Abstract
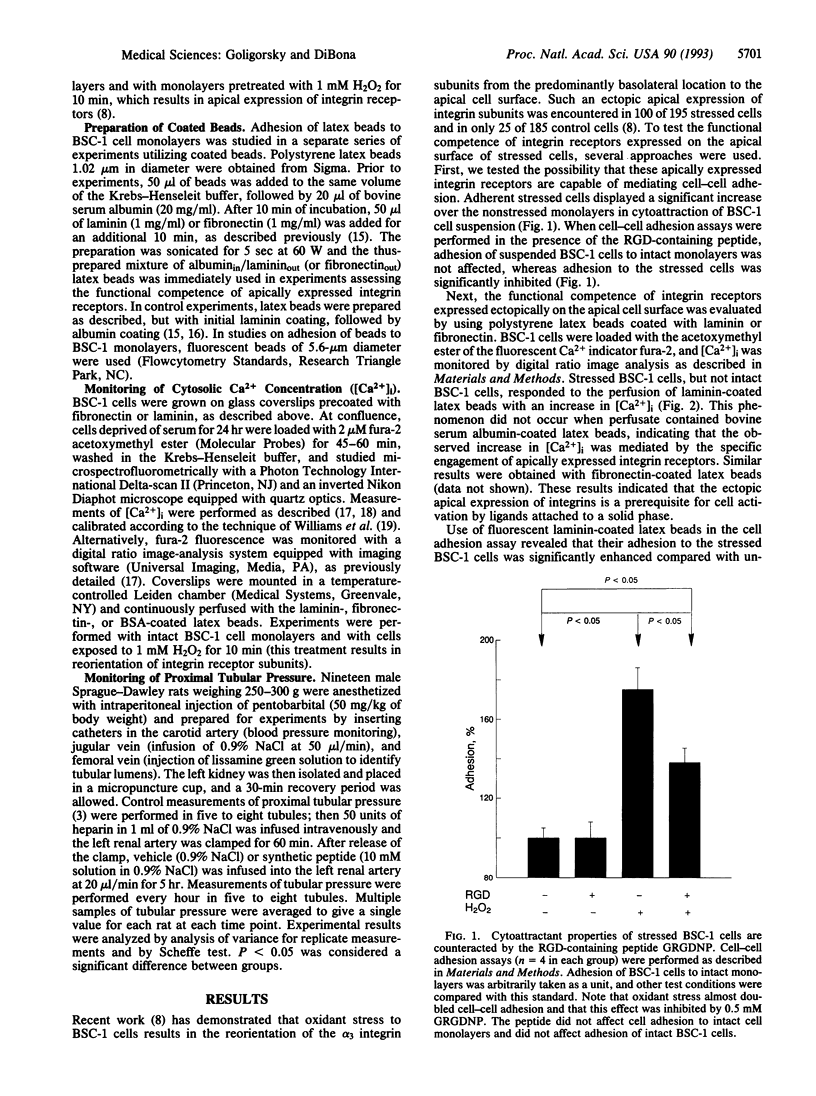
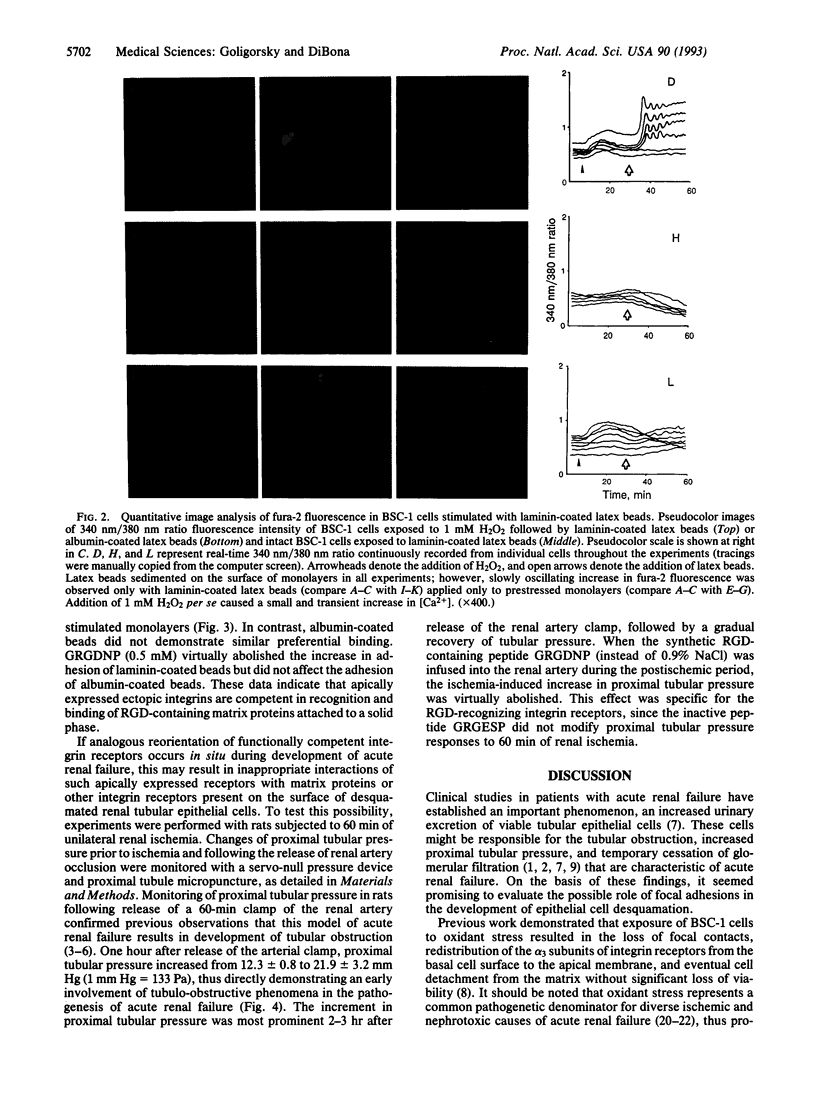
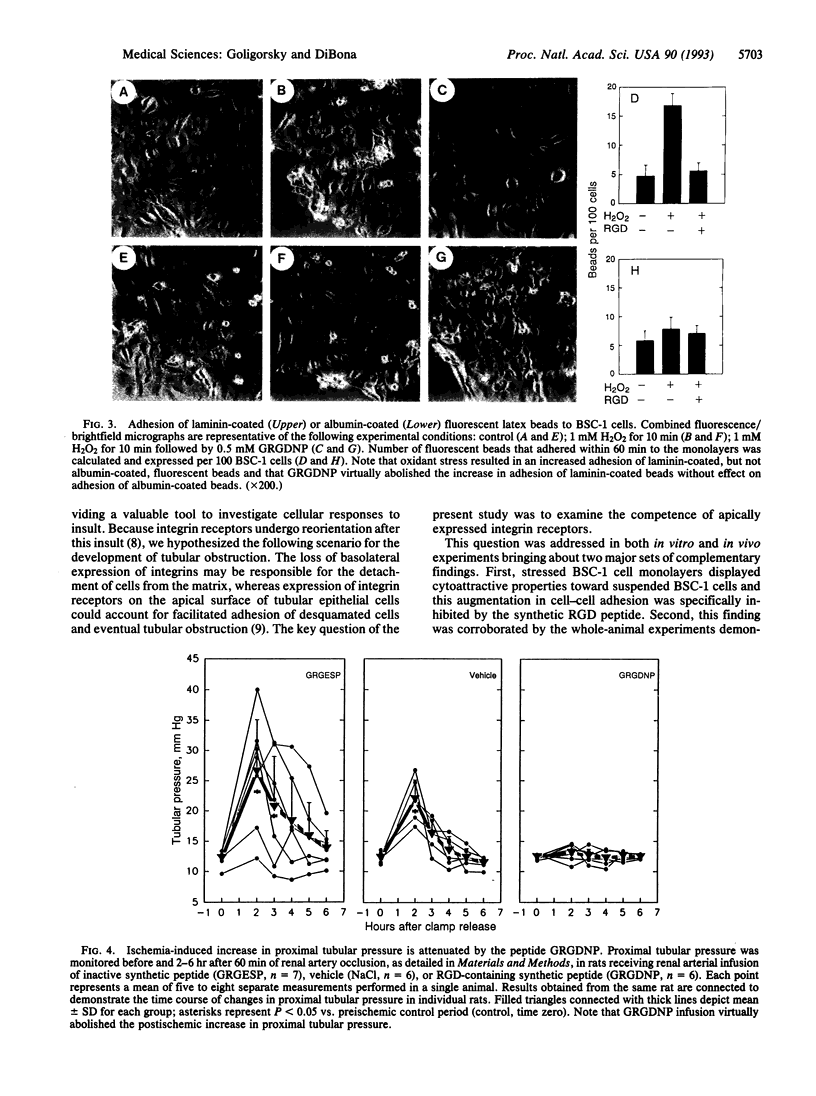
Reorientation of the alpha 3 subunit of integrins from predominantly basal to the apical cell surface of cultured renal tubular epithelial cells subjected to oxidant stress has previously been demonstrated. The present study was designed to assess functional competence of ectopically expressed apical integrins. Cell-cell adhesion assay revealed enhanced cytoatractant properties of stressed cells. Stressed epithelial cells exhibited specific recognition and binding of laminin-coated latex beads. These processes were inhibited with the peptide Gly-Arg-Gly-Asp-Asn-Pro (GRGDNP) suggesting a role of RGD-recognizing integrins in augmented adhesion to stressed cells. Given that such enhanced adhesion in in vivo acute renal failure may govern tubular obstruction by desquamated epithelium, a physiological marker of patency of tubular lumen, proximal tubular pressure, was monitored in rats subjected to 60 min of renal ischemia followed by reperfusion. Proximal tubular pressure increased 2-fold after 2 hr of reperfusion in animals that had undergone 60 min of ischemia. Infusion of GRGDNP into the renal artery during reperfusion period virtually abolished an increase in proximal tubular pressure observed in ischemic acute renal failure. These in vitro and in vivo findings are consistent with the hypothesis that RGD-recognizing integrins play an important role in the pathogenesis of tubular obstruction in ischemic acute renal failure.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Arendshorst W. J., Finn W. F., Gottschalk C. W. Micropuncture study of acute renal failure following temporary renal ischemia in the rat. Kidney Int Suppl. 1976 Oct;6:S100–S105. [PubMed] [Google Scholar]
- Baud L., Ardaillou R. Reactive oxygen species: production and role in the kidney. Am J Physiol. 1986 Nov;251(5 Pt 2):F765–F776. doi: 10.1152/ajprenal.1986.251.5.F765. [DOI] [PubMed] [Google Scholar]
- Becchetti A., Arcangeli A., Del Bene M. R., Olivotto M., Wanke E. Response to fibronectin-integrin interaction in leukaemia cells: delayed enhancing of a K+ current. Proc Biol Sci. 1992 Jun 22;248(1323):235–240. doi: 10.1098/rspb.1992.0067. [DOI] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Gailit J., Colflesh D., Rabiner I., Simone J., Goligorsky M. S. Redistribution and dysfunction of integrins in cultured renal epithelial cells exposed to oxidative stress. Am J Physiol. 1993 Jan;264(1 Pt 2):F149–F157. doi: 10.1152/ajprenal.1993.264.1.F149. [DOI] [PubMed] [Google Scholar]
- Goligorsky M. S., Lieberthal W., Racusen L., Simon E. E. Integrin receptors in renal tubular epithelium: new insights into pathophysiology of acute renal failure. Am J Physiol. 1993 Jan;264(1 Pt 2):F1–F8. doi: 10.1152/ajprenal.1993.264.1.F1. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast receptor for cell-substratum adhesion: studies on the interaction of baby hamster kidney cells with latex beads coated by cold insoluble globulin (plasma fibronectin). J Cell Biol. 1980 Jul;86(1):104–112. doi: 10.1083/jcb.86.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Iijima K., Moore L. C., Goligorsky M. S. Syncytial organization of cultured rat mesangial cells. Am J Physiol. 1991 Jun;260(6 Pt 2):F848–F855. doi: 10.1152/ajprenal.1991.260.6.F848. [DOI] [PubMed] [Google Scholar]
- OLIVER J. Correlations of structure and function and mechanisms of recovery in acute tubular necrosis. Am J Med. 1953 Oct;15(4):535–557. doi: 10.1016/0002-9343(53)90143-0. [DOI] [PubMed] [Google Scholar]
- OLIVER J., MacDOWELL M., TRACY A. The pathogenesis of acute renal failure associated with traumatic and toxic injury; renal ischemia, nephrotoxic damage and the ischemic episode. J Clin Invest. 1951 Dec;30(121):1307–1439. doi: 10.1172/JCI102550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller M. S., Hoidal J. R., Ferris T. F. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984 Oct;74(4):1156–1164. doi: 10.1172/JCI111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racusen L. C., Fivush B. A., Li Y. L., Slatnik I., Solez K. Dissociation of tubular cell detachment and tubular cell death in clinical and experimental "acute tubular necrosis". Lab Invest. 1991 Apr;64(4):546–556. [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991 Jan;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon E. E., McDonald J. A. Extracellular matrix receptors in the kidney cortex. Am J Physiol. 1990 Nov;259(5 Pt 2):F783–F792. doi: 10.1152/ajprenal.1990.259.5.F783. [DOI] [PubMed] [Google Scholar]
- Tanner G. A., Sophasan S. Kidney pressures after temporary renal artery occlusion in the rat. Am J Physiol. 1976 Apr;230(4):1173–1181. doi: 10.1152/ajplegacy.1976.230.4.1173. [DOI] [PubMed] [Google Scholar]
- Tanner G. A., Steinhausen M. Tubular obstruction in ischemia-induced acute renal failure in the rat. Kidney Int Suppl. 1976 Oct;6:S65–S73. [PubMed] [Google Scholar]
- Walker P. D., Shah S. V. Gentamicin enhanced production of hydrogen peroxide by renal cortical mitochondria. Am J Physiol. 1987 Oct;253(4 Pt 1):C495–C499. doi: 10.1152/ajpcell.1987.253.4.C495. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Fogarty K. E., Tsien R. Y., Fay F. S. Calcium gradients in single smooth muscle cells revealed by the digital imaging microscope using Fura-2. Nature. 1985 Dec 12;318(6046):558–561. doi: 10.1038/318558a0. [DOI] [PubMed] [Google Scholar]
- Yamada K. M. Adhesive recognition sequences. J Biol Chem. 1991 Jul 15;266(20):12809–12812. [PubMed] [Google Scholar]