Abstract
The mechanism underlying the effect of drought on the photosynthetic traits of leaves in forest ecosystems in subtropical regions is unclear. In this study, three limiting processes (stomatal, mesophyll and biochemical limitations) that control the photosynthetic capacity and three resource use efficiencies (intrinsic water use efficiency (iWUE), nitrogen use efficiency (NUE) and light use efficiency (LUE)), which were characterized as the interactions between photosynthesis and environmental resources, were estimated in two species (Schima superba and Pinus massoniana) under drought conditions. A quantitative limitation analysis demonstrated that the drought-induced limitation of photosynthesis in Schima superba was primarily due to stomatal limitation, whereas for Pinus massoniana, both stomatal and non-stomatal limitations generally exhibited similar magnitudes. Although the mesophyll limitation represented only 1% of the total limitation in Schima superba, it accounted for 24% of the total limitations for Pinus massoniana. Furthermore, a positive relationship between the LUE and NUE and a marginally negative relationship or trade-off between the NUE and iWUE were observed in the control plots. However, drought disrupted the relationships between the resource use efficiencies. Our findings may have important implications for reducing the uncertainties in model simulations and advancing the understanding of the interactions between ecosystem functions and climate change.
Water deficit is the primary factor that limits ecosystem productivity in most terrestrial biomes1. The physiological responses of trees to drought (i.e., carbon uptake) are directly related to vegetation growth2, ecosystem productivity3,4, frequency of fires5,6 and tree mortality7,8. The subtropical region experiences frequent seasonal droughts9 that result in declines in terrestrial carbon sequestration10. However, the mechanism underlying the effects of drought on the carbon uptake of subtropical ecosystems at the leaf level remains unclear11.
The carbon uptake of forest ecosystems is driven by leaf photosynthesis, the responses of which to drought are mediated by three physiological processes. First, stomatal closure is recognized as the main driver of the photosynthetic response to water stress by limiting CO2 diffusion from the atmosphere to the substomatal cavities to slow photosynthesis12,13. Second, the mesophyll conductance (gm) may rapidly decrease, thereby limiting CO2 diffusion from the substomatal cavities to the chloroplast stroma during water stress14,15. Finally, photosynthesis may be limited by biochemical processes in long-lasting, severe droughts, resulting in decreased photosynthetic enzyme activity (i.e., the maximum rate of Rubisco carboxylation, Vcmax), ribulose-1,5-bisphophate (RuBP) regeneration capacity (i.e., the maximum rate of photosynthetic electron transport, Jmax) and triose-phosphate utilization (TPU)16,17,18. As a result, drought stress directly influences CO2 diffusion and/or the biochemical process of photosynthesis, which in turn limits the net CO2 assimilation rate (An). For example, Maseda and Fernandez (2006) found that the rapid closure of stomata during water stress resulted in a decline in transpiration and the An19. Increasing evidence has shown that mesophyll conductance is finite20 and plays an important role in limiting the photosynthetic capacity12. Additionally, drought-stressed plants exhibit significant reductions in Vcmax, Jmax and TPU relative to plants with sufficient water21, indicating that biochemical processes dramatically inhibit photosynthesis during long-term severe droughts. These apparent discrepancies may arise from the fact that photosynthesis induced by drought stress is not limited by a single process. Instead, the combined effect of the stomatal, mesophyll, and biochemical limitations simultaneously regulates the decrease in photosynthesis in response to water stress21,22,23. However, a quantitative limitation analysis is needed to separate the three physiological processes in subtropical climatic zones12,22,24.
Drought not only decreases the leaf photosynthetic rate but also regulates the interaction of plant carbon uptake and environmental resources, which is termed the resource use efficiency. Three resource use efficiencies (water use efficiency (WUE), carbon gain at the expense of water loss; nitrogen use efficiency (NUE), carbon gain per nitrogen content; and light use efficiency (LUE), carbon gain per available light quantum flux density) are important functional parameters that intimately couple the uptake of carbon with the major growth limiting factors (water, nitrogen and light). In general, the leaf-level WUE has been reported to increase during soil water stress25,26, which suggests that stomata closure in response to H2O flux is more sensitive than the response to carbon flux27. Apart from increasing the WUE, stomatal closure during drought stress has an effect on photosynthesis but no effect on leaf nitrogen, leading to a decline in the NUE26,27. During drought stress periods, the LUE generally decreases with increasing drought intensity; indeed, no change was observed in the electron transport rate under mild and moderate water stress28, or the electron transport rate declined to a lesser extent than the net CO2 assimilation rate29. The changes in single resource use efficiency induced by drought have been well documented25,26,28, but the trade-off among the multiple resource use efficiencies of plants requires investigation.
Based on a recent integration of eddy covariance observations, subtropical forests in the East Asian region exhibit a high carbon dioxide uptake rate (362 g C m−2 year−1) compared with Asian tropical and temperate forests30. A model simulation indicated that drought caused the net exchange of carbon in the subtropical forests in Southern China to decrease by 63% and 47% in 2003 and 2004, respectively31. Despite the ecological importance of this region, the carbon uptake response of subtropical forests to drought is poorly constrained. To address this knowledge gap, a rainfall exclusion experiment was established in 2010 using two gradients of soil moisture content and three replicates. The three limiting processes (i.e., stomatal limitation, mesophyll limitation and biochemical limitation) that control photosynthesis and the three resource use efficiencies (iWUE, NUE and LUE) that represent the interaction of photosynthesis and environmental resources were estimated after a 3-year drought. We focused on (1) testing the sensitivity of the photosynthetic characteristics of Schima superba and Pinus massoniana in subtropical regions of China during an experimental drought and (2) determining the changes in the iWUE, NUE and LUE in response to water stress and identifying whether a trade-off existed among resource use efficiencies.
Results
The response of soil water content and leaf chemical characteristics to drought
The experimental drought significantly reduced the soil water content by 38% (t = 9.840; P < 0.0001) (Table 1). In general, the leaf traits (i.e., SLA, C concentration, N concentration and C/N ratio) for each species were not affected by the drought based on an independent sample T-test, except that a significant difference in the C/N ratio for Schima superba (P = 0.044) occurred between the control and drought plots (Table 1).
Table 1. Soil water content and leaf traits of Schima superba and Pinus massoniana grown in control and drought plots.
| Treatments | SWC (g g−1) | Schima superba | Pinus massoniana | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SLA (m2 kg−1) | Carea (g C m−2) | Narea (g N m−2) | Leaf C/N ratio (g g−1) | SLA (m2kg−1) | Carea (g C m−2) | Narea (g N m−2) | Leaf C/N ratio (g g−1) | ||
| Control | 0.21 ± 0.01 | 10.81 ± 0.58 | 47.01 ± 2.19 | 1.67 ± 0.09 | 28.27 ± 0.62 | 6.88 ± 0.24 | 75.84 ± 3.10 | 2.25 ± 0.09 | 33.86 ± 1.03 |
| Drought | 0.13 ± 0.01 | 10.85 ± 0.32 | 46.27 ± 1.35 | 1.54 ± 0.04 | 30.08 ± 0.57 | 7.52 ± 0.41 | 70.50 ± 4.68 | 2.10 ± 0.13 | 33.90 ± 1.37 |
| p value | 0.000 | 0.959 | 0.777 | 0.191 | 0.044 | 0.262 | 0.351 | 0.351 | 0.982 |
Note: The drought effects on the soil water content (SWC), specific leaf area (SLA), C concentration (Carea), N concentration (Narea) and Leaf C/N ratio were analyzed for Schima superba and Pinus massoniana using an independent sample T-test. Significant values (P < 0.05) are shown bold (Mean ± SE, N=12).
The response of the carbon assimilation process to drought
Based on an independent sample T-test, significant effects of drought on the An of Schima superba (t = 3.080, P = 0.005) and Pinus massoniana (t = 3.769, P = 0.001) were observed, which showed significant reductions during drought treatment (Fig. 1a,e). However, no significant effects of soil moisture on the Rd were observed (both P > 0.05) for either species (Fig. 1b,f). For Schima superba and Pinus massoniana, the responses of the Ag to the soil water treatments were significant (t = 3.134, P = 0.005 and t = 3.867, P = 0.001) and resulted in significant decreases in the drought plots compared with the control plots (Fig. 1c,g). Although there were no significant differences in Rd/Ag between drought treatments for each species, a general increasing trend from the control to the drought plots was indicated (Fig. 1d,h). No significant differences in drought resistance for An, Rd, Ag, and Rd/Ag were observed between Schima superba and Pinus massoniana (Fig. 1i–l).
Figure 1. The effect of drought on the carbon assimilation process for the two species.
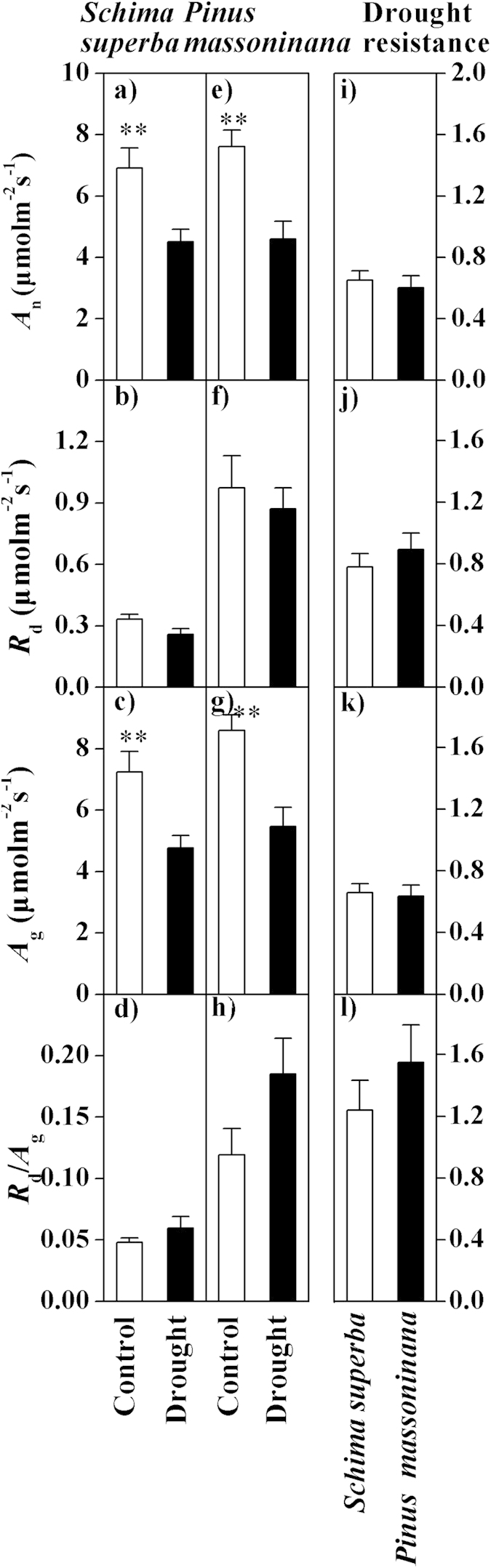
(a,e) The An (net CO2 assimilation rate, μmol CO2 m−2 s−1), (b,f) Rd (day respiration, μmol CO2 m−2 s−1), (c,g) Ag (gross CO2 assimilation, μmol CO2 m−2 s−1) and (d,h) ratio of Rd and Ag in the control and drought plots for Schima superba (a–d) and Pinus massoniana (e–h) are shown. The drought resistance of (i) An, (j) Rd, (k) Ag and (l) Rd/Ag in Schima superba and Pinus massoniana is indicated. ANOVA: *P < 0.05; **P < 0.01; and ***P < 0.001.
The response of the CO2 diffusion process to drought
Drought produced a 42% decrease in the gs (t = 2.709, P = 0.013) for Schima superba (Fig. 2a), whereas the effect of drought on the gs for Pinus massoniana was not significantly different between the control and drought plots (P > 0.05) (Fig. 2d). The responses of the gm to drought were not significantly different for either species, based on an independent sample T-test (both P > 0.05) (Fig. 2b,e). However, significant decreases in the gtot were observed (Schima superba: t = 2.618, P = 0.016; Pinus massoniana: t = 3.583, P = 0.002) in the drought plots relative to the control plots (Fig. 2c,f). The drought resistance of Pinus massoniana with regard to the gs appeared to be considerably higher than that of Schima superba (P = 0.065), whereas no significant differences in drought resistance related to the gm and gtot were found between the species (Fig. 2g–i).
Figure 2. The effect of drought on the CO2 diffusion process for the two species.
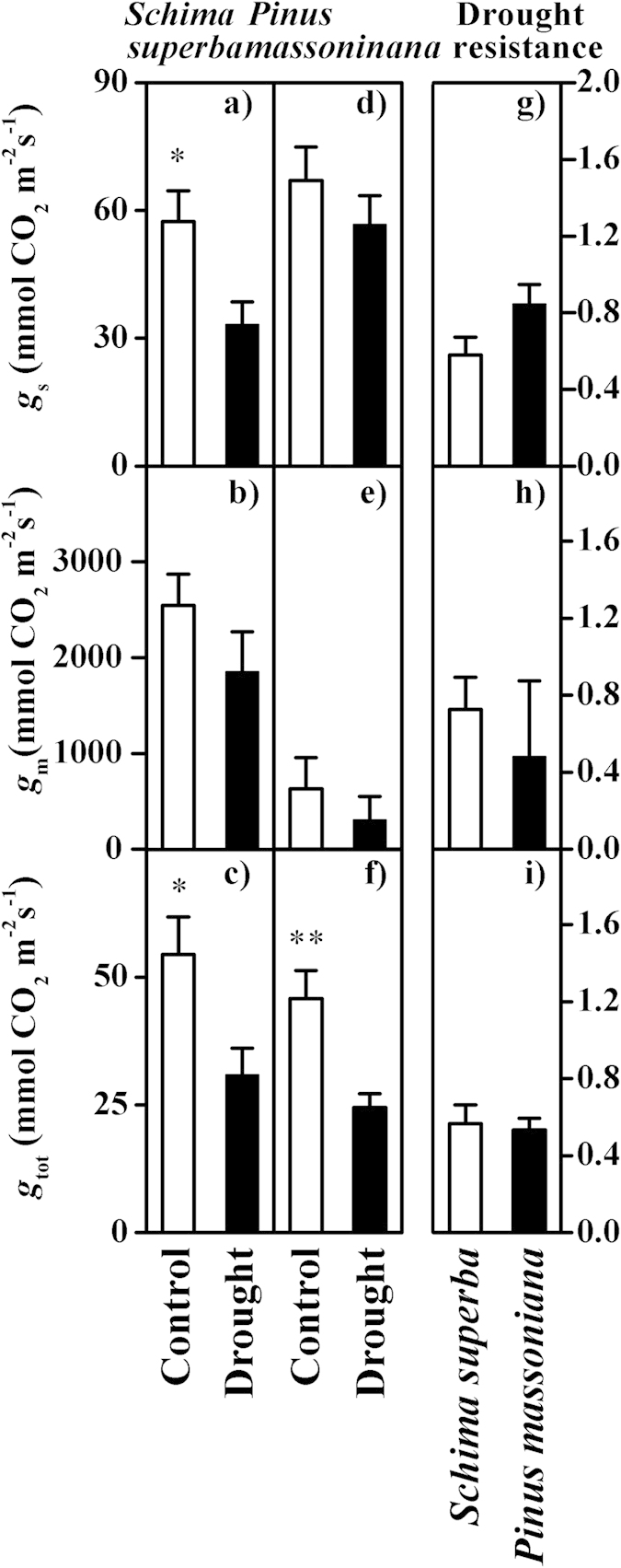
(a,d) The gs (stomatal conductance, mmol CO2 m−2 s−1), (b,e) gm (mesophyll conductance, mmol CO2 m−2 s−1) and (c,f) gtot (the total conductance, mmol CO2 m−2 s−1) in the control and drought plots of Schima superba (a–c) and Pinus massoniana (d–f) are shown. The drought resistance of (g) gs, (h) gm and (i) gtot in Schima superba and Pinus massoniana is indicated. ANOVA: *P < 0.05; **P < 0.01; and ***P < 0.001.
The response of biochemical processes to drought
The effects of drought on Vcmax and Jmax were not significantly different between the control and drought plots for Schima superba and Pinus massoniana (all P > 0.05, Figure S1a-b, e-f). For Schima superba, a significant increasing trend in Jmax/Vcmax (t = −2.229, P = 0.036) was observed from the control to the drought plots, whereas no significant effect of drought on Jmax/Vcmax was observed for Pinus massoniana (P > 0.05). No significant differences in drought resistance were observed for Vcmax, Jmax, TPU and Jmax/Vcmax between Schima superba and Pinus massoniana (Figure S1i-l).
Quantitative limitation analysis
For Schima superba, the values of SL, MCL and BL accounted for 87%, 1% and 12% of the limitations, respectively (Fig. 3). The contributions of the stomatal (SL) and non-stomatal limitations (NSL = MCL + BL) represented approximately seven-eighths and one-eighth of the total limitation, respectively. The role of the total diffusional limitation (DL = SL + MCL) was more important than that of the biochemical (BL) limitation.
Figure 3. The quantitative limitation of An (net CO2 assimilation rate, μmol CO2 m−2 s−1) for Schima superba (Ss) and Pinus massoniana (Pm) during drought stress.
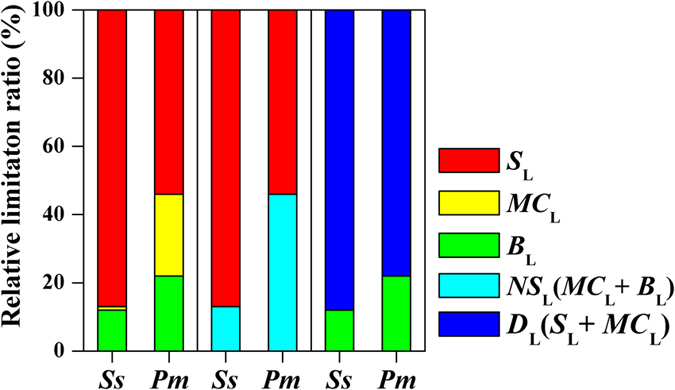
The stomatal limitation (SL, red area), mesophyll conductance limitation (MCL, yellow area) and biochemical limitation (BL, green area) are shown. The total diffusional limitation (DL = SL + MCL, blue area) and the non-stomatal limitation (NSL = MCL + BL, sky blue area) are also indicated.
For Pinus massoniana, the SL, MCL and BL values were equal to 54%, 24%, and 22% of the limitations, respectively. The stomatal (SL) and non-stomatal limitations (NSL) generally showed a similar magnitude. The contributions of the total diffusional limitation (DL) and biochemical limitation (BL) represented approximately two-thirds and one-third of the total limitation, respectively.
Resource use efficiency
A general increasing trend the in iWUE from the control to the drought plots was observed for Schima superba (P = 0.056), whereas significant declines in the iWUE due to the drought treatments (P = 0.021) were observed for Pinus massoniana (Fig. 4a,d). The effects of drought on the NUE of Schima superba and Pinus massoniana exhibited significant reductions from the control to drought plots (Fig. 4b,e). No significant differences were observed in the LUE for Schima superba and Pinus massoniana during drought treatments (all P > 0.05, Fig. 4c,f). The drought resistance of the iWUE exhibited significant differences between species, whereas no significant differences in drought resistance related to the LUE and NUE were observed between species (Fig. 4g-i).
Figure 4. The effect of drought on the resource use efficiency for the two species.
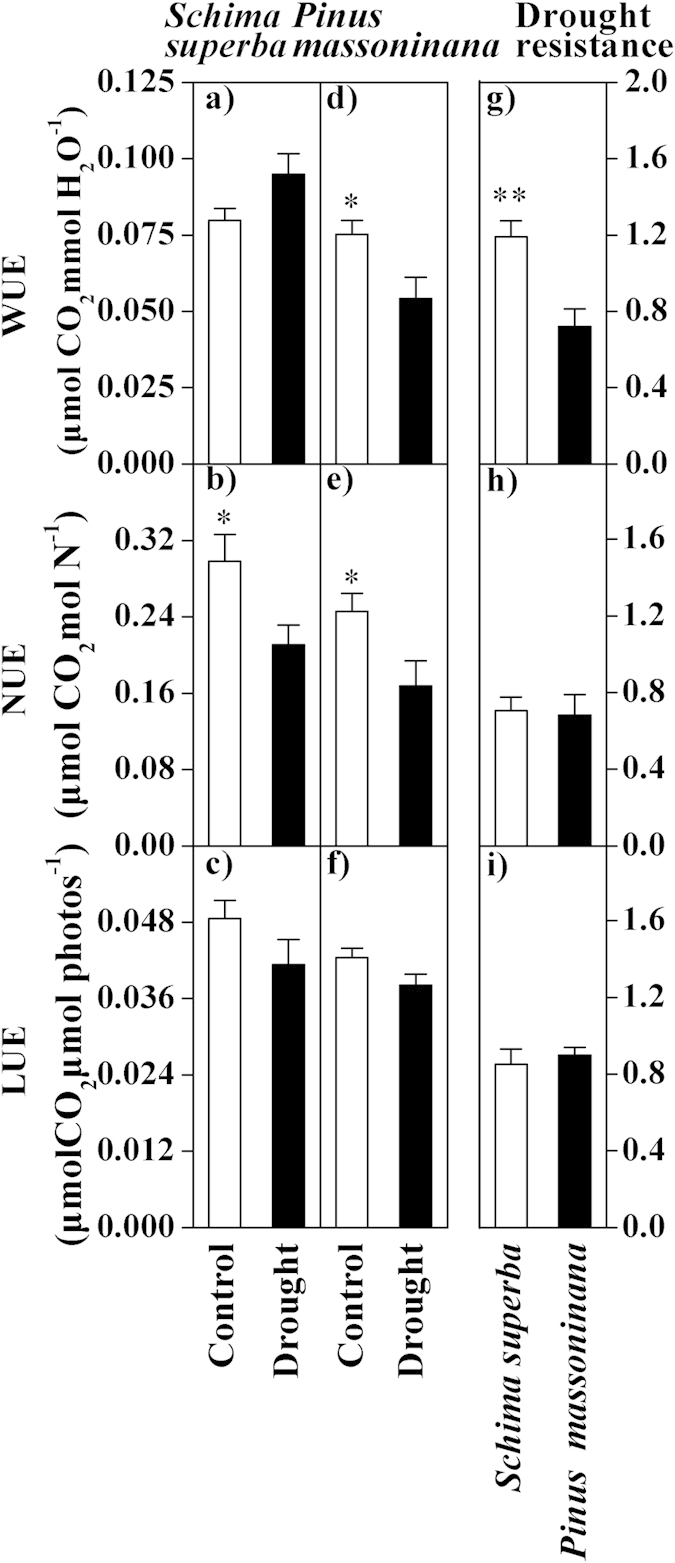
(a,d) The iWUE (the water use efficiency, μmol CO2 mmol H2O−1), (b,e) NUE (the nitrogen use efficiency, μmol CO2 mol N−1) and (c,f) LUE (the light use efficiency, μmol CO2 μmol Photons−1) in the control and drought plots of Schima superba (a–c) and Pinus massoniana (d–f) are shown. The drought resistance of the (g) iWUE, (h) NUE, and (i) LUE in Schima superba and Pinus massoniana is indicated. ANOVA: *P < 0.05; **P < 0.01; and ***P < 0.001.
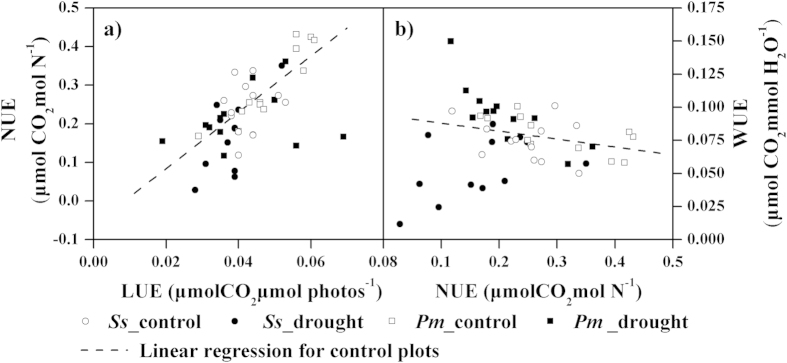
To determine the trade-off in resource use efficiency, simple linear regressions of LUE vs. NUE and NUE vs. iWUE were performed (Fig. 5). A significant positive correlation was found between the LUE and NUE in the control plots (y = 7.29x−0.06, R2 = 0.57, P < 0.0001). However, poor correlations between the LUE and NUE were found for all species in the drought plots (R2 = 0.06, P = 0.237). As a result, the regression slopes of the LUE and NUE were different between the control and drought treatments (P = 0.034). A marginally negative relationship between the NUE and iWUE was observed for all species in the control plots (y = −0.06x + 0.09, R2 = 0.12, P = 0.092), whereas the correlations between the NUE and iWUE were weak (R2 = 0.02, P = 0.481) in the drought plots. However, no significant difference was observed in the regression slopes of the NUE and iWUE for the control and drought treatments (P = 0.179).
Figure 5.
The relationships between the LUE (light use efficiency, μmol CO2 μmol Photons−1) and NUE (nitrogen use efficiency, μmol CO2 mol N−1) (a) or the NUE and iWUE (water use efficiency, μmol CO2 mmol H2O−1) (b) in the control and drought plots. Ss and Pm represent Schima superba and Pinus massoniana, respectively. The dotted line indicates the linear regression for the control plots.
Discussion
Quantitative limitation analysis of photosynthesis in response to drought
As expected, drought stress significantly decreased the leaf photosynthesis of the dominate species (Schima superba and Pinus massoniana) in subtropical forests. Our study reported that an approximately 37% decrease in the An in the drought plots was related to a decrease of approximately 38% in the SWC. The pattern of decreasing An with drought was similar to the pattern observed in forests under field conditions24,32,33. However, the degree to which drought affected the An did not significantly vary between Schima superba and Pinus massoniana (Fig. 1i). Two independent experiments on mesic and xeric species from diverse hydroclimates in Australia and Europe indicated interspecific differences in the drought response23. The velocity of the photosynthetic changes in response to water stress imposition were faster in evergreen forests than in semi-deciduous forests, although the declines in photosynthetic rate were similar in magnitude34. Although water stress is known to reduce the photosynthetic rate, the processes responsible for the key limitations are still a matter of debate35. Previous studies have demonstrated that the photosynthetic reaction to water stress is dominated by only one of the three physiological processes (stomatal conductance, mesophyll conductance and biochemical processes)36,37,38,39. Increasing evidence had shown that the combined effect of the stomatal, mesophyll, and biochemical limitations simultaneously regulates the decline in photosynthesis in response to drought. Thus, a quantitative limitation analysis of the changes in the photosynthetic rate in response to water stress was necessary12,40,41. Our quantitative limitation analysis demonstrated that the drought-induced limitation of photosynthesis in Schima superba was mainly due to the stomatal limitation (87%), whereas the stomatal (SL, 54%) and non-stomatal limitations (NSL, 46%) for Pinus massoniana generally showed similar magnitudes. A study of temperate deciduous forests reported a maximum value of 50% for the SL during drought stress39. A study of tropical evergreen forests (Campsiandra laurifolia, Symmeria paniculata, Acosmium nitens and Eschweilera tenuifolia) indicated that the SL accounted for 30–39% of the limitations in the dry season (March 2004)42. Therefore, the magnitude of the photosynthetic reaction to water stress for the three types of limitations varied between species.
Mesophyll conductance is typically absent in gas exchange measurements, which are assumed to be infinite43. However, studies have demonstrated that changes in mesophyll conductance in response to stress and limit photosynthesis are an important physiological process12,44. For a variety of climate zones and species, the MCL is responsible for approximately 14-30% of the limitations12,22,23,24. Similarly, Grassi and Magnani (2005) found a maximum value of 14% for the MCL for an ash forest under seasonal drought12. Another rainfall exclusion experiment in a Quercus ilex forest showed a maximum value of 20% for the MCL22. Our quantitative limitation analysis showed that the MCL was responsible for only 1% of the total limitation of Schima superba, although this limitation accounted for 24% of the total limitation for Pinus massoniana. Therefore, it is important to include mesophyll conductance into any detailed study of the gas exchange response to drought and the processed model.
An explanation for the discrepancies in the MCL between both species in the subtropical region may be related to the different phylogenetic clades (gymnosperms vs. angiosperms)45. The SLA was reported to strongly separate gymnosperms from angiosperms based on 305 North American woody species that spanned boreal to subtropical climates46. The gm can be influenced by changes in leaf anatomical characteristics, such as the thickness of leaf/mesophyll cell walls/chloroplasts and the stomata density47,48,49. Gymnosperms have a lower SLA value50, lower mesophyll porosity, thicker mesophyll cell wall51, and lower gm51,52 than angiosperms. In our study, the gymnosperm species (Pinus massoniana) had lower SLA and gm values than angiosperms, which might contribute to the high MCL in evergreen conifers.
The balance between Jmax and Vcmax
The Jmax and Vcmax relationship represents the resource allocation between the two photosynthetic cycles: electron transport and the Calvin cycle53. In the biochemically-based photosynthesis model, Vcmax was scaled to Jmax based on the hypothesis that the average Jmax/Vcmax ratio was 2.154. However, some studies have demonstrated that the Jmax to Vcmax ratio is not constant but varies with temperature55, leaf nitrogen56, and species57. The underlying processes responsible for the changes in the Jmax/Vcmax ratio due to water stress are still a matter of debate. We found that the Jmax/Vcmax ratio varied considerably among drought treatments in Schima superba (Fig. S1d), which was consistent with previous studies24,58. The hypothesis that droughts modify the balance between RuBP carboxylation and regeneration was supported by our study (i.e., the resource allocation between the two photosynthetic cycles (the Calvin cycle and electron transport) was changed). Current ecosystem models are less capable of accounting for climate extremes (warming, nitrogen deposition or drought), which attributes to model calibration using data collected under standard climate conditions59. The findings from manipulation experiments could reduce the uncertainties of model parameter estimates and the predictions made by the models.
The trade-off between resource use efficiencies (iWUE, NUE and LUE)
In the present study, we found a significant positive correlation between the LUE and NUE for all species in the control plots (Fig. 5a). Generally, the plants tended to obtain the maximum NUE with an increase in the LUE60. However, a poor correlation was observed between the LUE and NUE in the drought plots, indicating that drought stress might have weakened the relationship between the LUE and NUE in these plots (Fig. 5a). The slopes of the regression lines (LUE vs. NUE) from the control and drought plots were significantly different (P = 0.034). Furthermore, the NUE but not the LUE was significantly decreased in the drought treatments for each species (Fig. 4b,c,e,f). These results suggested that the responses of the LUE and NUE to drought were uncoupled. The use of resources (light and nitrogen) for plants may also be influenced by other factors, such as water stress.
Additionally, a marginally negative correlation was observed between the NUE and iWUE in the control plots for the two species studied here, which provided evidence for the existence of a trade-off between the uses of resources. An increase in the NUE together with a decrease in the iWUE indicated that compromises existed between the iWUE and NUE. This finding was consistent with previous studies of a large number of plants species61,62,63. An increase in the efficiency of the use of one resource can lead to a reduction in the efficiency of use of another resource60, suggesting that maximization of resource use efficiency depends on the most limited resources33. This trade-off may help enforce the species distribution across moisture and nutrient gradients62.
Methods
Experimental site
The experimental site was located in the Qianyanzhou (QYZ) Forest Experimental Station (26°44′N, 115°03′E) in Jiangxi Province of South China and belongs to Chinaflux. The mean annual air temperature is approximately 17.9 °C, and the highest and lowest daily temperatures recorded are 39.5 and −5.8 °C, respectively64. The annual average precipitation is 1,489 mm and mainly occurs from March to June (52% of total). The annual air temperature and annual precipitation in 2012 were 18.5 °C and 1741 mm, respectively. These characteristics are typical of the prevailing subtropical monsoon climate. The soil mainly consists of the red soil type formed principally from red sandstone, sand gravel or mudstone and river alluvial deposits. The main forest types include Masson’s pine (Pinus massoniana), slash pine (Pinus elliottii), Chinese fir (Cunninghamia lanceolata), and Schima (Schima superba). Pinus massoniana and Schima superba are the pioneer species and the dominant species in this region, respectively. Both species were mixed as conifer-broadleaf forests, with a tree density of stems of 700 ha−1.
Rainfall exclusion experiment
The rainfall exclusion experiment was initiated in January 2010. Rainfall was withheld for the entire year with no changes in other meteorological variables (Fig. 6). We used transparent polyester film placed at a height of 2 m on the trees to partially exclude throughfall drops onto the floor and allow light penetration. Trenches were dug around the perimeters of the plots to reduce the lateral inflow of water from the surrounding forest into the plots. Three rainfall exclusion plots (12 m × 12 m) were used. Three control plots were established in the same environment. An average of three Schima superba and five Pinus massoniana were growing in each plot. The height and diameter at breast height for each species in the control and drought plots are listed in Table S1. The plots were established in theW middle of the forests, and we attempted to select trees in the middle of the plots.
Figure 6.
Rainfall exclusion experiments at the QYZ station: (a) Control plot and (b) Drought plot. Photo credit: Lei Zhou.
CO2 response curve
We measured the foliar gas exchange of two species (Schima superba and Pinus massoniana) using a portable photosynthesis system (LI-6400, LI-COR Inc., USA) during the growing season (early September) of 2012 (Figure S2). Measurements were performed with two portable photosynthesis systems on clear sunny days between 8:00 a.m. to 2:00 p.m. Twelve individuals (4 individuals per plot) for each species were measured in each treatment (control and drought plots). All measurements were performed on fully expanded leaves with no signs of senescence or immaturity. Branches with sun-facing leaves were excised from the middle of the crown with the help of a lopper (3 m) affixed to a bamboo shoot (approximately 10 m) (Figure S3) and then immediately stored in a bottle with fresh water. The gas exchange measurements were conducted soon after branch excision. The time period from branch excision to the completion of the measurement was typically less than 30 min. Typical An/Ci curves (An versus the calculated intercellular CO2 concentrations, Ci) were measured at the ambient CO2 concentration (Ca) (ranging from 50 to 1400 μmol mol−1). The Ca was lowered stepwise from 400 to 50 μmol mol−1 and then increased again from 50 to 1400 μmol mol−1, with a total of 10 points. Photosynthesis was induced for 10 min at the saturating photosynthetically active photon flux density (PPFD) (1500 μmol photons m−2 s−1) at a given leaf temperature (25 °C). The CO2 concentrations in the cuvette were controlled using an injector system (LI-6400-01, LI-COR Inc.) that used a CO2 mixer and compressed CO2 cartridges. The PPFD was provided by the red/blue LED light source built into the foliar cuvette (LI-6400-02B, LI-COR Inc.) and was calibrated against an internal photodiode. The average value of the air temperature on the measurement days was 24.1 °C. The leaf temperature in the cuvette, which was controlled by the thermoelectric cooling elements of the Li-6400, was 25 °C. The cuvette was sealed with plasticine to prevent leakage. We placed twelve needles of Pinus massoniana side by side into a 2×3 cm sealed cuvette. The cuvette was sealed with plasticine to prevent leakage65,66.
An/Ci curves were fitted to estimate the Vcmax, Jmax, TPU and gm using spreadsheet-based software by minimizing the root mean square error (RMSE) of each curve67. The gs (mmolH2O m−2 s−1) was initially measured with the Li-6400. Due to the differences in diffusion coefficients between water vapor and CO2, the stomatal conductance to H2O was 1.6-fold higher than the stomatal conductance to CO268. To achieve consistency with the gm, we converted the gs,w to gs,c. The An measured at the 400 μmol mol−1 CO2 concentration and 25 °C leaf temperature from each An/Ci curve was used to track the differences between treatments for each species. The total conductance (gtot) was calculated from the sum of the gs and gm.
Light response curve
The light response curves of Schima superba and Pinus massoniana were measured using the LI-6400 after each An/Ci curve measurement. The PPFD was sequentially lowered from 1800 to 0 μmol m−2 s−1, with a total of 14 points. During each measurement, the CO2 concentration was maintained at 400 μmol mol−1, and the leaf temperature was maintained at 25 °C.
A non-rectangular hyperbola model69 solved for its negative root was used to describe the light response curves. In our study, the leaf maximum apparent quantum yield of CO2 uptake (AQY, μmol CO2 m−2s−1) and Rd (μmol CO2 m−2 s−1) were derived from the light response curve. The gross CO2 assimilation (Ag) was calculated by adding Rd to An.
Soil water content and leaf chemical characteristics
The gravimetric soil water content (SWC) was measured at the depth of 0–20 cm in the field. Soil samples from each plot were placed into aluminum boxes and then dried in an oven at 106 °C for 24 h. The SWC in this study was expressed as follows:
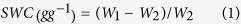 |
where W1 is the sample fresh weight, and W2 is the sample dry weight. Finally, eight soil moisture contents were averaged to represent the water content of each plot.
The foliage covered in the cuvette during the gas exchange measurements was used to measure the leaf C and N concentrations (Carea and Narea) with respect to area. Foliage was removed from branches after the gas exchange measurements, and then the area was measured with an area meter (LI-3100, Li-Cor Inc.). Foliage samples were dried at 65 °C for 48 h, and the leaf characteristics were measured with a CN analyzer. The leaf C/N ratio with respect to area was calculated using the leaf C and N concentrations. The specific leaf area (SLA) was calculated based on the measurements of the leaf area and dry mass.
Data analyses
The relative limitation to assimilation imposed by the stomatal conductance (SL), mesophyll conductance (MCL) and biochemical processes (BL) were separated using the approach12 proposed by Grassi and Magnani (2005). This approach makes it possible to compare relative limitations to assimilation, which partitions photosynthetic limitations into components related to stomatal conductance, mesophyll conductance, and leaf biochemical characteristics. The non-stomatal limitation (NSL) was defined as the sum of the contribution due to the mesophyll conductance and leaf biochemistry (NSL = MCL + BL). The total diffuse limitation (DL) was the sum of the stomatal and mesophyll conductance components (DL = SL + MCL).
The leaf-level intrinsic WUE (iWUE, μmol CO2 mmol H2O−1) was expressed as the ratio of the net CO2 assimilation rate versus the stomatal conductance. The NUE (μmol CO2 mol N−1) for leaf photosynthesis was defined as the ratio of the net photosynthesis rate to the leaf nitrogen content. The AQY derived from the light response curve was the proxy for the leaf-level LUE (μmol CO2 μmol Photons−1)70 in this study.
The drought resistance in this study was expressed as the ratio of the variables in drought plots to those in control plots71,72 (i.e., variable_drought/variable_control). Values closer to 1 imply greater drought resistance.
We performed independent sample T-tests with a 95% confidence level to examine the differences in each variable among treatments. Previously, the homogeneity of variables was evaluated with Levene’s test (P < 0.05). If the homogeneity test failed, the variables were log-transformed or sin-transformed prior to analysis. Regression models were used to determine the relationship between different resource use efficiencies. The general linear model (GLM) was used to test the significance of the slopes of the linear regression among resource use efficiencies. All statistical analyses were performed using SPSS Version 14.0 (SPSS Inc. Chicago, IL, USA).
Additional Information
How to cite this article: Zhou, L. et al. Responses of photosynthetic parameters to drought in subtropical forest ecosystem of China. Sci. Rep. 5, 18254; doi: 10.1038/srep18254 (2015).
Supplementary Material
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant No. 41401110 and 31400393), Strategic Priority Research Program of the CAS (Grant No. XDA05050602 and XDA05050702), the Key Project in the National Science & Technology Pillar Program of China (Grant No. 2013BAC03B00), the National Natural Science Foundation of China (Grant No. 30970484), and the National Key Technologies R & D Program (Grant NO. 2012BAC11B05).
Footnotes
Author Contributions L.Z., Y.G.C. and Q.K.L. conceived and designed the experiment. L.Z., S.Q.W. and Y.G.C. analyzed the data and wrote the paper. L.Z., Y.G.C., K.H. and Q.Z.Y. performed the field investigation and sample analyses and contributed to the draft of the manuscript.
References
- Nemani R. R. et al. Climate-driven increases in global terrestrial net primary production from 1982 to 1999. Science 300, 1560–1563 (2003). [DOI] [PubMed] [Google Scholar]
- Pasho E., Camarero J. J., de Luis M. & Vicente-Serrano S. M. Impacts of drought at different time scales on forest growth across a wide climatic gradient in north-eastern Spain. Agr. Forest Meteorol. 151, 1800–1811 (2011). [Google Scholar]
- Ciais P. et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 437, 529–533 (2005). [DOI] [PubMed] [Google Scholar]
- Zhao M. S. & Running S. W. Drought-Induced reduction in global terrestrial net primary production from 2000 through 2009. Science 329, 940–943 (2010). [DOI] [PubMed] [Google Scholar]
- Nepstad D. C., Tohver I. M., Ray D., Moutinho P. & Cardinot G. Mortality of large trees and lianas following experimental drought in an Amazon forest. Ecology 88, 2259–2269 (2007). [DOI] [PubMed] [Google Scholar]
- Phillips O. L. et al. Drought Sensitivity of the Amazon Rainforest. Science 323, 1344–1347 (2009). [DOI] [PubMed] [Google Scholar]
- Adams H. D. et al. Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought. Proc. Natl. Acad. Sci. USA 106, 7063–7066 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderegg W. R. L., Kane J. M. & Anderegg L. D. L. Consequences of widespread tree Mortality triggered by drought and temperature stress. Nat. Clim. Change 3, 30–36 (2013). [Google Scholar]
- Sun X. M., Wen X. F., Yu G. R., Liu Y. F. & Liu Q. J. Seasonal drought effects on carbon sequestration of a mid-subtropical planted forest of southeastern China. Sci. China Ser. D 49, 110–118 (2006). [Google Scholar]
- Wen X. F., Wang H. M., Wang J. L., Yu G. R. & Sun X. M. Ecosystem carbon exchanges of a subtropical evergreen coniferous plantation subjected to seasonal drought, 2003–2007. Biogeosciences 7, 357–369 (2010). [Google Scholar]
- Domingues et al. Seasonal patterns of leaf-level photosynthetic gas exchange in an eastern Amazonian rain forest. Plant Ecol. Divers. 7, 189–203 (2014). [Google Scholar]
- Grassi G. & Magnani F. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ 28, 834–849 (2005). [Google Scholar]
- Egea G., Verhoef A. & Vidale P. L. Towards an improved and more flexible representation of water stress in coupled photosynthesis-stomatal conductance models. Agr. Forest Meteorol. 151, 1370–1384 (2011). [Google Scholar]
- Flexas J., Ribas-Carbo M., Diaz-Espejo A., Galmes J. & Medrano H. Mesophyll conductance to CO2: current knowledge and future prospects. Plant Cell Environ. 31, 602–621 (2008). [DOI] [PubMed] [Google Scholar]
- Flexas J. et al. Mesophyll diffusion conductance to CO2: An unappreciated central player in photosynthesis. Plant Sci. 193–194, 70–84 (2012). [DOI] [PubMed] [Google Scholar]
- Bota J., Medrano H. & Flexas J. Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytol. 162, 671–681 (2004). [DOI] [PubMed] [Google Scholar]
- Keenan T. et al. Improved understanding of drought controls on seasonal variation in Mediterranean forest canopy CO2 and water fluxes through combined in situ measurements and ecosystem modelling. Biogeosciences 6, 1423–1444 (2009). [Google Scholar]
- Galmés J., Flexas J., Savé R. & Medrano H. Water relations and stomatal characteristics of Mediterranean plants with different growth forms and leaf habits: responses to water stress and recovery. Plant Soil 290, 139–155 (2007). [Google Scholar]
- Maseda P. H. & Fernandez R. J. Stay wet or else: three ways in which plants can adjust hydraulically to their environment. J. Exp Bot. 57, 3963–3977 (2006). [DOI] [PubMed] [Google Scholar]
- Loreto F., Harley P. C., Dimarco G. & Sharkey T. D. Estimation of Mesophyll Conductance to CO2 Flux by 3 Different Methods. Plant Physiol. 98, 1437–1443 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-StPaul N. K. et al. Photosynthetic sensitivity to drought varies among populations of Quercus ilex along a rainfall gradient. Funct. Plant Biol. 39, 25–37 (2012). [DOI] [PubMed] [Google Scholar]
- Limousin J. M., Misson L., Lavoir A. V., Martin N. K. & Rambal S. Do photosynthetic limitations of evergreen Quercus ilex leaves change with long-term increased drought severity? Plant Cell Environ. 33, 863–875 (2010). [DOI] [PubMed] [Google Scholar]
- Zhou S. X., Medlyn B., Sabaté S., Sperlich D. & Prentice I. C. Short-term water stress impacts on stomatal, mesophyll and biochemical limitations to photosynthesis differ consistently among tree species from contrasting climates. Tree Physiol. 34, 1035–1046. (2014). [DOI] [PubMed] [Google Scholar]
- Wilson K. B., Baldocchi D. D. & Hanson P. J. Spatial and seasonal variability of photosynthetic parameters and their relationship to leaf nitrogen in a deciduous forest. Tree Physiol. 20, 565–578 (2000). [DOI] [PubMed] [Google Scholar]
- Delucia E. H. & Heckathorn S. A. The Effect of Soil Drought on Water-Use Efficiency in a Contrasting Great-Basin Desert and SierranMontane Species. Plant Cell Environ. 12, 935–940 (1989). [Google Scholar]
- Reich P. B., Walters M. B. & Tabone T. J. Response of Ulmus-Americana Seedlings to Varying Nitrogen and Water Status. 2. Water and Nitrogen Use Efficiency in Photosynthesis. Tree Physiol. 5, 173–184 (1989). [DOI] [PubMed] [Google Scholar]
- Warren C. R. & Adams M. A. Internal conductance does not scale with photosynthetic capacity: implications for carbon isotope discrimination and the economics of water and nitrogen use in photosynthesis. Plant Cell Environ. 29, 192–201 (2006). [DOI] [PubMed] [Google Scholar]
- Flexas J., Bota J., Escalona J. M., Sampol B. & Medrano H. Effects of drought on photosynthesis in grapevines under field conditions: an evaluation of stomatal and mesophyll limitations. Funct. Plant Biol. 29, 461–471 (2002). [DOI] [PubMed] [Google Scholar]
- Flexas J., Escalona J. M. & Medrano H. Water stress induces different levels of photosynthesis and electron transport rate regulation in grapevines. Plant Cell Environ. 22, 39–48 (1999). [Google Scholar]
- Yu G. R. et al. High carbon dioxide uptake by subtropical forest ecosystems in the East Asian monsoon region. Proc. Natl. Acad. Sci. USA 111, 4910–4915 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu F. X. et al. Drought effects on carbon exchange in a subtropical coniferous plantation in China. J. Plant Ecol. 32, 1041–1051 (2008)(in chinese with english abstract). [Google Scholar]
- Ni B. R. & Pallardy S. G. Stomatal and Nonstomatal Limitations to Net Photosynthesis in Seedlings of Woody Angiosperms. Plant Physiol. 99, 1502–1508 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L. K. & Baldocchi D. D. Seasonal trends in photosynthetic parameters and stomatal conductance of blue oak (Quercusdouglasii) under prolonged summer drought and high temperature. Tree Physiol. 23, 865–877 (2003). [DOI] [PubMed] [Google Scholar]
- Galle A., Florez-Sarasa I., Aououad H. E. & Flexas J. The Mediterranean evergreen Quercus ilex and the semi-deciduous Cistus albidus differ in their leaf gas exchange regulation and acclimation to repeated drought and re-watering cycles. J. Exp. Bot. 62, 5207–5216 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flexas J. & Medrano H. Drought-inhibition of photosynthesis in C-3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 89, 183–189 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornic G. [Drought stress and high light effects on leaf photosynthesis]Photoinhibition of photosynthesis [ Baker N. R. & Bowyer J. R. (ed.)] [297–313] (Bios Scientific Pubishers, Oxford, 1994). [Google Scholar]
- Lawlor D. W. [The effects of water deficit on photosynthesis] Enviroment and Plant metabolism [ Smirnoff N. (ed.)] [129–160] (Bios Science Publishers, Oxford, 1995). [Google Scholar]
- Chaves M. M. et al. How plants cope with water stress in the field. Photosynthesis and growth. Ann. Bot. 89, 907–916 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K. B., Baldocchi D. D. & Hanson P. J. Quantifying stomatal and non-stomatal limitations to carbon assimilation resulting from leaf aging and drought in mature deciduous tree species. Tree Physiol. 20, 787–797 (2000). [DOI] [PubMed] [Google Scholar]
- Diaz-Espejo A., Nicolás E. & Enrique Fernández J. E. Seasonal evolution of diffusional limitations and photosynthetic capacity in olive under drought. Plant Cell Environ. 30, 922–933 (2007). [DOI] [PubMed] [Google Scholar]
- Varone L. et al. Stomatal and non-stomatal limitations to photosynthesis in seedlings and saplings of Mediterranean species pre-conditioned and aged in nurseries: Different response to water stress. Environ. Exp. Bot. 75, 235– 247 (2012). [Google Scholar]
- Herrera A., Tezara W., Marı´n O. & Rengifo E. Stomatal and non-stomatal limitations of photosynthesis in trees of a tropical seasonally flooded forest. Physiol. Plantarum 134, 41–48 (2008). [DOI] [PubMed] [Google Scholar]
- Farquhar G. D., Caemmerer S. V. & Berry J. A. A Biochemical-Model of Photosynthetic Co2 Assimilation in Leaves of C-3 Species. Planta 149, 78–90 (1980). [DOI] [PubMed] [Google Scholar]
- Niinemets U., Cescatti A., Rodeghiero M. & Tosens T. Leaf internal diffusion conductance limits photosynthesis more strongly in older leaves of Mediterranean evergreen broad-leaved species. Plant Cell Environ. 28, 1552–1566 (2005). [Google Scholar]
- Flexas J. et al. Stomatal and mesophyll conductances to CO2 in different plant groups: Underrated factors for predicting leaf photosynthesis responses to climate change? Plant Sci. 226, 41–48 (2014). [DOI] [PubMed] [Google Scholar]
- Stahl U. et al. Whole-plant trait spectra of North American woody plant species reflect fundamental ecological strategies. Ecosphere 4, 128. http://dx.doi.org/10.1890/ES13-00143.1 (2013). [Google Scholar]
- Tholen D. & Zhu X. G. The mechanistic basis of internal conductance. Plant Physiol. 156, 90–105 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosens T., Niinemets Ü., Vislap V., Eichelmann H. & Castro-Díez P. Developmental changes in mesophyll diffusion conductance and photosynthetic capacity under different light and water availabilities in Populus tremula: how structure constrains function. Plant Cell Environ. 35, 839–856 (2012). [DOI] [PubMed] [Google Scholar]
- Scafaro A. P., von Caemmerer S., Evans J. R. & Atwell B. J. Temperature response of mesophyll conductance in cultivated and wild Oryza species with contrasting mesophyll cell wall thickness. Plant Cell Environ. 34, 1999–2008 (2011). [DOI] [PubMed] [Google Scholar]
- Lusk C. H., Wright I. J. & Reich P. B. Photosynthetic differences contribute to competitive advantage of evergreen angiosperms over evergreen conifers. New Phytol. 160, 329–336 (2003). [DOI] [PubMed] [Google Scholar]
- De Lucia E. H., Whitehead D. & Clearwater M. J. The relative limitation of photosynthesis by mesophyll conductance in co-occurring species in a temperate rainforest dominated by the conifer Dacrydiumcupressinum. Funct. Plant Biol. 30, 1197–1204 (2003). [DOI] [PubMed] [Google Scholar]
- Tomás et al. Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: quantitative limitations and scaling up by models. J. Exp. Bot. 64, 2269–2281 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A. P. et al. The relationship of leaf photosynthetic traits–Vcmax and Jmax–to leaf nitrogen, leaf phosphorus, and specific leaf area: a meta-analysis and modeling study. Ecol. Evol. 4, 3218–3235 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullschleger S. D. Biochemical limitations to carbon assimilation in C3 plants – a retrospective analysis of the A/ci curves from 109 species. J. Exp. Bot. 44, 907–920 (1993). [Google Scholar]
- Chi Y. et al. Acclimation of foliar respiration and photosynthesis in response to experimental warming in a temperate steppe in northern China. PLOS One 8, e56482, doi: 10.1371/journal.pone.0056482 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamori W., Nagai T. & Makino A. The rate-limiting step for CO2 assimilation at different temperatures is influenced by the leaf nitrogen content in several C3 crop species. Plant Cell Environ. 34, 764–777 (2011). [DOI] [PubMed] [Google Scholar]
- Miao Z. W., Xu M., Lathrop R. G. & Wang Y. F. Comparison of the A-Cc curve fitting methods in determining maximum ribulose 1.5-bisphosphate carboxylase/oxygenase carboxylation rate, potential light saturated electron transport rate and leaf dark respiration. Plant Cell Environ. 32, 109–122 (2009). [DOI] [PubMed] [Google Scholar]
- Medlyn B. E. et al. Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant Cell Environ. 25, 1167–1179 (2002). [Google Scholar]
- Misson L., Limousin J. M., Rodriguez R. & Letts M. G. Leaf physiological responses to extreme droughts in Mediterranean Quercus ilex forest. Plant Cell Environ. 33, 1898–1910 (2010). [DOI] [PubMed] [Google Scholar]
- Hirose T. & Bazzaz F. A. Trade-off between light- and nitrogen-use efficiency in canopy photosynthesis. Ann. Bot. 82, 195–202 (1998). [Google Scholar]
- Field C., Merino J. & Mooney H. A. Compromises between Water-Use Efficiency and Nitrogen-Use Efficiency in 5 Species of California Evergreens. Oecologia 60, 384–389 (1983). [DOI] [PubMed] [Google Scholar]
- Patterson T. B., Guy R. D. & Dang Q. L. Whole-plant nitrogen- and water-relations traits, and their associated trade-offs, in adjacent muskeg and upland boreal spruce species. Oecologia 110, 160–168 (1997). [DOI] [PubMed] [Google Scholar]
- Chen S. P., Bai Y. F., Zhang L. X. & Han X. G. Comparing physiological responses of two dominant grass species to nitrogen addition in Xilin River Basin of China. Environ. Exp. Bot. 53, 65–75 (2005). [Google Scholar]
- Wang S. Q., Liu J. Y., Zhang C., Yi C. X. & Wu W. X. Effects of afforestation on soil carbon turnover in China’s subtropical region. J. Geogr. Sci, 21, 118–134 (2011). [Google Scholar]
- Tjoelker M. G., Oleksyn J., Lorenc-Plucinska J. & Reich P. B. Acclimation of respiratory temperature responses in northern and southern populations of Pinus banksiana. New Phytol. 181, 218–229 (2009). [DOI] [PubMed] [Google Scholar]
- Tjoelker M. G., Oleksyn J., Reich P. B. & Zytkowiak R. Coupling of respiration, nitrogen, and sugars underlies convergent temperature acclimation in Pinus banksiana across wide-ranging sites and populations. Global Change Biol. 14, 782–797 (2008). [Google Scholar]
- Sharkey T. D., Bernacchi C. J., Farquhar G. D. & Singsaas E. L. Fitting photosynthetic carbon dioxide response curves for C-3 leaves. Plant Cell Environ. 30, 1035–1040 (2007). [DOI] [PubMed] [Google Scholar]
- Damour G., Simonneau T., Cochard H. & Urban L. An overview of models of stomatal conductance at the leaf level. Plant Cell Environ. 33, 1419–1438 (2010). [DOI] [PubMed] [Google Scholar]
- Prioul J. L. & Chartier P. Partitioning of transfer and carboxylation components of intracellular resistance to photosynthetic CO2 fixation: a critica1 analysis of the methods used. Ann. Bot. 41, 789–800 (1977) [Google Scholar]
- Milcu A. et al. Functional diversity of leaf nitrogen concentrations drives grassland carbon fluxes. Ecol Lett. 17, 435–444 (2014). [DOI] [PubMed] [Google Scholar]
- Tilman D. & Downing J. A. Biodiversity and Stability in Grasslands. Nature 367, 363–365 (1994). [Google Scholar]
- Lan Z. C. & Bai Y. F. Testing mechanisms of N-enrichment-induced species loss in a semiarid Inner Mongolia grassland: critical thresholds and implications for long-term ecosystem responses. Philos. T. R. SOC. B. 367, 3125–3134 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.