Abstract
Background
Chlamydia pneumoniae illness is poorly characterized, particularly as a sole causative pathogen. We investigated a C. pneumoniae outbreak at a federal correctional facility.
Methods
We identified inmates with acute respiratory illness (ARI) from 1 November 2009 to 24 February 2010 through clinic self-referral and active case finding. We tested oropharyngeal and/or nasopharyngeal swabs for C. pneumoniae by real-time polymerase chain reaction (qPCR) and serum samples by microimmunofluorescence. Cases were inmates with ARI and radiologically confirmed pneumonia, positive qPCR, or serological evidence of recent infection. Swabs from 7 acutely ill inmates were tested for 18 respiratory pathogens using qPCR TaqMan Array Cards (TACs). Follow-up swabs from case patients were collected for up to 8 weeks.
Results
Among 33 self-referred and 226 randomly selected inmates, 52 (20.1%) met the case definition; pneumonia was confirmed in 4 by radiology only, in 9 by qPCR only, in 17 by serology only, and in 22 by both qPCR and serology. The prison attack rate was 10.4% (95% confidence interval, 7.0%–13.8%). White inmates and residents of housing unit Y were at highest risk. TAC testing detected C. pneumoniae in 4 (57%) inmates; no other causative pathogens were identified. Among 40 inmates followed prospectively, C. pneumoniae was detected for up to 8 weeks. Thirteen (52%) of 25 inmates treated with azithromycin continued to be qPCR positive >2 weeks after treatment.
Conclusions
Chlamydia pneumoniae was the causative pathogen of this outbreak. Higher risk among certain groups suggests that social interaction contributed to transmission. Persistence of C. pneumoniae in the oropharynx creates challenges for outbreak control measures.
Keywords: Chlamydia pneumoniae, Chlamydophila, atypical pneumonia, community-acquired pneumonia, prison
Chlamydia (Chlamydophila) pneumoniae is an obligate intracellular bacterium commonly associated with both upper and lower respiratory tract infections including pharyngitis, bronchitis, and pneumonia. First described in 1986, C. pneumoniae infections generally consist of low-grade fever, prolonged cough, coryza, headache, myalgias, and laryngitis [1, 2]. Macrolides are a common first-line treatment; however, tetracyclines and fluoro-quinolones are also effective. Symptoms may resolve without antibiotics and asymptomatic infection can occur [3–5].
Chlamydia pneumoniae is endemic world-wide. Outbreaks occur periodically and without a clear seasonal pattern, primarily in close-contact settings among high-risk populations such as in long-term care facilities [6–8]. Chlamydia pneumoniae is frequently detected alongside other respiratory pathogens, making its role as a primary disease-causing pathogen unclear [9–11]. The diagnosis of C. pneumoniae infection is commonly made through commercially available serological assays, despite poor sensitivity and specificity due to high background seroprevalence and potential cross-reaction with other Chlamydia species [12]. More recently, real-time polymerase chain reaction (qPCR) assays are being used to identify C. pneumoniae from respiratory specimens that are highly sensitive and specific for acute C. pneumoniae infection but are unable to provide a retrospective diagnosis [13–15].
During November 2009–February 2010, an outbreak of pneumonia was identified in a male federal correctional institution in Texas. Symptoms included low-grade fever, dry cough, and body aches, and illness appeared to occur in previously healthy inmates. Four inmates were hospitalized. Sputum and blood cultures, sputum testing for acid-fast bacilli, and urine testing for Legionella and Histoplasma were negative. This report describes the outbreak’s laboratory and epidemiological investigation, and illustrates the challenges of implementing outbreak control interventions in this setting.
METHODS
Outbreak Setting and Pathogen Identification
The facility’s 1574 inmates resided in 2 housing units, each with 3 floors. Although housing units were racially mixed, inmates comingled along ethnic (or gang) lines in common areas. Healthcare services were available for a nominal fee and smoking was not permitted on the premises. Upon suspicion of the outbreak, ill inmates were placed in single-celled housing units until their symptoms improved. Nasopharyngeal (NP) and oropharyngeal (OP) specimens available from 7 acutely ill inmates were sent to the Centers for Disease Control and Prevention (CDC) for multiple respiratory pathogen testing to rapidly identify the etiology.
Case Finding and Estimation of Attack Rate
We reviewed available prison medical records on 33 of 36 inmates who had self-referred to the facility’s infirmary during the outbreak period (1 November 2009–24 February 2010) and had been diagnosed with suspected or confirmed pneumonia. To establish a facility-wide attack rate and identify C. pneumoniae infection among inmates who did not seek medical care, we conducted active case finding among a systematic random sample of inmates. The sample size was calculated based on an expected maximum attack rate of 20% and 10% refusal rate; 270 beds were selected (17.5% of the inmate population). Beds were selected from a list of all bed numbers at the facility (minus known pneumonia cases) sorted by building, floor, and bunk (upper or lower).
Consenting inmates, both randomly selected and self-referred, were interviewed using a standardized questionnaire to collect demographic and clinical information, details on housing assignments, and general activities within the prison. Race categories were assigned by the Federal Bureau of Prisons (BOP). Information on past medical history was obtained via inmate report, prison records, and BOP’s electronic medical records. Inmates provided NP and OP, or combined NP/OP, swabs, except in cases where collection of 1 type of swab (NP or OP) was refused. Acute and convalescent sera for C. pneumoniae–specific serologies (immunoglobulins M [IgM] and G [IgG]) were collected from consenting participants who were symptomatic during the interview or who had experienced an acute respiratory infection within the previous month.
Case Definitions
We defined a case of C. pneumoniae illness as acute respiratory illness (ARI) in an inmate residing within the facility during the outbreak period supported by either radiological confirmation of pneumonia (noted by 2 independent readers) or laboratory evidence of acute C. pneumoniae infection. ARI was defined as myalgias, headache, or sore throat in the presence of cough lasting ≥3 days, self-reported fever ≥99°F, or self-reported chills. Laboratory evidence of acute infection was defined as C. pneumoniae detected by qPCR (from NP, OP, or NP/OP swab), an IgM titer of ≥1:10, or a ≥4-fold increase in IgG antibody titer between paired sera obtained 3–6 weeks apart. Persons with laboratory evidence of acute C. pneumoniae infection without ARI were defined as asymptomatic cases.
Risk Factor Analyses
To identify known risk factors for C. pneumoniae infection, we conducted 2 independent analyses using (1) self-referred cases, and (2) randomly selected cases. Persons identified in the systematic random sample who were not identified as cases and had neither an ARI nor laboratory evidence of infection served as controls; persons reporting an ARI without laboratory evidence of infection were excluded from these analyses.
Study of Persistence of C. pneumoniae After Infection
We tracked C. pneumoniae in the oropharynx after infection by weekly follow-up OP swabs among cases. During each swab collection, inmates reported current symptoms and antibiotics usage within the preceding 7 days. Participants were followed for up to 8 weeks, until they had ≥2 consecutive negative samples, or until they declined participation.
Laboratory Analysis
The initial 7 NP/OP swabs from acutely ill inmates were tested using qPCR at the CDC’s Respiratory Diseases Branch Laboratory in Atlanta, Georgia, using the TaqMan Array Card (TAC) for influenza (A and B), respiratory syncytial virus, human parainfluenza virus (types 1, 2, and 3), human metapneumovirus, rhinovirus, enterovirus, human parechovirus, adenovirus, Legionella pneumophila, Haemophilus influenzae, Streptococcus pneumoniae, Streptococcus pyogenes, Mycoplasma pneumoniae, Chlamydia pneumoniae, and Bordetella pertussis. Subsequent testing of respiratory and serological specimens was performed at the CDC using methods that have been previously described [15]. In brief, all NP and OP specimens were collected using Dacron swabs and immediately placed in refrigerated universal transport media (Becton Dickinson, Franklin Lakes, New Jersey). Serum samples and swabs were frozen at −20°C within 12 hours and sent to the CDC for analysis. Swabs were tested using a real-time multiplex PCR assay for detection of C. pneumoniae, M. pneumoniae, Legionella species, and a human DNA internal control [16]. Acute and convalescent sera were tested for C. pneumoniae–specific IgM and IgG antibodies using commercially available microimmunofluorescence kits (FOCUS Diagnostics, Cypress, California).
Statistical Analysis
Data were analyzed using SAS software, version 9.2 (SAS Institute, Cary, North Carolina). Weighted attack rates were calculated using the proportion of inmates represented by the sample. A suspected pneumonia attack rate was calculated for cases who self-referred for care and was added to the weighted sample attack rate to estimate the facility-wide attack rate. The variance of the estimated facility-wide attack rate included the variance of the weighted sample attack rate and the self-referred cases’ attack rate. Odds ratios and 95% confidence intervals comparing self-referred cases to randomly selected controls were calculated from univariate logistic regression models. Relative risks and 95% confidence intervals comparing randomly selected cases to randomly selected controls were calculated from univariate log binomial models.
RESULTS
Descriptive Epidemiology of the Outbreak
Of 36 inmates who self-referred for care during the outbreak, 3 did not meet case criteria. Among the remaining 33, 15 (45.5%) had radiologically confirmed pneumonia and 29 (87.9%) had laboratory confirmation of C. pneumoniae infection. Clinical characteristics were incomplete for 6 (Table 1), including 3 who refused to participate in the survey. Among 270 beds randomly selected for active case finding, 254 were occupied at the time of the survey; of these, 223 (87.8%) inmates consented to participate. The random sample identified 19 additional cases, bringing the total number to 52.
Table 1.
Characteristics of Respiratory Illness Among Inmates Who Self-Referred for Care and Were Identified by a Random Sample During an Outbreak of Chlamydia pneumoniae in a Federal Correctional Facility (N = 52)
| Characteristic | Self-Referred Cases (%) n = 33 |
Random Sample–Identified Cases (%) n = 19 |
All Cases (%) N = 52 |
|---|---|---|---|
| Demographics | |||
| Age, y, median (range)a | 42 (22–78) | 35 (24–59) | 40 (22–78) |
| Race and ethnicity | |||
| White | 15 (45.5) | 9 (47.4) | 24 (46.2) |
| Asian/Pacific Islander | 2 (6.0) | 1 (5.3) | 3 (5.8) |
| African American | 9 (27.3) | 4 (21.0) | 13 (25.0) |
| Hispanic | 1 (3.0) | 5 (26.3) | 6 (11.5) |
| Missing race data | 6 (18.2) | 0 | 6 (11.5) |
| Past medical historyb | |||
| Current or past smoker | 17 (56.7) | 9 (47.4) | 26 (53.1) |
| Self-reported asthma diagnosis | 3 (13.0) | 5 (26.32) | 8 (19.0) |
| Any self-reported chronic illnessc | 14 (42.4) | 10 (52.6) | 24 (46.2) |
| Unit of residence | |||
| Housing unit X | 10 (30.3) | 11 (57.9) | 21 (40.4) |
| Housing unit Y | 23 (69.7) | 8 (42.1) | 31 (59.6) |
| Clinical characteristics and laboratory findings | |||
| Pulmonary infiltrate on chest radiographyb | 15 (45.5) | NA | 15 (45.5) |
| Any laboratory test positiveb | 29 (87.9) | 19 (100) | 48 (92.3) |
| PCR positive | 23 (69.7) | 8 (42.1) | 31 (59.6) |
| 4-fold increase in IgGd | 5 (35.7) | 1 (25.0) | 6 (33.3) |
| Single IgM ≥10e | 24 (85.7) | 14 (87.5) | 38 (86.4) |
| Symptomsb,f | |||
| Cough | 29 (96.7) | 19 (100) | 48 (98.0) |
| Runny nose | 18 (62.1) | 17 (89.5) | 35 (72.9) |
| Sore throat | 23 (79.3) | 12 (63.2) | 35 (72.9) |
| Sputum | 19 (65.5) | 14 (73.7) | 33 (68.8) |
| Fever or chills | 25 (86.2) | 7 (38.9) | 32 (68.1) |
| Shortness of breath | 24 (82.8) | 4 (21.1) | 28 (58.3) |
| Wheezing | 21 (72.4) | 6 (35.3) | 27 (58.7) |
| Headache | 18 (60.0) | 4 (21.1) | 22 (44.9) |
| Sinus pain/pressure | 11 (44.0) | 8 (42.1) | 19 (43.2) |
| Joint or muscle aches | 14 (48.3) | 4 (21.1) | 18 (37.5) |
| Ear pain/pressure | 6 (23.1) | 2 (10.5) | 8 (17.8) |
| Diarrhea | 6 (20.7) | 0 | 6 (12.5) |
| Vomiting | 3 (10.3) | 1 (5.3) | 4 (8.3) |
| Median duration of cough, d | 29 (1–64) | 7 (3–42) | 21 (1–64) |
Abbreviations: IgG, immunoglobulin G; IgM, immunoglobulin M; NA, not applicable, none of the randomly selected inmates had x-rays taken; PCR, polymerase chain reaction.
Data missing for 1 participant.
Cases with missing data were excluded from these figures.
Includes diabetes, asthma, chronic obstructive pulmonary disease, chronic liver disease, hypercholesterolemia, human immunodeficiency virus infection, obesity, atherosclerosis, and heart failure.
Convalescent sera were available from 14 self-presenting cases and 4 surveyed cases.
Acute sera were available from 28 self-presenting cases and 16 surveyed cases.
Clinical symptoms obtained from the questionnaire were not available for 3 self-referred inmates; partial data were available for 4 self-referred inmates who completed a pilot questionnaire.
Inmates who self-referred for care were older (median age, 42 years) than cases identified by the systematic random sample (median age, 35 years; Table 1). Self-referred cases were also more often white (55.6%) or African American (33.3%), or lived in housing unit Y (69.7%). In contrast, random sample cases were more frequently Hispanic (26.3%) or reported a history of asthma (26.3%). The most common symptoms among cases were cough ≥3 days (98.0%), runny nose (72.9%), and sore throat (72.9%). The median duration of cough at time of case detection was 21 days (range, 1–64). In general, symptoms were reported more frequently among cases who self-referred compared to those identified through random selection.
Forty-eight cases had laboratory confirmation of C. pneumoniae infection; 29 (60.4%) self-referred for care. Among the 15 self-referred cases with radiologically confirmed pneumonia, 9 (60%) tested positive by qPCR for C. pneumoniae. Eight (42.1%) cases identified by random selection had a qPCR-positive swab, 14 (87.5%) had elevated IgM, and 1 (25%) of 4 who provided paired sera demonstrated a 4-fold rise in IgG. Eight asymptomatic C. pneumoniae–infected inmates were identified during the survey; 1 through a qPCR-positive OP swab and 7 through IgM-positive serologies.
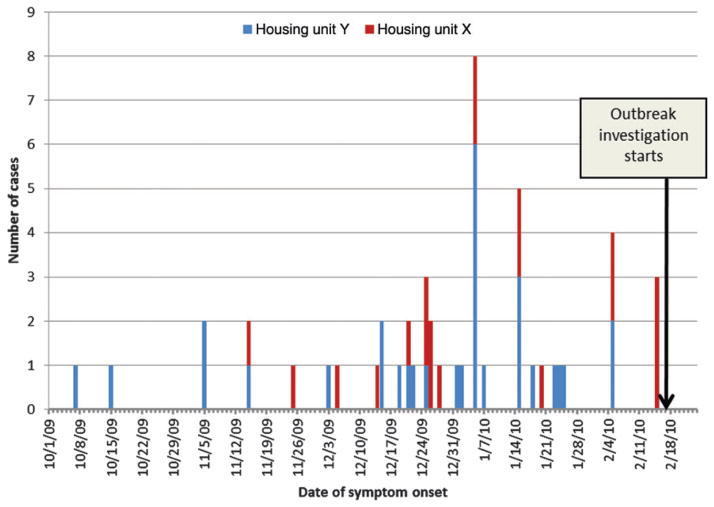
Weighted attack rates for C. pneumoniae infection ranged by floor and unit (Table 2), with a facility-wide attack rate of 10.4% (95% confidence interval, 7.0%–13.8%) and the highest attack rate on the second floor of housing unit Y (13.6%). The onset of illness among cases in housing unit Y generally preceded those in housing unit X (Figure 1).
Table 2.
Weighted Attack Rates of Chlamydia pneumoniae Infection Among 1574 Inmates in a Federal Correctional Facility by Housing Location
| Housing Location | Weighted Attack Rate (95% CI) |
|---|---|
| Facility-wide | 10.4 (7.0–13.8) |
| Housing unit X (all floors) | 10.0 (5.3–14.7) |
| Floor 1 | 4.8 (0–12.0) |
| Floor 2 | 9.7 (2.2–17.3) |
| Floor 3 | 12.8 (4.6–21.0) |
| Housing unit Y (all floors) | 10.9 (5.9–15.8) |
| Floor 1 | 0.8 (0–2.2) |
| Floor 2 | 13.6 (4.3–23.0) |
| Floor 3 | 12.5 (5.0–20.0) |
Abbreviation: CI, confidence interval.
Figure 1.
Distribution of Chlamydia pneumoniae cases by date of symptom onset and residential housing unit during an outbreak at a federal correctional facility (N = 52). The last laboratory-confirmed C. pneumoniae case was detected on 3 March 2010; however, symptom onset data were not available.
Risk Factor Analysis Findings
A total of 155 inmates from the random sample were without ARI or laboratory evidence of C. pneumoniae infection and were identified as controls and independently compared to the 33 self-referred and 19 randomly selected cases. Both self-referred and randomly selected cases were more often of white race compared to Hispanic and reside in housing unit Y, although these findings were statistically significant only for self-referred cases (Table 3). Latino-Hispanic inmates were at lowest risk of infection for self-referred cases. We found no associations with self-reported history of asthma, preincarceration smoking status, educational level, or time spent in any specific areas of the facility.
Table 3.
Univariate Comparison of Demographic and Clinical Characteristics Among Self-Referred and Randomly Selected Cases Compared to 155 Randomly Selected Controls During an Outbreak of Chlamydia pneumoniae Respiratory Illness in a Federal Correctional Facility
| Characteristic | Controls (n = 155) No. (%) |
Self-Referred Cases (n = 33)
|
Randomly Selected Cases (n = 19)
|
||||
|---|---|---|---|---|---|---|---|
| No. (%) | OR (95% CI) | P Value | No. (%) | RR (95% CI) | P Value | ||
| Age >55 ya | 8 (5.2) | 4 (12.5) | 2.6 (.7–9.2) | .14 | 3 (15.8) | 2.7 (.9–8.0) | .065 |
|
| |||||||
| Racea | |||||||
|
| |||||||
| White | 39 (25.5) | 15 (55.6) | 23.8 (3.0–187.7) | <.01 | 9 (47.4) | 2.5 (.9–7.0) | .079 |
|
| |||||||
| African-American | 44 (28.8) | 9 (33.3) | 12.7 (1.5–103.7) | .02 | 4 (21.1) | 1.1 (.3–3.9) | .864 |
|
| |||||||
| Latino-Hispanic | 62 (40.5) | 1 (3.7) | Referent | … | 5 (26.6) | Referent | … |
|
| |||||||
| Otherb | 8 (5.2) | 2 (7.4) | 15.5 (1.3–190.9) | .03 | 1 (5.3) | 1.5 (.2–11.3) | .701 |
|
| |||||||
| No education beyond high school diploma/GEDa | 109 (73.6) | 15 (65.2) | 0.7 (.3–1.7) | .40 | 10 (55.6) | 0.5 (.2–1.2) | .110 |
|
| |||||||
| Smoker prior to incarcerationa | 106 (68.8) | 17 (56.7) | 0.6 (.3–1.3) | .20 | 9 (47.4) | 0.5 (.2–1.1) | .066 |
|
| |||||||
| History of asthmaa | 19 (12.3) | 3 (13.0) | 1.1 (.3–3.9) | .92 | 5 (26.3) | 2.2 (.9–5.6) | .092 |
|
| |||||||
| Unit Y residence compared to unit X | 69 (44.5) | 23 (69.7) | 2.9 (1.3–6.4) | .01 | 8 (42.1) | 0.9 (.4–2.2) | .842 |
|
| |||||||
| Floor within unit Y | |||||||
|
| |||||||
| Floor 1 | 12 (17.4) | 1 (4.3) | 0.2 (.0–1.3) | .08 | 0 | NA | NA |
|
| |||||||
| Floor 2 | 26 (37.7) | 5 (21.7) | 0.4 (.1–1.1) | .07 | 5 (62.5) | 1.8 (.5, 7.0) | .3798 |
|
| |||||||
| Floor 3 | 31 (44.9) | 17 (74.0) | Referent | … | 3 (37.5) | Referent | … |
Abbreviations: CI, confidence interval; GED, graduate equivalent degree; NA, not applicable; OR, odds ratio; RR, relative risk.
Cases with missing data were excluded from these figures.
Includes American Indian, Alaska Native, Asian, and Pacific Islander.
Laboratory Results
From the 7 NP/OP specimens available for TAC testing, 4 (57.1%) tested positive for C. pneumoniae (1 was also positive for S. pneumoniae). One each was weakly positive for S. pneumoniae and H. influenzae, which frequently colonize the upper respiratory tract. Results for all other 15 pathogens were negative. Based on these findings, C. pneumoniae was determined to be the sole causative pathogen in the outbreak.
Among 256 randomly sampled and self-referred inmates, we collected combined NP/OP swabs from 66 (25.5%), paired separate NP and OP swabs from 29 (11.2%), only NP from 1 (0.3%), and only OP from 160 (61.8%). Positivity rates were 19.7% among combined NP/OPs, 10% among NPs, and 3.7% among OPs. Among 29 inmates who had NP and OP swabs collected and tested separately, only 2 had discrepant results; in both cases, OP swabs were positive and NP swabs were negative (data not shown).
Among 122 (47.1%) inmates who submitted single serum samples, 33 (12.7%) submitted paired serum samples 4–6 weeks later. This included 65 inmates who reported an ARI during the outbreak period but who were negative for C. pneumoniae infection by qPCR. Of these, 35 (59.3%) had a single IgG titer for C. pneumoniae ≥512 and 17 (28.8%) had a single IgM titer ≥10 (Table 4). Among 37 inmates with no ARI symptoms and who were negative by qPCR for C. pneumoniae, 23 (62.2%) had a single IgG assay and 7 (18.9%) had a single IgM assay showing elevated antibody levels.
Table 4.
Agreement of Antibody Testing With Real-Time Polymerase Chain Reaction Results During an Outbreak of Chlamydia pneumoniae in a Federal Correctional Facility, Among Samples From Inmates With and Without Acute Respiratory Illnessa
| C. pneumoniae qPCR Result | Single IgM ≥1:10 | Single IgG ≥1:512 | 4-Fold Increase in IgG |
|---|---|---|---|
| No ARI | |||
| qPCR-positive (n = 1) | 0/1 (0%) | 1/1 (100%) | 0/0 |
| qPCR-negative (n = 37) | 7/37 (18.9%) | 23/37 (62.2%) | 0/1 (0%) |
| ARI | |||
| qPCR-positive (n = 25) | 21/25 (84.0%) | 24/25 (96.0%) | 4/10 (40.0%) |
| qPCR-negative (n = 59) | 17/59 (28.8%) | 35/59 (59.3%) | 2/22 (9.1%) |
Results shown are the number positive/number tested (%).
Abbreviations: ARI, acute respiratory illness; IgG, immunoglobulin G; IgM, immunoglobulin M; qPCR, real-time polymerase chain reaction.
ARI defined as follows: cough lasting ≥3 days AND 1 or more of the following: myalgias, headache, sore throat; OR fever ≥37.2°C or chills AND 1 or more of the following: myalgias, headache, sore throat, cough ≥3 days.
Persistence of C. pneumoniae After Infection
We collected additional OP swabs on 40 inmates for up to 8 weeks after the initial investigation: 36 individuals met our case definition, 3 described an ARI but did not meet case criteria, and 1 self-referred for care after conclusion of the investigation and tested positive for C. pneumoniae by qPCR. For 12 (30.0%) inmates, no C. pneumoniae was detected at any visit (Figure 2). Among 28 inmates who tested qPCR positive for C. pneumoniae at least once, 6 were positive only on 1 swab; 22 others were positive up to 8 weeks after the outbreak investigation (median, 5 weeks). Sixteen (57.1%) received antibiotic treatment >2 weeks prior to their positive swab (13 with azithromycin; 3 with nonspecified antibiotic). A positive qPCR test was associated with cough in 35 of 69 (50.1%) carriage episodes. Six (30.0%) persons tested positive by qPCR following a single negative swab, and 4 (10.0%) tested positive after 2 consecutive negative swabs. At the time of their last swab, 16 (40%) inmates were both asymptomatic and qPCR-negative.
Figure 2.
Antibiotic usage and persistence of Chlamydia pneumoniae carriage and symptoms over time among 40 cases of C. pneumoniae respiratory infection at a federal correctional facility. *Indicates chest radiography–confirmed pneumonia. Abbreviations: AC, symptomatic, cleared (real-time polymerase chain reaction [qPCR] negative); AP, symptomatic, persistent infection (or reinfection); D, doxycycline; NS, not specified; SC, symptomatic, cleared (qPCR negative); SP, symptomatic, persistent infection (or reinfection); Z, azithromyccin.
Public Health Interventions
To stop transmission among inmates, the facility posted educational notices around the prison and encouraged sick inmates to present for care and early initiation of treatment by waiving the usual clinic fee and discontinuing single-cell housing of ill persons. A 5-day course of azithromycin was recommended for persons presenting with signs and symptoms of C. pneumoniae infection; a 14-day course of doxycycline was recommended for those who remained symptomatic after completing azithromycin. The last confirmed C. pneumoniae case was detected on 3 March 2010, 9 days after the outbreak investigation concluded; demographic and clinical data were not available for this inmate.
DISCUSSION
This report summarizes the largest C. pneumoniae outbreak confirmed by qPCR. The long duration of the outbreak (over 4 months) is consistent with C. pneumoniae outbreaks described in military barracks [17, 18] and contrasts with the shorter duration (1–2 months) often seen in long-term care facilities [6, 7]. The attack rates seen in this prison were lower than the median reported among long-term care facilities (46%), possibly due to lower prevalence of comorbid conditions as this facility was rated care level 2 by the BOP, and inmates did not have medical conditions that required more than routine appointments for monitoring [8]. The clinical presentation and spectrum of illness parallels those seen in other C. pneumoniae outbreaks [11, 19], although we did not find a large number of asymptomatic qPCR-positive persons.
Inmates generally spent a large portion of the day together and slept on bunks spaced approximately 3–5 feet apart, facilitating transmission of C. pneumoniae. The increased risk of C. pneumoniae infection among white inmates may have been due to social interaction; inmates frequently congregated along racial or ethnic lines, increasing likelihood of transmission within.
Several studies have demonstrated an increased risk of C. pneumoniae illness, particularly severe disease, among the elderly and persons with asthma or chronic obstructive pulmonary disease (COPD) [7, 11, 20]. In this investigation, inmates with C. pneumoniae tended to be older than those who were not ill; however, this trend was not statistically significant. In addition, none of the 4 persons hospitalized during the outbreak were >45 years of age. We did not see any increased risk among persons with asthma, COPD, or other chronic medical conditions. The lack of association between C. pneumoniae and previously established risk factors may be attributed to a lack of statistical power to detect one or to the lower prevalence of chronic medical conditions in our inmate population than that seen at long-term health facilities and greater variability in age than military barracks. In previous studies, smoking has also been associated with an earlier illness onset; however, its prohibition in federal prisons did not allow us to determine current smoking status for inmates.
Postinfection carriage of C. pneumoniae is not uncommon, and several studies have used culture to document persistence of the organism for up to 12 months after acute infection [2, 21]. Although these studies were small, shedding of C. pneumoniae continued after completion of treatment with tetracycline or doxycycline (range, 5–31 days). Here we document persistence of the organism in the oropharynx by qPCR for up to 8 weeks following appropriate antibiotic therapy. Most participants in our follow-up study continued to experience cough symptoms for several weeks after their first qPCR-positive swab. Persistence of respiratory symptoms and C. pneumoniae detection after acute infection has been theorized to be due to chronic intracellular infection and sustained airway irritation [22]. In this outbreak, close living quarters and poor compliance with medications may have led to reexposure to C. pneumoniae and contributed to ongoing transmission.
Our findings may not fully represent the spectrum of C. pneumoniae illness because we were unable to identify all symptomatic inmates and, although qPCR is highly sensitive for detecting acute shedding, it was not used on cases that occurred earlier in the outbreak. We also utilized serological markers to identify C. pneumoniae infection, which may have cross-reacted with other Chlamydia species, specifically C. trachomatis [14, 15, 23]. Our results again demonstrate problems with specificity, as several inmates without symptoms who were negative by qPCR nonetheless had elevated IgM antibody levels. Although IgG measurements in paired sera are generally more reliable, timing is essential to diagnosis. Here, sera collected outside the acute and convalescent phases of illness may not have demonstrated a 4-fold increase distinguishing recent from past exposure.
Over the past several decades, US correctional facilities have become a congregate setting of increasing importance to public health. The number of incarcerated persons in US jails and prisons has reached historic highs, compounded by inmate overcrowding [24]. A disproportionate number of inmates have health problems that put them at risk for serious complications of respiratory infections, making the early detection and containment of outbreaks a priority for correctional healthcare and public health authorities [25]. Despite constitutional entitlement of all inmates to basic healthcare, correctional facilities present unique public health challenges: close quarters facilitate droplet transmission, and medical illness is often underreported due to fear of stigmatization or isolation. Control of respiratory outbreaks can be difficult because of poor compliance with recommended behavioral modifications (eg cough etiquette, medication compliance) and because routine respiratory precautions (eg masks, alcohol-based sanitizers, soap dispensers) are often not feasible in this setting.
Key lessons learned from this outbreak investigation include (1) diagnostic advantages of using qPCR to identify C. pneumoniae during respiratory outbreaks; (2) recognition that antibiotic therapy for C. pneumoniae may not readily reduce coughing or eliminate carriage; (3) importance of inmate education and elimination of medical copay to maximize case identification during outbreaks in the correctional setting; and (4) prioritization of hand hygiene as the key infection control measure in jails and prisons. Last, this investigation highlights the value of collaboration between public health and corrections officials to advance our understanding of respiratory outbreaks and their management.
Acknowledgments
Financial support. This work was funded by the Centers for Disease Control and Prevention.
Footnotes
Disclaimer. Opinions expressed in this article are those of the authors and do not necessarily represent the opinions of the Federal Bureau of Prisons, the Department of Justice, or the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Miyashita N, Fukano H, Okimoto N, et al. Clinical presentation of community-acquired Chlamydia pneumoniae pneumonia in adults. Chest. 2002;121:1776–81. doi: 10.1378/chest.121.6.1776. [DOI] [PubMed] [Google Scholar]
- 2.Grayston JT, Kuo C-c, Wang S-p, Altman J. A new Chlamydia psittaci strain, TWAR, isolated in acute respiratory tract infections. N Engl J Med. 1986;315:161–8. doi: 10.1056/NEJM198607173150305. [DOI] [PubMed] [Google Scholar]
- 3.File TM, Jr, Tan JS. Chlamydia pneumoniae pneumonia. Semin Respir Crit Care Med. 2000;21:285–94. doi: 10.1055/s-2000-9857. [DOI] [PubMed] [Google Scholar]
- 4.Blasi F, Tarsia P, Aliberti S. Chlamydophila pneumoniae. Clin Microbiol Infect. 2009;15:29–35. doi: 10.1111/j.1469-0691.2008.02130.x. [DOI] [PubMed] [Google Scholar]
- 5.Miyashita N, Niki Y, Nakajima M, Fukano H, Matsushima T. Prevalence of asymptomatic infection with Chlamydia pneumoniae in subjectively healthy adults. Chest. 2001;119:1416–9. doi: 10.1378/chest.119.5.1416. [DOI] [PubMed] [Google Scholar]
- 6.Troy CJ, Peeling RW, Ellis AG, et al. Chlamydia pneumoniae as a new source of infectious outbreaks in nursing homes. JAMA. 1997;277:1214–8. [PubMed] [Google Scholar]
- 7.Miyashita N, Ouchi K, Shoji H, et al. Outbreak of Chlamydophila pneumoniae infection in long-term care facilities and an affiliated hospital. J Med Microbiol. 2005;54:1243–7. doi: 10.1099/jmm.0.46191-0. [DOI] [PubMed] [Google Scholar]
- 8.Utsumi M, Makimoto K, Quroshi N, Ashida N. Types of infectious outbreaks and their impact in elderly care facilities: a review of the literature. Age Ageing. 2010;39:299–305. doi: 10.1093/ageing/afq029. [DOI] [PubMed] [Google Scholar]
- 9.Marrie TJ, Peeling RW, Reid T, De Carolis E Canadian Community-Acquired Pneumonia Investigators. Chlamydia species as a cause of community-acquired pneumonia in Canada. Eur Respir J. 2003;21:779–84. doi: 10.1183/09031936.03.00095403. [DOI] [PubMed] [Google Scholar]
- 10.Dawood F, Ambrose J, Russell B, et al. Outbreak of pneumonia in the setting of fatal pneumococcal meningitis among US Army trainees: potential role of Chlamydia pneumoniae infection. BMC Infect Dis. 2011;11:157. doi: 10.1186/1471-2334-11-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kauppinen MT, Herva E, Kujala P, Leinonen M, Saikku P, Syrjälä H. The etiology of community-acquired pneumonia among hospitalized patients during a Chlamydia pneumoniae epidemic in Finland. J Infect Dis. 1995;172:1330–5. doi: 10.1093/infdis/172.5.1330. [DOI] [PubMed] [Google Scholar]
- 12.Dowell SF, Peeling RW, Boman J, et al. Standardizing Chlamydia pneumoniae assays: recommendations from the Centers for Disease Control and Prevention (USA) and the Laboratory Centre for Disease Control (Canada) Clin Infect Dis. 2001;33:492–503. doi: 10.1086/322632. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell SL, Budhiraja S, Thurman KA, Lanier Thacker W, Winchell JM. Evaluation of two real-time PCR chemistries for the detection of Chlamydophila pneumoniae in clinical specimens. Mol Cell Probes. 2009;23:309–11. doi: 10.1016/j.mcp.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein EJC, Kumar S, Hammerschlag MR. Acute respiratory infection due to Chlamydia pneumoniae: current status of diagnostic methods. Clin Infect Dis. 2007;44:568–76. doi: 10.1086/511076. [DOI] [PubMed] [Google Scholar]
- 15.Benitez AJ, Thurman KA, Diaz MH, Conklin L, Kendig NE, Winchell JM. Comparison of real-time PCR and a microimmunofluorescence serological assay for detection of Chlamydophila pneumoniae infection in an outbreak investigation. J Clin Microbiol. 2012;50:151–3. doi: 10.1128/JCM.05357-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thurman KA, Warner AK, Cowart KC, Benitez AJ, Winchell JM. Detection of Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella spp. in clinical specimens using a single-tube multiplex real-time PCR assay. Diagn Microbiol Infect Dis. 2011;70:1–9. doi: 10.1016/j.diagmicrobio.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ekman M-R, Grayston JT, Visakorpi R, Kleemola M, Kuo C-c, Saikku P. An epidemic of infections due to Chlamydia pneumoniae in military conscripts. Clin Infect Dis. 1993;17:420–5. doi: 10.1093/clinids/17.3.420. [DOI] [PubMed] [Google Scholar]
- 18.Kleemola M, Saikku P, Visakorpi R, Wang SP, Grayston JT. Epidemics of pneumonia caused by TWAR, a new Chlamydia organism, in military trainees in Finland. J Infect Dis. 1988;157:230–6. doi: 10.1093/infdis/157.2.230. [DOI] [PubMed] [Google Scholar]
- 19.File TM, Plouffe JF, Breiman RF, Skelton SK. Clinical characteristics of Chlamydia pneumoniae infection as the sole cause of community-acquired pneumonia. Clin Infect Dis. 1999;29:426–8. doi: 10.1086/520227. [DOI] [PubMed] [Google Scholar]
- 20.Metz G, Kraft M. Effects of atypical infections with Mycoplasma and Chlamydia on asthma. Immunol Allergy Clin North Am. 2010;30:575–85. doi: 10.1016/j.iac.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammerschlag MR, Chirgwin K, Roblin PM, et al. Persistent infection with Chlamydia pneumoniae following acute respiratory illness. Clin Infect Dis. 1992;14:178–82. doi: 10.1093/clinids/14.1.178. [DOI] [PubMed] [Google Scholar]
- 22.Blasi F, Aliberti S, Allegra L, et al. Chlamydophila pneumoniae induces a sustained airway hyperresponsiveness and inflammation in mice. Respir Res. 2007;8:83. doi: 10.1186/1465-9921-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuuminen T, Varjo S, Ingman H, Weber T, Oksi J, Viljanen M. Prevalence of Chlamydia pneumoniae and Mycoplasma pneumoniae immunoglobulin G and A antibodies in a healthy Finnish population as analyzed by quantitative enzyme immunoassays. Clin Diagn Lab Immunol. 2000;7:734–8. doi: 10.1128/cdli.7.5.734-738.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guerino P. Prisoners in 2010. US Department of Justice, Bureau of Justice Statistics Bulletin; 2011. NCJ 236096. Available at: http://www.bjs.gov/content/pub/pdf/p10.pdf. [Google Scholar]
- 25.John MR. The disease profile of Texas prison inmates. Ann Epidemiol. 2000;10:71–3. doi: 10.1016/s1047-2797(00)00034-x. [DOI] [PubMed] [Google Scholar]