Abstract
Aim:
Wheatgrass (WG) is the shoot of Triticum aestivum Linn. belongs to the family Gramineae, and possess high chlorophyll content and essential vitamins, minerals, vital enzymes, amino acids, dietary fibers etc., It has been shown to possess anti-cancer, anti-ulcer, antioxidant, and anti-arthritic activity due to the presence of biologically active compounds, and minerals. Therefore, in the present study, high-performance thin layer chromatography (HPTLC), and high-performance liquid chromatography (HPLC) methods for qualitative and quantitative analysis have been proposed, which will help in quality evaluation of wheat grass extract.
Materials and Methods:
Samples for analysis were prepared in methanol and water simply by sonication. These were applied on pre-coated silica plate and chromatograms were developed using toluene: Ethyl acetate: Formic acid. HPLC analysis was done on Waters HPLC system using water, methanol, and acetonitrile as mobile phase. Merck C18 column has been used.
Results:
HPTLC finger printing of alcoholic extracts of WG was carried out and found 10–11 spots at different wavelengths 254, 366, and 435 nm. HPLC fingerprinting produced 22 peaks at 256 nm. Quantitative HPTLC analysis was done to determine the gallic acid content, and was found to be 0.077% w/w in aqueous extract. By HPLC, the content of gallic acid and rutin was found to be 0.07%, and 0.04% w/w in aqueous extract of WG.
Conclusion:
The developed HPLC and HPTLC fingerprinting method can be used for the quality control, and standardization of WG and its extracts used as nutritional supplement.
KEY WORDS: High-performance liquid chromatography, high-performance thin layer chromatography, quality control, wheatgrass
Triticum aestivum (family: Gramineae) is a young grass of common wheat plant, generally referred as wheatgrass (WG). It is a good source of mineral nutrients, contains significant amount of iron, phosphorus, magnesium, manganese, copper, and zinc and also a rich source of tocopherol with high vitamin E potency,[1] The therapeutic potentials of WG have been attributed to its nutritional content, including chlorophyll, vitamins (A, C, and E), bioflavonoids, iron, minerals (calcium, and magnesium), and 17 amino acids, eight of which are essential.[2] The WG is reported to be effective in the treatment of chronic diseases such as cancer,[3] ulcerative colitis,[4] rheumatoid arthritis,[5] and bronchial disorders.[6]
Therefore, in the present study, a simple high-performance thin layer chromatography (HPTLC), and high-performance liquid chromatography (HPLC) method for qualitative and quantitative analysis has been proposed, which will helps in the quality evaluation of WG extract.
Materials and Methods
Plant material
T. aestivum grass was procured from Girme's WG, Sholapur, Maharashtra, India. The characterized, commercially available in the plant material was used as such in the present study. Leaves of T. aestivum, were cleaned, and washed thoroughly with the water, and re-washed with the distilled water, and dried under shade in a clean, dust free environment, and crushed by using a laboratory blender. Aqueous and alcoholic extract of T. aestivum was prepared by the accelerated solvent extraction system equipped with a solvent controller unit (ASE 350, Dionex Corporation, Sunnyvale, CA, USA).
Sample preparation
Extractions were carried out by using either the distilled water or 70% aqueous ethanol. The percent-yield of T. aestivum aqueous, and the alcoholic extract was 15.0% and 25.5% respectively.[7] The dried aqueous and alcoholic extracts of WG (200 mg) was weighed accurately and dissolved in 5.0 mL of methanol to make final concentration 40 mg/mL.
Ten mg of gallic acid, and rutin each was dissolved in 10 mL of methanol to give 1.0 mg/mL standard solution.
High-performance thin layer chromatography fingerprinting
Samples for analysis were applied on precoated silica gel plates using Linomat V sample applicator (CAMAG, Switzerland). For optimization of HPTLC solvent system, numbers of solvent systems were tried, but the most satisfactory resolution was obtained in the solvent toluene: ethyl acetate: formic acid; 8:2:0.5, v/v/v. The chromatograms were developed and scanned at λmax using Camag Scanner III.
High-performance liquid chromatography fingerprinting
HPLC analysis of the extracts was performed using Waters HPLC system (Waters Corporation, USA) equipped with Waters 2998 Photodiode Array Detector. Separation was performed in a Merck C18 (250 × 4.6, 5.0 μm) column by maintaining the isocratic flow rate (1.0 mL/min) of the mobile phase (acetonitrile: methanol; ratio) and the peaks were detected at 256 nm.
Quantitative estimation of marker constituents
Estimation of gallic acid by high-performance thin layer chromatography
The HPTLC method has been developed for the estimation of gallic acid in different extracts of WG. The psrecoated HPTLC silica gel plates were used as a stationary phase and toluene: ethyl acetate: formic acid; 8:2:0.5, v/v/v was used as a mobile phase. The developed chromatogram was then scanned by using Camag scanner III at 269 nm using slit dimension 5 mm × 0.30 mm.
Estimation of gallic acid and rutin by high-performance liquid chromatography
Gallic acid and rutin concentrations were determined by HPLC using Merck C18 (250 × 4.6, 5.0 µm) column as the stationary phase and water: Acetonitrile (50:50, v/v), as the mobile phase. The flow rate was kept at 1.0 mL/min, and the column temperature was 30°C. The chromatogram was monitored by ultraviolet (UV) absorbance at 254 nm.
Results and Discussion
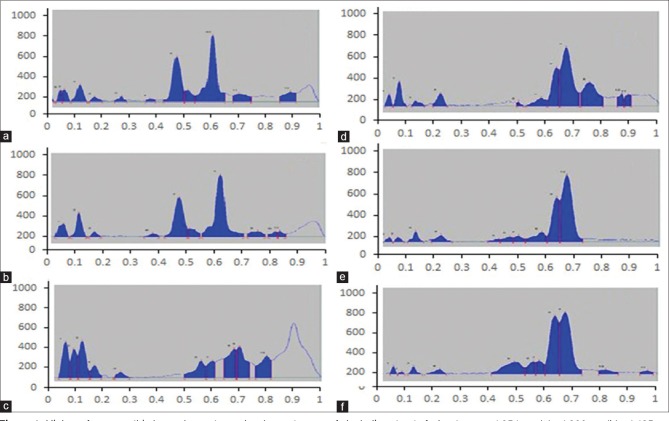
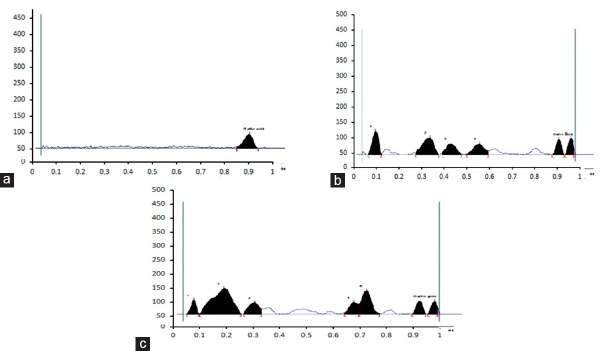
HPTLC and HPLC fingerprints were developed and quantitative estimation of gallic acid, and rutin in aqueous, and alcoholic extracts of WG was done. The chromatographic analysis provided qualitative insights into the constituents of the both extracts. Thin layer chromatography characterization of both extracts, showed many spots with different Rf values. Detection was done at UV 254, 366, and 435 nm for resolving metabolites such as flavonoids, and polyphenols etc. In HPTLC, the chromatogram of alcoholic extracts showed the presence of 11, 10, and 11 spots at 254, 366, and 435 nm, respectively [Figure 1]. The quantitative estimation of gallic acid was carried out in standard, aqueous extracts, and alcoholic extracts of WG by HPTLC at 254 nm [Figure 2]. The calibration curve in a range between 100 and 1000 ng/mL with a linearity of 0.996 was plotted, and the content of gallic acid was found to be 0.077%, and 0.049% w/w in aqueous and alcoholic extracts, respectively.
Figure 1.
High-performance thin layer chromatography chromatogram of alcoholic extract of wheatgrass; at 254 nm (a); at 366 nm (b); at 435 nm (c) for aqueous extract; at 254 nm (d); at 366 nm (e); at 435 nm (f)
Figure 2.
High-performance thin layer chromatography chromatogram of gallic acid at 269 nm: Standard (a); aqueous extract of wheatgrass (b); alcoholic extract of wheatgrass (c)
In present scenario, use of herbal extracts is under scrutiny, quality control of herbal extracts is a topic of continuous scientific interest. With the introduction of modern chromatographic systems, there is an ever growing demand to produce, and develop easy, fast, suitable, and economic methods for quality control.[8] For standardization of aqueous, and alcoholic extracts of WG, HPLC is a sensitive, and accurate tool that achieves the above said requirements, and is commonly used tool for the quality assessment of plant extract. Two-dimensional HPLC chromatogram fingerprints of aqueous and alcoholic extracts of WG were developed at 256 nm. The aqueous extract showed the presence of 14 peaks at various retention times (2.210, 2.841, 3.954, 6.377, 10.826, 12.348, 12.581, 15.148, 15.361, 15.437, 15.983, 16.698, 17.199, and 17.716 min) [Figure 3a]. Similarly, at 256 nm the alcoholic extract showed 22 peaks at 2.221, 2.872, 4.697, 6.408, 10.739, 11.505, 12.242, 13.434, 13.902, 14.343, 14.487, 15.101, 15.435, 15.942, 16.082, 16.436, 16.625, 16.889, 17.180, 17.697, 18.068, and 20.155 min [Figure 3b]. Based on the fingerprints, it can be concluded that this analytical technique is a convenient method to identify the presence of numerous constituents present in the extracts of WG. Simultaneous estimation of gallic acid and rutin was also carried out by HPLC method [Figure 4]. The linearity ranges were found in the range of 10–500 ng/ml and 25–500 ng/ml for gallic acid and rutin, respectively. The assay was judged to be linear as the correlation coefficient was >0.997 by the least square method. The content of gallic acid, and rutin was found to be 0.07% and 0.04% w/w in aqueous extract of WG. The rutin content in WG alcoholic extract was found to be 0.11% w/w, whereas gallic acid is absent.
Figure 3.
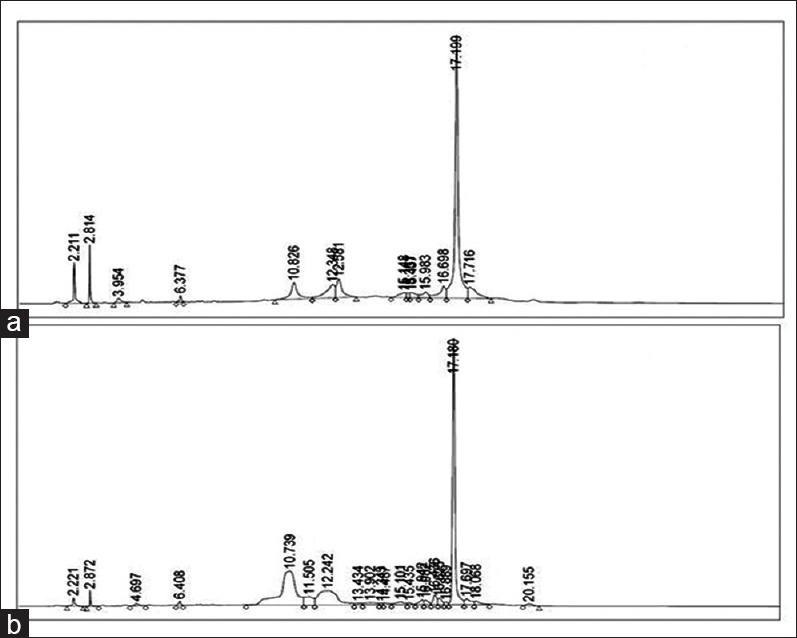
High-performance liquid chromatography chromatogram at 256 nm; wheatgrass aqueous extract (a); wheatgrass alcoholic extract (b)
Figure 4.
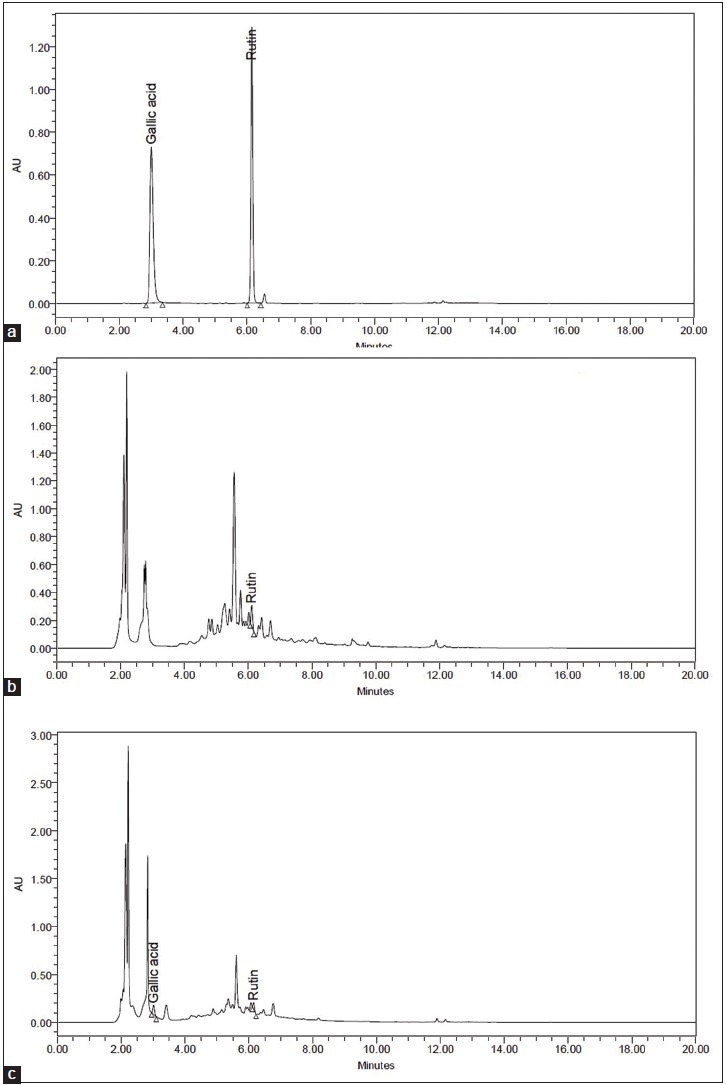
High-performance liquid chromatography chromatogram of gallic acid and rutin at 254 nm: Standard (a); wheatgrass alcoholic extract (b); wheatgrass aqueous extract (c)
Conclusion
The developed HPLC and HPTLC fingerprinting method can be used for the quality control and standardization of WG and its extracts, used as a nutritional supplement. Gallic acid and rutin estimation provides an additional advantage in the standardization of WG extracts.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Fahey JW, Stephenson KK, Dinkova-Kostova AT, Egner PA, Kensler TW, Talalay P. Chlorophyll, chlorophyllin and related tetrapyrroles are significant inducers of mammalian phase 2 cytoprotective genes. Carcinogenesis. 2005;26:1247–55. doi: 10.1093/carcin/bgi068. [DOI] [PubMed] [Google Scholar]
- 2.Walters R. New York: Avery Publishing Group; 1992. The Alternative Cancer Therapy Book. [Google Scholar]
- 3.Dey S, Sarkar R, Ghosh P, Khatun R, Ghorai K, Choudhari R, et al. Effect of wheat grass juice in supportive care of terminally ill cancer patients – A tertiary cancer centre Experience from India. Journal of Clinical Oncology, 2006 ASCO Annual Meeting Proceedings (Post-Meeting Edition) 2006;24 No 18S (June 20 Supplement) 8634. [Google Scholar]
- 4.Ben-Arye E, Goldin E, Wengrower D, Stamper A, Kohn R, Berry E. Wheat grass juice in the treatment of active distal ulcerative colitis: A randomized double-blind placebo-controlled trial. Scand J Gastroenterol. 2002;37:444–9. doi: 10.1080/003655202317316088. [DOI] [PubMed] [Google Scholar]
- 5.Nenonen MT, Helve TA, Rauma AL, Hänninen OO. Uncooked, lactobacilli-rich, vegan food and rheumatoid arthritis. Br J Rheumatol. 1998;37:274–81. doi: 10.1093/rheumatology/37.3.274. [DOI] [PubMed] [Google Scholar]
- 6.Fedoseev GB, Smirnov AI, Zukheir A, Ivanova OA, Smirnova OI, Alekseeva EF, et al. Absolute diet therapy and antibiotic tolerance in bronchial asthma patients. Ter Arkh. 1996;68:28–31. [PubMed] [Google Scholar]
- 7.Misra K, Tulsawani R, Shyam R, Meena DK, Morlock G. Hyphenated high-performance thin-layer chromatography for profiling of some Indian natural efficiency enhancers. J Liq Chromatogr Relat Technol. 2012;35:1364–87. [Google Scholar]
- 8.Selvamani P, Sen DJ, Gupta JK. Pharmacognostical standardization of Commiphora berryi (Arn) Engl and phytochemical studies on its crude extracts. Afr J Pharm Pharmacol. 2009;3:37–46. [Google Scholar]