Abstract
The National Ophthalmic Disease Genotyping and Phenotyping Network (eyeGENE®) was established in an effort to facilitate basic and clinical research of human inherited eye disease. In order to provide high quality genetic testing to eyeGENE®’s enrolled patients which potentially aids clinical diagnosis and disease treatment, we carried out a pilot study and performed Next-generation sequencing (NGS) based molecular diagnosis for 105 Retinitis Pigmentosa (RP) patients randomly selected from the network. A custom capture panel was designed, which incorporated 195 known retinal disease genes, including 61 known RP genes. As a result, disease-causing mutations were identified in 52 out of 105 probands (solving rate of 49.5%). A total of 82 mutations were identified, and 48 of them were novel. Interestingly, for three probands the molecular diagnosis was inconsistent with the initial clinical diagnosis, while for five probands the molecular information suggested a different inheritance model other than that assigned by the physician. In conclusion, our study demonstrated that NGS target sequencing is efficient and sufficiently precise for molecular diagnosis of a highly heterogeneous patient cohort from eyeGENE®.
Retinitis Pigmentosa (RP) is the most common form of inherited retinal degeneration, which has an estimated prevalence of 1 in 3,500–4,000 individuals1. RP patients first experience night blindness, followed by impaired daytime vision with visual fields gradually reduced from mid-periphery to the center due to the degeneration of rod photoreceptors followed by cone photoreceptors over time2. RP is genetically heterogeneous and more than 60 genes have been linked to the disease (RetNet)3. Molecular diagnosis is particularly challenging for RP patients for two reasons. First, the inheritance pattern is complex, including autosomal dominant, autosomal recessive, X-linked, and digenic as well as mitochondrial inherited forms4. More than half of RP cases are simplex, where the mode of inheritance is unclear, making the interpretation of variants more challenging. Second, there is extensive phenotypic and genetic overlap between RP and other retinal diseases or syndromic diseases with an eye phenotype, making it necessary to screen mutations in a large number of genes, not just those strictly associated with RP5,6.
The traditional diagnostic test for genetic and allelic heterogeneous diseases such as RP has been limited to Sanger sequencing and arrayed primer extension (APEX)7. However, Sanger sequencing, while accurate, is prohibitively costly and time consuming for large-scale screening. On the other hand, APEX can only detect known mutations, resulting in a low diagnostic yield for genetically heterogeneous conditions like RP. For example, a typical genetic diagnostic rate for autosomal recessive RP using APEX is reported to be 10% in a recent study8. In comparison, Next-generation sequencing (NGS) based technology, which allows multiple genes to be sequenced at the same time, has emerged as a robust, cost effective and accurate method. Recent studies utilizing NGS based method for molecular diagnosis of RP achieved a diagnostic rate ranging from 36% to 60%9,10,11. As a result, NGS based genetic testing is gradually being adopted as the method of choice for molecular diagnosis of RP patients, an important step towards better clinical diagnosis, prognosis, and identifying patients who may benefit from therapeutic interventions such as gene therapy10,12,13.
The National Ophthalmic Disease Genotyping and Phenotyping Network (eyeGENE®) is a multicenter genomic medicine initiative started by the National Eye Institute (NEI) at the National Institutes of Health (NIH) in 2006. eyeGENE® aims to promote studies of inherited eye diseases and their genetic causes. The program includes a CLIA-level DNA repository, a database linking genotype and phenotype data, and a patient registry. eyeGENE® not only expands patients’ access to diagnostic testing, but also allows registered researchers to gain access to the research database and samples for continued studies such as genotype-phenotype correlations, disease causing mutation prevalence and novel disease gene discovery14,15. Additionally, eyeGENE® is able to contact specific patient populations from the registry for recruitment of additional clinical studies.
The largest patient population in eyeGENE® is RP with total over 2,000 patients, of which about 70% are simplex cases. Due to the high cost and complexity of molecular diagnosis of RP, the vast majority of the simplex RP cases and some of the multiplex cases had not been tested, representing one of the biggest challenges for fulfilling eyeGENE®’s mission. To address this issue, we conducted a pilot study and performed NGS based mutation screening of 105 RP probands from eyeGENE® whose molecular diagnosis remained unknown. This cohort was tested using a custom designed 195-gene panel, which included 61 known RP causative genes and 19 genes that cause syndromic RP such as Usher and Bardet-Biedl syndrome. Through NGS based sequencing analysis we assigned causative mutations to 52 patients, achieving a solving rate of 49.5%. While 49 patients carried mutations in known RP genes, 3 patients were found to carry mutations in retinal disease genes that have not been associated with nonsyndromic RP previously. In addition, 5 of the 6 RP families initially labeled as autosomal dominant were found to carry compound heterozygous or homozygous mutations in known RP disease genes. Taken together, our results indicate that an NGS based approach is effective in providing a diagnosis for the highly heterogeneous patient collection at eyeGENE®.
Materials and Methods
Clinical identification of RP patients
Patients with inherited eye disease were enrolled in the eyeGENE® program (protocol #06-EI-0236) by approved certified eye care specialist. Clinical details and family history were provided by referring clinicians and entered into the eyeGENE® database (https://nationaleyegene.nei.nih.gov/eyeGENE). Clinical information and family history were further reviewed by members of the eyeGENE® Working Group to corroborate the patient’s diagnosis of RP. For this study, 105 unrelated RP probands were randomly selected from the eyeGENE® database. Informed consent was obtained from tested individuals or from parents or guardians for individuals under age 18. All experimental protocols were approved by the Institutional Review Board of Baylor College of Medicine. This study adhered to the Declaration of Helsinki.
DNA extraction, library preparation and capture sequencing
For each patient enrolled in eyeGENE®, a blood sample was collected and shipped to the eyeGENE® Coordinating Center CLIA (Clinical Laboratory Improvement Amendments) laboratory on the NIH campus in Bethesda, MD. Genomic DNA was extracted from whole blood either manually or automatically using the Gentra Puregene (Qiagen). DNA concentration was measured by a NanoDrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE) and samples were stored indefinitely in the eyeGENE® Biobank at NEI. A fraction of de-identified DNA was send to Baylor College of Medicine for diagnostic research testing. Genomic DNA from each sample was mechanically sheared, end repaired, and ligated to molecularly bar-coded adaptors to generate sequencing libraries following the manufacturer’s standard protocol (Illumina). Co-capture was performed on pooled DNA libraries in groups of up to 48 samples. Captured sample DNA was sequenced on an Illumina HiSeq 2000 according to the standard operating protocol.
Capture panel design
A capture panel enriched of the retinal disease genes was developed and assessed as described previously16. The panel covers coding exons and flanking splicing junctions for 195 known retinal disease genes at the time of design (Supplemental Table 1). A total of 61 nonsyndromic RP associated genes were included in the panel including 18 adRP genes and 33 arRP genes, 3 X-linked RP genes, and 7 RP genes that can be both dominant and recessive (Supplementary Table S1).
Bioinformatics analysis
An automated pipeline previously described was used to process sequencing data with reads mapping, recalibration, realignment, variant calling, variant filtering and annotation17. Since RP is a rare Mendelian disease, recessive variants with an allele frequency >0.5% or dominant variants with an allele frequency >0.1% in the following databases were filtered out: the 1000 genome database18, dbSNP135 (National Center for Biotechnology Information, http://www.ncbi.nlm.nih.gov/SNP/), the NHLBI Exome Sequencing database (http://evs.gs.washington.edu/EVS/), the NIEHS Exome Sequencing database (http://evs.gs.washington.edu/niehsExome/), as well as an internal control database of 997 exomes. The pathogenicity of these rare variants was assessed based on three criteria. First of all, variants reported in the Human Gene Mutation Database (HGMD)19 or the primary literature were identified. Secondly, variants that lead to severe loss of function mutations such as stopgain, stoploss, frameshift and splicing defects were identified. Third, missense variants that result in protein coding changes were evaluated by the in silico prediction program dbNSFP20 and only deleterious ones predicted by 3 out of the 6 algorithms (SIFT, Polyphen2, LRT, MutationTaster, MutationAssessor, and PhyloP) were considered as candidates.
Validation and Report
All putative causative mutations identified were validated by Sanger sequencing. A 500-bp flanking sequence at each side of the mutation was obtained from the UCSC genome browser. RepeatMasker was used to mask the repetitive region. Primer 3 was used to design a pair of primers at least 50 bp upstream and downstream from the mutation. After PCR amplification, the amplicons were sequenced on an ABI 3730xl or 3500XL Genetic Analyzer. Reports of the high-confidence genetic testing results were sent back to eyeGENE® and positive results were confirmed through direct sequencing by the CLIA certified laboratories in the eyeGENE® Network. Confirmed results were then shared with the referring clinician.
Results
The RP patient cohort
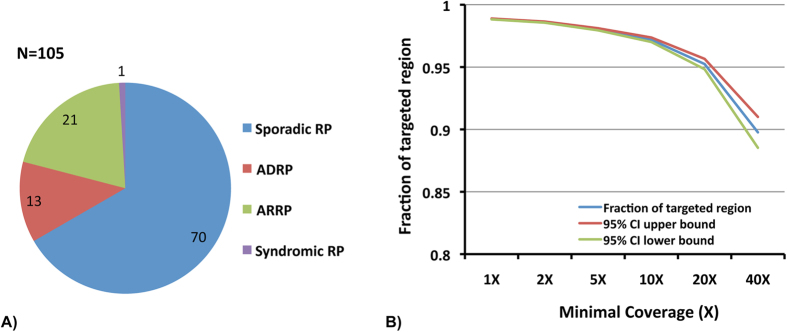
A total of 105 unrelated RP probands whose mutations remained unknown were randomly selected from the eyeGENE® database. Based on inheritance information documented in the database, most of the cases were simplex or unknown (67%), followed by autosomal recessive (20%), and autosomal dominant (13%). There was also one case of syndromic RP with hearing loss (Fig. 1a). The majority of the probands is Caucasian (65%), followed by unknown descent (19%), Asian (9%), African American (4%), and multiple races (2%). Among them, sixteen of the probands had been screened for mutations using Sanger direct sequencing in a subset of known RP genes, including ABCA4, CDH23, CLRN1, DFNB31, IMPDH1, KLHL7, NR2E3, PCDH15, PRPF3, PRPF8, PRPF31, RDS, RHO, RP1, RP2, RPGR, TOPORS, USH1C, USH1G, and USH2A (Supplementary Table S2).
Figure 1. The model of inheritance of the 105 RP probands and the sequencing quality.
(A) Majority of the 105 RP probands were simplex or unknown. (B) Fractions of targeted region with minimal coverage from 1X to 40X showed high quality sequencing results.
Identification of pathogenic mutations
To identify the pathogenic mutations in the 105 RP patients, NGS based panel sequencing that covers all coding exons and flanking splicing junctions of 195 known retinal disease genes was performed. Ten and twenty fold average coverage was achieved for 97% and 95% of the targeted regions, respectively (Fig. 1b). Sequencing results were analyzed using the bioinformatics pipeline as described in the method section. Known mutations were identified by searching the public databases, such as the HGMD database19, while novel variants were annotated for their impact on protein coding. As a result, putative mutations were found in 52 cases with a solving rate of 49.5% (Table 1).
Table 1. Pathogenic mutations were identified in 52 patients.
| ID | Gene | NM ID | Genotype | cDNA change | Protein change | References |
|---|---|---|---|---|---|---|
| ADRP | ||||||
| VGM+V.35 | EYS | NM_001142800 | Heterozygous | c.8984T>A | p.(Ile2995Asn) | PMID: 2053739433 |
| Heterozygous | c.7095T>G | p.(Tyr2365*) | PMID: 2053739433 | |||
| 3HV+M.66 | MERTK | NM_006343 | Homozygous | c.1787-2A>C | p.? | Novel |
| 3H5+K.42 | PDE6B | NM_000283 | Heterozygous | c.173C>T | p.(Ala58Val) | Novel |
| Heterozygous | c.2401C>T | p.(Gln801*) | Novel | |||
| 3WP+3.68 | RDH12 | NM_152443 | Homozygous | c.295C>A | p.(Leu99Ile) | PMID: 1532298221 |
| 5A2+H.62 | RHO | NM_000539 | Heterozygous | c.1040C>T | p.(Pro347Leu) | PMID: 221561734 |
| RC+V.27 | USH2A | NM_206933 | Heterozygous | c.9815C>T | p.(Pro3272Leu) | PMID: 1828161335 |
| Heterozygous | c.10342G>A | p.(Glu3448Lys) | PMID: 2426569336 | |||
| ARRP | ||||||
| VGJ+4.64 | CRB1 | NM_201253 | Homozygous | c.2401A>T | p.(Lys801*) | PMID: 1138948337 |
| 3UF+P.83 | CRB1 | NM_201253 | Homozygous | c.3961T>C | p.(Cys1321Arg) | Novel |
| 5WL+S.22 | CRB1 | NM_201253 | Heterozygous | c.3997G>A | p.(Glu1333Lys) | Novel |
| Heterozygous | c.3853T>C | p.(Cys1285Arg) | Novel | |||
| 59H+2.32 | PDE6B | NM_000283 | Heterozygous | c.2116A>T | p.(Lys706*) | PMID: 772454738 |
| Heterozygous | c.292C>T | p.(Arg98Cys) | Novel | |||
| Heterozygous | c.2093_2094insCCTGT | p.(Leu701Cysfs*14) | Novel | |||
| 3JY+V.17 | RDH12 | NM_152443 | Homozygous | c.295C>A | p.(Leu99Ile) | PMID: 1532298221 |
| 57R+R.78 | RDH12 | NM_152443 | Homozygous | c.377C>T | p.(Ala126Val) | PMID: 1914018039 |
| 347+7.8 | RPE65 | NM_000329 | Heterozygous | c.310G>A | p.(Gly104Ser) | Novel |
| Heterozygous | c.432C>G | p.(Tyr144*) | Novel | |||
| Heterozygous | c.2299delG | p.(Glu767Serfs*21) | PMID: 962405340 | |||
| U92+K.87 | USH2A | NM_206933 | Heterozygous | c.4714C>T | p.(Leu1572Phe) | PMID: 2202557941 |
| Heterozygous | c.11105G>A | p.(Trp3702*) | PMID: 235914059 | |||
| JX+6.76 | USH2A | NM_206933 | Homozygous | c.5012G>A | p.(Gly1671Asp) | Novel |
| Simplex/unknown RP | ||||||
| 5WY+Y.91 | CEP290 | NM_025114 | Heterozygous | c.5409A>C | p.(Glu1803Asp) | Novel |
| Heterozygous | c.5850delT | p.(Phe1950Leufs*15) | PMID: 1734560430 | |||
| 8G+Y.78 | CNGB1 | NM_001297 | Homozygous | c.3150delG | p.(Phe1051Leufs*12) | PMID: 2404377742 |
| 3XC+7.8 | CNGB1 | NM_001297 | Heterozygous | c.2805delG | p.(Glu935Aspfs*2) | Novel |
| Heterozygous | c.2544_2545insG | p.(Leu849Alafs*3) | Novel | |||
| U7U+9.12 | CRB1 | NM_201253 | Homozygous | c.2501G>A | p.(Gly834Asp) | Novel |
| UEW+W.58 | CRB1 | NM_201253 | Heterozygous | c.3712T>C | p.(Cys1238Arg) | Novel |
| Heterozygous | c.252_253insTG | p.(Asn87*) | Novel | |||
| 3XM+J.87 | CRX | NM_000554 | Heterozygous | c.682C>T | p.(Gln228*) | Novel |
| U6H+2.34 | EYS | NM_001142800 | Heterozygous | c.6078G>T | p.(Gln2026His) | Novel |
| Heterozygous | c.6416G>A | p.(Cys2139Tyr) | PMID: 2033377043 | |||
| TW+H.97 | EYS | NM_001142800 | Heterozygous | c.4350_4356del | p.(Ile1451Profs*3) | PMID: 2053739433 |
| Heterozygous | c.6714delT | p.(Ile2239Serfs*17) | PMID: 1897672544 | |||
| 3U6+9.42 | EYS | NM_001142800 | Heterozygous | c.904C>T | p.(Leu302Phe) | Novel |
| Heterozygous | c.8860T>C | p.(Phe2954Leu) | Novel | |||
| Homozygous | c.3250A>C | p.(Thr1084Pro) | Novel | |||
| VNM+T.47 | EYS | NM_001142800 | Homozygous | c.4402G>C | p.(Asp1468His) | Novel |
| Homozygous | c.3443+1G>T | p.? | Novel | |||
| 5ES+3.87 | GPR98 | NM_032119 | Heterozygous | c.2285G>A | p.(Arg762His) | Novel |
| Heterozygous | c.4349A>G | p.(Lys1450Arg) | Novel | |||
| UFC+7.74 | GRM6 | NM_000843 | Heterozygous | c.727G>T | p.(Val243Phe) | Novel |
| Heterozygous | c.2240C>T | p.(Ser747Leu) | Novel | |||
| 3XN+K.89 | IMPG2 | NM_016247 | Heterozygous | c.1589C>A | p.(Ser530*) | Novel |
| Heterozygous | c.3030_3031insTTTTAGGTGATGAA | p.(Ala1011Phefs*2) | Novel | |||
| 5VR+W.92 | MERTK | NM_006343 | Heterozygous | c.390G>A | p.(Trp130*) | PMID: 2415466210 |
| Heterozygous | c.2287C>A | p.(Pro763Thr) | Novel | |||
| 3V5+8.13 | NR2E3 | NM_014249 | Heterozygous | c.995-2A>C | p.? | Novel |
| Heterozygous | c.226C>T | p.(Arg76Trp) | PMID: 1065505645 | |||
| 3U3+6.63 | PDE6B | NM_000283 | Heterozygous | c.2193+1G>A | p.? | PMID: 772454738 |
| Heterozygous | c.299G>A | p.(Arg100His) | PMID: 2233437046 | |||
| UGQ+Q.72 | PDE6B | NM_000283 | Heterozygous | c.892C>T | p.(Gln298*) | PMID: 839417447 |
| Heterozygous | c.2116A>T | p.(Lys706*) | PMID: 772454738 | |||
| MK+W.33 | PDE6B | NM_000283 | Homozygous | c.1540delC | p.(Leu514Trpfs*61) | Novel |
| N6+A.15 | PROM1 | NM_006017 | Heterozygous | c.1117C>T | p.(Arg373Cys) | PMID: 2039311624 |
| 8J+Y.4 | PRPH2 | NM_000322 | Heterozygous | c.514C>T | p.(Arg172Trp) | PMID: 848557623 |
| 34U+F.88 | RDH12 | NM_152443 | Homozygous | c.805_809del | p.(Ala269Glyfs*2) | Novel |
| S7+G.76 | RHO | NM_000539 | Heterozygous | c.491C>T | p.(Ala164Val) | PMID: 798170125 |
| 5VY+V.14 | RHO | NM_000539 | Heterozygous | c.512C>T | p.(Pro171Leu) | PMID: 183377726 |
| U6Z+5.73 | RP2 | NM_006915 | Hemizygous | c.718delT | p.(Leu240Tyrfs*14) | Novel |
| 9C+Y.10 | RPGR | NM_001034853 | Hemizygous | c.2245G>T | p.(Glu749*) | Novel |
| U2C+J.77 | RPGR | NM_001034853 | Hemizygous | c.3039_3040del | p.(Glu1014Glyfs*64) | PMID: 2368134248 |
| UNM+T.54 | RPGR | NM_000328 | Hemizygous | c.1495_1496insA | p.(Ile499Asnfs*14) | Novel |
| 59R+5.99 | RPGRIP1 | NM_020366 | Heterozygous | c.1753C>T | p.(Pro585Ser) | PMID: 2115384149 |
| Heterozygous | c.2302C>T | p.(Arg768*) | PMID: 2007993150 | |||
| Heterozygous | c.973T>C | p.(Phe325Leu) | Novel | |||
| 5FP+L.15 | TULP1 | NM_003322 | Heterozygous | c.1213G>C | p.(Ala405Pro) | Novel |
| Heterozygous | c.1495C>T | p.(Pro499Ser) | Novel | |||
| 5FV+T.56 | USH2A | NM_206933 | Heterozygous | c.9921T>G | p.(Cys3307Trp) | PMID: 2156929851 |
| Heterozygous | c.13010C>T | p.(Thr4337Met) | PMID: 2050792452 | |||
| SS+6.62 | USH2A | NM_206933 | Heterozygous | c.2276G>T | p.(Cys759Phe) | PMID: 1077552953 |
| Heterozygous | c.10073G>A | p.(Cys3358Tyr) | PMID: 2050792452 | |||
| 32V+Y.3 | USH2A | NM_206933 | Heterozygous | c.842C>A | p.(Thr281Lys) | PMID: 2213527654 |
| Heterozygous | c.6795_6797del | p.(Glu2265_Tyr2266delinsAsp) | PMID: 1827389855 | |||
| P9+A.52 | USH2A | NM_206933 | Heterozygous | c.6172_6173insA | p.(Val2059Glyfs*44) | Novel |
| Heterozygous | c.2276G>T | p.(Cys759Phe) | PMID: 1077552953 | |||
| 5ZU+U.41 | USH2A | NM_206933 | Homozygous | c.5012G>A | p.(Gly1671Asp) | Novel |
| VHM+Y.45 | USH2A | NM_206933 | Heterozygous | c.5167G>C | p.(Gly1723Arg) | Novel |
| Heterozygous | c.4370C>A | p.(Ser1457*) | Novel | |||
| c.14792-2A>G | p.? | PMID: 2202557941 | ||||
| 8X+A.29 | USH2A | NM_206933 | Heterozygous | c.6779C>A | p.(Ser2260Tyr) | Novel |
| Heterozygous | c.12094G>A | p.(Gly4032Arg) | Novel | |||
| Heterozygous | c.2299delG | p.(Glu767Serfs*21) | PMID: 962405340 | |||
| 5CV+J.77 | USH2A | NM_206933 | Heterozygous | c.4714C>T | p.(Leu1572Phe) | PMID: 2202557941 |
| Heterozygous | c.9433C>T | p.(Leu3145Phe) | Novel | |||
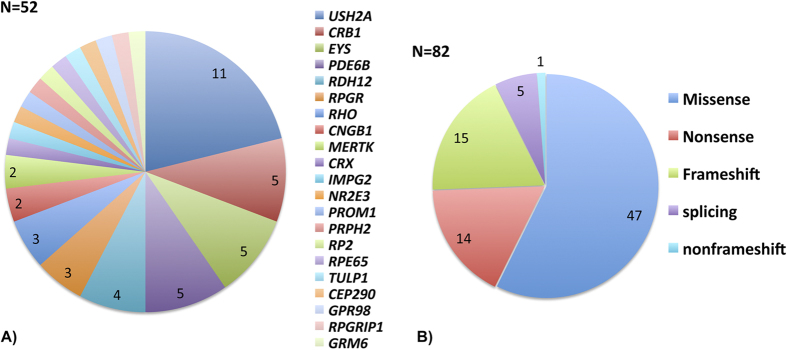
The mutations are distributed across 21 retinal disease genes with USH2A as the most frequently mutated gene, accounting for 11 solved cases (22%). In addition, mutations have been found in EYS (10%), CRB1 (10%), PDE6B (10%), RDH12 (8%), RPGR (6%), RHO (6%), CNGB1 (4%), MERTK (4%), and the rest of cases in 12 genes with one case for each gene (Fig. 2a). A total of 82 mutant alleles have been identified in our study, all of which have been confirmed by the CLIA certified laboratories in the eyeGENE® Network. Among them, missense mutations account for 58%, followed by frameshift (18%), nonsense (17%), and splicing (7%) (Fig. 2b). Interestingly, close to 60% of the mutant alleles have not been previously reported (48/82).
Figure 2. Disease-causing mutations were found for 52 probands and the majority of the mutations were missense.
(A) 21 retinal disease genes were assigned causal in the 52 solved cases. (B) A total of 82 mutations were identified along with their different types.
Identification of pathogenic mutations in dominant and recessive cases
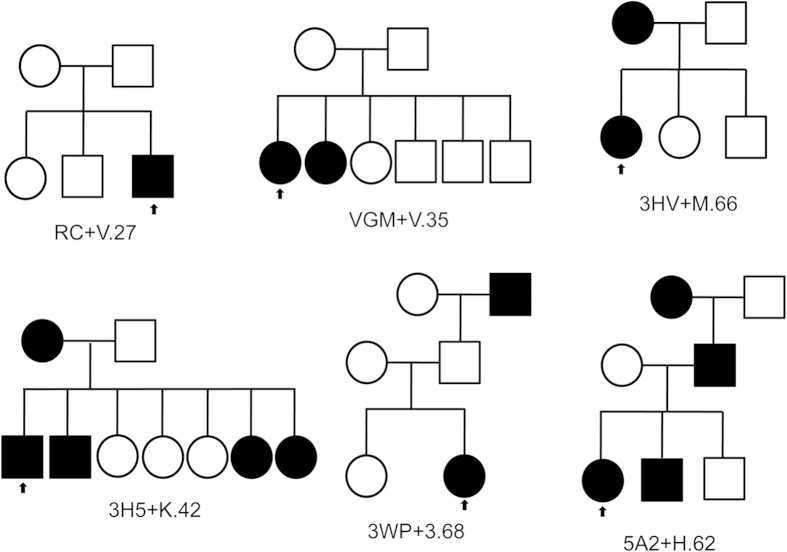
As shown in Fig. 1, a total of 13 RP cases were labeled as dominant inheritance based on the information documented in the eyeGENE® database. Among them, mutations were identified for 6 patients (Table 1). Consistent with the dominant inheritance model, a previously reported dominant mutation was found in RHO for one patient 5A2+H.62. In addition, homozygous or compound heterozygous mutations in recessive RP disease genes including USH2A, EYS, MERTK and PDE6B were identified for patients RC+V27, VGM+V.35, 3HV+M.66 and 3H5+K.42, respectively. Finally, for patient 3WP+3.68, a causal homozygous mutation was identified in RDH12, which can either cause recessive or dominant RP (Table 1). These patients have been assigned with a dominant inheritance model based on the initial diagnosis provided by the physician. Our molecular diagnosis results did not support this except for patient 5A2+H.62, so we contacted the clinicians for further information. Investigation of the pedigree information indicated that indeed some of the families were likely to be misclassified (Fig. 3). For example, in both RC+V.27 and VGM+V.35 families all affected individuals appear in the same generation while their parents are normal, suggesting that the inheritance model for these two families is indeed recessive. For the other three families, evidence of a dominant inheritance model is weak, because the pedigrees either lack male to male transmission (3WP+3.68), or only show affected members in 2 generations (3HV+M.66 and 3H5+K.42).
Figure 3. Pedigrees for 6 probands labeled dominant inheritance in the eyeGENE® database.
The small arrow indicates the proband sequenced in each family.
There were total 21 patients labeled as recessive inheritance and 9 of them were solved (Table 1). Consistent with the diagnosis of arRP, all genes found were known arRP genes, including CRB1, PDE6B, RDH12, RPE65, and USH2A. Of the solved arRP cases, one interesting case was the c.295C>A, p.(Ile2995Asn) missense mutation in homozygous state in RDH12 found in proband 3JY+V.17, which is also the causal mutation for the proband 3WP+3.68 (Table 1). This missense mutation leads to LCA21 when combined with a nonsense mutation, while severe RP22 is observed when combined with a second missense allele. Consistent with the idea that the c.295C>A, p.(Ile2995Asn) is a hypomorphic allele, both probands 3WP+3.68 and 3JY+V.17 show RP phenotype at age 11 and 3 years old, respectively. Therefore, both cases turned out to have relatively earlier onset age compared to typical RP patients and should be classified as juvenile RP. This is also in consistent with a recent research shown that RDH12 was the most frequently mutated gene in the juvenile RP group in a large Spanish cohort8.
Identification of pathogenic mutations in simplex/unknown cases
In this study, 70 (67%) of the RP cases were simplex or unknown, for which molecular diagnosis is most valuable. In the 70 simplex cases, causative mutations were identified in 37 samples. Specifically, we identified mutations in autosomal dominant retinal disease genes for 5 cases, in X-linked disease genes for 4 cases, and in recessive disease genes for 28 cases.
Dominant mutations in RP genes PROM1, PRPH2, RHO, and CRX were found in 5 probands (Table 1). While one novel nonsense mutation in CRX was found in patient 3XM+J.87, four mutations previously reported were found in genes PRPH2, PROM1, and RHO. For example, the p.(Arg172Trp) mutation in PRPH2 was assigned causative mutation for proband 8J+Y.4. In a previous study, the p.(Arg172Trp) mutation in PRPH2 was found to segregate in two independent families with affected members showing symptoms with blurred central vision and photophobia, while no complain of night blindness or restricted peripheral vision23. A closer investigation of the clinical exam result for proband 8J+Y.4 showed that this patient had both macular and peripheral retinal degeneration and that he experienced visual acuity loss (at 24 years) before night blindness (at 46 years). This is similar to the phenotype described in the previous study supporting that p.(Arg172Trp) in PRPH2 is likely the causative mutation. For another example, proband N6+A.15 was assigned the p.(Arg373Cys) mutation in PROM1. This mutation has been reported in a four-generation Italian family with autosomal dominant RP and affected members showing reduced central vision first and with night blindness progressing over time24. In proband N6+A.15, however, night blindness and visual acuity loss occurred at the same time (at age 30 years). It is possible that genetic background or environment factors could influence the onset of development of night blindness in patients with the p.(Arg373Cys) mutation in PROM1. Finally, p.(Ala164Val) and p.(Pro171Leu) mutations in RHO were found in patients S7+G.76 and 5VY+V.14, respectively. Both have been previously reported to be causal mutations and segregate in dominant RP families25,26, and both affect folding of rhodopsin protein by biochemistry studies27.
X-linked RP is estimated to account for 10% ~ 20% of all RP, of which the males typically show an early age of onset and a rapid course of vision loss. RPGR and RP2, the genes most often associated with X-linked RP, explain more than 15% of isolated male RP cases28. In this study, we identified hemizygous mutations in RPGR in 3 probands and RP2 in 1 proband (Table 1). Two out of the three RPGR mutations, p.(Glu749*) and p.(Glu1014Glyfs*64), were novel and were located at the mutation hot spot RPGR ORF15. All three mutations are likely to be loss of function mutations that either result in truncated proteins or no protein through nonsense mediated decay. The p.(Leu240Tyrfs*14) identified in RP2 was also novel and predicted to produce a prematurely truncated protein.
For 28 of the simplex/unknown cases, mutations were identified in 10 arRP genes (Table 1). As expected, the most frequently mutated genes were USH2A and EYS, accounting for 8 and 4 cases, respectively. Additionally, for three probands we found deleterious mutations in retinal disease genes other than those associated with RP (Table 1). For example, compound heterozygous mutations c.4349A>G, p.(Lys1450Arg) and c.2285G>A , p.(Arg762His) that are novel and predicted to be damaging, were found in Usher gene GPR98 in patient 5ES+3.87. Although the age of diagnosis of hearing loss in type II Usher patients can be variable, it is generally during childhood with a median age of 5 years29. However, patient 5ES+3.87 did not show any hearing loss at the time of the clinical visit when he was 39 years old, it is thus unlikely that patient 5ES+3.87 could be a typical type II Usher patient (Table 2). In another proband UFC+7.74, novel missense mutations p.(Val243Phe) and p.(Ser747Leu), predicted to be damaging, were found in the complete type of Congenital Stationary Night-blindness (CSNB) gene GRM6. Electroretinogram (ERG) responses for this patient were not recordable in either eye under scotopic and photopic conditions (Table 2 and supplementary Fig. S2). Finally, compound heterozygous mutations p.(Phe1950Leufs*15) and p.(Glu1803Asp) in CEP290 were found in proband 5WY+Y.91. The first allele, p.(Phe1950Leufs*15) in CEP290 has been previously reported in two LCA families in compound heterozygous state with either a splicing mutation or a non-frameshift mutation, and the second allele p.(Glu1803Asp) was novel30. A closer investigation of the clinical information of this patient showed that she had first experienced night-blindness at age 18 and vision loss at age 22. Also, her best corrected visual acuity was 20/20 in both eyes at the time of clinical visit when she was 39 years of age.
Table 2. Clinical information for 3 probands in which mutations in other retinal disease genes not previously associated with non-syndromic RP were found.
| ID | Gene | Disease previously associated | Patient Clinical Phenotype | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) when patient first aware of | Best corrected visual acuity | Hearing defects | Electroretinogram (Amplitude μV, Implicit time ms) | |||||||
| Night blindness | Vision loss | Dark-adapted | Light-adapted | |||||||
| OD | OS | OD | OS | |||||||
| 5WY+Y.91 | CEP290 | Leber congenital amaurosis | 18 | 22 | OD 20/20 OS 20/20 | No | 12, 33 | 16, 35 | 11, 37 | 12, 36 |
| 5ES+3.87 | GPR98 | Usher syndrome | 37 | 37 | OD 20/20 OS 20/20 | No | 43, 12 | 40, 24 | 30, 37 | 30, 38 |
| UFC+7.74 | GRM6 | Congenital stationary night blindness | 25 | 25 | OD 20/20 OS 20/25 | No | NR | NR | NR | NR |
All three patients underwent electroretinogram (ERG) tests following the ISCEV (International Society for Clinical Electrophysiology of Vision) standard. NR: not recordable
Discussion
One of the biggest challenges for fulfilling eyeGENE®’s mission is that most of its enrolled patients were not introduced to a clear plan of genetic testing that would be both financially efficient and result in a likely associated genetic cause. Of these patients, the majority (70%) is RP, which is highly heterogeneous at multiple levels. First, RP is both genetically and clinically heterogeneous with multiple genes contributing to the disease, and phenotypes vary greatly among patients. Second, the inheritance pattern is heterogeneous and not always clear based on the pedigree information alone. Finally, as a national network, the eyeGENE® patients were recruited and examined by many physician groups across the country thus the clinical tests performed and the diagnosis criteria are not always the same. The information for each proband available also varies significantly. As a result, molecular diagnosis of this highly heterogeneous collection is challenging. Here, we performed a pilot study using NGS based panel sequencing for molecular diagnosis of eyeGENE® RP patients and achieved a similar yield of solved cases (~50%) in comparison to previous studies that adopted similar approaches10,11. In addition, of the 82 mutations identified, 48 (~60%) of them were novel, which is also comparable to previous studies10,11.
One interesting finding of this study is the inconsistency between inheritance patterns assigned and the genetic test results for five out of six adRP solved cases (Table 1 and Fig. 3). While two of the families were misclassified (RC+V.27 and VGM+V.35), the other three families are inconclusive for dominant inheritance as members from less than three generations were affected. Indeed, assigning inheritance patterns based solely on pedigree information could be prone to error. For example, 8.5% of the families thought to have adRP truly have X-linked RP31. Using the NGS approach, however, helps to clarify this issue since all variants, regardless of inheritance, are considered simultaneously.
Our study also showed that NGS based molecular diagnosis can potentially reveal novel genotype phenotype associations. For example, in three cases, we identified potential new associations for mutations in retinal disease genes GPR98, CEP290 and GRM6 with an RP phenotype. Although the documented clinical information for these three patients supported RP phenotype, it is possible that these patients had Usher syndrome, LCA, or CSNB and were misdiagnosed as general RP. Segregation tests as well as clinical diagnosis refinements will be required to confirm the genetic testing results. Nevertheless, these findings are particularly important for the family, especially family members at risk. With the identification of many more mutations causing inherited retinal diseases and their associated phenotype clearly documented in the eyeGENE® database, clinicians and counselors will feel more confident in providing guidance to affected families.
In conclusion, our study showed that NGS based approach is robust and effective in providing precise molecular diagnosis for the highly heterogeneous collection of RP patients from eyeGENE®. The results from this study are essential for fulfilling the goals of eyeGENE® to advance vision research and to contribute to the shared resources for the research community. First, the novel mutations identified in these RP patients will be documented in the database and accessible to other research groups to continue the research cycle, which will provide valuable information for genotype-phenotype correlation studies in the future. Secondly, patient samples without assigned mutations represent a valuable resource for novel RP gene discovery. In fact, novel RP genes have been identified from these samples and have lead to existing publications32. Last but not least, genetic testing results will provide registered eyeGENE® patients the information and opportunity to participate in gene-specific clinical trials.
Additional Information
How to cite this article: Ge, Z. et al. NGS-based Molecular diagnosis of 105 eyeGENE® probands with Retinitis Pigmentosa. Sci. Rep. 5, 18287; doi: 10.1038/srep18287 (2015).
Supplementary Material
Acknowledgments
We would like to thank the patients and families who participated in this study by participating the eyeGENE® network and the eyeGENE® Working Group (https://nei.nih.gov/eyegene/staff_eyegene). The samples described are available to researchers through request and approval by the eyeGENE® Research Access Subcommittee. Additional information can be found at the eyeGENE® website nei.nih.gov/eyegene. The eyeGENE® study was supported by the Department of Health and Human Services/National Institutes of Health/National Eye Institute intramural program under eyeGENE® – Protocol 06-EI-0236 and 10-EI-N164 which has been funded in part under Contract No. HHS-N-260-2007-00001-C. This work is also supported by grants from the Retinal Research Foundation, Foundation Fighting Blindness (BR-GE-0613-0618-BCM), and the National Eye Institute (R01EY022356) (RC). Next-generation sequencing was conducted at the Functional Genomic Core (FGC) facility at Baylor College of Medicine supported by National Institutes of Health Shared Instrument Grant 1S10RR026550 (RC). We thank Ms. Li Zhao and Mr. Evan M. Jones for reviewing and editing the manuscript. FW was supported by a predoctoral fellowship funded by the Burroughs Wellcome Trust Fund: The Houston Laboratory and Population Sciences Training Program in Gene Environment Interaction. ZG is supported by the NIH T32 training grant (2T32EY007102-21A1).
Footnotes
Author Contributions Z.G. performed the majority of the data analysis and wrote the manuscript. K.B. and H.S. performed the clinical evaluation and ERG testing of the patient UFC+7.74. K.G. is in charge of the eyeGENE® sample management and communication with the clinicians. X.W. lead the efforts of confirming mutant alleles identified in this study in the CLIA certified laboratories in the eyeGENE® Network. F.W. analyzed some of the data. S.X. and H.W. did NGS sequencing. K.W. did Sanger validation. R.C. is in charge of the project design and revised the manuscript.
References
- Bunker C. H., Berson E. L., Bromley W. C., Hayes R. P. & Roderick T. H. Prevalence of retinitis pigmentosa in Maine. Am J Ophthalmol 97, 357–365 (1984). [DOI] [PubMed] [Google Scholar]
- Hamel C. Retinitis pigmentosa. Orphanet J Rare Dis 1, 40 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- SP Daiger B. R., J. G., A Christoffels, W Hide, Retnet, http://www.sph.uth.tmc.edu/RetNet/. Published 1996, Accessed April 1 2015.
- Hartong D. T., Berson E. L. & Dryja T. P. Retinitis pigmentosa. Lancet 368, 1795–1809 (2006). [DOI] [PubMed] [Google Scholar]
- Daiger S. P., Bowne S. J. & Sullivan L. S. Perspective on genes and mutations causing retinitis pigmentosa. Arch Ophthalmol 125, 151–158 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiger S. P., Sullivan L. S. & Bowne S. J. Genes and mutations causing retinitis pigmentosa. Clin Genet 84, 132–141 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurg A. et al. Arrayed primer extension: solid-phase four-color DNA resequencing and mutation detection technology. Genet Test 4, 1–7 (2000). [DOI] [PubMed] [Google Scholar]
- Avila-Fernandez A. et al. et al. Mutation analysis of 272 Spanish families affected by autosomal recessive retinitis pigmentosa using a genotyping microarray. Mol Vis 16, 2550–2558 (2010). [PMC free article] [PubMed] [Google Scholar]
- Glockle N. et al. Panel-based next generation sequencing as a reliable and efficient technique to detect mutations in unselected patients with retinal dystrophies. Eur J Hum Genet 22, 99–104 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F. et al. Next generation sequencing-based molecular diagnosis of retinitis pigmentosa: identification of a novel genotype-phenotype correlation and clinical refinements. Hum Genet 133, 331–345 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L. et al. Next-generation sequencing-based molecular diagnosis of 82 retinitis pigmentosa probands from Northern Ireland. Hum Genet 134, 217–230 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks M. E. et al. Next-generation sequencing (NGS) as a diagnostic tool for retinal degeneration reveals a much higher detection rate in early-onset disease. Eur J Hum Genet 21, 274–280 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. et al. Dependable and efficient clinical utility of target capture-based deep sequencing in molecular diagnosis of retinitis pigmentosa. Invest Ophthalmol Vis Sci 55, 6213–6223 (2014). [DOI] [PubMed] [Google Scholar]
- Blain D., Goetz K. E., Ayyagari R. & Tumminia S. J. eyeGENE(R): a vision community resource facilitating patient care and paving the path for research through molecular diagnostic testing. Clin Genet 84, 190–197 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz K. E., Reeves M. J., Tumminia S. J. & Brooks B. P. eyeGENE(R): a novel approach to combine clinical testing and researching genetic ocular disease. Curr Opin Ophthalmol 23, 355–363 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. et al. Comprehensive molecular diagnosis of 179 Leber congenital amaurosis and juvenile retinitis pigmentosa patients by targeted next generation sequencing. J Med Genet 50, 674–688 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenekoop R. K. et al. Mutations in NMNAT1 cause Leber congenital amaurosis and identify a new disease pathway for retinal degeneration. Nat Genet 44, 1035–1039 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abecasis G. R. et al. A map of human genome variation from population-scale sequencing. Nature 467, 1061–1073 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenson P. D. et al. The Human Gene Mutation Database: building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum Genet 133, 1–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Jian X. & Boerwinkle E. dbNSFP: a lightweight database of human nonsynonymous SNPs and their functional predictions. Hum Mutat 32, 894–899 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault I. et al. Retinal dehydrogenase 12 (RDH12) mutations in leber congenital amaurosis. Am J Hum Genet 75, 639–646 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacon-Camacho O. F., Jitskii S., Buentello-Volante B., Quevedo-Martinez J. & Zenteno J. C. Exome sequencing identifies RDH12 compound heterozygous mutations in a family with severe retinitis pigmentosa. Gene 528, 178–182 (2013). [DOI] [PubMed] [Google Scholar]
- Wells J. et al. Mutations in the human retinal degeneration slow (RDS) gene can cause either retinitis pigmentosa or macular dystrophy. Nat Genet 3, 213–218 (1993). [DOI] [PubMed] [Google Scholar]
- Michaelides M. et al. The PROM1 mutation p.R373C causes an autosomal dominant bull’s eye maculopathy associated with rod, rod-cone, and macular dystrophy. Invest Ophthalmol Vis Sci 51, 4771–4780 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs S. et al. Three novel rhodopsin mutations (C110F, L131P, A164V) in patients with autosomal dominant retinitis pigmentosa. Hum Mol Genet 3, 1203 (1994). [DOI] [PubMed] [Google Scholar]
- Dryja T. P., Hahn L. B., Cowley G. S., McGee T. L. & Berson E. L. Mutation spectrum of the rhodopsin gene among patients with autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci U S A 88, 9370–9374 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoczy E. P., Kiel C., McKeone R., Stricher F. & Serrano L. Analysis of disease-linked rhodopsin mutations based on structure, function, and protein stability calculations. J Mol Biol 405, 584–606 (2011). [DOI] [PubMed] [Google Scholar]
- Branham K. et al. Mutations in RPGR and RP2 account for 15% of males with simplex retinal degenerative disease. Invest Ophthalmol Vis Sci 53, 8232–8237 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abadie C. et al. Audiological findings in 100 USH2 patients. Clin Genet 82, 433–438 (2012). [DOI] [PubMed] [Google Scholar]
- Perrault I. et al. Spectrum of NPHP6/CEP290 mutations in Leber congenital amaurosis and delineation of the associated phenotype. Hum Mutat 28, 416 (2007). [DOI] [PubMed] [Google Scholar]
- Churchill J. D. et al. Mutations in the X-linked retinitis pigmentosa genes RPGR and RP2 found in 8.5% of families with a provisional diagnosis of autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci 54, 1411–1416 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M. et al. ATF6 Is Mutated in Early Onset Photoreceptor Degeneration With Macular Involvement. Invest Ophthalmol Vis Sci 56, 3889–3895 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littink K. W. et al. Mutations in the EYS gene account for approximately 5% of autosomal recessive retinitis pigmentosa and cause a fairly homogeneous phenotype. Ophthalmology 117, 2026–2033, 2033 e2021-2027 (2010). [DOI] [PubMed] [Google Scholar]
- Dryja T. P. et al. Mutations within the rhodopsin gene in patients with autosomal dominant retinitis pigmentosa. N Engl J Med 323, 1302–1307 (1990). [DOI] [PubMed] [Google Scholar]
- Herrera W. et al. Retinal disease in Usher syndrome III caused by mutations in the clarin-1 gene. Invest Ophthalmol Vis Sci 49, 2651–2660 (2008). [DOI] [PubMed] [Google Scholar]
- Eisenberger T. et al. Increasing the yield in targeted next-generation sequencing by implicating CNV analysis, non-coding exons and the overall variant load: the example of retinal dystrophies. PLoS One 8, e78496 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander A. I. et al. Leber congenital amaurosis and retinitis pigmentosa with Coats-like exudative vasculopathy are associated with mutations in the crumbs homologue 1 (CRB1) gene. Am J Hum Genet 69, 198–203 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin M. E., Ehrhart T. L., Berson E. L. & Dryja T. P. Mutation spectrum of the gene encoding the beta subunit of rod phosphodiesterase among patients with autosomal recessive retinitis pigmentosa. Proc Natl Acad Sci USA 92, 3249–3253 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benayoun L. et al. Genetic heterogeneity in two consanguineous families segregating early onset retinal degeneration: the pitfalls of homozygosity mapping. Am J Med Genet A 149A, 650–656 (2009). [DOI] [PubMed] [Google Scholar]
- Eudy J. D. et al. Mutation of a gene encoding a protein with extracellular matrix motifs in Usher syndrome type IIa. Science 280, 1753–1757 (1998). [DOI] [PubMed] [Google Scholar]
- Song J. et al. High-throughput retina-array for screening 93 genes involved in inherited retinal dystrophy. Invest Ophthalmol Vis Sci 52, 9053–9060 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiguchi K. M. et al. Whole genome sequencing in patients with retinitis pigmentosa reveals pathogenic DNA structural changes and NEK2 as a new disease gene. Proc Natl Acad Sci USA 110, 16139–16144 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audo I. et al. EYS is a major gene for rod-cone dystrophies in France. Hum Mutat 31, E1406–1435 (2010). [DOI] [PubMed] [Google Scholar]
- Collin R. W. et al. Identification of a 2 Mb human ortholog of Drosophila eyes shut/spacemaker that is mutated in patients with retinitis pigmentosa. Am J Hum Genet 83, 594–603 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider N. B. et al. Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat Genet 24, 127–131 (2000). [DOI] [PubMed] [Google Scholar]
- Neveling K. et al. Next-generation genetic testing for retinitis pigmentosa. Hum Mutat 33, 963–972 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin M. E., Sandberg M. A., Berson E. L. & Dryja T. P. Recessive mutations in the gene encoding the beta-subunit of rod phosphodiesterase in patients with retinitis pigmentosa. Nat Genet 4, 130–134 (1993). [DOI] [PubMed] [Google Scholar]
- Zahid S. et al. Phenotypic conservation in patients with X-linked retinitis pigmentosa caused by RPGR mutations. JAMA Ophthalmol 131, 1016–1025 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiszniewski W. et al. Potential involvement of more than one locus in trait manifestation for individuals with Leber congenital amaurosis. Hum Genet 129, 319–327 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walia S. et al. Visual acuity in patients with Leber’s congenital amaurosis and early childhood-onset retinitis pigmentosa. Ophthalmology 117, 1190–1198 (2010). [DOI] [PubMed] [Google Scholar]
- Bonnet C. et al. Complete exon sequencing of all known Usher syndrome genes greatly improves molecular diagnosis. Orphanet J Rare Dis 6, 21 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee T. L., Seyedahmadi B. J., Sweeney M. O., Dryja T. P. & Berson E. L. Novel mutations in the long isoform of the USH2A gene in patients with Usher syndrome type II or non-syndromic retinitis pigmentosa. J Med Genet 47, 499–506 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivolta C., Sweklo E. A., Berson E. L. & Dryja T. P. Missense mutation in the USH2A gene: association with recessive retinitis pigmentosa without hearing loss. Am J Hum Genet 66, 1975–1978 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Quesne Stabej P. et al. Comprehensive sequence analysis of nine Usher syndrome genes in the UK National Collaborative Usher Study. J Med Genet 49, 27–36 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer B. et al. Spectrum of USH2A mutations in Scandinavian patients with Usher syndrome type II. Hum Mutat 29, 451 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.