Abstract
Objectives
To present our institutional experience with adult prostate sarcoma over 30 years.
Methods
We reviewed 38 cases of adult prostate sarcoma diagnosed and treated at our institution between 1982 and 2012. Univariate Cox proportional hazards regression was used to determine if there was an association between specific disease characteristics (tumor size, histology, AJCC stage, and metastasis at diagnosis) and cancer-specific survival (CSS).
Results
A total of 38 patients were included, with a median age of 50 (range 17–73). Most men presented with lower urinary tract symptoms (45%), hematuria (24%), or acute urinary retention (21%). Diagnosis was established with prostate needle biopsy (68%) or transurethral resection of the prostate (18%). The predominant histologic subtypes were leiomyosarcoma (13 cases, 34%) and rhabdomyosarcoma (12 cases, 32%). Rhabdomyosarcoma was associated with poorer CSS (hazard ratio = 3.00; 95% CI 1.13, 7.92; p = 0.027) compared to leiomyosarcoma. We did not observe a significant relationship between tumor size and CSS. Overall, median CSS was 2.9 years (95% CI 1.5, 5.4), with 7.7 years for clinically localized disease (95% CI 2.5, not reached) and 1.5 years for metastatic disease (95% CI 1.1, 2.7).
Conclusions
Adult prostate sarcoma has a poor prognosis, especially in cases of metastatic disease at the time of diagnosis. Surgery remains the standard of care, but it provides limited benefit to those with metastatic disease or as a consolidation therapy after partial response to systemic therapy.
Keywords: disease-free survival, prostate neoplasm, soft tissue sarcoma, survival analysis
INTRODUCTION
Soft tissue sarcomas include a variety of unique neoplasms that arise from tissues of mesodermal origin. They are rare, comprising 1% of all cancers1 and only 0.7% of primary malignancies of the prostate.2 Twenty percent of soft tissue sarcomas arise from the abdomen or retroperitoneum.3 Approximately 50 percent of patients will die of their disease by 2 years,2 with sarcoma survival rates that have changed very little over time.4 Sarcomas of the prostate carry a particularly poor prognosis, as the majority are fatal.5 Significant risk factors for tumor recurrence and progression have been identified (primarily in the extremity sarcoma literature) including tumor grade, size, depth of invasion, and surgical margin status, but since sarcoma of the prostate is rare, clinical variables affecting prognosis are primarily based on single reports and small case series. Previous reports suggest that RMS may be a favorable subtype,2 while others have reported no survival difference between subtypes.5 The largest and most recent report of prostate sarcoma had a sample size of only 25 patients, and previous series were even smaller. 2,5,6 Here we present the largest series of adult prostate sarcomas in the literature, in an effort to identify and analyze clinical features that may be used to predict outcomes.
MATERIALS AND METHODS
Patients
After receiving institutional review board approval, we searched a prospectively maintained institutional database to identify all adult patients (aged ≥16 years) who had received a diagnosis of prostate sarcoma at Memorial Sloan Kettering Cancer Center between 1982 and 2012. This age group was used to maintain consistency with previous reports on adult prostate sarcoma. Medical records were reviewed for age at diagnosis, presenting symptoms, methods used for diagnosis, histologic subtype, tumor size, grade, treatments employed, disease recurrence, and cause of death. Tumors were retrospectively staged according to contemporary AJCC staging for soft tissue sarcoma.7
Statistical Analysis
We calculated CSS and RFS as a function of tumor size, histology, AJCC stage, and presence or absence of metastasis at diagnosis using the Kaplan-Meier method. To determine if any of these four characteristics was associated with CSS or RFS, we employed a univariate Cox proportional hazards regression model. Patients who did not undergo extirpative surgery (n = 11, 29%), as well as one patient who did not demonstrate any response to treatment, were excluded from the recurrence analysis. Patient CSS was determined from the date of diagnosis until death from prostate sarcoma or death from other causes or until the most recent patient contact, while RFS was determined from date of surgery until the date of recurrence, the most recent patient contact, or date of death. All analyses were conducted using Stata 12 (Stata Corp., College Station, TX).
RESULTS
A summary of patient characteristics are displayed in table 1. Additional detailed characteristics are presented in the Supplemental Table. Of 38 patients overall, 26 (68%) died of their disease, with a median follow-up of 1.8 years (IQR 1.3, 4.2). The median follow-up for patients who did not die of their disease was 4.1 years (IQR 1.0, 7.6). The median CSS among all patients was 2.9 years (95% CI 1.5, 5.4). Among patients with localized disease, median CSS was 7.7 years (95% CI 2.5, upper bound not estimable), while for patients with metastatic disease at diagnosis, median CSS was 1.5 years (95% CI 1.1, 2.7).
Table 1.
Patient and disease characteristics. Values are displayed as median (interquartile range) or frequency (percentage).
| Characteristics | No. (%) |
|---|---|
| No. pts | 38 (100) |
| Age at diagnosis | 50 (27, 57) |
| Tumor diagnosis modality | |
| Prostate biopsy | 26 (68) |
| TURP | 7 (18) |
| Other | 5 (13) |
| Tumor size | |
| <5 cm | 7 (19) |
| 5–10 cm | 19 (53) |
| >10 cm | 10 (28) |
| Tumor histology | |
| Leiomyosarcoma | 13 (34) |
| Rhabdomyosarcoma | 12 (32) |
| Other sarcomas* | 13 (34) |
| Tumor grade | |
| High grade | 35 (92) |
| Low grade | 3 (8) |
| AJCC stage IV | 10 (26) |
| No. pts with metastasis at diagnosis | 17 (45%) |
| Total PSA (ng/mL) | 1.0 (0.8, 2.2) |
| Radiation | 24 (77) |
| Unknown | 7 (18) |
| Chemotherapy | 31 (86) |
| Unknown | 2 (5) |
| Surgical approach | |
| Cystoprostatectomy† | 13 (48) |
| Pelvic exenteration† | 8 (30) |
| Prostatectomy† | 6 (22) |
| No surgery | 11 (29) |
| Positive surgical margins† | 5 (22) |
| Unknown | 4 (15) |
| Progressed through treatment‡ | 5 (13) |
Includes osteosarcoma, synovial, undifferentiated, and mixed sarcomas.
Values include only those who underwent surgery.
Patients who progressed through treatment were excluded from the recurrence analysis.
Primary treatment strategies employed depended on whether or not metastatic disease was present at the time of diagnosis. Twenty-one patients (55%) had no clinical evidence of metastatic disease at time of diagnosis; of these, 19 were treated with extirpative surgery including cystoprostatectomy (n = 9), radical prostatectomy, (n = 6) or pelvic exenteration (n = 4), while 2 refused surgery. Figure 1 shows an example of imaging and endoscopic appearance of 7cm clinically localized prostate sarcoma which was managed with cystoprostatectomy. Seven also received neoadjuvant systemic chemotherapy and radiation and another 4 received adjuvant chemotherapy, while 8 were treated with surgery alone. Agents most commonly utilized for neoadjuvant therapy included combination gemcitabine and docetaxel, or Adriamycin with Ifosfamide or cyclophosphamide, vincristine and doxorubicin. Of the 19 patients treated surgically, 8 remained disease-free after treatment, with a median follow-up of 81 months. Three patients had positive surgical margins, all of whom experienced recurrence and died as a result of disease progression (local disease in 1 and systemic in all 3). Of the 2 patients with clinically localized disease who were managed without surgery, 1 remained free of disease 55 months after treatment with chemotherapy and radiation, while the other progressed systemically and died of the disease 14 months after diagnosis.
Figure 1.


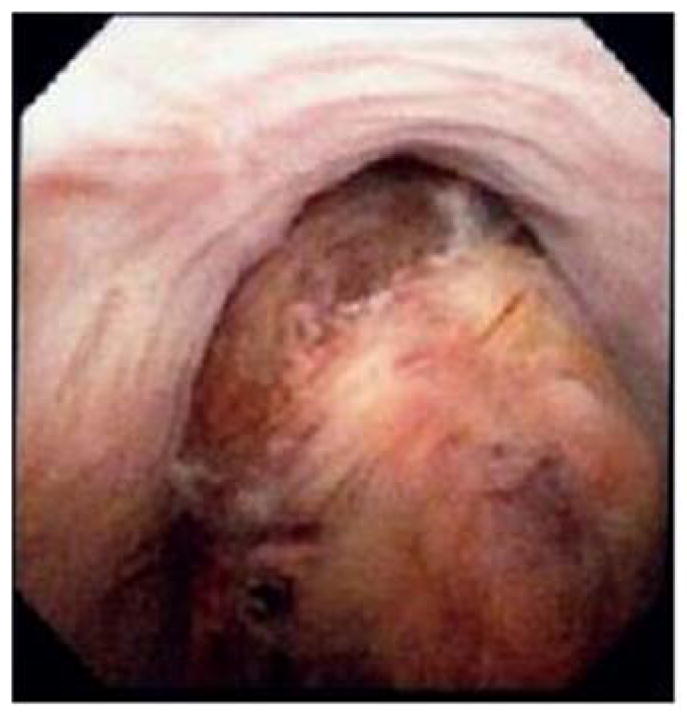
T2 weighted MRI axial (a) and coronal (b) images, and an endoscopic view (c) of a 7cm prostate sarcoma. The patient presented with acute urinary retention and underwent cystoprostatectomy for local control.
Seventeen patients (45%) had metastatic disease identified at the time of diagnosis, including 6 with limited nodal disease only. Chemotherapeutic regimens utilized most commonly included combination protocols VAC (vincristine, dactinomycin, cyclophosphamide), or MAID (doxorubicin (Adriamycin), ifosfamide, dacarbazine, mesna). Durable response in the setting of systemic disease was not seen with any regimen. Fifteen of the 17 died of their disease, with a median CSS of 1.5 years (95% CI 1.1, 2.7), while the remaining 2 had progressive disease but were lost to follow-up at 11 and 13 months. Eight of the patients with metastatic disease had extirpative surgery, including 4 with limited nodal disease, 2 after a partial response to upfront systemic therapy, 1 with a solitary pulmonary metastasis that was resected, and 1 for palliation of local symptoms. Two had positive surgical margins. All 8 of these patients progressed systemically and died of their disease at a median of 1.7 years (95% CI 0.7, 3.6) after surgery.
Figure 2 displays Kaplan-Meier curves for the probability of CSS stratified by tumor histology. The supplemental figures display Kaplan-Meier curves for the probability of CSS stratified by tumor stage, primary tumor size, and presence or absence of metastasis at diagnosis. Table 2 displays the results of univariate Cox proportional hazards regression analyses of factors associated with CSS and RFS. Tumor stage and metastasis at diagnosis were significantly associated with worse CSS (p = 0.002 and 0.0002, respectively). Stage IV disease at the time of diagnosis was associated with a hazard ratio of 4.13 (95% CI 1.72, 9.96) compared to lower stage disease; the hazard ratio for metastasis at diagnosis was 5.91 (95% CI 2.31, 15.11). We also found that, compared to LMS, RMS was associated with worse CSS, with a hazard ratio of 3.00 (95% CI 1.13, 7.92; p = 0.027). While we found no difference in any other pair-wise comparison between tumor histologies, because of limited data and the resulting wide confidence intervals, we cannot exclude survival differences of these other histologies compared to LMS. There was no significant difference in rates of disease-specific death between those with RMS versus other sarcomas, or between those with a tumor 5–10 cm and >10 cm (p = 0.2 and 0.7, respectively). Metastasis at diagnosis was associated with a higher risk of recurrence, with a hazard ratio of 3.60 (95% CI 1.27, 10.16; p = 0.016). We also observed a higher risk of recurrence among those with a stage IV tumor compared to those with a lower stage tumor, with a hazard ratio of 4.39 (95% CI 0.85, 22.75; p = 0.078). However, this difference was not statistically significant and only 2 patients presented with stage IV disease. We did not observe any statistically significant differences in recurrence rates based on tumor histology or size, but because of the wide confidence intervals we cannot exclude the possibility of an effect based on tumor size. We also found no significant difference in rates of recurrence between those with a tumor 5–10 cm versus >10 cm, or between those with RMS versus other sarcomas, (p = 0.8 and 0.7, respectively).
Figure 2.
Probability of cancer-specific survival by tumor histology (dashed line: leiomyosarcoma; solid black line: rhabdomyosarcoma; gray line: other sarcomas).
Table 2.
Factors associated with cancer-specific and recurrence-free survival
| Predictor | Cancer-Specific Survival* (n=38) | Recurrence-Free Survival† (n=26) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Hazard Ratio | 95% CI | p Value | Hazard Ratio | 95% CI | p Value | |
|
| ||||||
| AJCC Stage | ||||||
| Stage I–III | Ref. | - | - | Ref. | - | - |
| Stage IV | 4.13 | 1.72, 9.96 | 0.002 | 4.39 | 0.85, 22.75 | 0.078 |
|
| ||||||
| Tumor histology | ||||||
| Leiomyosarcoma | Ref. | - | - | Ref. | - | - |
| Rhabdomyosarcoma | 3.00 | 1.13, 7.92 | 0.027 | 1.21 | 0.31, 4.68 | 0.8 |
| Other sarcomas | 1.57 | 0.58, 4.22 | 0.4 | 1.56 | 0.55, 4.40 | 0.4 |
|
| ||||||
| Tumor size | ||||||
| <5 cm | Ref. | - | - | Ref. | - | - |
| 5–10 cm | 1.14 | 0.40, 3.21 | 0.8 | 1.32 | 0.40, 4.37 | 0.7 |
| >10 cm | 1.37 | 0.42, 4.54 | 0.6 | 1.12 | 0.28, 4.53 | 0.9 |
|
| ||||||
| Metastasis | ||||||
| No | Ref. | - | - | Ref. | - | - |
| Yes | 5.91 | 2.31, 15.11 | 0.0002 | 3.60 | 1.27, 10.16 | 0.016 |
Univariate Cox proportional hazards regression was used for the analyses.
CSS was analyzed among all patients.
RFS analysis excluded those who had no response to treatment or did not undergo surgery.
COMMENT
While the rarity of adult prostate sarcoma is a significant barrier to clinical research, this report represents the largest series yet in the literature and highlights the particularly poor prognosis associated with this disease. In general, patients with genitourinary sarcomas have increased rates of local recurrence and decreased survival compared to those with extremity soft tissue sarcomas,4, 5 likely a result of multiple factors including variations in tumor biology, advanced stage at time of presentation, and difficulty in achieving complete resection.8
Although not statistically significant, there was a trend towards shorter time to recurrence and CSS with larger tumor size, consistent with findings from the extremity soft tissue sarcoma literature,3 although limited by the small sample size. Contrary to some previous reports, especially in the pediatric literature, RMS did not confer a survival benefit in our cohort, and in fact these patients had worse overall and cancer-specific survival compared to LMS. Similarly, while there were too few events for statistical comparison, 5 patients had positive surgical margins, all of whom recurred and died of their disease.
Not surprisingly, the most important prognostic variable in our cohort was the presence of metastatic disease at the time of diagnosis, underscoring the limitations of current systemic therapy for metastatic sarcoma in adults. This is in contrast with pediatric RMS, where the majority of children with localized disease and nearly half with metastatic disease are alive 5 years after diagnosis.7 The mechanisms for this difference are still unknown, though it may be that RMS is more chemo-resistant in adults, or older patients may be unable to tolerate the doses needed to achieve the superior outcomes described in the pediatric literature. It is important to note that previous staging systems categorized nodal involvement as stage IV disease, but due to the slightly better prognosis associated with nodal-only metastases,7 contemporary AJCC staging categorizes nodal involvement as stage III (together with large high-grade tumors).7 In our series, no patient with metastatic disease achieved a long-term remission; and even patients with only limited nodal involvement and those who demonstrated an initial response to systemic therapy ultimately progressed, despite consolidative surgery in eight patients. Wang et al reported a similar limited response to therapy for systemic disease in the adult population.2 While there remains the possibility of achieving cure with resection of nodal metastases, our data indicate that most patients will progress systemically. Based on these findings, we would advise caution when contemplating extirpative surgery in the metastatic setting and recommend a frank discussion with patients and their families of the likelihood of limited success in achieving cure in this setting.
For patients with clinically localized disease, complete surgical resection with negative margins remains the mainstay of sarcoma treatment and represents the best chance of achieving cure.3 Some smaller studies have suggested a possible survival benefit to multimodal therapy over surgical resection alone, 6, 7 but because of small sample sizes and variations in treatment, the advantages are unclear. In a pathologic review of 11 cases of pelvic sarcomas, preoperative treatment with cisplatin and doxorubicin caused >95% necrosis of the primary tumor, although radiographic response was limited.9 Although efficacy of radiotherapy for soft tissue sarcoma has been described,10 it does not compensate for the presence of unplanned positive histologic margins.11 Similarly, the use of adjuvant chemotherapy is controversial, as its apparent impact is limited. A large meta-analysis of adjuvant chemotherapy for localized resectable soft tissue sarcoma demonstrated improved progression-free probability but unchanged overall survival.12 In our series, 4 of 8 patients with clinically localized disease were disease-free after surgery alone, while 5 of 12 were disease-free after surgery combined with neoadjuvant chemotherapy and/or radiotherapy. Our experience is similar to that reported by Sexton et al,5 that in select cases, neoadjuvant systemic therapy along with radiotherapy occasionally results in downsizing of the primary tumor, thereby permitting a complete resection. Because surgical cure is difficult and the majority of patients will eventually die of metastatic disease,13 innovative systemic treatments will be required to make a significant impact on survival in the majority of patients with adult prostate sarcoma. Novel targeted agents such as imatinib, which is used to treat metastatic gastrointestinal stromal tumors, may provide an alternative treatment approach for adult prostate sarcoma in the future.14
This study contains several notable limitations. While data were collected in a prospectively maintained database, retrospective review of clinical variables and outcomes was required for analysis and could introduce bias. Because a variety of radiation and chemotherapy doses and regimens were utilized, this information was not included as part of the survival analysis, and therefore we cannot comment on the efficacy of specific regimens or doses. Additionally, the thirty year span included in this series allows for potential heterogeneity between patients treated during this period. However, given the limited progress in the management of this rare disease, it is unfortunately not clear that results have markedly changed over this interval. Although this study was limited by a small sample size, it represents the largest single-institutional experience and provides important information about this rare disease.
CONCLUSIONS
Adult prostate sarcoma has a poor prognosis, especially in patients presenting with metastatic disease. Complete surgical resection, whenever possible, is the most effective treatment and is the mainstay of care for clinically localized disease. For patients with regional or distant metastatic disease, radical surgical resection, with or without adjuvant systemic chemotherapy and radiation therapy, unfortunately provides limited therapeutic benefit. Novel approaches based on an improved understanding of the molecular mechanisms responsible for this lethal disease are urgently needed.
Supplementary Material
Supplementary figure 1: Probability of cancer-specific survival by tumor stage (dashed line: stages I–III; solid black line: stage IV).
Supplementary figure 2: Probability of cancer-specific survival by tumor size (dashed line: <5 cm; solid black line: 5 – 10 cm; gray line: >10 cm).
Supplementary figure 3: Probability of cancer-specific survival by metastasis status at diagnosis (dashed line: no metastasis at diagnosis; solid black line: metastasis at diagnosis).
Acknowledgments
Funding: Supported by the Sidney Kimmel Center for Prostate and Urologic Cancers, by funds provided by David H. Koch through the Prostate Cancer Foundation, and by NIH/NCI Cancer Center Support Grant to MSKCC under award number P30 CA008748.
ABBREVIATIONS AND ACRONYMS
- AJCC
American Joint Committee on Cancer
- CSS
cancer-specific survival
- RFS
recurrence-free survival
- LMS
leiomyosarcoma
- RMS
rhabdomyosarcoma
- TURP
transurethral resection of the prostate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Liu L, Tang H, et al. Twenty-five cases of adult prostate sarcoma treated at a high-volume institution from 1989 to 2009. Urology. 2013;82:160–165. doi: 10.1016/j.urology.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 3.Clark MA, Fisher C, Judson I, et al. Soft-tissue sarcomas in adults. The New England journal of medicine. 2005;353:701–711. doi: 10.1056/NEJMra041866. [DOI] [PubMed] [Google Scholar]
- 4.Dotan ZA, Tal R, Golijanin D, et al. Adult genitourinary sarcoma: the 25-year Memorial Sloan-Kettering experience. The Journal of urology. 2006;176:2033–2038. doi: 10.1016/j.juro.2006.07.021. discussion 2038–2039. [DOI] [PubMed] [Google Scholar]
- 5.Sexton WJ, Lance RE, Reyes AO, et al. Adult prostate sarcoma: the M. D. Anderson Cancer Center Experience. The Journal of urology. 2001;166:521–525. doi: 10.1016/s0022-5347(05)65974-5. [DOI] [PubMed] [Google Scholar]
- 6.Janet NL, May AW, Akins RS. Sarcoma of the prostate: a single institutional review. American journal of clinical oncology. 2009;32:27–29. doi: 10.1097/COC.0b013e31817b6061. [DOI] [PubMed] [Google Scholar]
- 7.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of surgical oncology. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 8.Behranwala KA, A’Hern R, Omar AM, et al. Prognosis of lymph node metastasis in soft tissue sarcoma. Annals of surgical oncology. 2004;11:714–719. doi: 10.1245/ASO.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 9.Ahlering TE, Weintraub P, Skinner DG. Management of adult sarcomas of the bladder and prostate. The Journal of urology. 1988;140:1397–1399. doi: 10.1016/s0022-5347(17)42054-4. [DOI] [PubMed] [Google Scholar]
- 10.Strander H, Turesson I, Cavallin-Stahl E. A systematic overview of radiation therapy effects in soft tissue sarcomas. Acta oncologica. 2003;42:516–531. doi: 10.1080/02841860310014732. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz DL, Einck J, Bellon J, et al. Fast neutron radiotherapy for soft tissue and cartilaginous sarcomas at high risk for local recurrence. International journal of radiation oncology, biology, physics. 2001;50:449–456. doi: 10.1016/s0360-3016(00)01586-8. [DOI] [PubMed] [Google Scholar]
- 12.Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet. 1997;350:1647–1654. [PubMed] [Google Scholar]
- 13.Cheville JC, Dundore PA, Nascimento AG, et al. Leiomyosarcoma of the prostate. Report of 23 cases. Cancer. 1995;76:1422–1427. doi: 10.1002/1097-0142(19951015)76:8<1422::aid-cncr2820760819>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 14.Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2003;21:4342–4349. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1: Probability of cancer-specific survival by tumor stage (dashed line: stages I–III; solid black line: stage IV).
Supplementary figure 2: Probability of cancer-specific survival by tumor size (dashed line: <5 cm; solid black line: 5 – 10 cm; gray line: >10 cm).
Supplementary figure 3: Probability of cancer-specific survival by metastasis status at diagnosis (dashed line: no metastasis at diagnosis; solid black line: metastasis at diagnosis).



