Summary
Detailed measures of infant body composition are needed for understanding the impact of genes and environment on growth early in life. The purpose of this study was to compare the accuracy and bias of body composition in infants from dual energy X-ray absorptiometry (DXA) using MRI as the criterion method. The depots measured were total fat mass (FM), total fat-free mass (FFM) and trunk FM and FFM using DXA and MRI in 14 infants. None of the regression lines between DXA and MRI significantly deviate from the line of identity for any of the depots studied. However, Bland-Altman analyses revealed bias for trunk FM and trunk FFM. Our data showed DXA to be accurate (regression not significantly deviating from the line of identity), with high agreement (indicated by high R2) and without bias (non-significant Bland-Altman) when estimating total FM and FFM. This could not be said for trunk estimates.
Keywords: Body composition, growth, validation, fat mass, fat-free mass
Approximately 12% of children under the age of 2 years are obese [1], with 70% remaining so into adulthood [2]. Further, overweight/obese children are five times more likely to be overweight/obese as adults [3]. The strong link between obesity and future health risk precipitates the need for accurate tools for measuring and tracking body composition early in life.
Magnetic resonance imaging (MRI) is currently considered the most accurate technique for measurement of body composition in children and adults, but is not validated in neonates and infants [4]. However, MRI can be technically challenging and expensive, or inappropriate for many studies. Dual energy X-ray absorptiometry (DXA) is a commonly used alternate approach to measure body composition in children starting at birth [5]. Like MRI, DXA provides estimates of regional body composition but costs less and is easier to operate. To our knowledge, there are no published studies that have validated measures of infant body composition by DXA against a criterion standard like MRI. Therefore, the purpose of this study was to examine the accuracy and bias for measurements of whole body and trunk composition acquired using DXA compared to the same measurements acquired using MRI.
Fourteen healthy infants (3 girls, 11 boys; gestational age 38.8 ± 0.8 wks; birth weight 3,570.1 ± 768.1 g; birth length 49.2 ± 2.8 cm; 34.2 ± 5.0 days old at time of testing) were enrolled. The study was approved by the University of Oklahoma Health Sciences Center and Tribal IRB's. DXA and MRI scans were performed on the same day typically within 2-3 hours of each other.
Whole (less head) and regional (trunk) body composition was determined by DXA using a Lunar i DXAv11-30.062 (Infant whole body analysis enCore 2007 software, GE, Fairfield, CT) scanner as described by our group previously [5]. The effective radiation exposure associated with this scan is 0.22 mR. Further, total (less head) and regional (trunk) body composition were measured by MRI (Hitachi Aris II, 0.3 Tesla; slice thickness 5 mm; no spacing between slices) as described previously [6, 7]. Scans were analyzed by image segmentation and the Dixon method was used to quantify regional tissue fat content [8]. The Image Reading Center, Inc (New York, NY) read and interpreted all MRI scans.
The utility of DXA to determine whole body and trunk composition was assessed using MRI as the criterion method. Linear regression analysis was used to calculate the slope of the regression line between MRI and DXA for each depot. The coefficient of determination was assessed by the (R2) and the standard error of the estimate (SEE) was used as a measure of the accuracy in the prediction.
Potential bias between DXA and MRI for each depot was evaluated using the Bland-Altman analysis [9], which examines the difference between measures as a function of their means. All statistical analyses were performed using SPSS 20 with statistical significance set at p ≤ 0.05.
A summary of the total FM, FFM and trunk FM and FFM for both DXA and MRI are reported in Table 1, with DXA consistently underestimating the mass for each depot by 17-25%. Further, weight-for-age-length, weight-for-age and BMI-for-age z-scores are presented.
Table 1.
Mean and standard deviation for each depot measured using DXA, MRI and skinfolds. Additionally, weight-for-length, weight-for-age and BMI-for-age-z scores are reported.
| Variable | z-score | MRI | DXA | % diff |
|---|---|---|---|---|
| % total body fat | 26.8 ± 4 | 26.2 ± 4 | ||
| Total fat mass (g) | 1,170 ± 327 | 939 ± 275 | 19.7 | |
| Total fat-free mass (g) | 3,213 ± 412 | 2,640 ± 364 | 17.8 | |
| Trunk fat mass (g) | 497 ± 187 | 372 ± 122 | 25.2 | |
| Trunk fat-free mass (g) | 2,179 ± 325 | 1,807 ± 250 | 17.1 | |
| WHO WLZ | 0.38 ± 1.1 | |||
| WHO WAZ | 0.39 ± 1.2 | |||
| WHO BMIZ | 0.48 ± 1.1 |
Values for total fat and fat-free mass do not include the head for either method.
% diff is the difference between MRI and DXA.
WHO WLZ (Weight-for-age-length z-score; z-scores based on WHO 2006 growth charts).
WHO WAZ (Weight-for-age z-score; z-scores based on WHO 2006 growth charts).
WHO BMIZ (BMI-for-age z-score; z-scores based on WHO 2006 growth charts).
The calculated accuracy of measurement in each depot is shown in Figure 1 panels A-D with the regression line presented at the top right hand corner with none of the regressions deviating from the line of identity.
Figure 1.
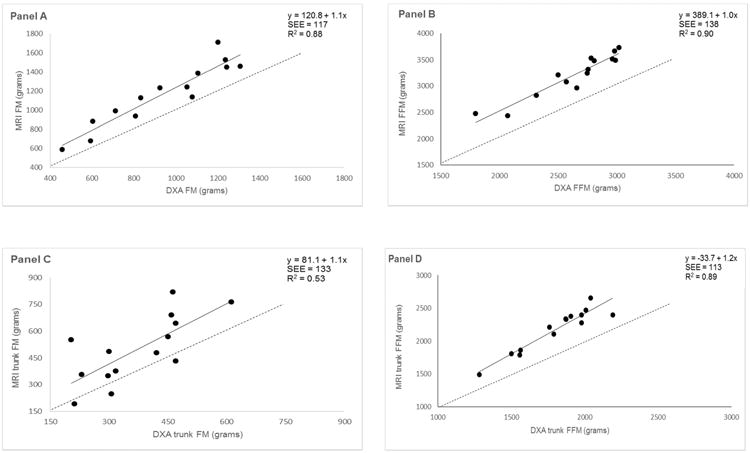
Regression comparison of FM and FFM content measured with DXA and MRI in one-month old infants: A) total body FM, B) total body FFM, C) trunk FM and D) trunk FFM. Solid line is regression. Dashed line is line of identity.
Total FM and FFM Bland-Altman analysis revealed no bias across the range of measured values while a significant negative bias for DXA-based measurements of trunk FM and trunk FFM compared to the measurement with MRI.
To our knowledge, this study is among the first to cross-validate body composition by DXA against MRI in such a young cohort. Our principal finding was DXA measures of total FM (18%) and FFM (20%) though lower than MRI estimates did not deviate from the line of identity using regression analysis. Furthermore, Bland-Altman analysis revealed no bias across the range of measured values in our cohort. Therefore, a high coefficient of determination and lack of bias of DXA is sufficiently high to recommend its use to track the growth and development of infants longitudinally or perform comparison studies among groups of infants. In agreement with our findings, Karlsson et al [10] reported a high correlation (r=0.95) and lack of bias between measures of total body FM in 5-year old children using DXA versus MRI. Collectively, DXA appears to be an appropriate alternative method to MRI for measurements of total body composition in infants.
We found estimates of total body FM and FFM by DXA to be valid, though they may underestimate the total of each by approximately 18% - 20%. The reason for the lower estimates by DXA is unclear, but perhaps reflects dimensionality differences between DXA (two dimensions) and MRI (three dimensions) [11].
In contrast to the results for whole body FM, we found the measurement of trunk FM by DXA to have a lower R2 while demonstrating bias. Bland-Altman analyses for trunk FM showed that the magnitude of underestimation with DXA increased as trunk FM increased. This methodological limitation of DXA was previously cited by Micklesfield and colleagues, “DXA is limited by its projectional nature and consequently visceral fat cannot be directly measured by DXA because subcutaneous fat lies above and below it” [11]. However, the inability of DXA to distinguish subcutaneous from visceral fat cannot be the sole explanation for the poor correlation because 70-90% of the trunk fat in a one-month-old is subcutaneous, not visceral. [12-14].
Our study demonstrated large absolute differences between the methods (17-25%) based upon the depot of interest. However, the sample size is small, and precludes a deeper understanding of these differences. Regression and Bland-Altman analyses demonstrated DXA to be accurate, without bias when estimating total body FM and FFM. This was not so for trunk estimates. The explanation for the poor relationships between DXA and MRI measures of trunk FM and FFM are unclear. Our data is a first step in better understanding both the strengths and weaknesses of using DXA in infants.
Acknowledgments
This work was supported by NIDDK (5R01DK089034-04) and by the CMRI Metabolic Research Program. The opinions expressed are those of the authors and not necessarily those of the NIH or any other organization with which the authors are affiliated. Olufolake Olufowote, Justin Fowler, Mary Ayn Tullier and Shelly Hopper served as study coordinators.
Supported By: CMRI Metabolic Research Program, National Institute of Diabetes and Digestive and Kidney Diseases (5R01DK089034-04)
Footnotes
Conflict of Interest Statement: None of the authors have conflicts of interest to report.
References
- 1.Mei Z, Ogden CL, Flegal KM, Grummer-Strawn LM. Comparison of the prevalence of shortness, underweight, and overweight among US children aged 0 to 59 months by using the CDC 2000 and the WHO 2006 growth charts. J Pediatr. 2008;153:622–628. doi: 10.1016/j.jpeds.2008.05.048. [DOI] [PubMed] [Google Scholar]
- 2.Reilly JJ. Obesity in childhood and adolescence: evidence based clinical and public health perspectives. Postgrad Med J. 2006;82:429–437. doi: 10.1136/pgmj.2005.043836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC, editor. CDC. AAP, APHA, NCR for helath and safety in child care and early education. 2009. pp. 1–26. 1-26. [Google Scholar]
- 4.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85:115–122. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 5.Fields DA, Demerath EW, Pietrobelli A, Chandler-Laney PC. Body Composition at 6 Months of Life:Comparison of Air-Displacement Plethysmography and Dual Energy X-Ray Absorptiometry. Obesity (Silver Spring) 2012;20:2302–2306. doi: 10.1038/oby.2012.102. [DOI] [PubMed] [Google Scholar]
- 6.Olhager E, Flinke E, Hannerstad U, Forsum E. Studies on human body composition during the first 4 months of life using magnetic resonance imaging and isotope dilution. Pediatr Res. 2003;54:906–912. doi: 10.1203/01.PDR.0000088064.63106.5E. [DOI] [PubMed] [Google Scholar]
- 7.Olhager E, Thuomas KA, Wigstrom L, Forsum E. Description and evaluation of a method based on magnetic resonance imaging to estimate adipose tissue volume and total body fat in infants. Pediatr Res. 1998;44:572–577. doi: 10.1203/00006450-199810000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Kovanlikaya A, Mittelman SD, Ward A, Geffner ME, Dorey F, Gilsanz V. Obesity and fat quantification in lean tissues using three-point Dixon MR imaging. Pediatr Radiol. 2005;35:601–607. doi: 10.1007/s00247-005-1413-y. [DOI] [PubMed] [Google Scholar]
- 9.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;8:307–310. [PubMed] [Google Scholar]
- 10.Karlsson AK, Kullberg J, Stokland E, Allvin K, Gronowitz E, Svensson PA, Dahlgren J. Measurements of total and regional body composition in preschool children: A comparison of MRI, DXA, and anthropometric data. Obesity (Silver Spring) 2013;21:1018–1024. doi: 10.1002/oby.20205. [DOI] [PubMed] [Google Scholar]
- 11.Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL. Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity (Silver Spring) 2012;20:1109–1114. doi: 10.1038/oby.2011.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibanez L, Lopez-Bermejo A, Suarez L, Marcos MV, Diaz M, de Zegher F. Visceral adiposity without overweight in children born small for gestational age. J Clin Endocrinol Metab. 2008;93:2079–2083. doi: 10.1210/jc.2007-2850. [DOI] [PubMed] [Google Scholar]
- 13.Goran MI, Nagy TR, Treuth MS, et al. African American prepubertal children. Am J Clin Nutr. 1997;65:1703–1708. doi: 10.1093/ajcn/65.6.1703. [DOI] [PubMed] [Google Scholar]
- 14.Asayama K, Dobashi K, Hayashibe H, Kodera K, Uchida N, Nakane T, Araki T, Nakazawa S. Threshold values of visceral fat measures and their anthropometric alternatives for metabolic derangement in Japanese obese boys. Int J Obes Relat Metab Disord. 2002;26:208–213. doi: 10.1038/sj.ijo.0801865. [DOI] [PubMed] [Google Scholar]


