 2001-2006. NCHS Data Brief 2011(59), 1–8. [PubMed] [Google Scholar]