Abstract
Background:
Nucleosides are supportive in the regulation and modulation of various physiological processes in body, they acts as precursors in nucleic acid synthesis, enhance immune response, help in absorption of iron and influence the metabolism of fatty acids. Cordyceps sinensis and Ganoderma lucidum are well-known for its use in traditional medicine of China, Nepal and India. They are rich in nucleosides such as adenine, adenosine, cordycepin, etc. Hence, a simple, economic and accurate high-performance liquid chromatography (HPLC) analytical method was proposed for determination of adenine and adenosine for the quality control of plants.
Materials and Methods:
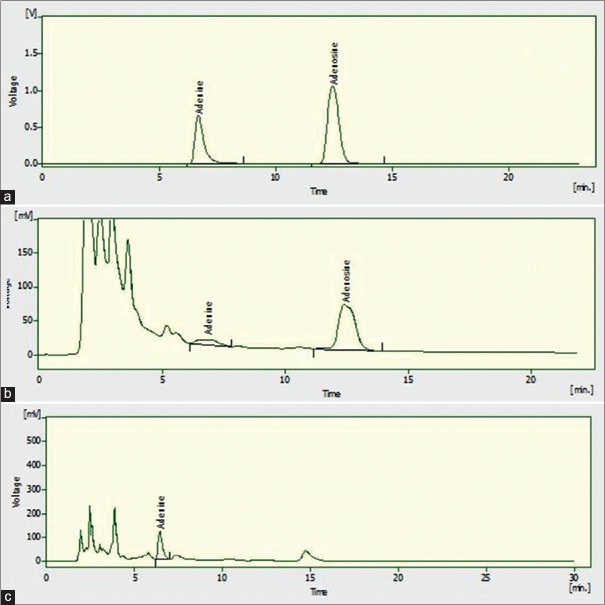
Chromatographic experiments were conducted on YL9100 HPLC system (South Korea). Reversed-phase chromatography was performed on a C18 column with methanol and dihydrogen phosphate as the mobile phase in isocratic elution method at a flow rate of 1.0 mL/min. Detection was carried out at 254 nm, which gives a sharp peak of adenine and adenosine at a retention time of 6.53 ± 0.02 min and 12.41 ± 0.02, respectively.
Results and Discussion:
Linear regression analysis data for the calibration plot showed a good linear relationship between response and concentration in the range of 25–200 µg/mL for adenosine and 100–800 µg/mL for adenine with regression coefficient of 0.999 and 0.996, respectively. The adenine was found 0.16% and 0.71% w/w in G. lucidum and in C. sinensis, respectively, and adenosine was found to be 0.14% w/w in G. lucidum whereas absent in C. sinensis.
Conclusion:
The developed HPLC method for the quantification of adenosine and adenine can be used for the quality control and standardization of crude drug and for the different herbal formulations, in which adenine and adenosine are present as major constituents. The wide linearity range, sensitivity, accuracy, and simple mobile phase imply the method is suitable for routine quantification of adenosine and adenine with high precision and accuracy.
KEY WORDS: Adenine, adenosine, Cordyceps sinensis, Ganoderma lucidum, high performance liquid chromatography
Nucleosides are supportive in the regulation and modulation of various physiological processes in body, they act as precursors in nucleic acid synthesis, enhance immune response, help in absorption of iron and influence the metabolism of fatty acids.[1] In India, the folk healers of Sikkim use Cordyceps sinensis for the prevention of ailments such as cancer, asthma, tuberculosis, diabetics, cough, cold, and hepatitis.[2] This C. sinensis is reportedly effective in renal and cardiovascular diseases,[2,3,4,5] immunological disorders and stress,[6] and inhibits inflammation and airway hyper-responsiveness. Ganoderma lucidum, is one of the most well-known traditional Chinese herbal medicinal, mushroom-like fungi, has been reported as a potential preventive agent for human disorders by exhibiting its anti-allergy, anti-inflammatory and antioxidant properties.[7,8,9] Both the drugs are rich in nucleosides such as adenine, adenosine, cordycepin, etc. As an endogenous nucleoside, adenosine has been widely investigated in different products, for instance, an adenosine is an important index for quality assessment of G. lucidum and C. sinensis.[10,11] Hence, a simple, economic and accurate high-performance liquid chromatography (HPLC) method was proposed for determination of adenine and adenosine for the quality control of both the plants.
Materials and Methods
Reagents and chemicals
Adenosine and adenine were obtained as gift samples from Defence Institute of Physiology and Allied Sciences (Delhi, India). HPLC-grade acetonitrile and methanol were purchased from Merck (India). Milli-Q water, used throughout the experiment, was prepared using Millipore water purification system.
Instrumentation and general conditions
Chromatographic experiments were conducted on a YL9100 HPLC system (South Korea) that comprised quaternary YL9110 pumps, a variable wavelength programmable YL9120 ultraviolet (UV)-visible detector, YL9130 column oven and a system controller. The instrument was controlled by use of YL-clarity software installed with the equipment. Samples were injected by using a Rheodyne injector fitted with a 20 µL fixed loop. Standard and sample solutions were filtered through a 0.22 µm syringe filter before injection. The separation was achieved by using C18 reversed phase column (Merck LichroCART 250-4, LiChrosphere 100 RP 18e, 5.0 µm, sorbent lot number L57020637). The mobile phase consisted of methanol and potassium dihydrogen phosphate (KH2 PO4) as the mobile phase in isocratic elution method at a flow rate of 1.0 mL/min. All the analysis was performed at room temperature and detection was carried out at a wavelength of 254 nm using a UV-visible detector.
Preparation of sample
G. lucidum and C. sinensis were procured from Ethno-Botany Division of Field Research Laboratory, Leh, India, and Defence Institute of Bio-Energy Research, Haldwani, respectively, where the voucher specimen of the plant was preserved in the herbarium. Fruit body of C. sinensis, and G. lucidum were cleaned and washed thoroughly with water and re-washed with distilled water and dried under shade in a clean, dust-free environment and crushed using a laboratory blender. Aqueous extract of C. sinensis and G. lucidum were prepared by the accelerated solvent extraction system equipped with a solvent controller unit (ASE 350, Dionex Corporation, Sunnyvale, CA, USA). Extractions were carried out using distilled water.
The sample was prepared by taking 20 mg of previously dried G. lucidum aqueous extract and 20 mg of C. sinensis extract in 2.0 mL of methanol each. The solutions were filtered using MiniGen MG25020Ny 0.2 µm syringe filter and used for analysis.
Results and Discussion
Optimization of chromatographic conditions
A variety of mobile phases was investigated for the development of the HPLC method suitable for analysis of adenine and adenosine in crude drug extract. The investigated mobile phases included methanol:water, 50:50 (% v/v), acetonitrile:phosphate buffer (pH 3.5 adjusted with orthophosphoric acid), 80:20 (% v/v), acetonitrile:phosphate buffer (pH 3.5 with orthophosphoric acid), 60:40 (% v/v), acetonitrile:water, 50:50 (% v/v) and acetonitrile:water, 60:40 (% v/v). The methanol: KH2 PO4 at 10:90 resulted in sharp peaks with a good resolution [Figure 1].
Figure 1.
High-performance liquid chromatography chromatogram of adenosine and adenine at 254 nm: Standard (a); Cordyceps sinensis (b); Ganoderma lucidum (c)
Calibration curve for adenine and adenosine
Linear regression analysis data for the calibration plot showed a good linear relationship between response and concentration in the range of 25–200 µg/mL for adenosine and 100–800 µg/mL for adenine with regression coefficient of 0.999 and 0.996, respectively; The regression equation is y = 239.64x − 244 and y = 28.936x − 82.86, respectively. The slope ± standard deviation (SD) were found to be 238.98 ± 0.52 and 28.23 ± 0.43, respectively. Intercept ± SD were found to be 244 ± 1.2 and 82.86 ± 1.1, respectively [Figure 2].
Figure 2.
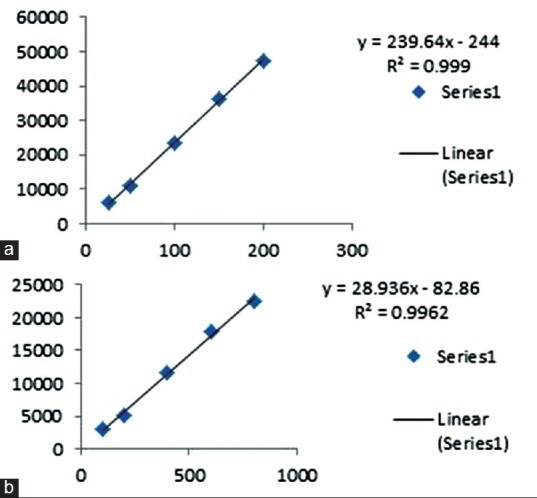
Calibration curve for (a): Standard adenosine (b): Standard adenine
Analysis of adenine and adenosine in samples
The quantitative estimation of adenine and adenosine content was carried out by HPLC by using calibration curve with respect to the peak area of standard peaks. The adenine was found to be 0.16% and 0.71% w/w in G. lucidum and in C. sinensis, respectively, and adenosine was found to be 0.14% w/w in G. lucidum whereas absent in C. sinensis.
Conclusion
The developed HPLC method was developed and validated and found to be simple, sensitive, and accurate in wide linearity range, which can be used for the quantification of adenosine in crude drugs and formulations. It can also be used for quality control and standardization of crude drugs for in which adenine and adenosine are present as a major constituent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kilfoil PJ, Tipparaju SM, Barski OA, Bhatnagar A. Regulation of ion channels by pyridine nucleotides. Circ Res. 2013;112:721–41. doi: 10.1161/CIRCRESAHA.111.247940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panda AK, Swain KC. Traditional uses and medicinal potential of Cordyceps sinensis of Sikkim. J Ayurveda Integr Med. 2011;2:9–13. doi: 10.4103/0975-9476.78183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo P, Kai Q, Gao J, Lian ZQ, Wu CM, Wu CA, et al. Cordycepin prevents hyperlipidemia in hamsters fed a high-fat diet via activation of AMP-activated protein kinase. J Pharmacol Sci. 2010;113:395–403. doi: 10.1254/jphs.10041fp. [DOI] [PubMed] [Google Scholar]
- 4.Wu MF, Li PC, Chen CC, Ye SS, Chien CT, Yu CC. Cordyceps sobolifera extract ameliorates lipopolysaccharide-induced renal dysfunction in the rat. Am J Chin Med. 2011;39:523–35. doi: 10.1142/S0192415X11009007. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Z, Wang X, Zhang Y, Ye G. Effect of Cordyceps sinensis on renal function of patients with chronic allograft nephropathy. Urol Int. 2011;86:298–301. doi: 10.1159/000323655. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Yu Y, Zhang Z, Ding Y, Dai X, Li Y. Effect of polysaccharide from cultured Cordyceps sinensis on immune function and anti-oxidation activity of mice exposed to 60Co. Int Immunopharmacol. 2011;11:2251–7. doi: 10.1016/j.intimp.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Mizuno, T . Seol, Korea: 1992. Comparative studies on the host-mediated antitumor polysaccharides isolated from the fruiting body and mycelium of the three Ganoderma species fungi, Ganoderma lucidum, G. tsugae and G. applanatum. 4th International Symposium on Ganoderma lucidum; pp. 21–9. [Google Scholar]
- 8.Xie JT, Wang CZ, Wicks S, Yin JJ, Kong J, Li J, et al. Ganoderma lucidum extract inhibits proliferation of SW 480 human colorectal cancer cells. Exp Oncol. 2006;28:25–9. [PubMed] [Google Scholar]
- 9.Chang ST. Mushroom research and development-equality and mutual benefit. In: Royes DJ, editor. Mushroom Biology and Mushroom Products. Pittsburg: Pennsylvania University Press; 1996. pp. 1–10. [Google Scholar]
- 10.Gao JL, Leung KS, Wang YT, Lai CM, Li SP, Hu LF, et al. Qualitative and quantitative analyses of nucleosides and nucleobases in Ganoderma spp. by HPLC-DAD-MS. J Pharm Biomed Anal. 2007;44:807–11. doi: 10.1016/j.jpba.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Gong YX, Li SP, Li P, Liu JJ, Wang YT. Simultaneous determination of six main nucleosides and bases in natural and cultured Cordyceps by capillary electrophoresis. J Chromatogr A. 2004;1055:215–21. doi: 10.1016/j.chroma.2004.09.015. [DOI] [PubMed] [Google Scholar]