Abstract
Natural killer (NK) cells are traditionally regarded as first-line effectors of the innate immune response, but they also have a distinct role in chronic infection. Here, we review the role of NK cells against hepatitis C virus (HCV) and hepatitis B virus (HBV), two agents that cause acute and chronic hepatitis in humans. Interest in NK cells was initially sparked by genetic studies that demonstrated an association between NK cell–related genes and the outcome of HCV infection. Viral hepatitis also provides a model to study the NK cell response to both endogenous and exogenous type I interferon (IFN). Levels of IFN-stimulated genes increase in both acute and chronic HCV infection and pegylated IFNα has been the mainstay of HCV and HBV treatment for decades. In chronic viral hepatitis, NK cells display decreased production of antiviral cytokines. This phenotype is found in both HCV and HBV infection but is induced by different mechanisms. Potent antivirals now provide the opportunity to study the reversibility of the suppressed cytokine production of NK cells in comparison with the antigen-induced defect in IFNγ and tumor necrosis factor-α production of virus-specific T cells. This has implications for immune reconstitution in other conditions of chronic inflammation and immune exhaustion, such as human immunodeficiency virus infection and cancer.
Keywords: HBV, HCV, Infection, Interferon, T Cell
Abbreviations used in this paper: CMV, cytomegalovirus; HBV, hepatitis B virus; HCV, hepatitis C virus; HLA, human leukocyte antigen; IFN, interferon; IL, interleukin; ILC, innate lymphoid cells; ISG, interferon-stimulated gene; KIR, killer-cell immunoglobulin-like receptor; LCMV, lymphocytic choriomeningitis virus; MHC, major histocompatibility complex; NCR, natural cytotoxicity receptor; NK, natural killer; STAT, signal transducer and activator of transcription; TGFβ, transforming growth factor β; TLR, Toll-like receptor; TNFα, tumor necrosis factor-α; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; TRAIL-R2, TRAIL death receptor 2
Summary.
This review focuses on the role of natural killer (NK) cells in acute and chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections and on cytokine-mediated mechanisms that contribute to alterations in NK cell phenotype and function.
Natural killer (NK) cells were identified in 1975 as large granular lymphocytes with an ability to kill without priming target cells that do not express major histocompatibility complexes (MHCs).1, 2 With the recent discovery of innate helper-like cells, NK cells are now considered part of the family of innate lymphoid cells (ILCs), a classification of innate immune cells that mirrors that of CD8 and CD4 T cells in the adaptive immune system. NK cells represent the cytotoxic arm of the ILC family and share expression of the transcription factor eomesodermin with cytotoxic CD8 T cells. Three groups of eomesodermin-negative helper-like ILCs are the innate counterparts to adaptive CD4 T cells. ILC1 express the transcription factor Tbet, have a TH1-like cytokine profile, and provides immunity against intracellular bacteria and parasites. ILC2s express the transcription factor GATA-3. Their cytokine profile of interleukin-4 (IL-4), IL-5, IL-9, and IL-13, and play a role in allergies and antihelminth responses that resembles that of TH2 cells. ILC3 express RORγt and is the counterpart of TH3 cells.3, 4, 5 An additional classification differentiates between conventional NK cells and tissue-resident NK cells. Liver-resident NK cells use Tbet rather than eomesodermin and are only weakly cytotoxic. As strong tumor necrosis factor-α (TNFα) producers, they are closer to ILC1 than to conventional NK cells.6, 7 They were initially described in mice, but an equivalent population of liver-resident NK cells has recently also been reported in humans.8
In viral infections, NK cells exert rapid innate responses by exerting cytotoxicity against infected target cells and by releasing antiviral cytokines. By killing immature dendritic cells and secreting proinflammatory cytokines and chemokines, NK cells support the priming of T cells and orchestrate the recruitment of other immune cells to the site of infection.9 These mechanisms enhance the adaptive arm of the immune response, which ultimately clears the infection and provides immune memory and protection upon reinfection. This classic division of labor between the innate and adaptive immune systems has recently been blurred, with some NK cells exhibiting features of adaptive cells, such as antigen-specific clonal expansion and contraction and development of long-lived memory. This feature was initially described for a subpopulation of liver-resident NK cells in mice that confers transferrable immunity against chemical haptens and viruses.10, 11 Further, mouse cytomegalovirus (CMV) is now known to induce memory NK cells via interaction of the viral glycoprotein with an NK cell receptor,12 and human CMV has been shown to induce oligoclonal expansions and epigenetic modifications of human NK cells with memory-like functions.13, 14, 15 A detailed review of the mechanisms of NK cell memory has been published elsewhere.16
NK cell responses are controlled by a large number of activating and inhibitory cell surface receptors. Activating receptors include natural cytotoxicity receptors (NKp30, NKp44, and NKp46), lectin-like receptors (NKG2C, NKG2D) that are expressed as dimers with CD94, and signaling lymphocyte activation molecule (SLAM) family receptors (2B4, CRACC, NTB-A), among others.17 Inhibitory receptors include killer-cell immunoglobulin-like receptors (KIRs) and NKG2A/CD94. Receptors are expressed in a combinatorial manner, which creates an estimated 6000 to 30,000 phenotypically distinct NK cell subpopulations in the blood of each individual and an even larger diversity among individuals.18
In the absence of infection, inflammation, and other diseases, NK cells mostly receive inhibitory signals. Expression of inhibitory receptors is genetically determined.18 It is thought that KIRs have coevolved with their ligands, the human leukocyte antigens (HLA), to ensure maintenance of self-tolerance. NK cells become activated when signals from inhibitory receptors decrease, such as when the expression of KIR-binding MHC molecules on virus-infected cells decreases, or when signals from activating receptors increase, such as when antibody-coated viral antigens and/or stress-induced ligands on infected cells are recognized. NK cells also respond to inflammatory cytokines such as type I interferons (IFNα and IFNβ), IL-2, IL-12, IL-15 and IL-18 that are commonly released in response to virus infections.19 NK cell activation increases the expression level of activating receptors,18 thereby allowing NK cells to acquire a more responsive state in the context of infection and inflammation.
This review discusses evidence for an antiviral role of NK cells in hepatitis C virus (HCV) infection, and then describes alterations of NK cell function in chronic HCV and hepatitis B virus (HBV) infection that are consistent with an increased regulatory role at the expense of antiviral function. The reversibility of this phenotype is discussed.
What Is the Role of Natural Killer Cells in Subclinical and Acute Hepatitis C Virus Infection?
Hepatitis C virus (HCV) is an RNA virus of the Flaviviridae family that establishes persistent infection in the majority of infected patients. A potential role for NK cells in viral hepatitis was first suggested by genetic studies that described a higher odds ratio of spontaneous HCV clearance20 and IFN-treatment-induced HCV clearance21 in KIR2DL3+ patients who are homozygous for HLA-C1 alleles as compared with patients who are homozygous or heterozygous for HLA-C2 alleles. HLA-C1 and HLA-C2 represent two groups of HLA-C alleles that differ in two amino acids in their respective HLA-Cw α1 domains. Because the interaction between KIRs on NK cells with HLA molecules on target cells plays a key role in NK cell inhibition, it has been suggested that the KIR2DL3/HLA-C1 compound genotype results in a lower activation threshold of NK cells, thereby allowing faster NK cell activation compared with less favorable genotypes. This is supported by data in an in vitro influenza A virus infection model that demonstrate a larger HLA-C–regulated NK cell subset with more rapid NK cell IFN-γ secretion and cytotoxicity in HLA-C1 than in HLA-C2 homozygous patients.22
An increased prevalence of KIR2DL3/HLA-C1 homozygosity is also observed in injection drug users who remain aviremic and antibody-negative despite high-risk behavior and frequent HCV exposure.21 The apparent immune protection in such individuals is associated with KIR2DL3 expression on NK cells23 and with an increased frequency of activated NK cells.24, 25 At the functional level, NK cells in the blood of exposed uninfected individuals display increased ex vivo IFNγ production24 and increased in vitro cytotoxicity.25 These results from cross-sectional cohorts are consistent with data from a prospective study of health care workers observed after an accidental needlestick.26 Accidental exposure to minute amounts of HCV-containing blood resulted in a transient increase the frequency of activated NK cells in the blood and their effector functions (both cytotoxicity and IFNγ production). The magnitude of the NK cell response correlated with that of the subsequent HCV-specific T-cell response. This likely represents an early innate response to an abortive or rapidly contained and cleared infection, because neither viremia nor HCV-specific antibodies are detected.26
Collectively, these studies demonstrate that NK cells are sensitive biomarkers of subclinical HCV exposure. While it is possible that NK cells—along with other components of the innate immune system—contribute to viral containment in this setting, it is obvious that innate immune responses on their own cannot clear the infection once high-level HCV viremia is established. Data from prospectively studied humans and experimentally infected chimpanzees demonstrate that high-level HCV viremia persists for weeks despite induction of a large set of intrahepatic interferon-stimulated genes (ISGs).27, 28 This immune response is initiated in the cytoplasm and in endosomes of infected cells by the pattern recognition receptors protein kinase, retinoic acid inducible gene-I, and toll-like receptor 3 (TLR3).29 Downstream signals, mediated by interferon regulatory factor 3 (IRF3) and nuclear factor-κB, result in the transcription of the IFNβ gene. IFNβ is released from infected cells, binds to the IFNα/β receptor (IFNAR1 and IFNAR2) on neighboring cells, and induces a diverse ISG set that includes many antiviral and proinflammatory genes.30 However, owing to HCV’s elaborate strategies to escape from IFN responses,29, 31 there is no decrease in viremia, just a plateau. Patients are typically clinically asymptomatic during this period and do not seek medical attention.
The onset of clinically symptomatic acute hepatitis with increased alanine aminotransferase levels occurs 8 to 10 weeks after infection. Without treatment, two-thirds of the infected patients develop chronic hepatitis C, which is associated with a 2–3 log10 reduction in viral titer. Because liver biopsies are clinically not indicated in the acute phase of hepatitis C, the intrahepatic effector responses responsible for the decrease in viremia have not been studied in patients. However, data from biopsy tissues of experimentally infected chimpanzees have clearly shown that the decrease in viremia coincides with an increase in intrahepatic IFNγ-mRNA levels.27, 28, 32
The relative contribution of T cells and NK cells to IFNγ production and antiviral response is not known at this time. Whereas the appearance and maintenance of HCV-specific T-cell responses in the blood, in particular CD4 T-cell proliferation and cytokine production, are the best predictors of viral clearance,32, 33, 34, 35, 36, 37 NK cells are also activated and display increased cytotoxicity and IFNγ production.38, 39, 40 Pelletier et al39 recently reported a correlation between the magnitude of T-cell response and the peripheral blood NK cell response in the acute phase of HCV infection, and Kokordelis et al40 found that NK cells from patients who later cleared the infection have a greater antiviral effect in vitro than NK cells from patients who developed chronic HCV infection. This opens the interesting question of whether the increased NK cell activity in acute HCV infection is an independent event or is triggered by CD4 T-cell–derived IL-2. The latter would render NK cells amplifiers and even downstream effectors of the virus-specific T-cell response.
How Does Chronic Hepatitis C Virus infection Alter Natural Killer Cell Function?
NK cells are activated not just in acute but also in chronic HCV infection. They express increased levels of CD69 and HLA-DR, indicating recent and more distant stimulation, respectively, and increased levels of NKp30,41 NKp44,22 NKp46,22, 42, 43 NKG2A, NKG2D,44 and the IL-2 receptor β chain CD122.22 Increased NKG2C expression has also been reported,22, 44 but has now been attributed to oligoclonal expansion of a highly differentiated NK cell subset during prior HCMV infection.45 NK cell activation is influenced by location, as NK cells are generally more activated in liver than in blood, even in uninfected individuals.41 NK cell activation is also influenced by additional factors; for example, NKp46 levels are strongly associated with female gender and Caucasian race.46
While exciting new information on liver-resident NK cells and NK cell memory is emerging in the general NK cell field, data on these topics are still sparse in HCV infection. This is mostly due to the limited number (about 100,000) of lymphocytes that can be isolated from subcutaneous liver biopsy tissues and the lack of liver tissue from uninfected controls. Only a single study performed a side-by-side comparison of intrahepatic NK cells from surgically resected liver tissue of patients with HCV infection and uninfected controls (who underwent cholecystectomy).41 In that study, HCV infection was associated with an increased frequency (compared with uninfected livers) of intrahepatic NK cells that shared some phenotypic and functional features with the unique liver-resident NK cell population that has been proposed to represent the human equivalent to mouse memory NK cells in subsequent studies.8, 47 This raises the interesting, but as yet unstudied question of whether HCV infection affects NK cells with memory functions in the liver.
Chronic HCV infection leaves a distinct signature also on peripheral blood NK cells, which has now been confirmed by several independent studies. Most remarkable is a dichotomy in effector functions that is characterized by increased cytotoxicity (evidenced by increased degranulation and production of tumor necrosis factor-related apoptosis-inducing ligand [TRAIL]) and, upon in vitro stimulation with IL-12/IL-15 or IL-12/IL-18, decreased production of antiviral cytokines (IFNγ and TNFα).22, 44, 48, 49 This is unexpected because the size of the CD56bright subset, which constitutes about 10% of peripheral blood NK cells in uninfected individuals, doubles in chronic HCV infection. CD56bright NK cells are known to produce large amounts of IFNγ and TRAIL with little perforin/granzyme-mediated cytotoxicity.50, 51 Most of the remaining NK cells belong to the more mature CD56dim subset. These cells contain high levels of perforin and granzyme, but can also rapidly produce chemokines and cytokines.50 The altered subset distribution and overall decrease in the number of intrahepatic and blood NK cells44, 48, 52, 53 should therefore not result in the selective decrease in cytokine production.
What is the mechanism underlying these divergent effector functions? Increasing signaling via NKp30, NKp44, and NKp46 may directly contribute to the increase in NK cell cytotoxicity because these molecules are natural cytotoxicity receptors (NCRs) and NCR+ NK cells have been shown to release more perforin/granzyme containing granules and exert greater in vitro antiviral effect than NCR− NK cells.40, 46 Increased expression of the activating receptor NKG2D and its ligands may also contribute to NK cell cytotoxicity,54 as may decreased expression of the inhibitory receptor NKG2A.
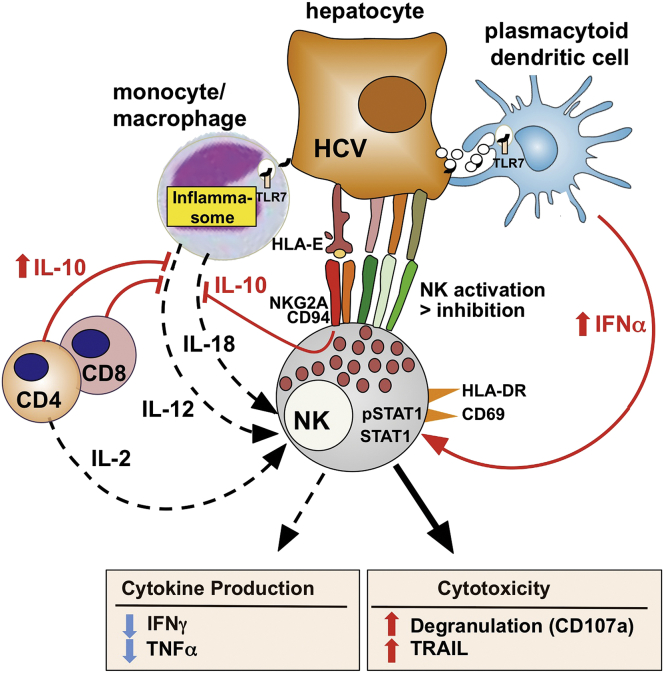
Cytokine-dependent signals are thought to play a major role in the increase in NK cell cytotoxicity and decrease in NK cell cytokine production in chronic HCV infection (Figure 1). This is evidenced by increased ex vivo levels of signal transducer and activator of transcription 1 (STAT1), a key molecule of IFNα signaling in NK cells. Because STAT1 itself is an ISG, increased STAT1 levels are consistent with type I IFN-mediated signaling in chronic HCV infection. This is supported by increased ex vivo levels of phosphorylated STAT1 and decreased levels of phosphorylated STAT4 in NK cells of HCV-infected patients compared with NK cells of uninfected patients.55, 56 Chronic exposure to virus-induced type I IFN can explain the NK cell phenotype of increased cytotoxicity and decreased cytokine production because induction of cytotoxicity and production of IFNγ require differential STAT1/4 signaling.
Figure 1.
Altered natural killer (NK) cell function in chronic hepatitis C virus (HCV) infection. NK cells are activated in HCV infection with greater signaling from activating than inhibitory receptors (see text for details). Polarization of NK cell function toward increased (solid line) cytotoxicity and decreased (dotted line) production of antiviral cytokines22, 44, 55 is driven by type I interferon (IFN), produced by Toll-like receptor 7 (TLR7)-stimulated plasmacytoid dendritic cells62, 63 and other nonparenchymal cells (not pictured). Mechanisms mediated by interleukin-10 (IL-10), which contribute to this NK cell phenotype in hepatitis B virus (HBV) infection, may also be operative in HCV infection because virus-specific T cells that secrete IL-10 have been demonstrated in the liver.113 IL-10 is also directly induced in NK cells via interaction of the inhibitory NKG2A/CD94 receptor with human leukocyte antigen E (HLA-E) on HCV-infected hepatocytes.74 HLA-E is thought to be stabilized by an endogenously processed HCVcore peptide and therefore expressed at increased levels.75 Suboptimal production (dotted line) of IL-18 and IL-2 may further decrease IFNγ production by NK cells. Monocytes from patients with chronic HCV infection produce less IL-18 than monocytes from healthy controls upon coculture with HCV-replicating hepatocytes and NK cells.70 HCV-specific CD4 T-cell responses and IL-2 production are reduced in HCV infection.114
Based on a model initially proposed by Miyagi et al57 for lymphocytic choriomeningitis virus (LCMV) infection, NK cells produce IFNγ in the early phase of a virus infection because of their constitutively high STAT4 expression. Chronic exposure to type I IFN results in increased expression of STAT1, and preferential STAT1 over STAT4 phosphorylation,57 increased STAT1-dependent cytotoxicity (with TRAIL itself being an ISG), and decreased STAT4-dependent IFNγ production.58 The observations of increased pSTAT1/pSTAT4 ratio and increased levels of the ISGs STAT1 and TRAIL in NK cells in chronic HCV infection therefore provide compelling evidence that type I IFN is produced locally in HCV infection even though it is detectable only rarely and at exceedingly low levels in the serum.59, 60, 61
Candidates for type I IFN production are nonparenchymal cells, such as liver-resident macrophages (Kupffer cells), plasmacytoid dendritic cells, and liver sinusoidal endothelial cells. Kupffer cells were identified as a local source of IFNα by immune-staining of liver sections.61 Plasmacytoid dendritic cells were shown to respond to short-range transfer of HCV RNA-containing exosomes from hepatoma cells with TLR7-induced production of type I IFN in in vitro experiments.62, 63 Finally, primary human liver sinusoidal endothelial cells and immortalized liver endothelial cells were shown to produce type I IFN after internalization of HCV and stimulation of TLR7 and retinoic acid-inducible gene 1 (RIG-I).64 The dichotomy of increased cytotoxicity and decreased cytokine production by NK cells is exacerbated when HCV-infected patients undergo IFN-based therapy.55, 65, 66, 67 This NK cell phenotype can be recapitulated when peripheral blood mononuclear cells of uninfected individuals are exposed to IFNα in vitro.22, 66
In addition to the local increase in type I IFN, a relative decrease in IL-12, IL-18, and IL-2 production is thought to contribute to the suppressed cytokine production of NK cells in chronic HCV infection (see Figure 1). HCV is known to activate the inflammasome in monocytes and macrophages in an infection-independent process that requires recognition of viral RNA by endosomal TLR7. This can be achieved by clathrin-mediated uptake and uncoating of HCV particles and release of their RNA in endosomes.68, 69 It can also be achieved by cell-to-cell transfer of RNA as shown in cocultures of primary human monocytes with hepatoma cells that contain replicating subgenomic HCV RNA but do not release infectious HCV particles.70 Inflammasome activation results in secretion of IL-18 and IL-1β in this coculture model,70 and both cytokines are also detected at increased levels in the blood of patients with acute HCV infection.71 IL-18 stimulates IFNγ production and down-regulation of HCV replication by NK cells in cocultures with HCV-replicon cells and monocytes.70 Interestingly, monocytes from healthy IL-18 production and IFNγ-mediated antiviral activity of NK cells improve when monocytes from HCV-infected patients are replaced with monocytes from healthy blood donors in this system.70 These data show that suboptimal monocyte activation and IL-18 production contribute to the defective cytokine production of NK cells in chronic HCV infection. A lack of IL-2 may further contribute to the decreased cytokine production of NK cells. This scenario in chronic HCV infection clearly contrasts with acute HCV infection where serum IL-18 levels peak,71 and where HCV-specific CD4 T-cell responses33, 36 and IFNγ producing NK cell responses40 are strong and predictive of HCV clearance.
In addition to monocytes, monocyte-derived dendritic cells have a reduced cytokine response in HCV infection. Specifically, they produce less IL-15 than those of healthy controls, which results in reduced expression of MHC class I-related chain A and B (MICA/B) and in reduced NK cell stimulation via the MICA/B ligand NKG2D.72, 73 Conversely, NK cells from HCV patients do not activate dendritic cells as much as NK cells from healthy donors.74 This is thought to be due to stabilization of HLA-E on HCV-infected hepatocytes by an endogenously processed HCVcore peptide.74, 75 HLA-E binds to the inhibitory NKG2A/CD94 receptor on NK cells and induces IL-10 and TGF-β production.74
What are the consequences of an NK cell phenotype that is biased toward an increase in cytotoxicity and a decrease in cytokine production? NK cells have been shown to exert antiviral effects both via perforin/granzyme-mediated cytotoxicity and via IFNγ-mediated down-regulation of HCV replication in in vitro models.66, 76, 77 As initially proposed by Guidotti and Chisari,78 a cytokine-mediated antiviral effect may be more efficient than cytotoxicity in the infected liver because secreted cytokines can reach many infected hepatocytes whereas cytotoxicity requires a 1:1 interaction between cells.78 The altered functional phenotype of NK cells in chronic HCV infection may therefore facilitate chronic inflammation via killing of infected cells but not allow viral clearance due to impaired IFNγ production. Because suppressed cytokine secretion is also a key characteristic of the T-cell response in chronic HCV infection,79 this may be a general mechanism to dampen the immune response of the host and to ameliorate disease pathogenesis.
Does the Altered Natural Killer Cell Phenotype Extend to Other Forms of Chronic Hepatitis?
To answer the question whether the altered NK cell phenotype represents a specific mechanism of HCV persistence or a general host response to decrease immunopathology, it is interesting to study NK cells in other viral infections. HBV is a good example because it belongs to a different family of viruses and employs different mechanisms to establish persistence.31 Most HCV infections occur in immune-competent adults whereas most HBV infections occur during the neonatal period and in early childhood when immune responses are less mature and the inflammatory response is lower than in adulthood. One of the most striking differences to HCV is the absence of detectable ISG responses, even in the acute phase of hepatitis.80, 81 This suggests that type I IFN—the main factor that drives the increase in NK cell cytotoxicity and the decrease in IFNγ production in HCV infection—does not play a major role in HBV infection.
However, despite the differential type I IFN response, alterations in the NK cell phenotype and function in HBV infection are strikingly similar to those in HCV infection. The overall percentage of NK cells in the peripheral blood mononuclear cell population is decreased,44, 82, 83 with a relative and absolute increase of the CD56bright NK cell subset.84 Likewise, NK cells display increased expression of activating receptors such as NKp30, NKp46, and NKG2C43 with decreased expression of the inhibitory marker NKG2A.43, 52
As in HCV infection, NK cells of chronic HBV patients have a reduced capacity to produce IFNγ compared with healthy controls.44, 84, 85 Conversely, NK cell cytotoxicity is maintained, and the percentage of TRAIL-expressing CD56bright NK cells is increased. This altered NK cell phenotype is already apparent in pediatric patients after perinatal acquisition of HBV infection from their mothers.86
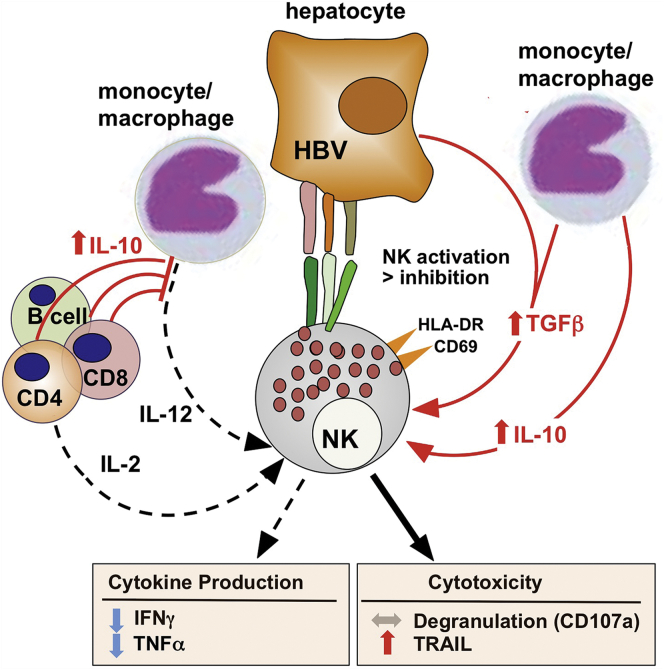
Although NK cell phenotype and function are similar in HCV and HBV infection, the underlying causative mechanisms are different (Figure 2). In HBV infection, alterations in NK cell function appear to be induced by IL-10 and TGFβ rather than IFNα.84 Accordingly, the cytokine-induced dichotomy in NK cell effector functions increases during phases of increased disease activity with increased IL-10 serum levels.84 Some of the IL-10 may be derived from regulatory B cells, which have been demonstrated in HBV infection.87 This NK cell phenotype can be recapitulated by in vitro exposure to IL-10, which suppresses IFNγ production without altering cytotoxicity and IL-10 production. Conversely, blockade of IL-10 and/or TGFβ restores IFNγ production of both CD56bright and CD56dim NK cells in vitro.84
Figure 2.
Altered natural killer (NK) cell function in chronic hepatitis B virus (HBV) infection. Activated NK cells display a similar functional phenotype of increased cytotoxicity (increased tumor necrosis factor-related apoptosis-inducing ligand [TRAIL] production, conserved cytotoxicity) and decreased cytokine production in HBV infection as seen in chronic hepatitis C virus (HCV) infection,44 but it appears to be induced by transforming growth factor β (TGFβ) and interleukin-10 (IL-10) rather than by type I interferon (IFN). Neutralization of TGFβ and IL-10 normalizes NK cell function in vitro.84 IL-10–producing regulatory B cells have been described in HBV infection and may contribute to the phenotype.87 In contrast to HCV infection, increased interferon-stimulated gene (ISG) levels are not a typical feature of chronic HBV infection.81
Thus, the common theme of preserved NK cell cytotoxicity and impaired IFNγ production is found in both chronic HBV and HCV infections. Like HCV, HBV is sensitive to the antiviral effects of IFNγ. IFNγ from adoptively transferred HBV-specific CD8+ T cells88 and from therapeutically activated NK cells has been shown to down-regulate HBV replication in transgenic mouse models.89 The lack of IFNγ production by NK cells in chronic HBV infection may therefore result in reduced viral control and inability to clear HBV, and the preserved cytotoxicity appears to contribute to liver inflammation. Along this line, polymorphisms in the IL-10 promoter are associated with increased IL-10 production, and with increased disease severity and progression in chronic HBV infection.90, 91 Furthermore, liver inflammation correlates with TRAIL expression on NK cells and TRAIL-expressing NK cells are enriched in the liver.92
What could be the reason for the preserved NK cell cytotoxicity in chronic viral hepatitis? NK cell cytotoxicity may serve a regulatory role, aimed at controlling virus-specific T-cell responses. HBV-specific T cells up-regulate TRAIL death receptor 2 (TRAIL-R2), which renders them susceptible to apoptosis by TRAIL-expressing NK cells. Increased TRAIL-R2 expression is specifically observed on intrahepatic HBV-specific CD8+ T cells in close contact with TRAIL-expressing NK cells, and it correlates with the HBV titer.93 In contrast, the majority of T cells of other specificity do not up-regulate TRAIL-R2, which indicates that T-cell receptor stimulation by cognate antigen rather than bystander activation is the underlying mechanism. Indeed, in vitro depletion of NK cells from peripheral blood mononuclear cells of HBV-infected patients increases HBV-specific but not CMV-specific CD8+ T-cell responses.93 A similar regulatory role of NK cells is also found in the mouse model of chronic LCMV-induced hepatitis.94 NK cells specifically kill LCMV-specific CD4 T cells in a perforin-dependent manner, and a reduced CD4 T-cell response is associated with a secondary reduction of the LCMV-specific CD8 T-cell response.94 In addition, activated CD8 T cells become targets of NKG2D+ NK cells because they up-regulate NKG2D ligands in LCMV infection.95 Consequently, depletion of NK cells restores CD4 T-cell help and thereby enhances the LCMV-specific CD8 T-cell response,94 and blocking of the activating receptor NKG2D on NK cells directly increases the frequency of functioning, antigen-specific CD8 T cells.95 It has therefore been proposed that NK cells function as “rheostats” for virus-specific T-cell responses and that therapeutic targeting of NK cells may have differential effects based on the degree of virus-driven T-cell exhaustion.96
Are Altered Immune Functions Reversible by Antiviral Therapy?
The observed down-regulation of IFNγ production by both NK cells and T cells in HCV and HBV infections raises the interesting question of whether their impaired function can be restored by antiviral therapy. As described in the previous sections, the suppression of NK cell IFNγ production is cytokine-driven—that is, driven by an excess of IFNα, IL-10, and transforming growth factor β (TGFβ) and by a relative lack of IL-12, IL-18, and IL-2. In contrast, impaired cytokine production of T cells is attributed to chronic T-cell receptor stimulation by viral antigens.97 Chronic T-cell receptor signaling induces inhibitory molecules such as programmed death 1 (PD-1), T-cell immunoglobulin domain and mucin domain-3 (Tim-3), and cytotoxic T lymphocyte antigen 4 (CTLA-4),28, 98, 99, 100, 101 and promotes an “exhausted” T-cell phenotype that is also observed in diseases such as HIV infection and cancer.102
Antiviral drugs that rapidly clear HCV infection and significantly reduce HBV viremia provide an excellent clinical model to answer the question of whether the described changes in NK cell and T-cell function are reversible. IFN-based treatment regimens are not suitable to answer these questions because IFN exerts not only antiviral, but also immune-modulatory function.103 Specifically, IFN-based therapies exacerbate the functional dichotomy of NK cells toward increased cytotoxic effector functions and reduced IFNγ production,65, 67 suppress proliferation of T-cell and IFNγ production of T cells,104, 105 and do not fully restore T-cell effector function even if they achieve viral clearance.106
In contrast, effective removal of HCV by an IFN-free regimen of direct-acting antivirals normalizes both phenotype and function of NK cells, as now shown in two independent studies on combination therapy with the HCV NS5A inhibitor daclatasvir and the NS3 protease inhibitor asunaprevir.49, 107 Successful treatment of IFN nonresponders with this IFN-free regimen is associated with a rapid decrease of NK cell activation within the first 24 hours of treatment initiation and with normalization of NK cell phenotype and function by week 8 of the 24-week regimen.49 Both NK cell cytotoxicity and IFNγ production reach levels that are indistinguishable from those of healthy controls.49 The concomitant decrease in the frequency of pSTAT1-expressing NK cells confirms the role of virus-induced type I IFN for the altered function of NK cell function in chronic HCV infection.49 This is consistent with a decrease in the expression level of the ISGs RSAD2, ISG15, OAS3, and IFIT1 in the total blood leukocyte population.107
The effect of treatment-induced HCV clearance on the T-cell response is quite different from its effect on NK cells. Spaan et al107 studied HCV-specific T-cell responses in parallel to NK cell responses in five HLA-A2+ patients treated with daclatasvir and asunaprevir. They used dextramers of HCV-peptide loaded HLA-A2 molecules to detect HCV-specific T cells ex vivo irrespective of their function, and they report an increase in the frequency of HCV-specific T cells in the blood at the treatment time point of week 12. This is consistent with an earlier report on an increased ex vivo frequency and improved in vitro proliferation of HCV-specific CD8 T cells in 51 HCV patients who were treated with the protease inhibitor faldaprevir, the HCV NS5B polymerase inhibitor deleobuvir with or without ribavirin.108 CD8 T-cell populations that target the sequence of the infecting virus showed a greater improvement in in vitro proliferation than those that target sequences in which the virus had escaped, thus excluding any bias for preexisting memory T cells. Notably, however, an improved ex vivo T-cell function has not yet been demonstrated in any study. In our own study of asunaprevir/daclatasvir-treated patients,49 we did not observe any recovery of the IFNγ production of HCV-specific T cells when ex vivo Elispot assays with sets of overlapping HCV peptides or minimal optimal T-cell epitopes were performed (B. Rehermann, unpublished results). Combined, these early results suggest that successful IFN-free therapy normalizes phenotype and function of NK cells, and that it restores the proliferative capacity but not necessarily the ex vivo effector function of HCV-specific T cells.
Treatment of HBV infection differs from treatment of HCV infection in the sense that IFN-free HBV-specific antiviral regimens significantly decrease viremia but do not induce complete viral clearance.109 This is because the covalently closed circular form of the HBV DNA persists in infected cells and serves as its transcriptional template. Consequently, only very few patients clear HBsAg with long-term nucleos(t)ide analogue treatment.110 Consistent with suppression but not complete elimination of HBV, antiviral therapy with nucleoside analogues corrects some but not all of the abnormal NK cell parameters. The treatment-induced decrease in HBV titer is paralleled by normalization of the percentage of NK cells in peripheral blood mononuclear cells, the size of the CD56bright NK cell subset, and TRAIL expression on NK cells. This is associated with reduced liver inflammation. However, consistent with low-level HBV persistence, there is no or only partial85 restoration of the NK cells’ capacity to produce IFNγ.
As in HCV infection, restoration of T cell responses in HBV infection is mainly detectable with T cell assays that require more than a week of in vitro stimulation with viral antigens. Restoration of T-cell proliferation is greater in patients who respond to long-term antiviral treatment with suppression of viremia and loss of HBsAg than in those who show a decrease in viremia but remain HBsAg positive.111 While the expanded T-cell lines also displayed detectable IL-2, TNFα, and IFNγ responses upon restimulation with their cognate antigen, T cells remain dysfunctional in ex vivo assays. Furthermore, even in vitro responses are not maintained when treatment is discontinued.112 The T-cell responses of successfully treated patients therefore remain inferior to those of spontaneously recovered patients.
Collectively, these results suggest, that cytokine-induced alterations in NK cell function do normalize when the virus is cleared whereas antigen-induced alterations in T-cell function are not fully reversible. The observation that complete normalization of the NK cell phenotype and function can be achieved in HCV-infected but not in HBV-infected patients fits the observation that HCV can be completely cleared with antiviral treatment whereas HBV persists at low levels.
Conclusions and Implications
Research over the past 10 years has expanded our view on NK cells as both an antiviral and a regulatory immune cell population in viral hepatitis. Chronic HCV and HBV infection impose similar alterations on NK cell phenotype and function. Impairment of IFNγ production is observed for both NK cells and T cells, but induced by different mechanisms. With new direct-acting antiviral treatment regimens now available as standard of care for HCV infection, research into the reversibility of these immune alterations has just begun. An expansion of this new area of research may provide useful information and strategies for immunotherapy of infections such as HBV that cannot be cleared with antiviral drugs alone and require an active immune response. Further research in the emerging field of ILC subtypes, liver-resident NK cells, and NK-cell-mediated memory functions is important to increase our knowledge of the immune surveillance in the liver and may lead to novel strategies for immunotherapy of hepatocellular carcinoma.
Footnotes
Conflicts of interest The author discloses no conflicts.
Funding This study was funded by the intramural research program of NIDDK, National Institutes of Health.
References
- 1.Kiessling R., Klein E., Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells: specificity and distribution according to genotype. Eur J Immunol. 1975;5:112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 2.Kiessling R., Klein E., Pross H. “Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975;5:117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- 3.Eberl G., Colonna M., Di Santo J.P. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science. 2015;348:aaa6566. doi: 10.1126/science.aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Artis D., Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- 5.Diefenbach A. Profiling the diversity of innate lymphoid cells. Nat Immunol. 2015;16:222–224. doi: 10.1038/ni.3107. [DOI] [PubMed] [Google Scholar]
- 6.Gordon S.M., Chaix J., Rupp L.J. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity. 2012;36:55–67. doi: 10.1016/j.immuni.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klose C.S., Flach M., Mohle L. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157:340–356. doi: 10.1016/j.cell.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 8.Marquardt N., Beziat V., Nystrom S. Cutting edge: identification and characterization of human intrahepatic CD49a+ NK cells. J Immunol. 2015;194:2467–2471. doi: 10.4049/jimmunol.1402756. [DOI] [PubMed] [Google Scholar]
- 9.Vivier E., Raulet D.H., Moretta A. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paust S., Gill H.S., Wang B.Z. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11:1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng H., Jiang X., Chen Y. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J Clin Invest. 2013;123:1444–1456. doi: 10.1172/JCI66381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun J.C., Beilke J.N., Lanier L.L. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J., Zhang T., Hwang I. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity. 2015;42:431–442. doi: 10.1016/j.immuni.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luetke-Eversloh M., Hammer Q., Durek P. Human cytomegalovirus drives epigenetic imprinting of the IFNG locus in NKG2Chi natural killer cells. PLoS Pathog. 2014;10:e1004441. doi: 10.1371/journal.ppat.1004441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlums H., Cichocki F., Tesi B. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42:443–456. doi: 10.1016/j.immuni.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beaulieu A.M., Madera S., Sun J.C. Molecular programming of immunological memory in natural killer cells. Adv Exp Med Biol. 2015;850:81–91. doi: 10.1007/978-3-319-15774-0_7. [DOI] [PubMed] [Google Scholar]
- 17.Bryceson Y.T., March M.E., Ljunggren H.G. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horowitz A., Strauss-Albee D.M., Leipold M. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci Transl Med. 2013;5:208ra145. doi: 10.1126/scitranslmed.3006702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biron C.A., Nguyen K.B., Pien G.C. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 20.Khakoo S.I., Thio C.L., Martin M.P. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 21.Knapp S., Warshow U., Ho K.M. A polymorphism in IL28B distinguishes exposed, uninfected individuals from spontaneous resolvers of HCV infection. Gastroenterology. 2011;141:320–325. doi: 10.1053/j.gastro.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahlenstiel G., Titerence R.H., Koh C. Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alfa-dependent manner. Gastroenterology. 2010;138:325–335. doi: 10.1053/j.gastro.2009.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thoens C., Berger C., Trippler M. KIR2DL3+NKG2A− natural killer cells are associated with protection from productive hepatitis C virus infection in people who inject drugs. J Hepatol. 2014;61:475–481. doi: 10.1016/j.jhep.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 24.Sugden P.B., Cameron B., Mina M. Protection against hepatitis C infection via NK cells in highly-exposed uninfected injecting drug users. J Hepatol. 2014;61:738–745. doi: 10.1016/j.jhep.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Golden-Mason L., Cox A.L., Randall J.A. Increased natural killer cell cytotoxicity and NKp30 expression protects against hepatitis C virus infection in high-risk individuals and inhibits replication in vitro. Hepatology. 2010;52:1581–1589. doi: 10.1002/hep.23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werner J.M., Heller T., Gordon A.M. Innate immune responses in hepatitis C virus-exposed healthcare workers who do not develop acute infection. Hepatology. 2013;58:1621–1631. doi: 10.1002/hep.26353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bigger C.B., Brasky K.M., Lanford R.E. DNA microarray analysis of chimpanzee liver during acute resolving hepatitis C virus infection. J Virol. 2001;75:7059–7066. doi: 10.1128/JVI.75.15.7059-7066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su A.I., Pezacki J.P., Wodicka L. Genomic analysis of the host response to hepatitis C virus infection. Proc Natl Acad Sci USA. 2002;99:15669–15674. doi: 10.1073/pnas.202608199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horner S.M., Gale M., Jr. Regulation of hepatic innate immunity by hepatitis C virus. Nat Med. 2013;19:879–888. doi: 10.1038/nm.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schoggins J.W., MacDuff D.A., Imanaka N. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature. 2014;505:691–695. doi: 10.1038/nature12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park S.H., Rehermann B. Immune responses to HCV and other hepatitis viruses. Immunity. 2014;40:13–24. doi: 10.1016/j.immuni.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thimme R., Bukh J., Spangenberg H.C. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc Natl Acad Sci USA. 2002;99:15661–15668. doi: 10.1073/pnas.202608299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diepolder H.M., Zachoval R., Hoffmann R.M. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet. 1995;346:1006–1007. doi: 10.1016/s0140-6736(95)91691-1. [DOI] [PubMed] [Google Scholar]
- 34.Missale G., Bertoni R., Lamonaca V. Different clinical behaviors of acute hepatitis C virus infection are associated with different vigor of the anti-viral cell-mediated immune response. J Clin Invest. 1996;98:706–714. doi: 10.1172/JCI118842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Urbani S., Amadei B., Fisicaro P. Outcome of acute hepatitis C is related to virus-specific CD4 function and maturation of antiviral memory CD8 responses. Hepatology. 2006;44:126–139. doi: 10.1002/hep.21242. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan D.E., Sugimoto K., Newton K. Discordant role of CD4 T-cell response relative to neutralizing antibody and CD8 T-cell responses in acute hepatitis C. Gastroenterology. 2007;132:654–666. doi: 10.1053/j.gastro.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 37.Thimme R., Oldach D., Chang K.M. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395–1406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amadei B., Urbani S., Cazaly A. Activation of natural killer cells during acute infection with hepatitis C virus. Gastroenterology. 2010;138:1536–1545. doi: 10.1053/j.gastro.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pelletier S., Drouin C., Bedard N. Increased degranulation of natural killer cells during acute HCV correlates with the magnitude of virus-specific T cell responses. J Hepatol. 2010;53:805–816. doi: 10.1016/j.jhep.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kokordelis P., Kramer B., Korner C. An effective interferon-gamma-mediated inhibition of hepatitis C virus replication by natural killer cells is associated with spontaneous clearance of acute hepatitis C in human immunodeficiency virus-positive patients. Hepatology. 2014;59:814–827. doi: 10.1002/hep.26782. [DOI] [PubMed] [Google Scholar]
- 41.Varchetta S., Mele D., Mantovani S. Impaired intrahepatic natural killer cell cytotoxic function in chronic hepatitis C virus infection. Hepatology. 2012;56:841–849. doi: 10.1002/hep.25723. [DOI] [PubMed] [Google Scholar]
- 42.De Maria A., Fogli M., Mazza S. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur J Immunol. 2007;37:445–455. doi: 10.1002/eji.200635989. [DOI] [PubMed] [Google Scholar]
- 43.Nattermann J., Feldmann G., Ahlenstiel G. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut. 2006;55:869–877. doi: 10.1136/gut.2005.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oliviero B., Varchetta S., Paudice E. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology. 2009;137:1151–1156. doi: 10.1053/j.gastro.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 45.Beziat V., Dalgard O., Asselah T. CMV drives clonal expansion of NKG2C+ NK cells expressing self-specific KIRs in chronic hepatitis patients. Eur J Immunol. 2012;42:447–457. doi: 10.1002/eji.201141826. [DOI] [PubMed] [Google Scholar]
- 46.Golden-Mason L., Stone A.E., Bambha K.M. Race- and gender-related variation in NKp46 expression associated with differential anti-HCV immunity. Hepatology. 2012;56:1214–1222. doi: 10.1002/hep.25771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hudspeth K., Donadon M., Cimino M. Human liver-resident CD56/CD16 NK cells are retained within hepatic sinusoids via the engagement of CCR5 and CXCR6 pathways. J Autoimmun. 2015 doi: 10.1016/j.jaut.2015.08.011. http://dx.doi.org/10.1016/j.jaut.2015.08.011 Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morishima C., Paschal D.M., Wang C.C. Decreased NK cell frequency in chronic hepatitis C does not affect ex vivo cytolytic killing. Hepatology. 2006;43:573–580. doi: 10.1002/hep.21073. [DOI] [PubMed] [Google Scholar]
- 49.Serti E., Chepa-Lotrea X., Kim Y.J. Successful interferon-free therapy of chronic hepatitis C virus infection normalizes natural killer cell function. Gastroenterology. 2015;149:190–200. doi: 10.1053/j.gastro.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fauriat C., Long E.O., Ljunggren H.G. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 2010;115:2167–2176. doi: 10.1182/blood-2009-08-238469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Maria A., Bozzano F., Cantoni C. Revisiting human natural killer cell subset function revealed cytolytic CD56dimCD16+ NK cells as rapid producers of abundant IFN-gamma on activation. Proc Natl Acad Sci USA. 2011;108:728–732. doi: 10.1073/pnas.1012356108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonorino P., Ramzan M., Camous X. Fine characterization of intrahepatic NK cells expressing natural killer receptors in chronic hepatitis B and C. J Hepatol. 2009;51:458–467. doi: 10.1016/j.jhep.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 53.Dessouki O., Kamiya Y., Nagahama H. Chronic hepatitis C viral infection reduces NK cell frequency and suppresses cytokine secretion: reversion by anti-viral treatment. Biochem Biophys Res Comm. 2010;393:331–337. doi: 10.1016/j.bbrc.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 54.Sene D., Levasseur F., Abel M. Hepatitis C virus (HCV) evades NKG2D-dependent NK cell responses through NS5A-mediated imbalance of inflammatory cytokines. PLoS Pathog. 2010;6:e1001184. doi: 10.1371/journal.ppat.1001184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edlich B., Ahlenstiel G., Azpiroz A.Z. Early changes in interferon signaling define natural killer cell response and refractoriness to interferon-based therapy of hepatitis C patients. Hepatology. 2012;55:39–48. doi: 10.1002/hep.24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miyagi T., Takehara T., Nishio K. Altered interferon-alpha-signaling in natural killer cells from patients with chronic hepatitis C virus infection. J Hepatol. 2010;53:424–430. doi: 10.1016/j.jhep.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 57.Miyagi T., Gil M.P., Wang X. High basal STAT4 balanced by STAT1 induction to control type 1 interferon effects in natural killer cells. J Exp Med. 2007;204:2383–2396. doi: 10.1084/jem.20070401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nguyen K.B., Cousens L.P., Doughty L.A. Interferon alpha/beta-mediated inhibition and promotion of interferon gamma: STAT1 resolves a paradox. Nat Immunol. 2000;1:70–76. doi: 10.1038/76940. [DOI] [PubMed] [Google Scholar]
- 59.Meissner E.G., Wu D., Osinusi A. Endogenous intrahepatic IFNs and association with IFN-free HCV treatment outcome. J Clin Invest. 2014;124:3352–3363. doi: 10.1172/JCI75938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shin E.C., Seifert U., Kato T. Virus-induced type I IFN stimulates generation of immunoproteasomes at the site of infection. J Clin Invest. 2006;116:3006–3014. doi: 10.1172/JCI29832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lau D.T., Negash A., Chen J. Innate immune tolerance and the role of Kupffer cells in differential responses to interferon therapy among patients with HCV genotype 1 infection. Gastroenterology. 2013;144:402–413. doi: 10.1053/j.gastro.2012.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dreux M., Garaigorta U., Boyd B. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe. 2012;12:558–570. doi: 10.1016/j.chom.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takahashi K., Asabe S., Wieland S. Plasmacytoid dendritic cells sense hepatitis C virus-infected cells, produce interferon, and inhibit infection. Proc Natl Acad Sci USA. 2010;107:7431–7436. doi: 10.1073/pnas.1002301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Giugliano S., Kriss M., Golden-Mason L. Hepatitis C virus infection induces autocrine interferon signaling by human liver endothelial cells and release of exosomes, which inhibits viral replication. Gastroenterology. 2015;148:392–402.e13. doi: 10.1053/j.gastro.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ahlenstiel G., Edlich B., Hogdal L.J. Early changes in natural killer cell function indicate virologic response to interferon therapy for hepatitis C. Gastroenterology. 2011;141:1231–1239. doi: 10.1053/j.gastro.2011.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stegmann K.A., Bjorkstrom N.K., Veber H. Interferon-alpha-induced TRAIL on natural killer cells is associated with control of hepatitis C virus infection. Gastroenterology. 2010;138:1885–1897. doi: 10.1053/j.gastro.2010.01.051. [DOI] [PubMed] [Google Scholar]
- 67.Oliviero B., Mele D., Degasperi E. Natural killer cell dynamic profile is associated with treatment outcome in patients with chronic HCV infection. J Hepatol. 2013;59:38–44. doi: 10.1016/j.jhep.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 68.Chattergoon M.A., Latanich R., Quinn J. HIV and HCV activate the inflammasome in monocytes and macrophages via endosomal Toll-like receptors without induction of type 1 interferon. PLoS Pathog. 2014;10:e1004082. doi: 10.1371/journal.ppat.1004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Negash A.A., Ramos H.J., Crochet N. IL-1beta production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog. 2013;9:e1003330. doi: 10.1371/journal.ppat.1003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Serti E., Werner J.M., Chattergoon M. Monocytes activate natural killer cells via inflammasome-induced interleukin 18 in response to hepatitis C virus replication. Gastroenterology. 2014;147:209–220. doi: 10.1053/j.gastro.2014.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chattergoon M.A., Levine J.S., Latanich R. High plasma interleukin-18 levels mark the acute phase of hepatitis C virus infection. J Infect Dis. 2011;204:1730–1740. doi: 10.1093/infdis/jir642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jinushi M., Takehara T., Kanto T. Critical role of MHC class I-related chain A and B expression on IFN-alpha-stimulated dendritic cells in NK cell activation: impairment in chronic hepatitis C virus infection. J Immunol. 2003;170:1249–1256. doi: 10.4049/jimmunol.170.3.1249. [DOI] [PubMed] [Google Scholar]
- 73.Jinushi M., Takehara T., Tatsumi T. Autocrine/paracrine IL-15 that is required for type I IFN-mediated dendritic cell expression of MHC class I-related chain A and B is impaired in hepatitis C virus infection. J Immunol. 2003;171:5423–5429. doi: 10.4049/jimmunol.171.10.5423. [DOI] [PubMed] [Google Scholar]
- 74.Jinushi M., Takehara T., Tatsumi T. Negative regulation of NK cell activities by inhibitory receptor CD94/NKG2A leads to altered NK cell-induced modulation of dendritic cell functions in chronic hepatitis C virus infection. J Immunol. 2004;173:6072–6081. doi: 10.4049/jimmunol.173.10.6072. [DOI] [PubMed] [Google Scholar]
- 75.Nattermann J., Nischalke H.D., Hofmeister V. The HLA-A2 restricted T cell epitope HCV core 35–44 stabilizes HLA-E expression and inhibits cytolysis mediated by natural killer cells. Am J Pathol. 2005;166:443–453. doi: 10.1016/S0002-9440(10)62267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kramer B., Korner C., Kebschull M. NKp46(High) expression defines a NK cell subset that is potentially involved in control of HCV replication and modulation of liver fibrosis. Hepatology. 2012;56:1201–1213. doi: 10.1002/hep.25804. [DOI] [PubMed] [Google Scholar]
- 77.Larkin J., Bost A., Glass J.I. Cytokine-activated natural killer cells exert direct killing of hepatoma cells harboring hepatitis C virus replicons. J Infect Dis. 2006;26:854–865. doi: 10.1089/jir.2006.26.854. [DOI] [PubMed] [Google Scholar]
- 78.Guidotti L.G., Chisari F.V. To kill or to cure: options in host defense against viral infection. Curr Opin Immunol. 1996;8:478–483. doi: 10.1016/s0952-7915(96)80034-3. [DOI] [PubMed] [Google Scholar]
- 79.Wedemeyer H., He X.S., Nascimbeni M. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J Immunol. 2002;169:3447–3458. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- 80.Dunn C., Peppa D., Khanna P. Temporal analysis of early immune responses in patients with acute hepatitis B virus infection. Gastroenterology. 2009;137:1289–1300. doi: 10.1053/j.gastro.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 81.Wieland S., Thimme R., Purcell R.H. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci USA. 2004;101:6669–6674. doi: 10.1073/pnas.0401771101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Duan X.Z., Wang M., Li H.W. Decreased frequency and function of circulating plasmocytoid dendritic cells (pDC) in hepatitis B virus infected humans. J Clin Immunol. 2004;24:637–646. doi: 10.1007/s10875-004-6249-y. [DOI] [PubMed] [Google Scholar]
- 83.Zou Z., Xu D., Li B. Compartmentalization and its implication for peripheral immunologically-competent cells to the liver in patients with HBV-related acute-on-chronic liver failure. Hepatol Res. 2009;39:1198–1207. doi: 10.1111/j.1872-034X.2009.00571.x. [DOI] [PubMed] [Google Scholar]
- 84.Peppa D., Micco L., Javaid A. Blockade of immunosuppressive cytokines restores NK cell antiviral function in chronic hepatitis B virus infection. PLoS Pathogens. 2010;6:e1001227. doi: 10.1371/journal.ppat.1001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tjwa E.T., van Oord G.W., Hegmans J.P. Viral load reduction improves activation and function of natural killer cells in patients with chronic hepatitis B. J Hepatol. 2011;54:209–218. doi: 10.1016/j.jhep.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 86.Heiberg I.L., Pallett L.J., Winther T.N. Defective natural killer cell anti-viral capacity in paediatric HBV infection. Clin Exp Immunol. 2015;179:466–476. doi: 10.1111/cei.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Das A., Ellis G., Pallant C. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J Immunol. 2012;189:3925–3935. doi: 10.4049/jimmunol.1103139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ando K., Moriyama T., Guidotti L.G. Mechanisms of class I restricted immunopathology. A transgenic mouse model of fulminant hepatitis. J Exp Med. 1993;178:1541–1554. doi: 10.1084/jem.178.5.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cavanaugh V.J., Guidotti L.G., Chisari F.V. Interleukin-12 inhibits hepatitis B virus replication in transgenic mice. J Virol. 1997;71:3236–3243. doi: 10.1128/jvi.71.4.3236-3243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cheong J.Y., Cho S.W., Hwang I.L. Association between chronic hepatitis B virus infection and interleukin-10, tumor necrosis factor-alpha gene promoter polymorphisms. J Gastroenterol Hepatol. 2006;21:1163–1169. doi: 10.1111/j.1440-1746.2006.04304.x. [DOI] [PubMed] [Google Scholar]
- 91.Miyazoe S., Hamasaki K., Nakata K. Influence of interleukin-10 gene promoter polymorphisms on disease progression in patients chronically infected with hepatitis B virus. Am J Gastroenterol. 2002;97:2086–2092. doi: 10.1111/j.1572-0241.2002.05926.x. [DOI] [PubMed] [Google Scholar]
- 92.Dunn C., Brunetto M., Reynolds G. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J Exp Med. 2007;204:667–680. doi: 10.1084/jem.20061287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peppa D., Gill U.S., Reynolds G. Up-regulation of a death receptor renders antiviral T cells susceptible to NK cell-mediated deletion. J Exp Med. 2013;210:99–114. doi: 10.1084/jem.20121172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Waggoner S.N., Cornberg M., Selin L.K. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2012;481:394–398. doi: 10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lang P.A., Lang K.S., Xu H.C. Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc Natl Acad Sci USA. 2012;109:1210–1215. doi: 10.1073/pnas.1118834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Welsh R.M., Waggoner S.N. NK cells controlling virus-specific T cells: Rheostats for acute vs. persistent infections. Virology. 2013;435:37–45. doi: 10.1016/j.virol.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rehermann B. Pathogenesis of chronic viral hepatitis. Differential roles of T cells and NK cells. Nat Med. 2013;19:859–868. doi: 10.1038/nm.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Raziorrouh B., Schraut W., Gerlach T. The immunoregulatory role of CD244 in chronic hepatitis B infection and its inhibitory potential on virus-specific CD8+ T-cell function. Hepatology. 2010;52:1934–1947. doi: 10.1002/hep.23936. [DOI] [PubMed] [Google Scholar]
- 99.Bengsch B., Seigel B., Ruhl M. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 2010;6:e1000947. doi: 10.1371/journal.ppat.1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nakamoto N., Cho H., Shaked A. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 2009;5:e1000313. doi: 10.1371/journal.ppat.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Golden-Mason L., Palmer B.E., Kassam N. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol. 2009;83:9122–9130. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wherry E.J. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 103.Rehermann B., Bertoletti A. Immunological aspects of antiviral therapy of chronic hepatitis B virus and hepatitis C virus infections. Hepatology. 2015;61:712–721. doi: 10.1002/hep.27323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rahman F., Heller T., Sobao Y. Effects of antiviral therapy on the cellular immune response in acute hepatitis C. Hepatology. 2004;40:87–97. doi: 10.1002/hep.20253. [DOI] [PubMed] [Google Scholar]
- 105.Lauer G.M., Lucas M., Timm J. Full-breadth analysis of CD8+ T-cell responses in acute hepatitis C virus infection and early therapy. J Virol. 2005;79:12979–12988. doi: 10.1128/JVI.79.20.12979-12988.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Missale G., Pilli M., Zerbini A. Lack of full CD8 functional restoration after antiviral treatment for acute and chronic hepatitis C virus infection. Gut. 2012;61:1076–1084. doi: 10.1136/gutjnl-2011-300515. [DOI] [PubMed] [Google Scholar]
- 107.Spaan M., van Oord G., Kreefft K. Immunological analysis during interferon-free therapy for chronic hepatitis C virus infection reveals modulation of the natural killer cell compartment. J Infect Dis. 2015 doi: 10.1093/infdis/jiv391. http://dx.doi.org/10.1093/infdis/jiv391 Published online. [DOI] [PubMed] [Google Scholar]
- 108.Martin B., Hennecke N., Lohmann V. Restoration of HCV-specific CD8+ T cell function by interferon-free therapy. J Hepatol. 2014;61 doi: 10.1016/j.jhep.2014.05.043. 1219–1219. [DOI] [PubMed] [Google Scholar]
- 109.Kwon H., Lok A.S. Hepatitis B therapy. Nat Rev Gastroenterol Hepatol. 2011;8:275–284. doi: 10.1038/nrgastro.2011.33. [DOI] [PubMed] [Google Scholar]
- 110.Wursthorn K., Jung M., Riva A. Kinetics of hepatitis B surface antigen decline during 3 years of telbivudine treatment in hepatitis B e antigen-positive patients. Hepatology. 2010;52:1611–1620. doi: 10.1002/hep.23905. [DOI] [PubMed] [Google Scholar]
- 111.Boni C., Laccabue D., Lampertico P. Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology. 2012;143:963–973. doi: 10.1053/j.gastro.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 112.Boni C., Penna A., Bertoletti A. Transient restoration of anti-viral T cell responses induced by lamivudine therapy in chronic hepatitis B. J Hepatol. 2003;39:595–605. doi: 10.1016/s0168-8278(03)00292-7. [DOI] [PubMed] [Google Scholar]
- 113.Accapezzato D., Francavilla V., Paroli M. Hepatic expansion of a virus-specific regulatory CD8+ T cell population in chronic hepatitis C virus infection. J Clin Invest. 2004;113:963–972. doi: 10.1172/JCI20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Semmo N., Krashias G., Willberg C. Analysis of the relationship between cytokine secretion and proliferative capacity in hepatitis C virus infection. J Viral Hepat. 2007;14:492–502. doi: 10.1111/j.1365-2893.2007.00842.x. [DOI] [PubMed] [Google Scholar]