Introduction
Mycobacterium tuberculosis (Mtb) is an extremely successful bacterium that is transmitted person-to-person by the aerosol route. The World Health Organization (WHO) has estimated that more than 2 billion persons are latently infected with Mtb. From this large reservoir of asymptomatic infected people emerges 8-10 million cases of active TB each year resulting in the deaths of nearly 1.7 million people each year 1. The increased incidence of TB has been attributed to three major factors: the HIV pandemic, the emergence of multidrug-resistant strains of Mtb, and the failure of the major vaccine, BCG, to prevent pulmonary tuberculosis 2,3,4.
The success of this pathogen is closely linked to its ability to alter the intracellular environment of the alveolar macrophage. When inhaled, Mtb enters the lower respiratory tract and is thought to be deposited in distal alveoli. One of their first interactions is with alveolar macrophages, which they infect. Although macrophages excel at phagocytizing and destroying biological particles including dead cells and bacteria, Mtb has adapted to the harsh intracellular environment, which allows it to survive and replicate within these phagocytic cells. Now there is more data that Mtb eventually kills the host cell. By subverting or avoiding critical components of macrophage immunity including phagolysosomal fusion, microbicidal effectors, and as will be discussed in this chapter, cell death pathways, Mtb evades both innate and adaptive immune responses. Therefore, delineating how Mtb and macrophages interact is fundamental to the host-pathogen relationship.
Manipulation of macrophage death pathways is one mechanism that allows Mtb to evade host defenses. Three major outcomes are observed following productive Mtb infection of human and murine macrophages in vitro: a) necrosis, a form of death characterized by plasma membrane disruption; b) apoptosis, a form of death in which the plasma membrane integrity is preserved; and c) survival of the infected macrophages. Characterization of these different phenotypes is challenging because of the asynchronous nature of intracellular infection and heterogeneity among the bacteria and macrophages. Other factors such as the percentage of infected macrophages and variation in the number of bacteria internalized by each macrophage can affect the kinetics of cell death when studied in vitro. Nevertheless, a spectrum of all three phenotypes can be observed following infection of normal macrophages with virulent Mtb. In general, highly virulent Mtb strains predominantly induce necrosis 5. The concept that virulent Mtb induce necrosis in part by actively inhibiting macrophage apoptosis 6, has gained additional support by the identification of mutants that induce apoptosis instead of necrosis 7,8. The different cellular fates of Mtb infected macrophages are of great interest as the death modality influences the outcome of infection. In particular, apoptotic death reduces the viability of different mycobacterial species 9,10 including Mtb 11,12. Here, we discuss the cellular mechanisms that regulate the death modality of Mtb infected macrophages and lead to important functional consequences.
Macrophage apoptosis is a host defense mechanism against Mtb
The discovery that many attenuated strains of mycobacteria induce more apoptosis than their wild type counterparts supports the hypothesis that virulent mycobacteria inhibit macrophage apoptosis. Indeed, there exists a reciprocal relationship between virulence and apoptosis. As such, Mtb infection predominantly results in necrosis, while attenuated mutant strains including BCG and H37Ra primarily induce apoptosis. Now, investigators are identifying single gene mutations in Mtb that shift the balance from necrosis to apoptosis 7,8. Although it is not yet clear whether virulent Mtb block the triggering of apoptosis or inhibit downstream events that give rise to the typical cellular changes associated with apoptosis, it can be argued that by inducing necrosis, Mtb evades host defenses and provides a pathway for its exit from the infected cell and its dissemination. Detailed analysis of “necrosis” reveals it to be heterogeneous and certain subtypes have been defined that have unique cellular triggers and molecular mechanisms. Thus, pyroptosis and necroptosis are forms of necrosis that are dependent on caspase-1 and receptor-interacting protein 1 and 3 (RIP-1/3), respectively 13,14,15. Thus, the idea that necrosis is a passive, accidental, and unregulated form of cell death is old dogma. How Mtb induces necrosis is a question that remains unanswered.
In contrast to necrosis, the past decades have made tremendous progress in unraveling the signaling pathways that lead to initiation of apoptosis. Hallmarks of apoptosis include the segmentation of DNA 16, exposure of phosphatidylserine on the outer leaflet of the plasma membrane and finally, packaging of cellular components into membrane-bound blebs 17,18. During apoptosis the dying cell produces ‘find me’ and ‘eat me’ signals that aid its rapid clearance by phagocytes through the process of efferocytosis 19.
Apoptosis is initiated by two major pathways
1) The extrinsic pathway
The induction of apoptosis by attenuated Mtb in human monocyte-derived macrophages is mediated by the executioner caspases 3 and 7 and requires two distinct signals: one is a lipid and the other is a protein 20,21. The protein signal can be reconstituted by TNF. Indeed, the induction of apoptosis in macrophages by Mtb requires the action of TNF to activate the extrinsic death receptor dependent pathway (Figure 1). Although both bacterial strains produce comparable amounts of TNF 22, the avirulent strain H37Ra much more potently induces apoptosis than the virulent H37Rv strain. One explanation for this observation is that soluble TNFR-2 is shed by macrophages infected with virulent Mtb, and neutralizes TNF, resulting in a “TNF-poor” microenvironment 23. This model is consistent with data that caspase 8 activation, which is an essential and early step in the induction of apoptosis by the extrinsic pathway, is inhibited in H37Rv-infected macrophages (H. Remold, unpublished observation). Recently some of the early components of the extrinsic apoptotic pathway activated by Mtb in the murine macrophage cell line RAW 264 have been identified 24. In Mtb-infected cells, TNF production induces reactive oxygen species (ROS) -dependent activation of apoptosis signal-regulating kinase (ASK1; A002816), a member of the mitogen-activated protein kinase family causing FLIPs phosphorylation. Phosphorylated FLIPs interacts with the E3 ubiquitin ligase c-CYBL facilitating proteasomal FLIPs degradation involving the tyrosine kinase c-Abl. FLIPs degradation then enables activation of caspase 8 leading to caspase 3 / 7 activation and apoptosis.
Figure 1. Two major pathways leading to macrophage death following M. tuberculosis infection.
Some macrophages death programs are triggered by the extrinsic pathway (surface receptor mediated): Ligation of death receptor (e.g. Fas or TNF-α receptors) is followed by activation of caspase-8 which leads to apoptosis. Alternatively, the Intrinsic Pathway (mitochondria-mediated) is activated: Permeabilization of mitochondria outer membrane potential (MOMP) leads to release of apoptotic mediators such as cytochrome c from the mitochondrial intermembrane space into the cytosol leading to formation of apoptosome complex and activation of caspases (9, 3, and 7) which in turn induce apoptosis. However, during necrosis mitochondrial permeability transition (MPT) causes mitochondrial inner membrane perturbation (MIMP), collapse of the membrane potential, uncoupling of the respiratory chain, and overproduction of reactive oxygen species (ROS). Cyclophilin D (CypD) is a mitochondrial protein which is involved in MPT and necrosis.
2) The intrinsic pathway
Induction of apoptosis in vertebrate cells most commonly proceeds through the intrinsic apoptotic pathway, which is functionally defined by mitochondrial outer membrane permeabilization (MOMP) 25. MOMP is a central event that can lead to apoptosis as it results in release of apoptotic mediators, including cytochrome c, Smac-DIABLO, AIF and other factors from the mitochondrial inter-membrane space and ultimately results in the activation of caspase 9, 3, and 7 (Figure 1). Although these events usually occur independently of other changes in the mitochondria, they can also be associated with the opening of the mitochondrial inner membrane pore (PT pore), which leads to mitochondrial permeability transition (MPT), loss of the mitochondrial inter-membrane potential (Δψm), and necrosis. We found that Mtb infection, whether virulent or avirulent, induces such changes in the mitochondrial membranes and that these changes are the key events that determine the death modality of infected macrophages.
Mitochondrial damage and macrophage death
The different combinations of MOMP and mitochondrial permeability transition (MPT, the opening of a pore in the inner mitochondrial membrane) in model experimental systems, and their effect on the cellular outcome are reviewed below. The changes in MOMP and MPT induced by virulent and avirulent Mtb infection will then be discussed in the context of these scenarios.
In Scenario I, MPT causes the mitochondria to become leaky to water, which results in swelling, dysfunction and eventually necrosis 25. Irreversible MPT can lead to outer mitochondrial membrane damage, which manifests itself as MOMP, in this case a byproduct of MPT. This scenario emerges when hepatocytes under oxidative stress or due to other toxic treatment undergo both necrosis and apoptosis 26.
However in Scenario II, MOMP and apoptosis can occur independently of MPT. This is the case when MOMP is induced by members of the Bcl-2 family of apoptosis-inducing proteins, which do not affect the mitochondrial inner membrane 27. Specifically, processing of the Bcl-2 protein by BID leads to activation of the pro-apoptotic Bcl-2 family proteins BAX and BAK causing MOMP and translocation of pro-apoptotic factors including cytochrome c into the cytosol, activation of caspase 9, and eventually caspase 3. This process neither induces nor requires MPT 28.
In Scenario III, effector molecules capable of damaging the mitochondrial inner membrane gain access to the mitochondrial inter-membrane space if the mitochondrial outer membrane is permeable. This seems to be the mechanism by which Ndufs1, the 30 kDa subunit of mitochondrial complex 1 of the electron transport chain, is damaged by caspase 3 29. Caspase 3 is thought to access the mitochondrial inter membrane space via pores generated in the mitochondrial outer membrane, which allow pro-apoptotic factors including cytochrome c to escape into the cytosol 28. Damage of Ndufs1 disrupts the electron transport chain in the inner membrane leading to ROS accumulation and necrosis.
Finally, MPT can also occur independently of MOMP (Scenario IV). This is thought to be how granzyme A damages components of the mitochondrial inner membrane 30. Hsp70 and Hsp90 are candidate molecules that serve as cytosolic chaperones for granzyme A and allow the protease to enter the mitochondrial inter-membrane space without damaging the mitochondrial outer membrane leading to cleavage of Ndufs3 30.
In macrophages infected with attenuated Mtb, apoptosis is associated with MOMP yet MPT is not induced, as described in scenario 2 28. Inhibition of MOMP diminishes only apoptosis, but does not affect MPT 5. Silencing of the gene for the pro-apoptotic Bcl-2 protein BAX, which is required for the release of cytochrome c and AIF from the mitochondrial inter-membrane space, abrogates Mtb-induced apoptosis, but does not affect MPT or necrosis 5. In contrast, virulent Mtb induce both MOMP and MPT leading to irreversible mitochondrial swelling and necrosis 5. MPT can be inhibited by CsA (which blocks the function of cyclophilin D in the mitochondrial inner membrane), has a requirement for mitochondrial Ca++ loading and is independent of Bcl-2 family member induced apoptosis 31. Inhibition of MPT, down-regulates only necrosis, but does not affect the degree of MOMP or apoptosis 32,33. It is not clear at present whether in Mtb infected macrophages MPT is dependent on opening of pores in the mitochondrial outer membrane (MOMP – Scenario III) or whether toxic molecular species enter the mitochondrial inter membrane space via chaperones (Scenario IV). The different mechanisms induced by virulent and avirulent Mtb indicate that in Mtb infected macrophages MOMP and MPT are independent phenomena; virulent Mtb are unique in their induction of MPT that leads to the destruction of the mitochondrial outer membrane causing secondary cytochrome c release (see Scenario I) 5. In summary induction of apoptosis or necrosis in Mtb infected macrophages depends on highly specific mechanisms leading to different types of mitochondrial membrane perturbation. Attenuated and virulent Mtb alike cause transient MOMP characterized by cytochrome c release from the mitochondrial inter-membrane space, which requires BAX. In contrast only virulent H37Rv causes MPT.
While cell death is a tightly regulated process, the host-pathogen interaction adds several layers of complexity. How the death modality of infected cells affects the outcome of infection, particularly during different clinical states in people (e.g., latency vs. disease), remains a pertinent question. Here, other investigators working on the genetics of susceptibility to Mtb provide an important perspective. Gene expression profiling finds that several genes related to apoptosis are expressed less in active TB patients than in latently infected people, suggesting that decreased apoptotic activity is associated with the reactivation of latent infection 34. Using a more targeted approach, Abebe et al found that patients with active TB in Ethiopia had elevated expression of genes associated with the extrinsic apoptosis pathway including TNF, Fas, FasL and caspase-8. However, the expression of FLIP, an intrinsic inhibitor of caspase-8, was also significantly elevated 35. Although the upregulation of TNF, Fas/FasL and caspase-8 may be the signature of an immune response capable of inducing apoptosis in infected cells, the authors propose a model in which Mtb inhibits the extrinsic apoptosis pathway by upregulating FLIP to evade an apoptotic death. Finally, the eicosanoid biosynthetic pathways, which regulate the death modality of infected human and murine macrophages, have now been identified as important genetic loci that regulate susceptibility to tuberculosis and leprosy in people 36,37,38. While the genetic and functional data require greater scrutiny and functional correlation, they independently provide scientific motivation to better understand how death is regulated in Mtb infected macrophages.
Host lipid mediators modulate Mtb infected macrophage death modality
As bacterial factors can affect the death modality, host factors also determine whether an infected cell undergoes apoptosis or necrosis. In particular, the eicosanoids appear to be critical regulators of apoptosis following Mtb infection 12,11,39. Mtb induces apoptosis and triggers concomitant antimycobacterial activity of human macrophages based on the activity of cytosolic phospholipase A2-γ (cPLA2-γ), a group IV cytosolic PLA2, which catalyzes the release of arachidonic acid from the sn-2 position of membrane phospholipids 21. Arachidonic acid and its diverse products regulate death in several cell types 40. For example, arachidonic acid products are second messengers in TNF-induced apoptosis 41, and oxygen radicals, which are produced during lipoxygenation of arachidonic acid, induce ROS production, which can induce cell death 42. Arachidonic acid also activates sphingomyelinase leading to ceramide production and apoptosis 43. Which of these mechanisms are important in vivo is not clear 44.
An interesting area of research focuses on the role of the eicosanoids prostaglandin E2 (PGE2) and lipoxin A4 (LXA4) in regulating programmed cell death of macrophages 12,11,39. The cyclooxygenases COX1 and COX2 convert arachidonic acid into the central intermediate PGH2 45, which is converted by specific synthases into diverse prostanoids 46. Interaction of these prostanoid species, which includes the prostaglandins PGD2, PGE2, PGF2α, PGI2 and thromboxane, with an array of specific prostanoid receptors affects many cellular pathways. In the case of PGE2, interaction with one of four receptors, EP1, EP2, EP3 and EP4 triggers intracellular pathways that either promote or inhibit inflammation 47. Importantly, the functional outcome of PGE2 signaling is largely determined by its interaction with its specific receptors 47. For example, EP1 mediates the elevation of intracellular Ca++. By contrast, EP2, which is involved in joint inflammation and neutrophil recruitment, and EP4, which induces cell migration in tumor invasion, both lead to an increase in intracellular cAMP levels. EP2 signaling results in PKA activation and triggering EP4 activates adenylate cyclase and phosphatidylinositol 3 kinase. Triggering EP3, decreases cAMP concentrations and is known to mediate fever and angiogenesis 47.
D'Avila et al find that lipid bodies form at distinct cytoplasmic sites following infection of murine macrophages with the attenuated M. bovis strain BCG. These lipid bodies are the site of COX2 activity and PGE2 generation 48. Indeed, PGE2 production has been a consistent finding following BCG infection of mice and macrophages 49. We find that macrophages infected with attenuated Mtb also activate the PGE2 production, which prevents necrosis and leads instead to an apoptotic death (Figure 2) 12. In contrast, virulent Mtb strains, such as H37Rv or Erdman, only minimally induce the production of PGE2 by macrophages 12. This raises the possibility that virulent Mtb actively inhibits PGE2 production. Thus, an important strategy that Mtb exploits to avoid death by apoptosis is the subversion of host eicosanoid biosynthetic pathways 12,11.
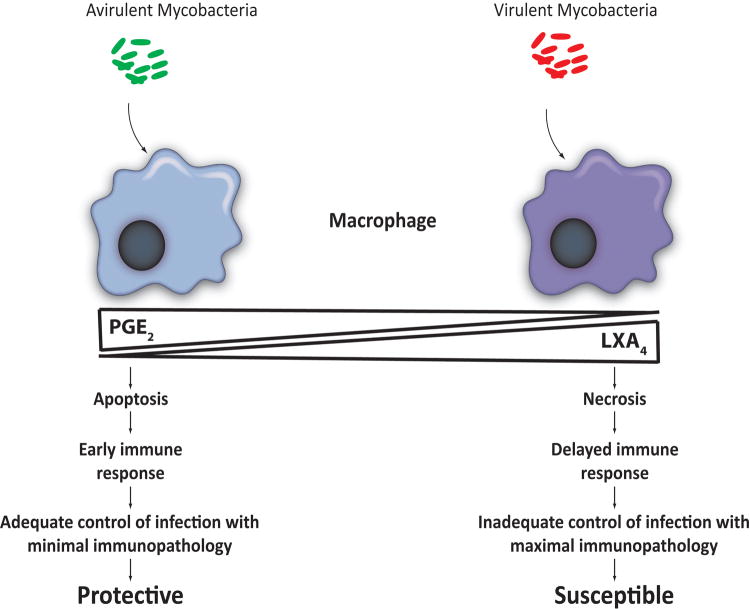
Figure 2. Virulent mycobacteria tip the balance between PGE2 and LXA4 production in macrophages.
Infection with virulent Mtb induces LXA4, which inhibits the production of COX-2 dependent PGE2. In the absence of PGE2 mitochondria are damaged and membrane microdisruptions remain unrepaired triggering macrophage necrosis. We hypothesize that bacterial inhibition of prostaglandin production is an immune evasion strategy that allows Mtb to avoid the consequences of apoptosis which leads to early immune response
Lipoxins are also generated from arachidonic acid but require the action of different enzymes including 5- and 15-lipoxygenases 50. Lipoxins are anti-inflammatory and modulate chemokine and cytokine expression, monocyte trafficking and efferocytosis (phagocytosis of apoptotic cells) 51. In contrast to attenuated strains, virulent Mtb induces LXA4 production, which inhibits cyclooxygenase-2 production effectively shutting down PGE2 biosynthesis, and provides an explanation for how Mtb inhibits PGE2 production 12,11. In a PGE2-poor microenvironment, the macrophage cannot prevent mitochondrial damage nor enable repair of plasma membrane disruptions effectively 5,12,11. Both processes are required to prevent macrophage necrosis and induce apoptotic cell death 12,11. Virulent Mtb in pre-necrotic macrophages continues to replicate and once the cells are lysed, propagate the infection by spreading to uninfected macrophages. Thus, the balance of PGE2 and LXA4 production by the infected macrophage regulates the relative amount of apoptosis and necrosis following Mtb infection and has important functional consequences for innate control of intracellular Mtb infection.
Induction of LXA4 by virulent Mtb inhibits PGE2 production and triggers mitochondrial permeability transition (MPT) leading to irreversible mitochondrial damage 12. By triggering LXA4 production in the host macrophage virulent Mtb inhibits prostanoid production by blocking COX2 mRNA accumulation. By contrast, attenuated Mtb induce only minimal amounts of LXA4 and cause instead production of substantial amounts of PGE2. We found that when macrophage are infected with attenuated Mtb PGE2 actively suppresses mitochondrial inner membrane perturbation, which is the outcome in an infection with virulent Mtb 12. Therefore infection with virulent H37Rv, a PGE2 non-inducer, causes MPT, which is suppressed by reconstitution with PGE2.
Our model that lipoxin production by Mtb infected macrophages is associated with increased bacterial replication and greater virulence is strengthened by the recent genetic analysis of zebrafish susceptibility to M. marinum 52. Multiple mutant classes with different innate susceptibilities to M. marinum were identified by Tobin et al 52. A hypersusceptible zebrafish mutant was found to map to the Ita4h locus, which encodes leukotriene A4 hydrolase (LTA4H), an enzyme that is required for the final step of leukotriene B4 (LTB4) synthesis. While LTA4H deficiency results in the loss of LTB4 production, addition of LTB4 did not complement the genetic defect nor increase host resistance. In the absence of LTA4H, its substrate, LTA4, accumulates and can lead to redirected eicosanoid synthesis and increase lipoxin synthesis. Therefore, Tobin et al hypothesize that the increased susceptibility of the zebrafish LTA4H mutant is due to an increase in lipoxin production. The same study presents human genetic data that polymorphisms in the LTA4H gene are associated with susceptibility to pulmonary and meningeal tuberculosis 52,38. Thus, from fish to man, the eicosanoids appear to play an unexpected role in susceptibility to tuberculosis.
Blocking plasma membrane repair
The ESAT-6 secretion system 1 (ESX-1), a specialized type VII secretion system, is required for the secretion of certain virulence factors including the immunodominant antigens early secreted antigen 6 kilodaltons (ESAT6) and culture filtrate protein 10 (CFP10). Although known to contribute to bacterial virulence, why ESX-1 is required for bacterial survival in the host is unknown. Some data indicate that ESAT6 damages host cell membranes 53,54. We hypothesized that disruption of the plasma membrane by Mtb is one mechanism that induces necrosis of the macrophage. Interaction of mycobacteria with the host macrophage results in plasma membrane microdisruptions. Microdisruptions induced by attenuated Mtb are rapidly resealed by plasma membrane repair mechanisms that include recruitment of lysosomal and Golgi apparatus–derived vesicles to the macrophage surface lesions 11,55,56. Lysosomal or Golgi membrane recruitment to the plasma membrane can be assessed by measuring LAMP1 or mannosidase II translocation to the macrophage surface 57,58. Active membrane repair prevents necrosis and is required for induction of apoptosis. By contrast, if resealing of the plasma membrane microdisruptions inflicted by the bacteria is inhibited, as is the case with virulent Mtb infection and necrosis ensues.
Ca++ sensors are of crucial importance for the recruitment of both lysosomal and Golgi vesicles to the membrane lesions. Gene silencing of the lysosomal Ca++ sensor synaptotagmin 7 (SYT7) impairs the recruitment of lysosomal, but not Golgi membranes to the cell surface 11,59. The recruitment of Golgi-derived vesicles to the cell surface, which occurs independently of lysosomal vesicle recruitment, requires the expression of neuronal calcium sensor 1 (NCS-1), a Ca++ sensor that is particularly abundant in the Golgi 11,60. Silencing NCS-1 gene expression, or the use of brefeldin A, a Golgi-specific transport inhibitor, both inhibit translocation of Golgi membranes. These data show that both lysosomal and Golgi membranes are involved in plasma membrane repair and are recruited independently to plasma membrane lesions of infected macrophage.
Plasma membrane resealing is cAMP dependent 61, and addition of forskolin, an activator of adenylate cyclase, results in greater translocation of lysosomal membranes to the cell surface 11. The protective effect of PGE2 on mitochondrial stability is mediated through the PGE2 receptor EP2 12 and binding of PGE2 to either EP2 or EP4 causes increased cAMP accumulation 62. Consistent with this, PGE2 treatment of human macrophages infected with virulent H37Rv reconstitutes repair mediated by lysosomal membranes. By contrast PGE2 does not affect Golgi mediated repair 11. Although the protective effects of PGE2 on mitochondria require the EP2 receptor, PGE2-dependent lysosomal membrane translocation requires PI3K activation, which indicates that signaling through EP4 is involved 11. These findings have important functional consequences for control of intracellular mycobacterial replication. First, Alox-5-/- mice (unable to produce LXA4 and other Alox-5-dependent products) survive longer than wild type (WT) control mice after low dose aerosol infection with virulent Mtb 63. Conversely, Ptges-/- (unable to produce PGE2) mice succumb earlier than WT mice (unpublished observation: M. Divangahi, S.M. Behar, and H. Remold). However, as many cell types produce eicosanoids, these results do not provide information about the role of eicosanoids during innate immunity. In experiments using macrophages from Ptges-/- and Alox-5-/- mice, we found that Ptges-/- macrophages were unable to control intracellular Mtb infection, while Alox5-/- macrophages limited Mtb replication better than WT macrophages 11. This phenotype is replicated in vivo when Mtb infected Ptges-/-, Alox5-/- and WT macrophages were adoptively transferred into the lungs of V(D)J recombination-activating protein 1-deficient (Rag-/-) recipient mice. Recipient mice that received infected Alox5-/- macrophages had a substantially lower mycobacterial lung burden than recipients that received infected Ptges-/- or WT macrophages. Since Rag1-/- mice lack B and T cells, the greater capacity of Rag1-/- mice to control pulmonary infection following transfer of Mtb infected Alox5-/- macrophages must be attributed to an intrinsic property of Alox5-/- macrophages or a unique interaction between Alox5-/- macrophages and the innate immune system 11.
One conceivable explanation for the role of PGE2 in fostering membrane repair is that PGE2 is required for the generation of SYT7, the lysosomal Ca++ sensor essential for plasma membrane repair. Virulent Mtb stimulate LXA4 production in macrophages, which inhibits PGE2 production by down regulation of COX2 mRNA accumulation 12. Indeed we find that in contrast to LAMP1 expression, SYT7 transcription is specifically induced by PGE2. Likewise, Alox5-/- macrophages infected with virulent Mtb express more SYT7 than WT or Ptges-/- macrophages 11. Although it is not known how PGE2 modulates SYT7 expression, collectively these data indicate that PGE2 is an essential mediator of SYT7 expression and is therefore of critical importance for the prevention of necrosis and induction of apoptosis. Cumulatively, these studies show that the balance of PGE2 and LXA4 production by infected macrophages affects the outcome of infection in the microenvironment of the lung (Figure 2).
The fate of Mtb-infected macrophages determines cross-presentation of mycobacterial antigens
As elegantly discussed in chapter 8 by Dr. Behar, an alternate possibility is that phagocytosis of dying infected macrophages leads to acquisition of bacterial antigens by DC, as has been shown for influenza and Listeria 64,65. The relevance of these processes to mycobacterial antigen presentation was first investigated by Schaible et al 66. Extracellular vesicles derived from infected DC and macrophages were identified that were free of viable bacteria but contained mycobacterial lipids and proteins. The origin of these vesicles was not entirely clear, but they appear to be apoptotic blebs or possibly exosomes. While infected macrophages were not efficient to directly stimulate CD8+ T cells, their co-culture with uninfected DC led to the transfer of mycobacterial antigens to DC, which became competent to cross-present the antigens to CD8+ T cells. Presentation was TAP-1-dependent and required an intact class I MHC pathway. Thus, the antigenic cargo contained in these vesicles could be cross-presented by DC to CD8+ T cells. As these studies were done with previously activated T cells, the observed T cell activation was not true cross-priming but would be more accurately categorized as cross-presentation. Nevertheless, the uptake of antigen-containing vesicles by DC provides a mechanism by which uninfected DC can acquire Mtb antigens and prime naïve T cells.
Winau et al used similar vesicles purified from BCG infected murine macrophages to immunize mice 67. Again, the purified apoptotic bodies contained bacterial antigens but no bacteria. CD8+ T cell priming was observed and required an intact class I MHC pathway. Successful T cell priming was associated with DC homing to the tissue sites where the purified vesicles were injected. Interestingly, initiation of the endosomal processing pathway abrogated CD8+ T cell priming– a feature that may be unique to cross-presentation of class I MHC-restricted peptides. The generation of CD8+ T cell responses in naïve mice indicates that true cross-priming occurred. Remarkably, not only did a CD8+ T cell response develop, but also vaccination with the vesicles generated immunity that protected mice against challenge with virulent Mtb.
The studies by Schaible and Winau provide the foundation for the “Detour Model” as proposed by Kaufmann 68. They convincingly show that the mycobacterial antigens contained in purified vesicles are taken up by both human and murine DC and enter the class I MHC pathway. However, these studies fall short of demonstrating whether apoptosis of infected macrophage is required for the transfer of antigens to DC and whether this process occurs in vivo indicating physiological significance. Additionally, the apoptotic vesicles used in the studies by Winau et al and Schaible et al were derived from BCG infected macrophages 67,68 and it isn't clear whether infection of macrophage with wild-type virulent Mtb would lead to apoptosis and enhanced T cell immunity. Finally, while immunization with purified vesicles cross-primes antigen-specific T cells, it is uncertain whether the generation of vesicles from infected macrophages is required for CD8+ T cell priming in vivo. The finding that Mtb infected DC traffic from the lung to the regional LN with kinetics mirroring T cell priming could be consistent with Mtb infected DC directly priming Mtb-specific T cells and could indicate the existence of a priming pathway independent of the “Detour Pathway” 68. Thus, the role of apoptosis and cross-priming in the generation of adaptive immunity during virulent Mtb infection remained an important unanswered question. To confirm the existence of these pathways and to begin to elucidate their relevance, a better understanding of the host factors regulating cell death during Mtb infection was required.
The role of eicosanoids in apoptosis-mediated cross-presentation
Eicosanoids have been identified as important host lipid mediators that regulate inflammation and susceptibility following mycobacterial infection. One effect of eicosanoids is the regulation of cell death in both human and murine macrophages infected with Mtb 11,12. As discussed above, prostanoids such as the host lipid mediator PGE2 induce plasma membrane repair and prevent mitochondrial damage; together these events protect infected macrophages against necrosis and instead promote apoptosis. Importantly, products of 5-lipoxygenase including LXA4 are produced by macrophages after infection with virulent Mtb. LXA4 inhibits COX-2 activity, which shuts down prostaglandin synthesis. As predicted, macrophages from mice that lack 5-lipoxygenase, produce prostaglandins even after infection with virulent Mtb and undergo more apoptosis than necrosis. Interestingly, Alox5-/- mice are more resistant to Mtb. Studies from Bafica et al found that a more pronounced Th1 cytokine response is detected in the lungs of infected Alox5-/- mice compared to WT controls mice 63.
In order to determine whether apoptotic macrophages contribute to adaptive immunity, we established a novel adoptive transfer model in which macrophages from wild-type or knockout mice were infected in vitro with Mtb and then transferred by intra-tracheal instillation into normal recipient mice. This strategy was used to determine whether the macrophage genotype influences the T cell response and control of infection 39. By using knockout macrophages that are prone to undergo either apoptosis (e.g., Alox5-/-) or necrosis (e.g., Ptges-/-) following infection, we determined how these two different cellular fates alter the course of infection in vivo. One advantage of this adoptive transfer infection model is that the development of tuberculosis occurs in a developmentally normal host with an intact immune system, which avoids the pitfalls of studying Mtb infection in knockout mice in which the genetic lesion affects multiple cell types and physiological processes.
We used the CD8+ T cell response to TB10.4, a mycobacterial antigen that elicits an immunodominant response following low dose aerosol Mtb infection, to track the CD8+ T cell response following intra-tracheal transfer of Mtb-infected macrophages 39. An earlier TB10.4-specific CD8+ T cell response was detected both in the draining pulmonary LN and in the lung following transfer of pro-apoptotic macrophages compared to wild-type macrophages. Importantly, the cellular fate of the infected macrophages was crucial; pre-treatment of the pro-apoptotic macrophages with inhibitors of caspase-8 and caspase-9, which prevented apoptosis of the infected macrophages, abrogated the enhancement of the CD8+ T cell response 39.
To determine how the Mtb infected macrophages enhanced the CD8+ T cell response, the infected macrophage adoptive transfer model was adapted for use with OT-I TCR-transgenic mice (carry a transgenic CD8 T-cell receptor (TCR) for the MHC class I– restricted OVA257–264 peptide), so early events in T cell priming could be easily assessed. Similar to the intra-tracheal adoptive transfer of Mtb-infected macrophages, OT-I CD8+ T cell priming was detected earlier after the transfer of Mtb-infected OVA-pulsed Alox5-/- macrophages compared to wild-type macrophages. Importantly, the infected macrophages did not directly activate CD8+ T cells; instead, T cell priming required endogenous DC, since DC depletion abrogated OT-I CD8+ T cell expansion. Similar to the results of Winau et al 67, CD8+ T cell priming required TAP-1 and an intact class I MHC pathway. These experiments show that CD8+ T cell priming requires cross-presentation of antigen acquired by DC from apoptotic Mtb-infected macrophage via the detour pathway. In addition, after the transfer of Mtb-infected pro-apoptotic Alox5-/- macrophages, not only was there an earlier and more robust Mtb-specific CD8+ T cell response, but the CD4+ T cell response to ESAT6 and Ag85B was also enhanced 39. This may not be too surprising if DC phagocytosis of apoptotic vesicles transfers their cargo of Mtb antigens to the endocytic system, which intersects with the MHC II processing pathway. However, the mechanisms that govern this potential transfer have yet to be elucidated. Thus, while apoptosis has been directly linked to increased CD8+ T cell responses via cross-presentation, it also enhances class II MHC-restricted antigen presentation. This has important implications for the finding that vaccination with apoptosis-inducing bacterial vaccines or apoptotic vesicles induces protection against virulent Mtb: namely, the protective immunity elicited may be due to a combination of Mtb-specific CD4+ and CD8+ T cells.
Moreover, the pro-apoptotic mutants of Mtb prime a greater T cell response and enhance host control of infection 7. This has generated considerable interest in whether pro-apoptotic mutants of Mtb could be used as a vaccine strategy. For example, vaccination with attenuated BCG or Mtb that induce greater macrophage apoptosis or with purified apoptotic bodies 67 may stimulate an enhanced T cell response. Collectively, these studies have provided important evidence that during pulmonary Mtb infection apoptosis of infected macrophages: 1) leads to innate control of early bacterial growth; and 2) acts as a reservoir of antigen that facilitates initiation of acquired T cell immunity via cross-priming by DC.
Conclusions
The finding that macrophages infected with virulent Mtb undergo necrosis while macrophages infected with attenuated mutant strains of Mtb undergo apoptosis, suggests that wild-type Mtb actively inhibits apoptosis. This forms the foundation for the concept that apoptosis is an innate macrophage defense mechanism. Apoptosis is associated with a reduction in the viability of intracellular Mtb and provides an important link to the establishment of T cell immunity. Investigation of the interaction between Mtb and macrophages finds that three distinct mechanisms contribute to macrophages necrosis. First, Mtb inhibits plasma membrane repair. Second, virulent Mtb causes inner mitochondrial membrane damage. Third, Mtb inhibits generation of the apoptotic cellular envelope. These three effects predispose the infected macrophages to necrosis. In part, these events occur because virulent Mtb inhibits the production of PGE2, a prostaglandin that is important for stimulation of membrane repair and protection of the mitochondrion. However, it is also important to notice that some investigators have found that PGE2 can impair immunity to other bacterial infections 69,70 or Influenza viral infection (M. Divangahi, unpublished observation). Thus how virulent Mtb subvert eicosanoid biosynthesis to alter the death modality of macrophages to foil both innate and adaptive immunity is an important area for future investigation. Given our capacity to manipulate eicosanoid-pathways, a better understanding of how their regulation is altered by mycobacteria may lead to novel approach to intervene therapeutically as well as to develop immunomodulatory strategies that can enhance vaccine efficacy.
Acknowledgments
M.D. is supported by the Canadian Institute of Health Research-New Investigator Award. Work in his laboratory is supported by the Canadian Institute of Health Research (CIHR) and The Natural Sciences and Engineering Research Council of Canada (NSERC).
Reference List
- 1.WHO. World Health Organization: Global Tuberculosis Control 2010. 2012 Ref Type: Generic. [Google Scholar]
- 2.Behar SM, Martin CJ, Nunes-Alves C, Divangahi M, Remold HG. Lipids, apoptosis, and cross-presentation: links in the chain of host defense against Mycobacterium tuberculosis. Microbes Infect. 2011;13:749–756. doi: 10.1016/j.micinf.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dye C, Williams BG. The population dynamics and control of tuberculosis. Science. 2010;328:856–861. doi: 10.1126/science.1185449. [DOI] [PubMed] [Google Scholar]
- 4.Kaufmann SH. How can immunology contribute to the control of tuberculosis? Nat Rev Immunol. 2001;1:20–30. doi: 10.1038/35095558. [DOI] [PubMed] [Google Scholar]
- 5.Chen M, Gan H, Remold HG. A mechanism of virulence: virulent Mycobacterium tuberculosis strain H37Rv, but not attenuated H37Ra, causes significant mitochondrial inner membrane disruption in macrophages leading to necrosis. J Immunol. 2006;176:3707–3716. doi: 10.4049/jimmunol.176.6.3707. [DOI] [PubMed] [Google Scholar]
- 6.Keane J, Remold HG, Kornfeld H. Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J Immunol. 2000;164:2016–2020. doi: 10.4049/jimmunol.164.4.2016. [DOI] [PubMed] [Google Scholar]
- 7.Hinchey J, et al. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J Clin Invest. 2007;117:2279–2288. doi: 10.1172/JCI31947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velmurugan K, et al. Mycobacterium tuberculosis nuoG is a virulence gene that inhibits apoptosis of infected host cells. PLoS Pathog. 2007;3:e110. doi: 10.1371/journal.ppat.0030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molloy A, Laochumroonvorapong P, Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guerin. J Exp Med. 1994;180:1499–1509. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fratazzi C, Arbeit RD, Carini C, Remold HG. Programmed cell death of Mycobacterium avium serovar 4-infected human macrophages prevents the mycobacteria from spreading and induces mycobacterial growth inhibition by freshly added, uninfected macrophages. J Immunol. 1997;158:4320–4327. [PubMed] [Google Scholar]
- 11.Divangahi M, et al. Mycobacterium tuberculosis evades macrophage defenses by inhibiting plasma membrane repair. Nat Immunol. 2009;10:899–906. doi: 10.1038/ni.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen M, et al. Lipid mediators in innate immunity against tuberculosis: opposing roles of PGE2 and LXA4 in the induction of macrophage death. J Exp Med. 2008;205:2791–2801. doi: 10.1084/jem.20080767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandenabeele P, Galluzzi L, Vanden BT, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 15.Duprez L, Wirawan E, Vanden BT, Vandenabeele P. Major cell death pathways at a glance. Microbes Infect. 2009;11:1050–1062. doi: 10.1016/j.micinf.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Cohen JJ. Apoptosis. Immunol Today. 1993;14:126–130. doi: 10.1016/0167-5699(93)90214-6. [DOI] [PubMed] [Google Scholar]
- 17.Fadok VA, et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 18.Fadok VA, et al. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 19.Henson PM, Tuder RM. Apoptosis in the lung: induction, clearance and detection. Am J Physiol Lung Cell Mol Physiol. 2008;294:L601–L611. doi: 10.1152/ajplung.00320.2007. [DOI] [PubMed] [Google Scholar]
- 20.Krysko DV, D'Herde K, Vandenabeele P. Clearance of apoptotic and necrotic cells and its immunological consequences. Apoptosis. 2006;11:1709–1726. doi: 10.1007/s10495-006-9527-8. [DOI] [PubMed] [Google Scholar]
- 21.Duan L, Gan H, Arm J, Remold HG. Cytosolic phospholipase A2 participates with TNF-alpha in the induction of apoptosis of human macrophages infected with Mycobacterium tuberculosis H37Ra. J Immunol. 2001;166:7469–7476. doi: 10.4049/jimmunol.166.12.7469. [DOI] [PubMed] [Google Scholar]
- 22.Christofferson DE, Yuan J. Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol. 2010;22:263–268. doi: 10.1016/j.ceb.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 24.Balcewicz-Sablinska MK, Keane J, Kornfeld H, Remold HG. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-alpha. J Immunol. 1998;161:2636–2641. [PubMed] [Google Scholar]
- 25.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 26.Kim JS, He L, Lemasters JJ. Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem Biophys Res Commun. 2003;304:463–470. doi: 10.1016/s0006-291x(03)00618-1. [DOI] [PubMed] [Google Scholar]
- 27.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bossy-Wetzel E, Newmeyer DD, Green DR. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 1998;17:37–49. doi: 10.1093/emboj/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ricci JE, et al. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell. 2004;117:773–786. doi: 10.1016/j.cell.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Martinvalet D, Dykxhoorn DM, Ferrini R, Lieberman J. Granzyme A cleaves a mitochondrial complex I protein to initiate caspase-independent cell death. Cell. 2008;133:681–692. doi: 10.1016/j.cell.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baines CP, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 32.Gan H, et al. Enhancement of antimycobacterial activity of macrophages by stabilization of inner mitochondrial membrane potential. J Infect Dis. 2005;191:1292–1300. doi: 10.1086/428906. [DOI] [PubMed] [Google Scholar]
- 33.Connern CP, Halestrap AP. Purification and N-terminal sequencing of peptidyl-prolyl cis-trans-isomerase from rat liver mitochondrial matrix reveals the existence of a distinct mitochondrial cyclophilin. Biochem J. 1992;284(Pt 2):381–385. doi: 10.1042/bj2840381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maertzdorf J, et al. Human gene expression profiles of susceptibility and resistance in tuberculosis. Genes Immun. 2011;12:15–22. doi: 10.1038/gene.2010.51. [DOI] [PubMed] [Google Scholar]
- 35.Abebe M, et al. Expression of apoptosis-related genes in an Ethiopian cohort study correlates with tuberculosis clinical status. Eur J Immunol. 2010;40:291–301. doi: 10.1002/eji.200939856. [DOI] [PubMed] [Google Scholar]
- 36.Herb F, et al. ALOX5 variants associated with susceptibility to human pulmonary tuberculosis. Hum Mol Genet. 2008;17:1052–1060. doi: 10.1093/hmg/ddm378. [DOI] [PubMed] [Google Scholar]
- 37.Tobin DM, et al. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell. 2010;140:717–730. doi: 10.1016/j.cell.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tobin DM, et al. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell. 2012;148:434–446. doi: 10.1016/j.cell.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Divangahi M, Desjardins D, Nunes-Alves C, Remold HG, Behar SM. Eicosanoid pathways regulate adaptive immunity to Mycobacterium tuberculosis. Nat Immunol. 2010;11:751–758. doi: 10.1038/ni.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolf LA, Laster SM. Characterization of arachidonic acid-induced apoptosis. Cell Biochem Biophys. 1999;30:353–368. doi: 10.1007/BF02738119. [DOI] [PubMed] [Google Scholar]
- 41.Chang DJ, Ringold GM, Heller RA. Cell killing and induction of manganous superoxide dismutase by tumor necrosis factor-alpha is mediated by lipoxygenase metabolites of arachidonic acid. Biochem Biophys Res Commun. 1992;188:538–546. doi: 10.1016/0006-291x(92)91089-9. [DOI] [PubMed] [Google Scholar]
- 42.Peterson DA, et al. Polyunsaturated fatty acids stimulate superoxide formation in tumor cells: a mechanism for specific cytotoxicity and a model for tumor necrosis factor? Biochem Biophys Res Commun. 1988;155:1033–1037. doi: 10.1016/s0006-291x(88)80600-4. [DOI] [PubMed] [Google Scholar]
- 43.Jayadev S, Linardic CM, Hannun YA. Identification of arachidonic acid as a mediator of sphingomyelin hydrolysis in response to tumor necrosis factor alpha. J Biol Chem. 1994;269:5757–5763. [PubMed] [Google Scholar]
- 44.Finstad HS, et al. Cell proliferation, apoptosis and accumulation of lipid droplets in U937-1 cells incubated with eicosapentaenoic acid. Biochem J. 1998;336(Pt 2):451–459. doi: 10.1042/bj3360451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rocca B, FitzGerald GA. Cyclooxygenases and prostaglandins: shaping up the immune response. Int Immunopharmacol. 2002;2:603–630. doi: 10.1016/s1567-5769(01)00204-1. [DOI] [PubMed] [Google Scholar]
- 46.Murakami M, et al. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J Biol Chem. 2000;275:32783–32792. doi: 10.1074/jbc.M003505200. [DOI] [PubMed] [Google Scholar]
- 47.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 48.D'Avila H, et al. Mycobacterium bovis bacillus Calmette-Guerin induces TLR2-mediated formation of lipid bodies: intracellular domains for eicosanoid synthesis in vivo. J Immunol. 2006;176:3087–3097. doi: 10.4049/jimmunol.176.5.3087. [DOI] [PubMed] [Google Scholar]
- 49.Almeida PE, et al. Mycobacterium bovis bacillus Calmette-Guerin infection induces TLR2-dependent peroxisome proliferator-activated receptor gamma expression and activation: functions in inflammation, lipid metabolism, and pathogenesis. J Immunol. 2009;183:1337–1345. doi: 10.4049/jimmunol.0900365. [DOI] [PubMed] [Google Scholar]
- 50.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 51.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tobin DM, et al. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell. 2010;140:717–730. doi: 10.1016/j.cell.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith J, et al. Evidence for pore formation in host cell membranes by ESX-1-secreted ESAT-6 and its role in Mycobacterium marinum escape from the vacuole. Infect Immun. 2008;76:5478–5487. doi: 10.1128/IAI.00614-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Jonge MI, et al. ESAT-6 from Mycobacterium tuberculosis dissociates from its putative chaperone CFP-10 under acidic conditions and exhibits membrane-lysing activity. J Bacteriol. 2007;189:6028–6034. doi: 10.1128/JB.00469-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roy D, et al. A process for controlling intracellular bacterial infections induced by membrane injury. Science. 2004;304:1515–1518. doi: 10.1126/science.1098371. [DOI] [PubMed] [Google Scholar]
- 56.Togo T, Alderton JM, Bi GQ, Steinhardt RA. The mechanism of facilitated cell membrane resealing. J Cell Sci. 1999;112(Pt 5):719–731. doi: 10.1242/jcs.112.5.719. [DOI] [PubMed] [Google Scholar]
- 57.Granger BL, et al. Characterization and cloning of lgp110, a lysosomal membrane glycoprotein from mouse and rat cells. J Biol Chem. 1990;265:12036–12043. [PubMed] [Google Scholar]
- 58.Novikoff PM, Tulsiani DR, Touster O, Yam A, Novikoff AB. Immunocytochemical localization of alpha-D-mannosidase II in the Golgi apparatus of rat liver. Proc Natl Acad Sci U S A. 1983;80:4364–4368. doi: 10.1073/pnas.80.14.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martinez I, et al. Synaptotagmin VII regulates Ca(2+)-dependent exocytosis of lysosomes in fibroblasts. J Cell Biol. 2000;148:1141–1149. doi: 10.1083/jcb.148.6.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burgoyne RD, O'Callaghan DW, Hasdemir B, Haynes LP, Tepikin AV. Neuronal Ca2+-sensor proteins: multitalented regulators of neuronal function. Trends Neurosci. 2004;27:203–209. doi: 10.1016/j.tins.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 61.Togo T, Alderton JM, Steinhardt RA. Long-term potentiation of exocytosis and cell membrane repair in fibroblasts. Mol Biol Cell. 2003;14:93–106. doi: 10.1091/mbc.E02-01-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Regan JW. EP2 and EP4 prostanoid receptor signaling. Life Sci. 2003;74:143–153. doi: 10.1016/j.lfs.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 63.Bafica A, et al. Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production. J Clin Invest. 2005;115:1601–1606. doi: 10.1172/JCI23949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Albert ML. Death-defying immunity: do apoptotic cells influence antigen processing and presentation? Nat Rev Immunol. 2004;4:223–231. doi: 10.1038/nri11308. [DOI] [PubMed] [Google Scholar]
- 65.Yrlid U, Wick MJ. Salmonella-induced apoptosis of infected macrophages results in presentation of a bacteria-encoded antigen after uptake by bystander dendritic cells. J Exp Med. 2000;191:613–624. doi: 10.1084/jem.191.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schaible UE, et al. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat Med. 2003;9:1039–1046. doi: 10.1038/nm906. [DOI] [PubMed] [Google Scholar]
- 67.Winau F, et al. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity. 2006;24:105–117. doi: 10.1016/j.immuni.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 68.Winau F, Kaufmann SH, Schaible UE. Apoptosis paves the detour path for CD8 T cell activation against intracellular bacteria. Cell Microbiol. 2004;6:599–607. doi: 10.1111/j.1462-5822.2004.00408.x. [DOI] [PubMed] [Google Scholar]
- 69.Aronoff DM, et al. E-prostanoid 3 receptor deletion improves pulmonary host defense and protects mice from death in severe Streptococcus pneumoniae infection. J Immunol. 2009;183:2642–2649. doi: 10.4049/jimmunol.0900129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Medeiros AI, Serezani CH, Lee SP, Peters-Golden M. Efferocytosis impairs pulmonary macrophage and lung antibacterial function via PGE2/EP2 signaling. J Exp Med. 2009;206:61–68. doi: 10.1084/jem.20082058. [DOI] [PMC free article] [PubMed] [Google Scholar]