Abstract
Objective:
The present study was envisaged to evaluate the protective effect of polyherbal formulation, DRDC/AY/8060, developed by Dabur India Ltd., against paracetamol and D-galactosamine induced hepatic toxicities in Wistar rats.
Materials and Methods:
The study was carried out in two different experiments of 10 and 14 days against paracetamol and D-galactosamine, respectively. Animals were divided into different treatment groups (n = 6). The control group received normal saline, a toxicant group in two experiments received paracetamol 750 mg/kg p.o. every 72 h for 10 days and D-galactosamine 400 mg/kg i.p. single dose. The test formulation was used at the two dose levels of 120 and 240 mg/kg/day. Treatment groups treated with test formulations were also administered D-galactosamine as given in toxicant group. At the end of the dosing schedule, blood was withdrawn from the retrobulbar plexus of the animals for serum estimation of serum glutamate oxaloacetate transferase (SGOT), serum glutamate pyruvate trasnferase (SGPT), albumin, bilirubin, and alkaline phosphatase (ALP). Following the withdrawal of blood animals was sacrificed, and liver tissue was excised for estimation of thiobarbituric acid reactive substances (lipid peroxidation, malondialdehyde), tissue glutathione (GSH) and histopathological studies.
Results:
It was evident from the biochemical estimation that both paracetamol and galactosamine caused hepatotoxicity in the toxicant groups. However, treatment with DRDC/AY/8060 significantly (P < 0.001, vs. toxicant) reduced the levels of SGOT, SGPT, serum bilirubin, and ALP, as well as decreased lipid peroxidation. In addition, treatment with test formulation also significantly (P < 0.001, vs. toxicant) elevated serum albumin and GSH levels compared to toxicant groups.
Conclusion:
On the basis of these studies and comparative evaluation it can be concluded that the formulation DRDC/AY/8060 showed hepatoprotective activity against paracetamol and D-galactosamine at 120 mg/kg and 240 mg/kg.
KEY WORDS: Hepatoprotective, liver disease, polyherbal formulation
Liver disorders are one of the major causes of morbidity and mortality all over the world. Drug-induced hepatic toxicity is one of the main cause of liver diseases, and accounts for increasing number of hospital admissions.[1] Natural plant products have been globally used for the prevention and treatment of hepatic disorders and scientifically proven for their medicinal efficacy.[2] DRDC/AY/8060 is a new polyherbal formulation developed for prevention and treatment of the liver disorder. It contains the Aqueous extracts of Amlaki bhumi (Phyllanthus niruri) Aerial part, Guduchi (Tinospora cordifolia), Neem (Azadirachta indica) bark, Kalmegh (Andrographis paniculata) whole plant, Harataki (Terminalia chebula) chhal, Amla (Emblica officinalis) dry, bhera (Terminalia belerica) chhal, and Kutki (Picrorhiza kurroa) roots. The present study was undertaken to evaluate the hepatoprotective potential of DRDC/AY/8060 against paracetamol and D-galactosamine induced hepatic toxicity in Wistar rats.
Materials and Methods
Animals
Male Wistar albino rats weighing 200–250 g were used for the study. The animals were maintained under standard laboratory conditions and were allowed free access to food pellets (Ashirwad Industries, Chandigarh) and water ad libitum. The study protocol was approved by Institutional Animal Ethics Committee of Jamia Hamdard.
Paracetamol induced hepatic toxicity
Animals were randomly divided into six groups (n = 6/group) and treated as follows:
Group 1 (control): Normal saline, 1 ml/kg for 10 days
Group 2 (toxicant): Paracetamol 750 mg/kg p.o. every 72 h for 10 days
Group 3 (standard 1): Liv52 p.o. for 10 days along with paracetamol 750 mg/kg p.o. every 72 h for 10 days
Group 4 (standard 2): Livfit p.o. for 10 days along with paracetamol 750 mg/kg p.o. every 72 h for 10 days
Group 5 (formulation dose 1): Formulation 8060, 120 mg/kg p.o. for 10 days, along with paracetamol 750 mg/kg p.o. every 72 h for 10 days
Group 6 (formulation dose 2): Formulation 8060, 240 mg/kg p.o. for 10 days along with paracetamol 750 mg/kg p.o. every 72 h for 10 days.
D-galactosamine induced hepatic toxicity
Animals were randomly divided into six groups (n = 6/group) and treated as follows:
Group I (control): Normal saline, 1 ml/kg for 14 days
Group II (toxicant): D-galactosamine 400 mg/kg i.p. single dose
Group III (standard 1): Liv52 p.o. for 14 days, D-galactosamine 400 mg/kg i.p. the single dose was co-administered on 14th day
Group IV (standard 2): Livfit p.o. for 14 days, D-galactosamine 400 mg/kg i.p. the single dose was co-administered on 14th day
Group V (formulation dose 1): Formulation 8060, 120 mg/kg p.o. for 14 days, D-galactosamine 400 mg/kg i.p. the single dose was co-administered on 14th day
Group VI (formulation dose 2): Formulation 8060, 240 mg/kg p.o. for 14 days, D-galactosamine 400 mg/kg i.p. the single dose was co-administered on 14th day.
Biochemical and histopathological evaluation
Blood was collected from the tail vein of animals 48 h after the completion of the treatment, allowed to coagulate and centrifuged to collect serum at 27°C. Following these animals were sacrificed, and liver tissue was removed for estimation of lipid peroxidation and glutathione (GSH) levels and histopathological studies. Biochemical estimation of serum glutamate oxaloacetate transferase (SGOT), serum glutamate pyruvate transferase (SGPT), albumin, bilirubin, and alkaline phosphatase (ALP) levels was carried out using commercially available kits.
Results and Discussion
Paracetamol induced hepatic toxicity
The effect of various treatments on hepatic biochemical parameters is shown in Table 1. Paracetamol caused hepatic toxicity in the toxicant group as evident from significantly (P < 0.001, vs. toxicant) elevated levels of SGOT, SGPT, serum bilirubin, and ALP, as well as increased lipid peroxidation and significantly reduced (P < 0.001, vs. toxicant) level of serum albumin and tissue GSH compared to toxicant. However, both the standard drugs (Liv52 and Livfit) significantly (P < 0.001) improved these parameters showing positive shifts of values toward control groups compared to toxicants [Table 1]. Treatment with formulation 8060 at 120 mg/kg and 240 mg/kg showed significant reduction (P < 0.001) of serum SGOT and SGPT levels in comparison to the toxicant group. Improvement in serum SGOT level was comparatively better (P < 0.001) at 240 mg/kg than 120 mg/kg dose. Similarly, treatment with formulation 8060 at doses of 120 and 240 mg/kg also reduced increased serum ALP and bilirubin levels significantly (P < 0.001 vs. toxicant). Treatment with paracetamol significantly reduced serum albumin and tissue GSH levels compared to control group (P < 0.001 vs. control), whereas, animals treated with formulation 8060 showed significant improvement (P < 0.001 vs. toxicant) in serum albumin and tissue GSH level. Lipid peroxidation was evident from significantly increased (P < 0.001) malondialdehyde (MDA) level in hepatic tissue of the paracetamol (toxicant) treated groups were significantly higher (P < 0.001 vs. control) compared to normal animals. However, treatment with test formulation (8060) significantly reduced (P < 0.001 vs. toxicant) elevated MDA level. These results histopathological evaluation [Figure 1] were in accord with biochemical parameters.
Table 1.
Results of estimation of hepatic biomarkers in different treatment groups against paracetamol-induced hepatic toxicity
Figure 1.
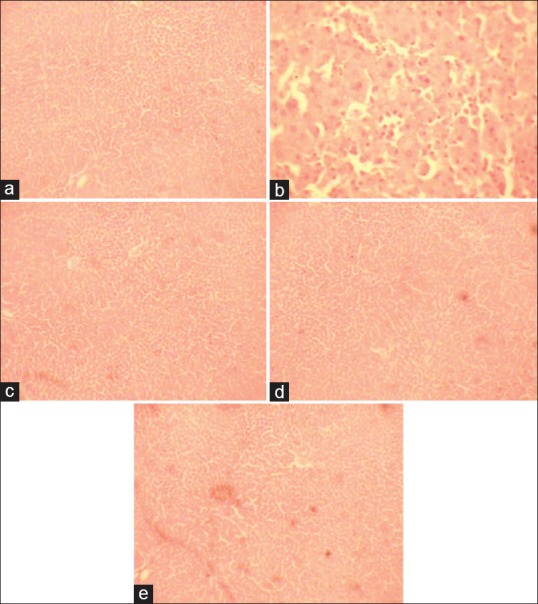
Histology of hepatic tissue of different treatment groups against paracetamol-induced hepatic toxicity. (a) Control group with normal histological features, (b) toxicant group necrotic areas and vacuole formation, (c) and (d) showing almost normal histology after treatment with DRDC/AY/8060 at 120 mg/kg and 240 mg/kg, respectively. (e) Normal architecture of hepatic tissue in animals treated with standard silymarin
D-galactosamine induced hepatic toxicity
The results of biochemical estimations of various treatment groups treated against D-galactosamine induced hepatic toxicity are shown in Table 2. Estimation of biochemical parameters showed that D-galactosamine caused hepatic in the toxicant group as evidenced from significantly (P < 0.001, vs. toxicant) elevated SGOT, SGPT, serum bilirubin, and ALP levels, as well as significantly reduced (P < 0.001, vs. toxicant) level of serum albumin. D-galactosamine also caused increased lipid peroxidation and reduced GSH levels in treated animals. Treatment with standard 1 and standard 2 (i.e., Liv52 and Livfit, respectively) significantly (P < 0.001) reduced the elevated levels of SGOT, SGPT, serum bilirubin, ALP, and tissue MDA. Formulation 8060 significantly reduced (P < 0.001) SGOT and SGPT levels in comparison to toxicant group at both 120 mg/kg and 240 mg/kg. Improvement in both SGOT and SGPT levels were comparatively better (P < 0.001) at the dose of 240 mg/kg vs. 120 mg/kg. Similarly, treatment with formulation 8060 at doses of 120 and 240 mg/kg also reduced increased serum ALP and bilirubin levels significantly (P < 0.001 vs. toxicant). Comparatively better reduction in the bilirubin levels was observed at the higher dose (240 mg/kg) of formulation 8060. Further treatment with formulation 8060 significantly improved (P < 0.001 vs. toxicant) the levels of albumin and GSH in serum and tissue, respectively. Elevated Lipid peroxidation measured as liver tissue MDA level showed significant reduction after treatment with formulation (8060) (P < 0.001 vs. toxicant) at 120 and 240 mg/kg doses. Histopathological observations were found in concurrence with the results of biochemical estimations [Figure 2].
Table 2.
Results of estimation of hepatic biomarkers in different treatment groups against D-galactosamine induced hepatic toxicity
Figure 2.
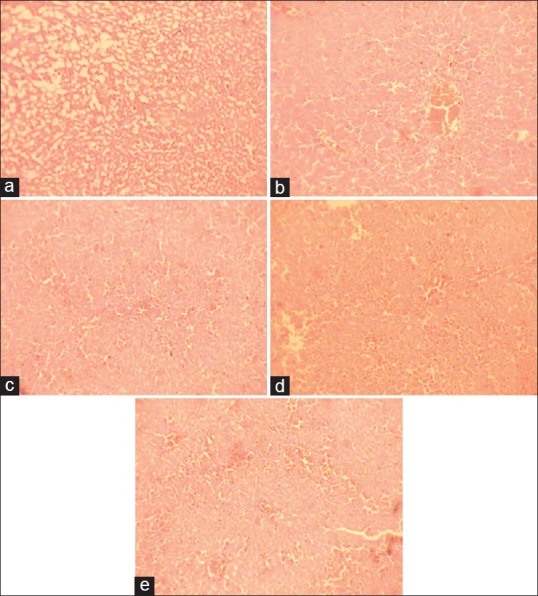
Histology of hepatic tissue of different treatment groups against D-galactosamine induced hepatic toxicity. (a) Control group with normal histological features, (b) toxicant group necrotic areas and vacuole formation, (c) and (d) showing almost normal histology after treatment with DRDC/AY/8060 at 120 mg/kg and 240 mg/kg, respectively. (e) The normal architecture of hepatic tissue in animals treated with standard silymarin
Formulation 8060 contains different established herbal drugs. Bishayi et al. have suggested that treatment by T. cordifolia extract may be the critical remedy for improving CCl4 induced liver dysfunction, as well as immune functions.[3] P. niruri and A. indica have also been shown to be protective against drug-induced hepatic oxidative stress.[4,5] Andrographolide, the major active antihepatotoxic principle present in A. paniculata are also reported to reduce CCl4-induced hepatic enzyme levels.[6] Similarly, T. chebula is also reported to have hepatoprotective activity, which is attributed to its prominent anti-oxidative and membrane stabilizing activities.[7] E. officinalis extract has been found to reduce elevated levels of hepatic biomarkers, indicating that the extract could inhibit the induction of fibrosis in rats.[8] Protective effects of T. belerica have been shown on microsomal lipid peroxidation and triglycerides in the liver suggest a restorative effect in the process of liver damage.[9] Like all other components, P. kurroa has shown hepatoprotective activity. It also reduced the lipid content liver more significantly the standard dose of the known hepatoprotective silymarin.[10] All these reports are suggestive of strong hepatoprotective activity of formulation 8060 against the two toxicants.
Conclusion
The polyherbal DRDC/AY/8060 possesses good hepatoprotective activity.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Døssing M, Sonne J. Drug-induced hepatic disorders. Incidence, management and avoidance. Drug Saf. 1993;9:441–9. doi: 10.2165/00002018-199309060-00007. [DOI] [PubMed] [Google Scholar]
- 2.Handa SS, Chakraborty KK, Sharma A. Antihepatotoxic activity of some Indian herbal formulations as compared to silymarin. Fitoterapia. 1986;57:307. [Google Scholar]
- 3.Chatterjee M, Sil PC. Hepatoprotective effect of aqueous extract of Phyllanthus niruri on nimesulide-induced oxidative stress in vivo. Indian J Biochem Biophys. 2006;43:299–305. [PubMed] [Google Scholar]
- 4.Chattopadhyay RR. Possible mechanism of hepatoprotective activity of Azadirachta indica leaf extract: Part II. J Ethnopharmacol. 2003;89:217–9. doi: 10.1016/j.jep.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Handa SS, Sharma A. Hepatoprotective activity of andrographolide from Andrographis paniculata against carbontetrachloride. Indian J Med Res. 1990;92:276–83. [PubMed] [Google Scholar]
- 6.Tasduq SA, Singh K, Satti NK, Gupta DK, Suri KA, Johri RK. Terminalia chebula (fruit) prevents liver toxicity caused by sub-chronic administration of rifampicin, isoniazid and pyrazinamide in combination. Hum Exp Toxicol. 2006;25:111–8. doi: 10.1191/0960327106ht601oa. [DOI] [PubMed] [Google Scholar]
- 7.Jose JK, Kuttan R. Hepatoprotective activity of Emblica officinalis and Chyavanaprash. J Ethnopharmacol. 2000;72:135–40. doi: 10.1016/s0378-8741(00)00219-1. [DOI] [PubMed] [Google Scholar]
- 8.Anand KK, Singh B, Saxena AK, Chandan BK, Gupta VN. Hepatoprotective studies of a fraction from the fruits of Terminalia belerica Roxb. On experimental liver injury in rodents. Phytother Res. 1994;8:287–92. [Google Scholar]
- 9.Shetty SN, Mengi S, Vaidya R, Vaidya AD. A study of standardized extracts of Picrorhiza kurroa Royle ex Benth in experimental nonalcoholic fatty liver disease. J Ayurveda Integr Med. 2010;1:203–10. doi: 10.4103/0975-9476.72622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bishayi B, Roychowdhury S, Ghosh S, Sengupta M. Hepatoprotective and immunomodulatory properties of Tinospora cordifoliain CCl4 intoxicated mature albino rats. J Toxicol Sci. 2002;27:139–46. doi: 10.2131/jts.27.139. [DOI] [PubMed] [Google Scholar]


