Abstract
Rift Valley fever virus (RVFv) is capable of causing dramatic outbreaks amongst economically important animal species and is capable of causing severe symptoms and mortality in humans. RVFv is known to circulate widely throughout East Africa; serologic evidence of exposure has also been found in some northern African countries, including Mauritania. This study aimed to ascertain whether RVFv is circulating in regions beyond its known geographic range. Samples from febrile patients (n = 181) and nonfebrile healthy agricultural and slaughterhouse workers (n = 38) were collected during the summer of 2014 and surveyed for exposure to RVFv by both serologic tests and PCR. Of the 219 samples tested, 7.8% of nonfebrile participants showed immunoglobulin G reactivity to RVFv nucleoprotein and 8.3% of febrile patients showed immunoglobulin M reactivity, with the latter samples indicating recent exposure to the virus. Our results suggest an active circulation of RVFv and evidence of human exposure in the population of Tunisia.
Keywords: Arbovirus, seroprevalence, Tunisia, unexplained acute fever, vectors
Introduction
Rift Valley fever virus (RVFv) is an arthropod-borne Phlebovirus in the family Bunyaviridae, with widespread epidemiology throughout sub-Saharan African regions first reported in the early 1900s by a veterinary surgeon in Kenya [1]. The virus is maintained and transmitted by mosquitoes, mainly Aedes and Culex species [1], [2]. Though the virus has been shown to be maintained in mosquito populations through vertical and horizontal transmission [3], there has also been evidence of its maintenance in mammalian reservoirs, and there remains the possibility that there are other uncharacterized wildlife reservoirs which allow transmission at the wildlife/domesticated livestock interface via mosquito [4].
The epidemiologic range of the virus has been increasing, with reported outbreaks in West Africa, Saudi Arabia, North Africa and southern Africa [5], [6], [7], [8], [9]. There is evidence that RVFv is capable of transmission through a varied repertoire of diverse anthropophilic mosquito species, primarily Aedes and Culex species [10]. Indications of increasing diversity of vector competence may lead to rapid expansions in the future, which may pose a risk to countries geographically farther from traditional areas considered endemic [11].
Increasing circulation of RVFv in competent mosquito populations is a common observation in endemic areas after increased rainfall; it is usually followed by increased transmission to domesticated animals, where it is capable of causing severe animal health problems such as abortion [11], [12], [13]. This can result in a significant economic burden on the affected communities, particularly in arid areas that rely on geographically limited agriculture. An increased prevalence in human disease is also observed, particularly amongst agricultural workers, who are at a higher risk of exposure via mosquito bite, and abattoir workers or butchers, who are at risk of exposure via contaminated blood when preparing meat from infected animals [13]. Exposure via the bodily fluids of infected animals and also via aerosolization of the virus during butchering has been previously suggested as a major route of exposure for high-risk groups [14] and may put an individual at higher risk of developing more severe clinical symptoms, such as haemorrhagic manifestations due to exposure to higher viral titres.
Rift Valley fever outbreaks are regularly reported in East Africa and have also been reported in Mauritania [8]. One recent study identified Tunisia as a high-risk country; the authors inferred that environmental conditions and the presence of the vector meant that an epizootic occurrence was possible if the virus was present—a constant and considerable risk throughout the year, with a particularly high probability in July, after the wettest months [15]. Previous studies looking for serologic evidence of exposure to Rift Valley fever in 2006–2007 found no positivity in the studied population [16]. Indeed, as a result of anthropogenic influences such as irrigation and well drilling, surface water levels in several arid regions have increased over the years, allowing the emergence of vector-borne diseases due to the wider distribution of competent mosquitos throughout the country [17], thus increasing potential risk since previous studies were performed in both human and animal populations. On the basis of mathematical modelling, Tunisia is considered at risk for RVFv [15]. In this study, we performed a seroepidemiologic survey to demonstrate circulation of RVFv in Tunisia.
Materials and Methods
Study sites
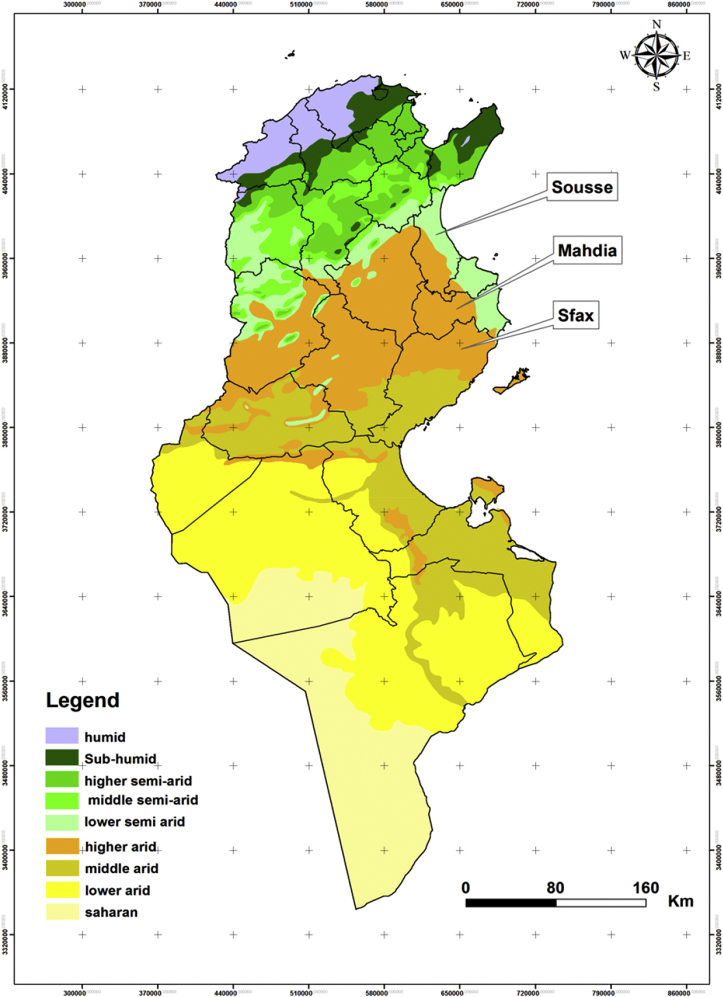
Tunisia covers a wide climatic range, from a Mediterranean climate, with its rainy winter, in the north to a Saharan climate in the south (Fig. 1). The northern part of the country is separated from the south by the Tunisian Ridge, a range of hills running northeast to southwest for approximately 220 km, marking the climatic boundary between the Mediterranean Sea to the north and the dry steppes of central Tunisia. Between the northern slopes of the Tunisian Ridge and the chains of hills bounding it on the south are extensive plateaus, called the High Tell. The Sahara is separated from the central steppe land by a series of salted areas called chotts.
Fig. 1.
Bioclimatic map of Tunisia showing sampling sites, with governorates from which samples were derived for this study highlighted.
Sample collection
Samples were collected from patients seeking care at hospitals who had reported unexplained acute fever (n = 181). Samples were also actively taken from abattoir workers (n = 38) in order to survey evidence of previous exposure amongst groups designated to be at high risk of infection. Most participants originated from the governorates of Sousse, Sfax and Mahdia (Fig. 1), and samples were collected during the summer of 2014. All participants were asked for history of tick and/or mosquito bites. Samples were collected by Vacutainer, and plasma was separated, frozen at −20°C and transported to Public Health England Porton Down, UK, for analysis. The study was conducted after receipt of ethical approval from the ethical committee (HHS-IRB 00008931; Farhat Hached University Hospital, protocol reference date 8 April 2013).
RNA extraction and PCR
Nucleic acid extraction was performed using a QiaAmp Viral RNA Mini kit (Qiagen) according to the manufacturer's instructions. Purified RNA was stored at −20°C until required. Amplification was performed using a previously published assay [18] as follows: forward: 5′-AAAGGAACAATGGACTCTGGTCA-3′; reverse: 5′-CACTTCTTACTACCATGTCCTCCAAT-3′, probe 5′-6FAM AAAGCTTTGATATCTCTCAGTGCCCCAA BHQ1-3′. Reverse transcriptase (RT)-PCR assays were conducted in one step using the Superscript III quantitative real-time PCR (qRT-PCR) kit (Life Technologies) according to the manufacturer’s instructions with cycling conditions of 50°C for 10 minutes and 95°C for 2 minutes, followed by 40 cycles of 95°C for 10 seconds and 60°C for 40 seconds, followed by holding at 40°C for 2 minutes. Data acquisition was performed using the ABI 7500 Real-Time PCR machine (Applied Biosystems) with 45 analysed cycles with a threshold of 0.05 and data analysed using the ABI 7500 on-board software.
Indirect immunofluorescence assay
Sera were tested for the presence of antibodies reactive to RVFv using commercially available indirect immunofluorescence testing kits (Euroimmun). All serum samples were tested for the presence of RVFv by qRT-PCR before serologic testing under Containment Level 2 conditions. In brief, sera samples were diluted 1/100 and incubated on irradiated/fixed RVFv infected and noninfected Vero cells for 60 minutes at room temperature before washing five times for 5 minutes in sample buffer including 0.1% Tween 20. Antibodies binding to the infected cells were detected and measured through a secondary antibody labelled with fluorescein isothiocyanate; 1/100 was set as the minimum cutoff. For immunoglobulin (Ig) M measurement, the process was modified by initial dilution of sera in EUROSORB IgG removal reagent (Euroimmun) before commencing the protocol as before. Slides were mounted in mounting media and viewed under a fluorescent microscope at excitation/emission peaks of 495/517 nm, respectively. Positive and negative controls provided with the commercial kit were tested to provide a benchmark to which samples were compared.
Results
Samples collected from patients with acute fever were initially tested according to local protocols for serologic reactivity to Brucella, Rickettsia and West Nile virus and the presence of these agents through blood and urine cultures. All samples in this study were shown to be negative during this testing phase and were sent to Public Health England Porton Down to be analysed for Rift Valley fever by both molecular and serologic testing.
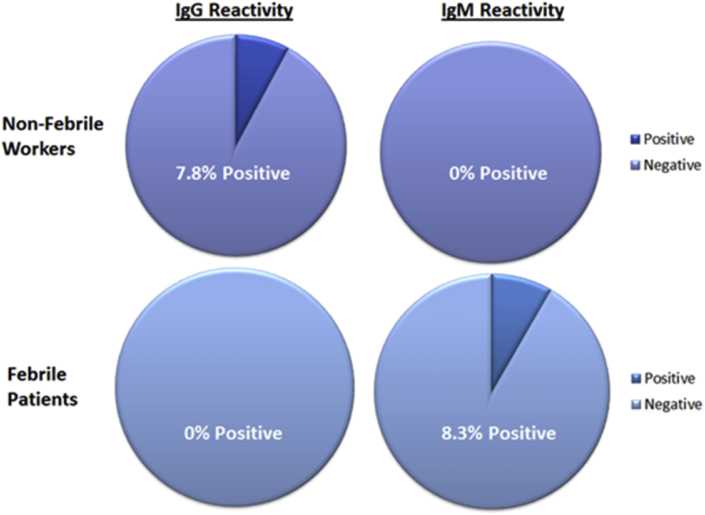
In total 219 samples were tested by qRT-PCR and indirect immunofluorescence to ascertain the level of exposure in the study population. Commercial immunofluorescence controls were tested to provide a benchmark to which samples could be compared. The commercial immunofluorescence assay test kit utilized in this study is European Community (CE) marked as a diagnostic kit and has been validated in house for diagnostic use against a panel of positive and negative samples. The presence of RVFv was not detected by qRT-PCR in either cohort; however, 7.8% of the tested nonfebrile cohort showed IgG serologic reactivity (n = 3) to RVFv at the screening dilution, a reaction shown to be repeatable on retesting (Fig. 2). Furthermore, 8.3% of febrile patients were found to have circulating IgM reactive to RVFv (n = 15). Samples which tested positive by IgG and IgM were titrated to 1/10 000 dilutions in phosphate-buffered saline to evaluate the abundance of reactive antibody.
Fig. 2.
Representation of percentage of immunoglobulin (Ig) G–positive and IgM-positive samples detected in febrile patient cohort collected at presentation to hospital, and nonfebrile worker cohort collected actively from volunteers in rural areas. Data show similar levels of IgG positivity amongst nonfebrile patients compared to IgM in febrile patients, 7.8% IgG and 8.3% IgM.
The 18 serologically reactive samples were derived from patients and participants in the governorates of Sousse, Mahdia and Sfax, areas highlighted in the bioclimatic map comprising Fig. 1. Fifteen samples drawn from febrile patients showed only IgM reactivity. In contrast, three seropositive samples drawn from afebrile farmers and abattoir workers showed only IgG reactivity, with IgM absent (Table 1). Clinical details were taken for all samples collected during this study, in line with normal clinical practice in Tunisia, including travel history.
Table 1.
Positive samples, cohort and associated results
| Sample ID | Type | IgG | IgM | Confirmation | PCR | Outcome | Origin |
|---|---|---|---|---|---|---|---|
| TUN/008 | Farmer Sera | 1:100 | Negative | IgG Positive | Negative | RVF IgG Positive | Sfax |
| TUN/026 | Farmer Sera | 1:100 | Negative | IgG Positive | Negative | RVF IgG Positive | Sousse |
| TUN/028 | Farmer Sera | 1:100 | Negative | IgG Positive | Negative | RVF IgG Positive | Sousse |
| TUN/042 | Febrile Patient | Negative | 1:10000 | IgM Positive | Negative | RVF IgM Positive | Sousse |
| TUN/048 | Febrile Patient | Negative | 1:100 | IgM Positive | Negative | RVF IgM Positive | Sousse |
| TUN/053 | Febrile Patient | Negative | 1:100 | IgM Positive | Negative | RVF IgM Positive | Sfax |
| TUN/078 | Febrile Patient | Negative | 1:100 | IgM Positive | Negative | RVF IgM Positive | Sfax |
| TUN/083 | Febrile Patient | Negative | 1:100 | IgM Positive | Negative | RVF IgM Positive | Sousse |
| TUN/086 | Febrile Patient | Negative | 1:100 | IgM Positive | Negative | RVF IgM Positive | Sousse |
| TUN/089 | Febrile Patient | Negative | 1:100 | IgM Positive | Negative | RVF IgM Positive | Sousse |
| TUN/094 | Febrile Patient | Negative | 1:100 | IgM Positive | Negative | RVF IgM Positive | Sfax |
| TUN/102 | Febrile Patient | Negative | 1:100 | IgM Positive | Negative | RVF IgM Positive | Mahdia |
| TUN/107 | Febrile Patient | Negative | 1:100 | IgM Positive | Negative | RVF IgM Positive | Sousse |
| TUN/120 | Febrile Patient | Negative | 1:100 | IgM Positive | Negative | RVF IgM Positive | Sousse |
| TUN/123 | Febrile Patient | Negative | 1:100 | IgM Positive | Negative | RVF IgM Positive | Sousse |
| TUN/129 | Febrile Patient | Negative | 1:100 | IgM Positive | Negative | RVF IgM Positive | Sousse |
| TUN/135 | Febrile Patient | Negative | 1:100 | IgM Positive | Negative | RVF IgM Positive | Sousse |
| TUN/188 | Febrile Patient | Negative | 1:100 | IgM Positive | Negative | RVF IgM Positive | Sousse |
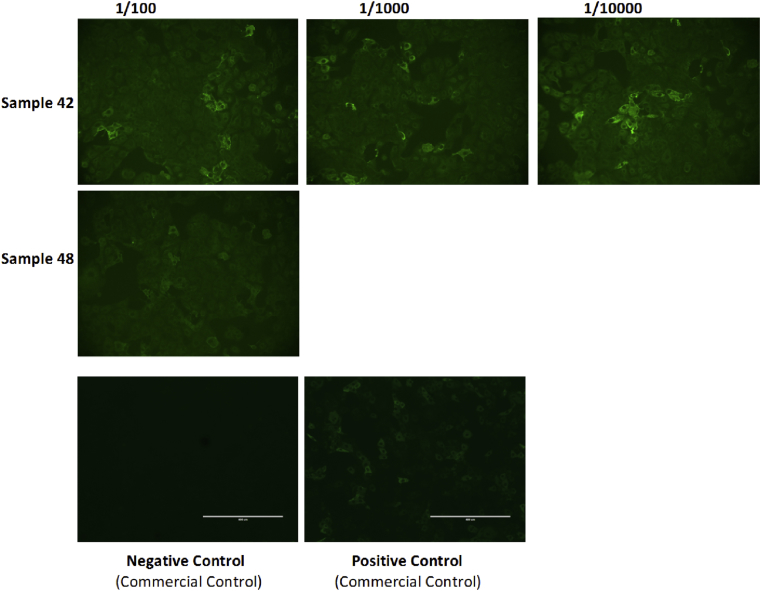
Two samples were titrated beyond the screening dilution (Fig. 3). Sample 48 showed some fluorescence at 1/1000 dilution; however, on retest, it failed to titrate beyond 1/100. Sample 42 titrated beyond this, to 1/10 000, making this the strongest IgM reaction detected in this study.
Fig. 3.
Immunofluorescence showing level of fluorescence across several titrations up to dilution 1/10 000 for sample 42 and 1/100 for sample 48. Images of positive and negative controls tested are also shown.
Discussion
Our results strongly suggest the presence of active RVFv circulation in Tunisia, confirming our expectation from a recent appraisal of North Africa for the relative risk of harbouring circulating RVFv that was recently undertaken [15]. This finding is not unexpected, with RVFv also being found in northwestern Africa [8], [11]. Tunisia is notable for its close proximity to European Mediterranean countries and the migratory patterns of birds through the country leading northwards into Europe [11]. There is also evidence of RVFv exposure in bird populations [19], which are a potential zoonotic risk for vector-borne disease. For example, birds infested with Hyalomma marginatum, the principal vector of Crimean-Congo haemorrhagic fever virus, have been detected as far north as the United Kingdom [20]. Accumulation of surface water and changing surface topology affect bird migratory routes and vector population size; both factors result in a high risk of an increase in prevalence of zoonotic disease [16]. The potential for cross-border transmission through the exchange of economically important animal species from neighbouring countries may also result in the importation of zoonotic pathogens at the wildlife–domesticated livestock interface. Circulation of West Nile virus in the region is recognized, and investigations have suggested an increasing prevalence which may be attributable to anthropogenic environmental changes [21]. These events illustrate the need for improved and comprehensive surveillance in areas where environmental change is recognized to track relative risk to human and animal health to arthropod-borne diseases such as RVFv and West Nile virus.
Rift Valley fever, while primarily being transmissible through the bite of the mosquito vector species, may also be transmitted through direct contact with blood. There are several anecdotal reports of veterinary workers engaged in animal necropsies being exposed to high levels of RVFv during the course of their duties [22], and surveillance activities have detected prevalence of reactive antibody and the virus itself in abattoir and slaughterhouse workers [7], [14]. Critical to the control measures imposed during the outbreak in Saudi Arabia in 2000 was the education of agricultural workers to avoid close contact sick animals and consumption of diseased meat in order to reduce risk of spread through direct contact [9]. This risk may be substantially increased during certain religious festivals in Tunisia, where the slaughter of domestic ruminants by individual families disseminates the risk to a much wider population. It is notable that in our study no abattoir or agricultural workers sampled tested positive for IgM, which is indicative of recent exposure; instead, they only showed signs of previous historical exposure. However, this may be an artefact of our results resulting from the small numbers of samples collected from these populations.
Other countries perceived as being at high risk of RVFv outbreaks are neighbouring Algeria and Libya [15]. Travel and trade across the border are common and risk disease importation. The strong IgM response detected in the course of this study is indicative of a recent and strong reaction to exposure to RVFv or a serologically related virus. It is notable that there was a lack of IgG response seen in any sample from febrile patients, suggesting collection soon after exposure before detectable IgG appearing or nonspecific reactivity with a closely related Phlebovirus. Additionally, the lack of a detectable virus genome complicates confirmatory testing, something which may be attributed to sampling after the viraemic stage of disease has occurred [23]. Detailed medical records were collected for all patients and high-risk participants in this study. Of the 18 seroreactive cases, records indicated that none had travelled abroad in the 2 months preceding sampling. Additionally there was no identifiable link between seropositive abattoir workers and the importation of livestock from abroad for slaughter, as the peak of importation occurred after the sampling period. Even so, the possibility remains of a case introduced through travel or movement of livestock in a country with optimal conditions for an outbreak.
This study provides evidence to support more detailed and thorough surveillance activities for RVFv in order to conclusively demonstrate whether these cases are autochthonous findings and to survey its prevalence in the region’s indigenous mosquito populations.
Conclusion
Because of the potential for RVFv to create a devastating agricultural burden, its control becomes critically important when it is discovered to be circulating in a region. A campaign of active surveillance, prevention strategies, increased research and vaccination of livestock all play a role in the control of RVFv and serve not only to reduce the potential economic burden but also, by interrupting the virus transmission amongst livestock, to interrupt transmission across the human–livestock interface, thus significantly reducing the risk to public health. Active collaboration between public health and animal health institutions of Tunisia is critical, as is increasing cohesive planning with the institutes of neighbouring countries. Though our study is limited in scope, we hope that it provides a foundation for further surveillance and investigation of RVFv in Tunisia.
Acknowledgements
Supported in part by a grant from the US Civilian Research and Development Foundation (CRDF) (16953) and with funding from the UK Biological Engagement Programme (UKBEP). The authors thank N. A. Sammour and D. Elliott, project managers, CRDF and UKBEP, respectively, for enabling this collaboration and providing funding for this work. The views expressed are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, the Department of Health, Public Health England or their partner institutions in Tunisia.
Conflict of Interest
None declared.
References
- 1.Daubney R., Hudson J.R., Garnham P.C. Enzootic hepatitis or Rift Valley fever. An undescribed virus disease of sheep cattle and man from East Africa. J Pathol. 1931;34:545–579. [Google Scholar]
- 2.Dar O., McIntyre S., Hogarth S., Heymann D. Rift Valley fever and a new paradigm of research and development for zoonotic disease control. Emerg Infect Dis. 2013;19:189–193. doi: 10.3201/eid1902.120941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chevalier V., de la Rocque S., Baldet T., Vial L., Roger F. Epidemiological processes involved in the emergence of vector-borne diseases: West Nile fever, Rift Valley fever, Japanese encephalitis and Crimean-Congo haemorrhagic fever. Rev Sci Tech. 2004;23:535–555. doi: 10.20506/rst.23.2.1505. [DOI] [PubMed] [Google Scholar]
- 4.Olive M.M., Goodman S.M., Reynes J.M. The role of wild mammals in the maintenance of Rift Valley fever virus. J Wildl Dis. 2012;48:241–266. doi: 10.7589/0090-3558-48.2.241. [DOI] [PubMed] [Google Scholar]
- 5.Arthur R.R., el-Sharkawy M.S., Cope S.E., Botros B.A., Oun S., Morrill J.C. Recurrence of Rift Valley fever in Egypt. Lancet. 1993;342(8880):1149–1150. doi: 10.1016/0140-6736(93)92128-g. [DOI] [PubMed] [Google Scholar]
- 6.Bukbuk D.N., Fukushi S., Tani H., Yoshikawa T., Taniguchi S., Iha K. Development and validation of serological assays for viral hemorrhagic fevers and determination of the prevalence of Rift Valley fever in Borno State, Nigeria. Trans R Soc Trop Med Hyg. 2014;108(12):768–773. doi: 10.1093/trstmh/tru163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durand J.P., Bouloy M., Richecoeur L., Peyrefitte C.N., Tolou H. Rift Valley fever virus infection among French troops in Chad. Emerg Infect Dis. 2003;9:751–752. doi: 10.3201/eid0906.020647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faye O., Ba H., Ba Y., Freire C.C., Faye O., Ndiaye O. Reemergence of Rift Valley fever, Mauritania, 2010. Emerg Infect Dis. 2014;20(2):300–303. doi: 10.3201/eid2002.130996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassan O.A., Ahlm C., Evander M. A need for one health approach—lessons learned from outbreaks of Rift Valley fever in Saudi Arabia and Sudan. Infect Ecol Epidemiol. 2014;4 doi: 10.3402/iee.v4.20710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turell M.J., Wilson W.C., Bennett K.E. Potential for North American mosquitoes (Diptera: Culicidae) to transmit Rift Valley fever virus. J Med Entomol. 2010;47:884–889. doi: 10.1603/me10007. [DOI] [PubMed] [Google Scholar]
- 11.Chevalier V. Relevance of Rift Valley fever to public health in the European Union. Clin Microbiol Infect. 2013;19:705–708. doi: 10.1111/1469-0691.12163. [DOI] [PubMed] [Google Scholar]
- 12.Shieh W.J., Paddock C.D., Lederman E. Pathologic studies on suspect animal and human cases of Rift Valley fever from an outbreak in Eastern Africa, 2006–2007. Am J Trop Med Hyg. 2010;83(2 suppl.):38–42. doi: 10.4269/ajtmh.2010.09-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coetzer J.A. The pathology of Rift Valley fever. II. Lesions occurring in field cases in adult cattle, calves and aborted foetuses. Onderstepoort J Vet Res. 1982;49:11–17. [PubMed] [Google Scholar]
- 14.Abu-Elyazeed R., el-Sharkawy S., Olson J., Botros B., Soliman A., Salib A. Prevalence of anti-Rift-Valley-fever IgM antibody in abattoir workers in the Nile delta during the 1993 outbreak in Egypt. Bull World Health Organ. 1996;74(2):155–158. [PMC free article] [PubMed] [Google Scholar]
- 15.Arsevska E., Hellal J., Mejri S., Hammami S., Marianneau P., Calavas D. Identifying areas suitable for the occurrence of Rift Valley fever in North Africa: implications for surveillance. Transbound Emerg Dis. 2015 Feb 6 doi: 10.1111/tbed.12331. In press. [DOI] [PubMed] [Google Scholar]
- 16.Ayari-Fakhfakh E., Ghram A., Bouattour A., Larbi I., Gribâa-Dridi L., Kwiatek O. First serological investigation of peste-des-petits-ruminants and Rift Valley fever in Tunisia. Vet J. 2011;187(3):402–404. doi: 10.1016/j.tvjl.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Barhoumi W., Qualls W.A., Archer R.S., Fuller D.O., Chelbi I., Cherni S. Irrigation in the arid regions of Tunisia impacts the abundance and apparent density of sand fly vectors of Leishmania infantum. Acta Trop. 2015;141(pt A):73–78. doi: 10.1016/j.actatropica.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drosten C., Göttig S., Schilling S., Asper M., Panning M., Schmitz H. Rapid detection and quantification of RNA of Ebola and Marburg viruses, Lassa virus, Crimean-Congo hemorrhagic fever virus, Rift Valley fever virus, dengue virus, and yellow fever virus by real-time reverse transcription–PCR. J Clin Microbiol. 2002;40(7):2323–2330. doi: 10.1128/JCM.40.7.2323-2330.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies F.G., Addy P.A. Rift Valley fever. A survey for antibody to the virus in bird species commonly found in situations considered to be enzootic. Trans R Soc Trop Med Hyg. 1979;73:584–585. doi: 10.1016/0035-9203(79)90059-2. [DOI] [PubMed] [Google Scholar]
- 20.Jameson L.J., Morgan P.J., Medlock J.M., Watola G., Vaux A.G. Importation of Hyalomma marginatum, vector of Crimean-Congo haemorrhagic fever virus, into the United Kingdom by migratory birds. Ticks Tick Borne Dis. 2012;3:95–99. doi: 10.1016/j.ttbdis.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Feki I., Marrakchi C., Ben Hmida M., Belahsen F., Ben Jemaa M., Maaloul I. Epidemic West Nile virus encephalitis in Tunisia. Neuroepidemiology. 2005;24(1–2):1–7. doi: 10.1159/000081042. [DOI] [PubMed] [Google Scholar]
- 22.Brett N.A., Juno T., Jacqueline W., Ayanda C., Dadja E., Charlene J. Epidemiologic investigations into outbreaks of Rift Valley fever in humans, South Africa, 2008–2011. Emerg Infect Dis J. 2013;19:1918. doi: 10.3201/eid1912.121527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mansfield K.L., Banyard A.C., McElhinney L., Johnson N., Horton D.L., Hernández-Triana L.M. Rift Valley fever virus: a review of diagnosis and vaccination, and implications for emergence in Europe. Vaccine. 2015;33(42):5520–5531. doi: 10.1016/j.vaccine.2015.08.020. [DOI] [PubMed] [Google Scholar]