Significance
Glucocorticoids are primary stress and immune mediators of several fundamental processes, including reproduction and development. However, the direct actions of glucocorticoid signaling in the uterus are largely unknown. We have discovered that the glucocorticoid receptor (GR) in the mouse uterus is essential for normal fertility. Female mice lacking GR specifically in the uterus are subfertile due to defects in implantation related to inadequate remodeling of the endometrial stroma. Moreover, the deficiency in uterine glucocorticoid signaling leads to aberrant gene regulation during tissue remodeling events. Our findings demonstrate previously unrecognized functions of GR in both uterine physiology and fertility.
Keywords: glucocorticoid receptor, uterus, decidualization, implantation
Abstract
In addition to the well-characterized role of the sex steroid receptors in fertility and reproduction, organs of the female reproductive tract are also regulated by the hypothalamic–pituitary–adrenal axis. These endocrine organs are sensitive to stress-mediated actions of glucocorticoids, and the mouse uterus contains high levels of the glucocorticoid receptor (GR). Although the presence of GR in the uterus is well established, uterine glucocorticoid signaling has been largely ignored in terms of its reproductive and/or immunomodulatory functions on fertility. To define the direct in vivo function of glucocorticoid signaling in adult uterine physiology, we generated a uterine-specific GR knockout (uterine GR KO) mouse using the PRcre mouse model. The uterine GR KO mice display a profound subfertile phenotype, including a significant delay to first litter and decreased pups per litter. Early defects in pregnancy are evident as reduced blastocyst implantation and subsequent defects in stromal cell decidualization, including decreased proliferation, aberrant apoptosis, and altered gene expression. The deficiency in uterine GR signaling resulted in an exaggerated inflammatory response to induced decidualization, including altered immune cell recruitment. These results demonstrate that GR is required to establish the necessary cellular context for maintaining normal uterine biology and fertility through the regulation of uterine-specific actions.
Mammalian reproduction is a complex process where defects occurring at any of the sequential steps can lead to poor outcomes or pregnancy demise (reviewed in ref. 1). During early pregnancy, uterine receptivity, implantation, and endometrial remodeling (termed decidualization) are critical to supporting embryonic growth. Signaling pathways tightly governing these stages are coordinated through well-known and extensively studied actions of the ovarian steroid hormones estrogen and progesterone. However, one of the most complex and least understood factors contributing to the early stages of pregnancy is the maternal hypothalamic–pituitary–adrenal axis (HPA). Glucocorticoids are primary stress hormones synthesized in response to activation of the HPA axis that function to maintain homeostasis of a wide variety of physiological processes, including inflammatory and immune responses, development, and reproduction (2, 3). Previous studies implicating glucocorticoid signaling in the female reproductive tract have focused largely on their function in the hypothalamus and pituitary, where chronic stress or glucocorticoid exposure can suppress gonadotropin synthesis and release (reviewed in refs. 2, 4, 5). However, the potential direct actions of glucocorticoids on the uterus and its nurturing environment have been largely ignored.
At the cellular level, the actions of glucocorticoids are mediated by the glucocorticoid receptor (GR), which functions as a ligand-dependent transcription factor. Upon ligand activation, GR regulates the expression of many genes in a tissue- and context-specific manner. Due to the ubiquitous nature of the GR, glucocorticoids can target cells throughout the body. Whole-animal knockouts of GR result in perinatal lethality, demonstrating the critical role GR plays in development but providing no insight into the role of GR in adult uterine physiology and fertility (6). Thus, to elucidate the role of glucocorticoid signaling in reproduction, we conditionally ablated GR in the uterus. These mice are subfertile, exhibiting defects in embryo implantation and subsequent decidualization. Moreover, the regulation of genes critical to decidualization was largely absent in uterine GR KO mice, revealing for the first time a crucial role for uterine GR signaling during the initiation of pregnancy.
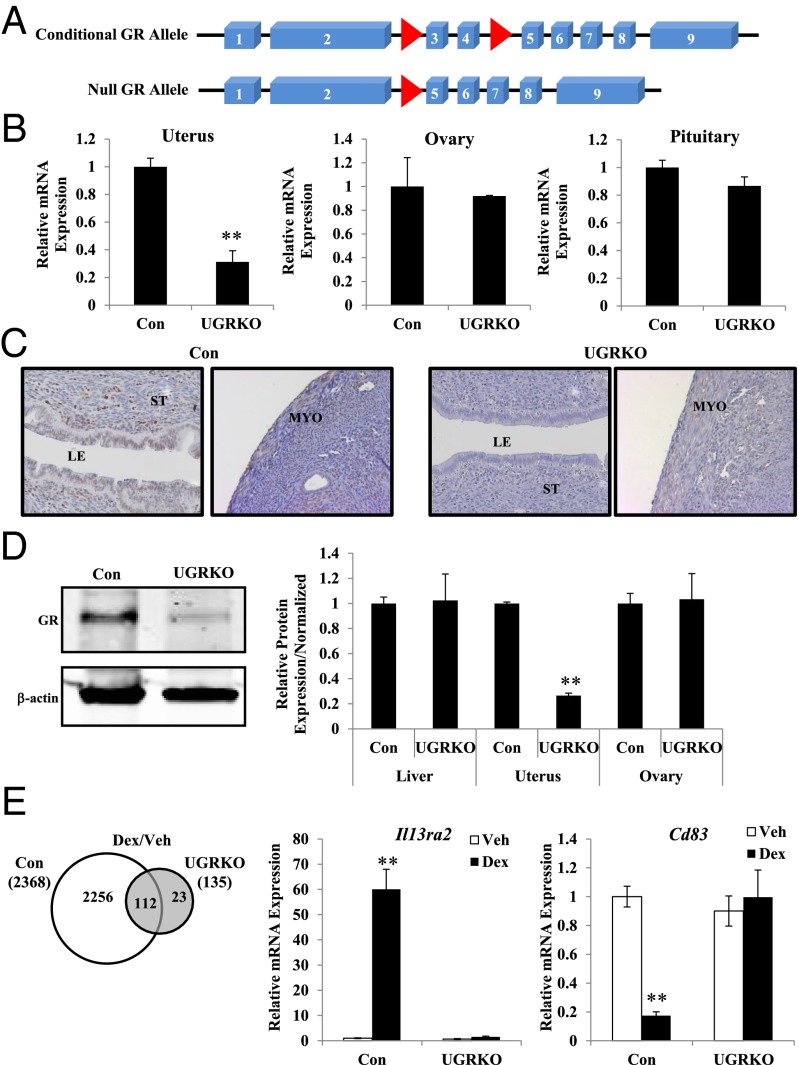
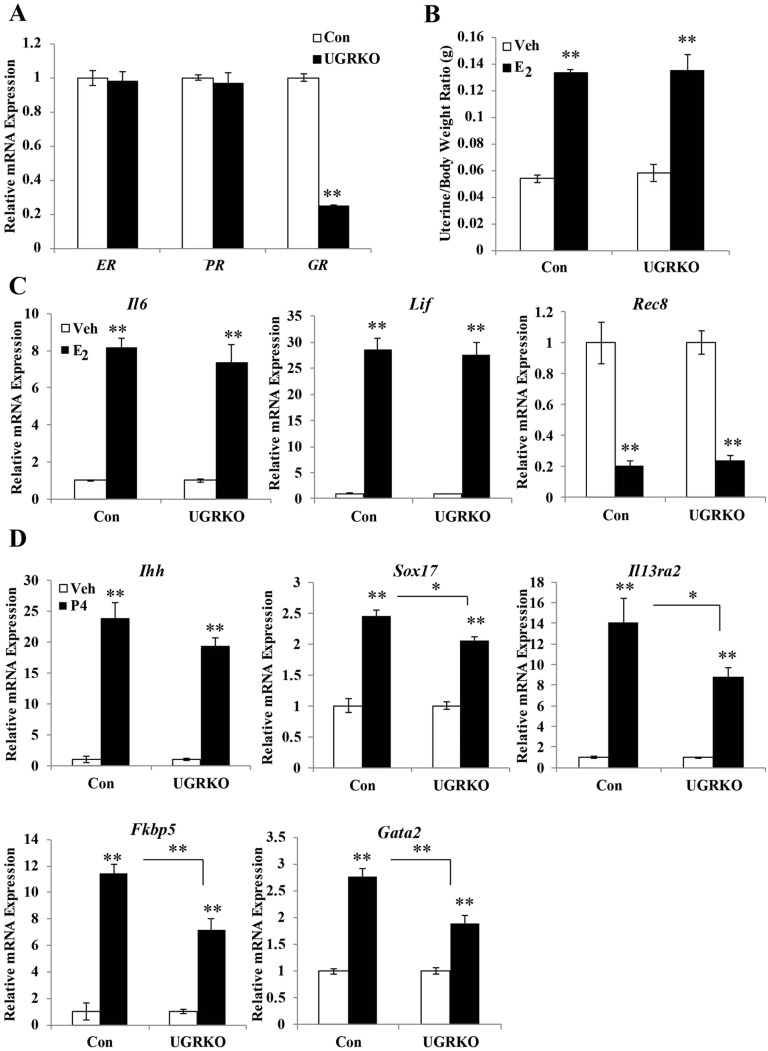
Results
Conditional ablation of GR was necessary to study its in vivo role in adult uterine function because global inactivation of GR leads to perinatal lethality (6). Therefore, GRloxP,loxP mice were crossed to the progesterone receptor (PR)cre mouse model, which recombines alleles in the PR-expressing epithelial and stromal compartments of the uterus (7) (Fig. 1A). Cre-negative littermates served as controls (GRloxP,loxP) in our studies. Compared with controls, GR mRNA was significantly reduced in the uteri but not ovaries or pituitaries from cycle-matched uterine GR KO mice (GRloxP,loxPPRcre/+) (Fig. 1B). Consistent with sites of cre expression in the PRcre mouse, immunohistochemistry revealed a loss of GR in the luminal epithelium and stromal cells but only a partial ablation in the uterine myometrium of uterine GR KO mice (Fig. 1C). Immunoblots of whole-uterine lysates showed a marked reduction in the level of GR protein from the uterine GR KO mice (Fig. 1D). No differences were seen between genotypes in GR protein levels in the liver or ovary, and no changes were apparent in uterine expression of ERα and PR mRNA or protein, indicating the tissue and receptor specificity of the knockdown (Fig. S1 A and B). To determine whether glucocorticoid responsiveness was diminished in the uterus, adrenalectomized/ovariectomized control and uterine GR KO mice were treated with the synthetic glucocorticoid dexamethasone (Dex) for 4 h, and genome-wide gene expression analysis was performed (Fig. 1E). In the absence of uterine GR, dexamethasone treatment resulted in only 135 significantly regulated genes compared with 2,368 in GR-containing control mice. The expression of two identified glucocorticoid-regulated genes was validated in independent biological samples (Fig. 1E). Interleukin 13 receptor, alpha 2 (Il13ra2) was induced, and cluster of differentiation-83 (CD83) was repressed in control mice in response to dexamethasone. In contrast, regulation of both genes was absent following Dex treatment in uterine GR KO mice. The absence of Dex-dependent gene regulation indicates uterine responsiveness to glucocorticoids is impaired in these mice.
Fig. 1.
Generation of mice with a conditional knockout of GR in the uterus. (A) Mice deficient in GR in the uterus were created by crossing mice with a floxed GR allele (GRloxP,loxP; red arrows) with mice expressing PRCre/+ recombinase. Cre-mediated deletion of exons 3 and 4 results in a null GR allele. A frameshift mutation and premature stop codon result if splicing occurs between exons 2 and 5. (B) Quantitative RT-PCR of GR mRNA from the uterus, ovary, and pituitary of control and uterine GR KO mice (n = 3–5 mice per genotype). The results represent the mean ± SEM. **P < 0.01. (C) Representative immunohistochemistry staining of uterine sections from control and uterine GR KO mice with anti-GR antibody (200× magnification). Counterstaining is hematoxylin. LE, luminal epithelium; MYO, myometrium; ST, stroma. (D) Representative immunoblot of GR from the uterus of control and uterine GR KO mice. GR protein expression in the liver, uterus, and ovary was quantified and normalized to levels of the housekeeping protein β-actin (n = 5–9 mice per genotype). The results represent the mean ± SEM. **P < 0.01. (E) Adrenalectomized/ovariectomized control and uterine GR KO mice were injected i.p. with vehicle (saline; white bar) or 1 mg/kg Dex (black bar) for 4 h, and microarray analysis was performed on isolated mRNA (n = 3 mice per group). The number of genes statistically different (P < 0.01) between control and uterine GR KO groups were sorted by treatment using Venn diagram. Quantitative RT-PCR was performed on independent mRNA samples (n = 4 mice per group) to validate Il13ra2 and Cd83 mRNA levels in total RNA isolated from the uterus. Data are mean ± SEM. **P < 0.01.
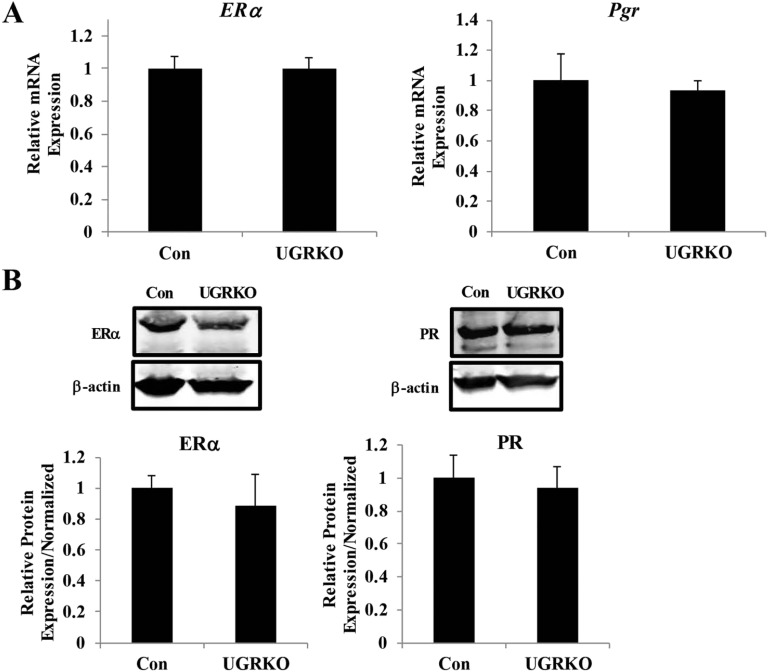
Fig. S1.
Ablation of GR does not alter uterine estrogen or progesterone receptor expression. (A) Quantitative RT-PCR of ERα and PR mRNA from the uterus of control and uterine GR KO mice (n = 3–5 mice per genotype). The results represent the mean ± SEM. (B) Representative immunoblot of ERα and PR from the uterus of control and uterine GR KO mice. Protein expression in the uterus was quantified and normalized to levels of the housekeeping protein β-actin (n = 3–6 mice per genotype). The results represent the mean ± SEM.
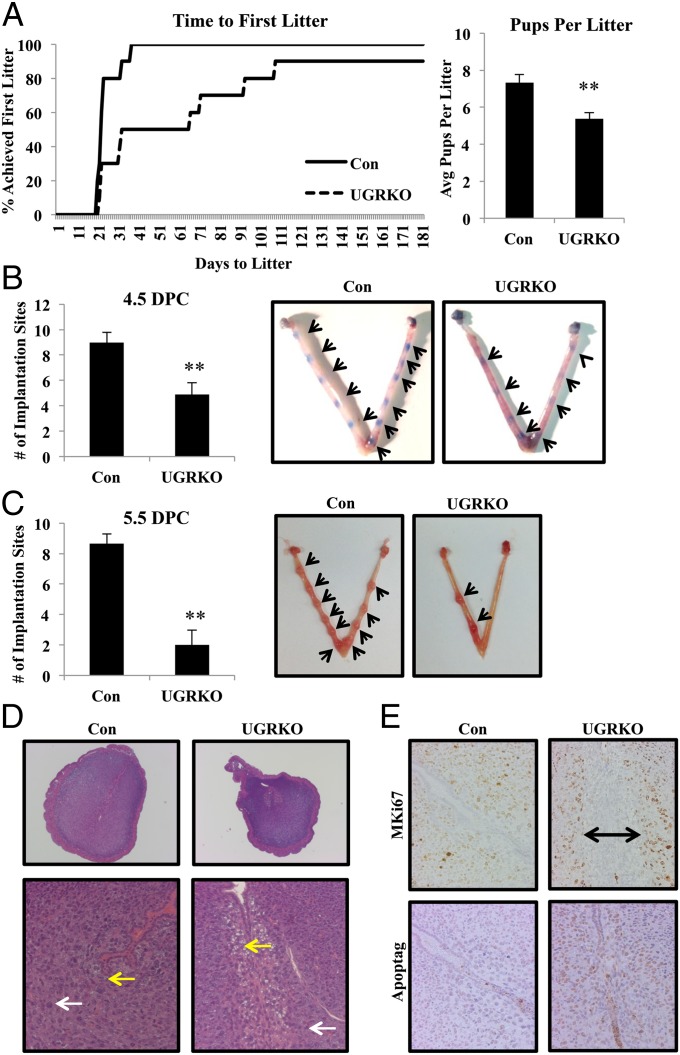
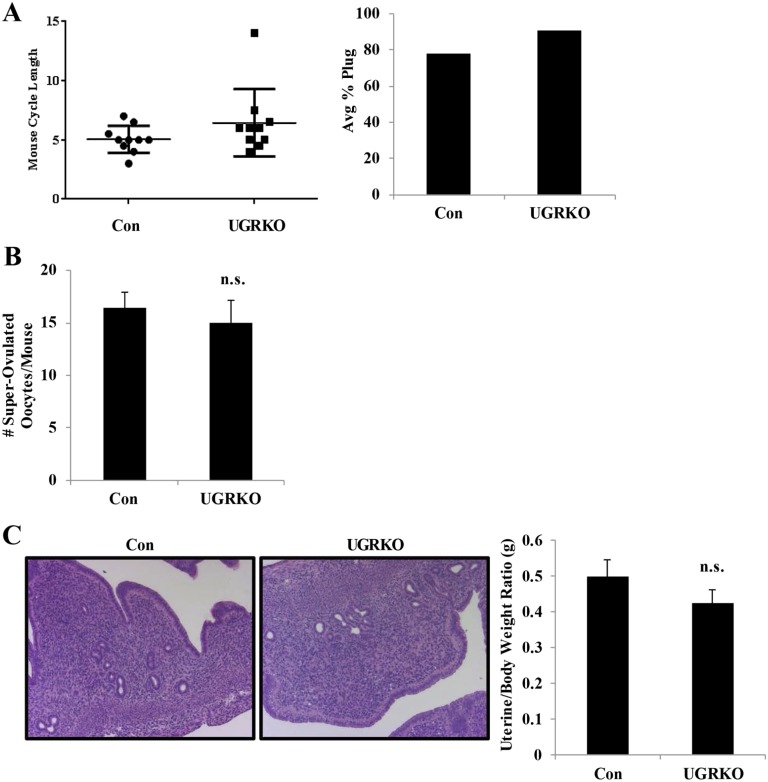
To elucidate the impact of uterine GR ablation on fertility, 8-wk-old sexually mature control and uterine GR KO female mice (n = 10 for each genotype) were mated with known fertile WT male mice for 6 mo (Fig. 2A). Whereas the control mice exhibited normal fertility and fecundity (25.2 ± 1.78 d to first litter and 7.34 ± 0.42 pups per litter), the uterine GR KO mice were considerably subfertile (66 ± 16.57 d to first litter and 5.37 ± 0.33 pups per litter). The estrous cycle was not significantly different in the uterine GR KO mice compared with controls (Fig. S2A), although one of the 10 uterine GR KO mice never completed an estrous cycle. Furthermore, vaginal plugs were found in uterine GR KO mice, indicating that the subfertility was not due to the absence of mating (Fig. S2A). To examine the ability to produce oocytes, 8-wk-old females were stimulated with gonadotropins and superovulated oocytes were retrieved from the oviduct. No differences in the number of oocytes present were observed between genotypes, suggesting that the subfertility observed is not due to impaired ovarian function (Fig. S2B). Histological examination of the uteri of 2-mo-old uterine GR KO mice showed no gross morphological changes, and no differences were observed in uterine weight (Fig. S2C).
Fig. 2.
Uterine GR deficiency results in subfertility. (A) A 6-mo breeding study reveals uterine GR KO mice are subfertile (n = 10 mice per genotype). The results represent the mean ± SEM. *P < 0.05; **P < 0.01. (B and C) Gross morphology and quantification of implantation sites in pregnant uteri. Control and uterine GR KO mice were mated with wild-type males. The morning observance of a vaginal plug was considered 0.5 dpc. The average number of observed implantation sites at 4.5 dpc (n = 9–10 mice per genotype) (B) and 5.5 dpc (n = 6–11 mice per genotype) (C) in control and uterine GR KO mice was quantified. The results represent the mean ± SEM. **P < 0.01. Images are representative uteri showing implantation sites. Arrows mark implantation sites. (D) Representative images of H&E-stained cross-sections of 5.5 dpc implantation sites (magnification: Upper, 40×; Lower, 200×). White arrows indicate decidualizing stromal cells. Yellow arrows denote cell vacuolization present in uterine GR KO mice but absent in control mice. (E) Measurement of uterine cell proliferation by MKi67 and cell death by TUNEL immunohistochemistry in control and uterine GR KO mice of 5.5 dpc implantation sites (200× magnification). Counterstaining is hematoxylin. Bar with arrows designates region with no proliferation.
Fig. S2.
Uterine GR KO mice exhibit normal mating behavior, ovarian function, and uterine morphology. (A) Average cycle length was calculated based on daily vaginal smears, where the time to complete one cycle was evaluated over 14 d. Each dot represents the cycle length in days for each of the 10 mice per genotype. Average percentage plug rate was calculated based on the number of female mice where a copulatory plug was present on 0.5 dpc following mating with a wild-type C57BL/6 male mouse. The results represent the mean of 10 female mice per genotype. (B) Control and uterine GR KO females were stimulated with gonadotropins, and oocytes were collected from the oviduct at 0.5 dpc (n = 10 mice per genotype). The number of superovulated oocytes was comparable (n.s., not significant) between hormone-stimulated control and uterine GR KO female mice (control, 16.5 ± 4.5; uterine GR KO, 15 ± 7.6). The results represent the mean ± SEM. (C) Representative images of H&E-stained longitudinal sections of control and uterine GR KO uteri (magnification 100×). Average uterine weight was comparable (n.s., not significant) between cycle-matched control and uterine GR KO mice (control, 0.49 ± 0.21; uterine GR KO, 0.42 ± 0.15). The results represent the mean ± SEM.
Actions of the ovarian hormones estrogen and progesterone in the uterus are critical to the establishment of pregnancy (reviewed in ref. 8). To investigate whether the subfertility observed in the uterine GR KO mice was due to deficient estrogen and progesterone signaling, adrenalectomized/ovariectomized control and uterine GR KO mice were treated with E2 or P4, and the estrogen-dependent induction of uterine weight (24 h postinjection) and target gene expression (4 h postinjection) were analyzed. The absence of uterine GR does not alter the mRNA expression of ER or Pgr (Fig. S3A). There was no significant difference in uterine weight gain (Fig. S3B) or E2 target gene expression (Fig. S3C) in control mice compared with uterine GR KO mice, indicating that GR ablation does not impair estrogen responsiveness. The expression of acute progesterone target genes Ihh, Sox17, Il13ra2, Fkbp5, and Gata2 was induced by progesterone injection in both control and uterine GR KO mice (Fig. S3D). However, there was modest attenuation of the extent of gene induction in uterine GR KO mice for some of these target genes, suggesting that progesterone action is preserved in a gene-specific fashion.
Fig. S3.
GR ablation does not impair the uterine response to estrogen and progesterone. (A) Quantitative RT-PCR of ERα, PR, GR mRNA from the uterus of control and uterine GR KO mice (n = 4 mice per genotype). The results represent the mean ± SEM. (B) Proportion uterine weight relative to body weight was determined in mice (n = 9–14 per group) following 24-h treatment with vehicle or 10 μg/kg of E2. Bar graphs show mean ± SEM. Quantitative RT-PCR measurement of target genes after (C) 4 h E2 or (D) P4 (n = 4 per treatment group). Bar graphs show mean ± SEM. *P < 0.05; **P < 0.01.
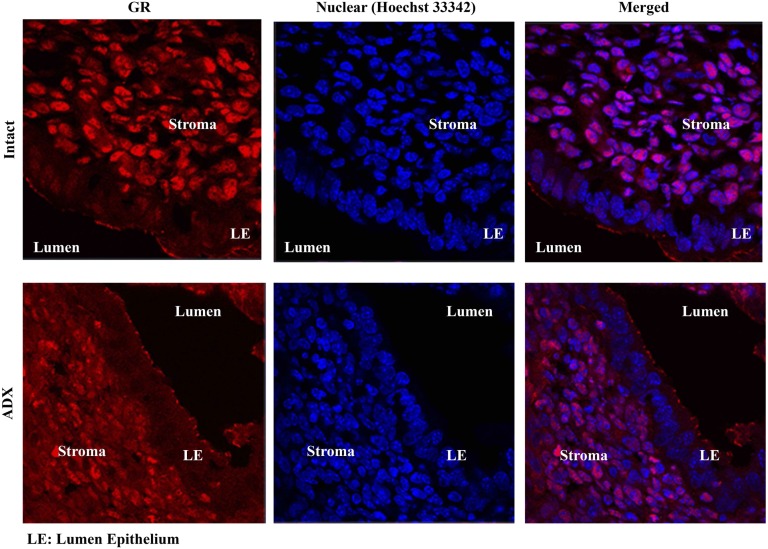
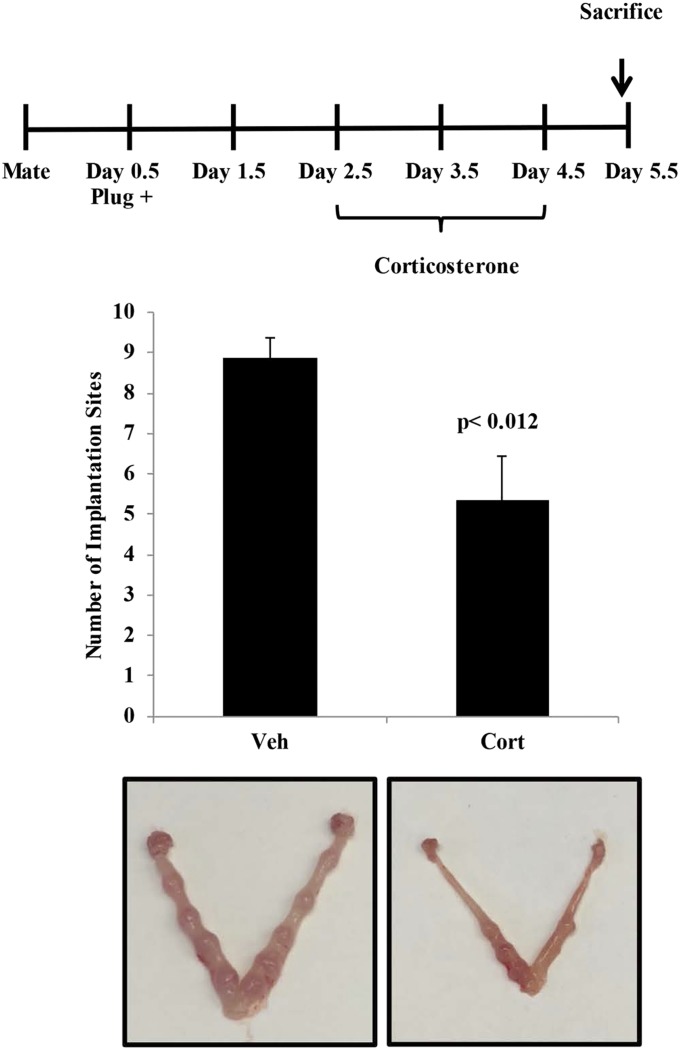
Based on these findings we sought to determine if the effects of uterine GR signaling or deletion were critical for reproductive events of early pregnancy. Embryo attachment and subsequent invasion into a receptive endometrium are indispensable for the establishment of pregnancy. To determine whether circulating glucocorticoids are sufficient to activate the glucocorticoid receptor during implantation, we evaluated translocation of the glucocorticoid receptor at 4.5 d postcoitum (dpc) in intact and adrenalectomized mice (Fig. S4). Circulating levels of glucocorticoids in intact mice lead to nuclear translocation of the glucocorticoid receptor, and this effect was largely abolished in adrenalectomized mice. These data suggest that actions of the uterine glucocorticoid receptor during early pregnancy are mediated by glucocorticoids. Maternal stress (9) or exposure of pregnant mice to high levels of glucocorticoids can significantly reduce implantation sites at 4.5 dpc (corticosterone: 5.36 ± 1.45; controls: 8.89 ± 1.45), suggesting that altered glucocorticoid signaling can interfere with implantation (Fig. S5). However, this approach is subject to both indirect and direct actions of glucocorticoids at peripheral sites outside the uterus. Thus, we examined the number of implantation sites in uterine GR KO mice and found the number to be significantly lower than in controls at 4.5 dpc (uterine GR KO: 4.88 ± 2.75; controls: 9.0 ± 2.44), indicating that uterine GR is necessary for successful implantation (Fig. 2B).
Fig. S4.
Endogenous glucocorticoids drive nuclear translocation of the glucocorticoid receptor during pregnancy. Immunofluorescence of the glucocorticoid receptor in cross-sections of 4.5 dpc pregnant intact and adrenalectomized mice. Glucocorticoid receptor expression is shown in red, and Hoechst staining of the nuclei is shown in blue. Images taken at 630×.
Fig. S5.
Maternal exposure to glucocorticoids impairs implantation. Pregnant mice were treated by i.p. injection of vehicle or 10 mg/kg corticosterone at 2.5, 3.5, and 4.5 dpc. The number of implantation sites was counted at 5.5 dpc. Bar graphs show mean ± SEM.
Moreover, the number of implanting embryos visualized was further decreased at 5.5 dpc (uterine GR KO: 2.0 ± 3.22; controls: 8.66 ± 1.50; Fig. 2C). Eight of 11 uterine GR KO mice that were plug-positive had no visible implantation sites at 5.5 dpc. Of those uterine GR KO mice able to implant, histological examination of the observed implantation sites at 5.5 dpc revealed altered endometrial architecture (Fig. 2D). At 5.5 dpc, the blastocyst has implanted and decidualization of the stromal cell compartment is characterized by proliferation and differentiation. Compared with controls, stromal cells of the uterine GR KO uterus failed to equivalently decidualize. Furthermore, at day 5.5 uterine GR KO mice exhibit vacuoles in the stromal cell compartment lining the luminal epithelium. This area of vacuolization is devoid of proliferating stromal cells, unlike the stromal compartment of control mice (Fig. 2E). Interestingly, the stromal cells lining the luminal epithelium in the uterine GR KO mice were TUNEL positive, indicating that this region of the uterus is undergoing cell death (Fig. 2E). These findings suggest that uterine GR plays a major role in both embryo implantation and normal uterine decidualization.
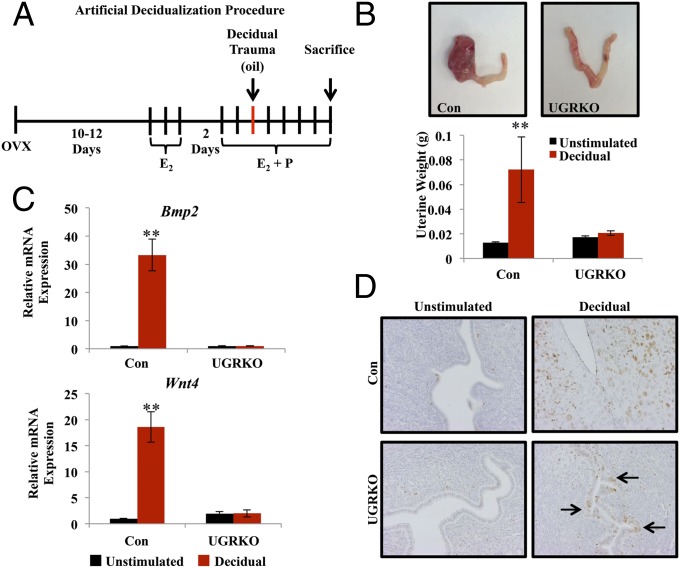
Uterine decidualization is a key event in embryo implantation and is essential to supporting the developing embryo. Decidualization requires hormone priming and an inciting event, either embryo attachment or an inflammatory stimulus. To elucidate the cause of defective implantation and subsequent decidualization failure, female control and uterine GR KO mice were assessed for their ability to mount a decidual response following exogenous hormone treatment and deciduogenic stimulus (Fig. 3A). For each genotype, one uterine horn received a deciduogenic stimulus, and the contralateral horn served as the unstimulated control. Five days after receiving a decidual trauma, the decidualized horn of the control mice exhibited a robust response (Fig. 3B, Left). In stark contrast, the stimulated horn of the uterine GR KO mice showed no significant increase in uterine size compared with the unstimulated horn. Previous studies have determined molecular markers of decidualization, including induction of the genes bone morphogenetic protein 2 (Bmp2) and wingless-related MMTV integration site 4 (Wnt4) (10, 11). The expression of Bmp2 and Wnt4 mRNA was highly induced in the decidual horn of the control mice (Fig. 3C). However, neither Bmp2 nor Wnt4 were induced in the stimulated horn of the uterine GR KO mice, consistent with the absence of a decidual response in these mice. Stromal cell proliferation was assessed by MKi67 staining (Fig. 3D). Robust stromal cell proliferation was observed in the decidual horn of the control mice. Interestingly, in addition to the absence of stromal cell proliferation in the decidual horn of uterine GR KO mice, the luminal epithelium was undergoing unexpected proliferation. Persistent epithelial cell proliferation, an estradiol-driven function, indicates that the antagonism of the estrogenic response required during the window of implantation is deficient in the uterine GR KO mice, implicating GR in this process (12).
Fig. 3.
Uterine GR is critical for decidualization of the mouse endometrial stroma. (A) Timeline of the artificial decidualization procedure. (B) Gross uterine morphology of uteri 5 d after deciduogenic stimulus. (Left) Horn received deciduogenic stimulus. (Right) Horn did not receive a stimulus (unstimulated) in both the control and uterine GR KO. The unstimulated (black bar) and decidualized horns (red bar) were weighed in the control and uterine GR KO mice (n = 4–5 mice per group). The results represent the mean ± SEM. **P < 0.01. (C) mRNA measurement of known decidual regulators Bmp2 and Wnt4 on the fifth day following deciduogenic stimulus by quantitative RT-PCR. The results represent the mean ± SEM. **P < 0.01. (D) Proliferative marker MKi67 by immunohistochemistry of the unstimulated and decidual horn of control and uterine GR KO mice 5 d after deciduogenic stimulus (magnification 200×). Counterstaining is hematoxylin.
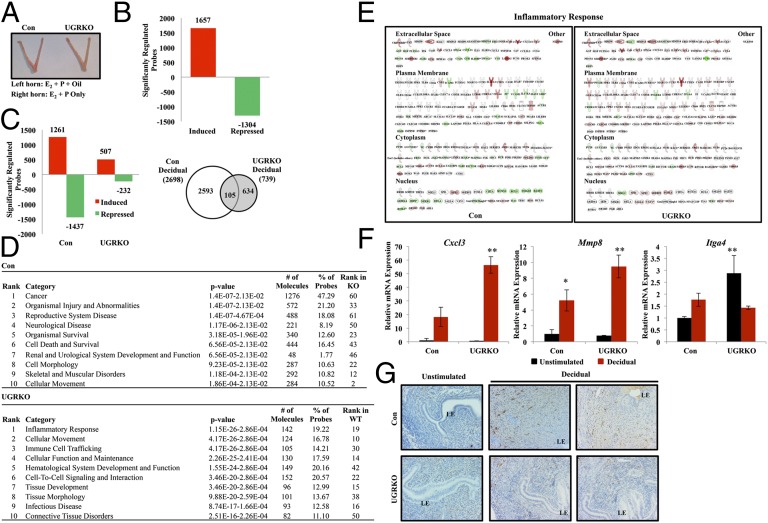
To investigate the role of GR in the coordinated orchestration of molecular events leading to decidualization, genome-wide microarray analysis was performed on the unstimulated and decidual horn of control and uterine GR KO mice 2 h following decidual trauma. Although no gross differences were apparent in uterine size at this early time point (Fig. 4A), gene expression differed vastly in the unstimulated and decidual horn of the control mice compared with the uterine GR KO mice (Fig. 4 B and C). When the unstimulated horns were compared between genotypes, there were 2,691 genes that were identified as significantly different in the uterine GR KO mice compared with controls (1,657 induced; 1,304 repressed), indicating that the response to the priming hormones was vastly different in the absence of uterine GR. This alteration in gene expression likely contributes to these mice being unable to respond to the deciduogenic stimulus (Fig. 4B). According to literature-based Ingenuity Pathway Analysis (IPA) analysis, the significantly different genes in the unstimulated horn were enriched for the biological functions organismal survival, reproductive system disease, and embryonic development (Table S1).
Fig. 4.
Transcriptional response to decidualization is blunted in uterine GR KO mice. (A) Gross uterine morphology of uteri 2 h after deciduogenic stimulus. (Left) Horn received stimulus (E2 + P + oil). (Right) Control (E2 + P). (B) Microarray analysis was performed on mRNA isolated from both the unstimulated horn and horn receiving deciduogenic stimulus (decidual) of control and uterine GR KO mice. Comparison of the unstimulated horn in control and uterine GR KO mice allowed the identification of gene probes differentially regulated in response to E2 + P. (C) Probes differentially expressed during decidualization were identified by comparing the unstimulated horn from the control and uterine GR KO mice to the decidual horn of the same genotype. Significantly induced and repressed probes are graphed by genotype. The number of probes statistically different (P < 0.05) between control and uterine GR KO were sorted by genotype using Venn diagram. (D) The differentially expressed decidual genes were analyzed by IPA software for each genotype. Shown are the top 10 diseases and biological functions most significantly associated with the genes differentially regulated in the uteri of control and uterine GR KO during decidualization. (E) Inflammatory response is the most significantly regulated pathway following the decidual stimulus in the uterine GR KO mice. The pathway is shown with genes regulated in control and uterine GR KO mice with expression values of each genotype overlaid (red indicates induced; green indicates repressed). (F) mRNA of significantly regulated genes Cxcl3, Mmp8, and Itga4 that are associated with the inflammatory response was independently validated by quantitative RT-PCR (n = 3–4 mice per group). Black bar, unstimulated horn; red bar, decidual horn. The results represent mean ± SEM. *P < 0.05; **P < 0.01. (G) Identification of macrophages by F4/80 immunoreactivity in the unstimulated and decidual horn of control and uterine GR KO mice 2 d after deciduogenic stimulus. Counterstaining is hematoxylin. 200× magnification. LE, luminal epithelium.
Table S1.
Deletion of uterine GR results in altered transcriptional response to preimplantation hormones
| Rank | Category | P value | No. of molecules | % of probes |
| 1 | Cancer | 1.94E-11–1.24E-02 | 1,245 | 46.26 |
| 2 | Gastrointestinal disease | 1.94E-11–7.78E-03 | 640 | 23.78 |
| 3 | Cellular growth and proliferation | 2.76E-10–6.96E-03 | 511 | 18.99 |
| 4 | Organismal survival | 1.86E-09–6.96E-03 | 351 | 13.04 |
| 5 | Organismal injury and abnormalities | 1.97E-08–1.16E-02 | 671 | 24.93 |
| 6 | Reproductive system disease | 1.97E-08–9.58E-03 | 537 | 19.95 |
| 7 | Embryonic development | 2.07E-07–1.20E-02 | 319 | 11.85 |
| 8 | Organismal development | 2.07E-07–1.14E-02 | 463 | 17.20 |
| 9 | Developmental disorder | 6.63E-07–1.22E-02 | 299 | 11.11 |
| 10 | Skeletal and muscular disorders | 6.63E-07–7.78E-03 | 219 | 8.13 |
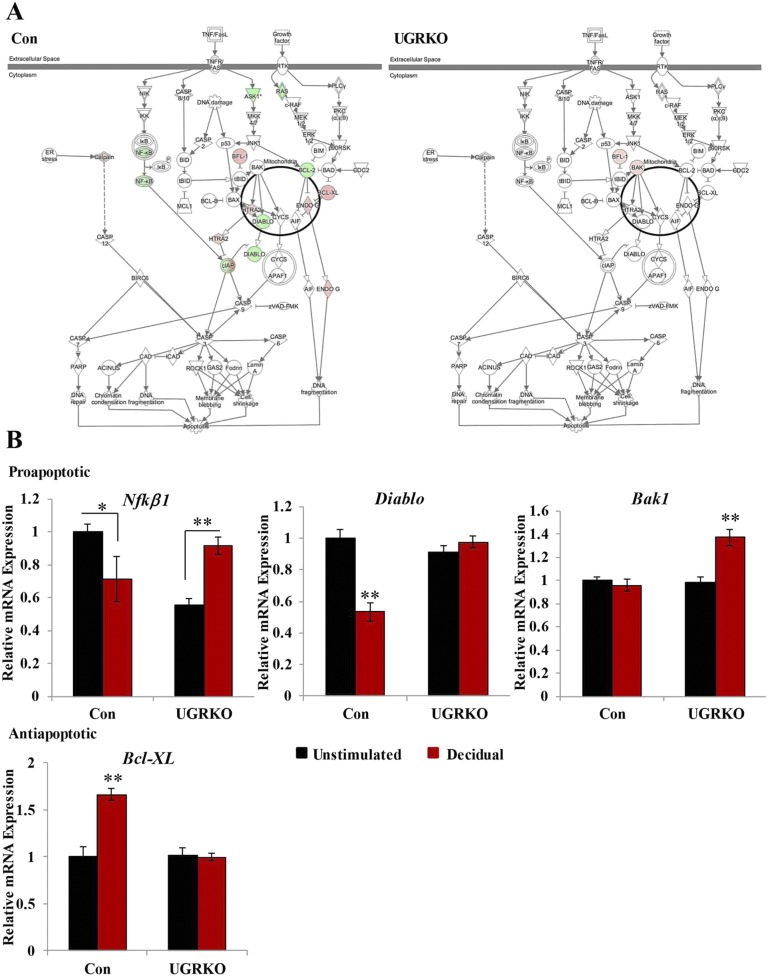
The genes significantly regulated in the decidual horn were then compared with those in the unstimulated horn to determine the uterine transcriptional response to decidualization in the presence and absence of uterine GR. In control mice, 2,698 genes were induced or repressed in response to decidualization, whereas only 739 genes were significantly regulated in uterine GR KO mice (Fig. 4C). Though the ratio of induced to repressed genes was similar in control mice, the balance shifts in uterine GR KO mice to favor induction. Approximately 70% of the genes significantly regulated in the uterine GR KO mice were induced, suggesting that GR signaling is necessary to limit expression of these genes during decidualization. Due to the considerable reduction in the number of genes significantly regulated in response to decidualization in the uterine GR KO mice, the gene lists were compared to determine if the response to decidualization in uterine GR KO mice represented a diminished overall response or genes regulated uniquely in the absence of GR. A comparison of the genes significantly regulated by decidualization in the uterine GR KO mice revealed only 14% (105 genes) overlapped with those significantly regulated in control mice, defining uniquely regulated genes and indicating that GR signaling is necessary to maintain the transcriptional context required for decidualization. Analysis of the significantly expressed genes in each genotype by IPA software identified considerably different biological functions (Fig. 4D). In fact, only one of the top 10 biological functions (cellular movement) was common between control and uterine GR KO mice. Data from this microarray may shed light on the observed apoptosis in the cross-sections of 5.5 dpc uterine GR KO mice (Fig. S6A). In control mice, decidualization induced the expression of anti-apoptotic genes and repressed several proapoptotic genes (Fig. S6B). In response to decidualization in uterine GR KO mice, only two genes are regulated in the apoptosis signaling pathway, suggesting GR signaling is required to balance the expression of genes regulating cell death and survival in the uterus. Analysis of this microarray was inconclusive as to the contribution of autophagy regulated genes to the presence of vacuoles in the cross-sections of 5.5 dpc uterine GR KO mice.
Fig. S6.
(A) The differentially expressed decidual genes were analyzed by IPA software, and the regulation of genes in the apoptosis signaling pathway was visualized for each genotype. (B) Quantitative RT-PCR to independently validate regulation of pro- and anti-apoptotic genes (n = 3–6 mice per group). Bar graphs show mean ± SEM. *P < 0.05; **P < 0.01.
Interestingly, many of the top biological functions enriched following the deciduogenic stimulus in uterine GR KO mice were related to inflammatory and immune responses. These findings were further substantiated using an independent functional annotation clustering analysis. Gene Ontology (GO) analysis found no common biological processes in control and uterine GR KO mice. The top biological functions identified by GO analysis of the genes regulated in uterine GR KO mice included chemotaxis, inflammatory response, defense response, immune response, and immune cell migration (Tables S2 and S3). As one of the most notably affected functions according to both IPA and GO analysis, the inflammatory response pathway was compared in control and uterine GR KO mice. There was a pronounced shift in the number of genes regulated and the ratio of induced to repressed genes following decidualization in uterine GR KO mice (Fig. 4E). In control mice, 102 genes (representing 4% of the total genes regulated in control mice) were significantly regulated with 45% induced and 55% repressed. In the uterine GR KO mice, 134 genes (representing 18% of the total genes regulated in uterine GR KO mice) were significantly regulated with 85% induced and 15% repressed. Furthermore, of the 212 genes annotated as part of the inflammatory response pathway, only 49 (23%) were in common in control and uterine GR KO mice, and 10% of these commonly regulated genes were anticorrelated in direction of regulation. Among the genes identified with differential expression between control and uterine GR KO mice were chemokine ligand 3 (Cxcl3), matrix metalloproteinase-8 (Mmp8), and integrin alpha 4 (Itga4). To independently confirm the changes in these three genes, we performed quantitative RT-PCR on the uteri from four additional control and uterine GR KO mice 2 h following the deciduogenic stimulus (Fig. 4F). The magnitude of induction of Cxcl3 and Mmp8 following the stimulus was significantly greater in the uterine GR KO mice, suggesting that uterine GR may dampen the inflammatory response during decidualization. Interestingly, expression of Itga4 mRNA, an integrin expressed in the uterus during early pregnancy, was significantly higher in the unstimulated horn of uterine GR KO mice compared with control mice (13). In response to decidualization, Itga4 was repressed in uterine GR KO mice, whereas it was induced in controls.
Table S2.
Gene ontology analysis of genes differentially expressed in response to decidualization in uterus of control and uterine GR KO mice: Control
| No. | Annotation | No. of genes | Bayes factor | P value |
| 1 | GO:0009581 [5]: detection of external stimulus | 37 | 35 | <0.0001 |
| 2 | GO:0007600 [5]: sensory perception | 37 | 34 | <0.0001 |
| 3 | GO:0007186 [6]: G protein coupled receptor protein signaling pathway | 71 | 30 | <0.0001 |
| 4 | GO:0050877 [4]: neurophysiological process | 49 | 30 | <0.0001 |
| 5 | GO:0007606 [6]: sensory perception of chemical stimulus | 34 | 30 | <0.0001 |
| 6 | GO:0007608 [7]: perception of smell | 32 | 29 | <0.0001 |
| 7 | GO:0009605 [4]: response to external stimulus | 94 | 26 | <0.0001 |
| 8 | GO:0050875 [3]: cellular physiological process | 743 | 18 | <0.0001 |
| 9 | GO:0050896 [3]: response to stimulus | 147 | 17 | <0.0001 |
| 10 | GO:0007166 [5]: cell surface receptor linked signal transduction | 116 | 17 | <0.0001 |
| 11 | GO:0050874 [3]: organismal physiological process | 124 | 16 | <0.0001 |
| 12 | GO:0008152 [3]: metabolism | 543 | 12 | <0.0001 |
| 13 | GO:0044237 [4]: cellular metabolism | 508 | 10 | <0.0001 |
| 14 | GO:0044238 [4]: primary metabolism | 490 | 10 | <0.0001 |
| 15 | GO:0007165 [4]: signal transduction | 187 | 9 | <0.0001 |
| 16 | GO:0051276 [6]: chromosome organization and biogenesis | 4 | 5 | 0.0003 |
Table S3.
Gene ontology analysis of genes differentially expressed in response to decidualization in uterus of control and uterine GR KO mice: UGRKO
| No. | Annotation | No. of genes | Bayes factor | P value |
| 1 | GO:0042330 [5]: taxis | 21 | 29 | <0.0001 |
| 2 | GO:0006935 [6]: chemotaxis | 21 | 29 | <0.0001 |
| 3 | GO:0006954 [5]: inflammatory response | 19 | 21 | <0.0001 |
| 4 | GO:0006952 [5]: defense response | 50 | 18 | <0.0001 |
| 5 | GO:0006955 [4]: immune response | 43 | 18 | <0.0001 |
| 6 | GO:0009607 [4]: response to biotic stimulus | 51 | 16 | <0.0001 |
| 7 | GO:0042221 [6]: response to chemical substance | 23 | 16 | <0.0001 |
| 8 | GO:0007154 [3]: cell communication | 114 | 12 | <0.0001 |
| 9 | GO:0007165 [4]: signal transduction | 100 | 12 | <0.0001 |
| 10 | GO:0009628 [5]: response to abiotic stimulus | 24 | 9 | <0.0001 |
| 11 | GO:0009611 [5]: response to wounding | 22 | 9 | <0.0001 |
| 12 | GO:0043207 [5]: response to external biotic stimulus | 28 | 7 | <0.0001 |
| 13 | GO:0050900 [6]: immune cell migration | 5 | 6 | <0.0001 |
| 14 | GO:0009613 [5]: response to pest, pathogen or parasite | 26 | 6 | <0.0001 |
| 15 | GO:0006950 [4]: response to stress | 34 | 5 | 0.0001 |
| 16 | GO:0045123 [6]: cellular extravasation | 3 | 5 | 0.0002 |
Because the regulation of genes involved in the inflammatory response and immune cell trafficking is abnormal following the deciduogenic stimulus in uterine GR KO mice, we wanted to determine whether immune cell populations differ in the decidual horn of control and uterine GR KO mice. Macrophage infiltration, apparent in the decidualizing control horn 2 d following the deciduogenic stimulus, is largely absent in the stimulated horn of the uterine GR KO mice (Fig. 4G). Together, these data indicate that disruption of GR signaling in the uteri of uterine GR KO mice not only leads to the disruption of the anti-inflammatory actions of GR but also dysregulation of multiple genes and pathways with critical roles during decidualization.
Discussion
Glucocorticoids are critical regulators of a wide variety of physiological processes largely due to their actions as transcriptional regulators, allowing signal amplification across many molecular networks. Initially recognized for their potent anti-inflammatory properties, glucocorticoids and the ubiquitous GR mediate transcriptional regulation and signal transduction throughout the body. Glucocorticoid signaling has been suggested to play a role centrally in regulating female fertility through modulation of hypothalamic and pituitary functions. However, only indirect evidence has indicated that glucocorticoids are able to modulate the actions of estradiol in the uterus (14, 15). To elucidate the direct contribution of glucocorticoid signaling in the uterus, GR was conditionally ablated using the PRcre mouse model (7). We have demonstrated that GR KO in the uterus results in early pregnancy failure, indicating that GR is required for appropriate uterine endometrial receptivity vital to implantation and decidualization. In the absence of uterine GR, the incidence of implantation was reduced at 4.5 dpc. A further decrease in the number of implantation sites was observed at 5.5 dpc, indicating that GR is necessary for implantation and the uterine response to implantation to support pregnancy. Early pregnancy loss is of considerable concern clinically, where it is estimated that ∼30–60% of conceptuses are lost before 12 wk (16, 17).
Successful implantation requires both interaction between the luminal epithelium and synchronous development of the endometrial stroma. The etiology of subfertility in the uterine GR KO mice suggests GR may play a role in both of these processes and is likely due to the combined dysregulation of many different genes. Integrins are important adhesion molecules present at the surface of the luminal epithelium during implantation. Immediately following the activation of the uterus by a decidualization stimulus expression of Itga4 was induced in control mice, though this same stimulus resulted in Itga4 repression in uterine GR KO mice. At the initiation of implantation, expression of Itga4 is induced in the uterus, and in vivo blocking of Itga4 in the uterus of pregnant mice on the day of implantation leads to implantation failure (13). Dexamethasone has been shown to induce Itga4 expression in eosinophils and bone-marrow cultures, although to our knowledge this is the first study implicating GR regulation of Itga4 in the uterus during pregnancy (18). In addition to Itga4, many other plasma membrane receptors exhibited altered expression levels in uterine GR KO mice. Prostaglandins also have an important role as signaling molecules in the early events of implantation and decidualization, and both prostaglandin I2 receptor (Ptgir) and prostaglandin E receptor 4 (Ptger4) were down-regulated in uterine GR KO mice following the decidualization stimulus (19). Although neither of these genes has been previously shown to be regulated by glucocorticoids, we found that Ptgir was significantly regulated by dexamethasone in our microarray in the uterus of control mice. Failure of glucocorticoids to maintain appropriate expression of these and other cell surface receptors may underlie defective implantation in the absence of uterine GR.
In control mice, activation of the uterus by the decidualization stimulus resulted in a transcriptional profile favoring functions related to cell survival and morphology. The biological response to decidualization in the control mice was characterized by abundant stromal cell proliferation and morphological changes. As indicated by the pathway analysis of genes regulated by decidualization in the uterine GR KO mice, cell survival and morphology were not highly regulated biological functions. Accordingly, stromal cell proliferation was significantly blunted in the GR-deficient uterus. Both bone morphogenic protein 2 (Bmp2) and wingless-related MMTV integration site 4 (Wnt4) are key regulators of stromal cell proliferation and differentiation because deletion of either gene confers fertility defects in early pregnancy (10, 11). In the absence of an intact GR signaling pathway in the uterus, the induction of these genes during decidualization was abolished. Uterine deletion of Wnt4 results in aberrant apoptosis in the stroma adjacent to the luminal epithelial cells, and this phenotype was recapitulated in the uterine GR KO mice. Despite the similarities in phenotypes, it is not clear whether Wnt4 and Bmp2 are direct GR target genes or alterations in their expression profiles are secondary to the defect in decidualization.
Strikingly, the genes that were significantly regulated in response to decidualization in the uterine GR KO mice were enriched for biological functions related to the immune system and inflammatory response. Historically, maternal tolerance of the semiallogenic fetus was thought to occur through immune suppression (20). It is now well recognized that the maternal immune system not only adapts during pregnancy but is also actively involved in all stages of the reproductive process. Animal models have shown that appropriate levels of immune cell activity in the uterus are necessary to establish and maintain a healthy pregnancy (21–23). Macrophages play an important role in the endometrium during early pregnancy, including uterine remodeling and inducing the expression of epithelial glycoproteins required for embryo attachment and implantation (24, 25). Macrophage depletion in mice results in early pregnancy loss due to implantation failure, which can be corrected by macrophage transfer (26). Macrophage recruitment into the endometrium is largely absent in the uterine GR KO mice, suggesting that altered expression of the signaling and transcriptional networks regulating the immune and inflammatory response may underlie subfertility in uterine GR KO mice.
Infertility in women may arise from a variety of causes, including inappropriate hormone levels, ovulation defects, damage or obstruction of the female reproductive tract, or an inadequate uterine environment. Understanding the mechanisms governing uterine receptivity, implantation, and uterine support of the developing embryo has been aided by the development of gene-targeting experiments in mice and uterine-specific deletion models. Such model systems have exposed the complex and redundant nature in which the uterus regulates early stages of pregnancy (27). Historically, stress or high levels of glucocorticoids have been suggested to regulate reproductive function through modulating hypothalamic and pituitary actions on the gonadotropins (5, 28). Our model indicates that uterine GR signaling plays a crucial role in establishing uterine receptivity, as evidenced by microarray analysis demonstrating deficiencies in the programming required to induce decidualization in the uterine GR KO mice. As a transcriptional regulator, GR is able to regulate thousands of genes and impact numerous signaling pathways. Identification and characterization of master regulators, such as GR, that are able to integrate many aspects of physiology are of significant clinical relevance and may lead to strategies to improve pregnancy rates.
Materials and Methods
Please see detailed methods in SI Material and Methods.
Generation of the Uterine GR KO Mice and Treatments.
Mice homozygous for the floxed GR allele (GRloxP,loxP) (29) were mated with mice expressing Cre recombinase under the direction of the progesterone receptor (PRCre) (7), provided by Franco DeMayo and John Lydon, Baylor College of Medicine, Houston (UGRKO; Fig. 1A). The artificial decidualization response was performed as previously described (30). All experiments were performed according to a protocol approved by the Institutional Animal Care and Use Committee at the National Institute of Environmental Health Sciences.
Real-Time RT-PCR.
Total RNA was extracted from mouse tissue using the Qiagen RNeasy Mini Kit (Qiagen), according to the manufacturer’s instruction. The abundance of mRNAs was determined from at least three biological replicates on a 7900HT sequence detection system (Applied Biosystems).
Immunohistochemistry.
Uterine sections from paraffin-embedded tissue (5 μm) were deparaffinized and rehydrated in graded alcohol series. Sections were either stained with H&E for histological analysis or with antibodies to GR (1:500) (31), MKi67 (1: 400; Cell Signaling Technology), or F4/80 (1:500; BioLegend).
Immunoblotting.
Tissues from control and uterine GR KO mice were lysed in RIPA buffer. Membranes with equivalent amounts of protein were incubated with polyclonal anti-GR antibodies (1:1,000), ERα (HC-20) antibodies (1:1,000; Santa Cruz Biotechnology), PR (C-19) antibodies (1:1,000; Santa Cruz Biotechnology), or monoclonal anti-β-actin antibodies (1:10,000; EMD Millipore).
Microarray Analysis.
Gene expression analysis was performed on RNA from uteri of control and uterine GR KO mice using the Agilent Whole Mouse Genome oligonucleotide arrays (014868; Agilent Technologies). Significant changes in gene expression were defined on the basis of P value (P < 0.01).
Statistical Analysis.
Data are presented as means ± SEM. Statistical significance was determined by ANOVA with Tukey’s post hoc analysis. Statistical significance was defined as *P < 0.05 or **P < 0.01.
SI Materials and Methods
Generation of the Uterine GR KO Mice and Treatments.
Mice homozygous for the floxed GR allele (GRloxP,loxP) (29) were mated with mice expressing Cre recombinase under the direction of the progesterone receptor (PRCre) (7), provided by Franco DeMayo and John Lydon, Baylor College of Medicine, Houston (UGRKO; Fig. 1A). For collecting uterine samples, control and uterine GR KO mice were cycle-matched or ovariectomized and adrenalectomized (OVX/ADX), which was required for experiments involving exogenous hormone treatment. OVX/ADX surgeries occurred at 6 wk of age, and mice were given 10–14 d of rest to deplete endogenous hormones. Hormones were prepared before injection as follows: Dex (Steraloids Inc) was prepared by dissolving in saline with sonication until into solution, estradiol (E2; Steraolids Inc) was prepared by first dissolving in 100% ethanol and then diluting in saline, progesterone (P4; Sigma-Aldrich) was prepared by dissolving in dimethyl sulfoxide. Hormone treatment occurred through i.p. injection with saline, 1 mg/kg of Dex, 10 μg/kg E2, or 40 mg/kg P4. Mice were culled 4 h following injection; uteri weighed and processed for mRNA. To determine estrous stage, vaginal smears were taken daily at approximately the same time over a period of 14 d. Fertility was assessed through paired mating of control or uterine GR KO females with wild-type males (1:1). Time to first litter, litter size, and number of litters per female were recorded over a 6-mo period. Stimulation of ovulation was induced in 8- to 10-wk-old female mice with 5 international units (IU) of pregnant mare’s serum gonadotropin (Calbiochem) i.p., followed by 5 IU human CG (hCG; Calbiochem) 48 h later. Ovulated eggs from oviducts were enumerated 18 h after hCG injection. To collect samples from pregnant mice, 8- to 10-wk-old females were paired with wild-type males, and copulatory plugs were checked in the morning. The morning when vaginal plug was observed was designated as 0.5 dpc. Implantation was examined at 4.5 and 5.5 dpc. Implantation at 4.5 dpc was visualized by tail-vein injection of 1% Chicago Sky Blue 6B (0.9% saline; Sigma Aldrich). The artificial decidualization response was performed as previously described (30). Briefly, OVX control and uterine GR KO were treated 3 d with 100 ng E2, followed by 2 d of rest and 3 d of 6.7 ng E2 and 1 mg P4. Mice were then given a deciduogenic stimulus of 50 μL intrauterine sesame oil injection. Mice were then given daily injections of the combined E2 and P4 until the day of sacrifice (2 h, 2 d, or 5 d after stimulus).
Immunohistochemistry.
Uterine sections from paraffin-embedded tissue (5 μm) were deparaffinized and rehydrated in graded alcohol series. Sections were either stained with H&E for histological analysis or overnight with antibodies to GR (1:500) (31), MKi67 (1:400; Cell Signaling Technology), or F4/80 (1:500; BioLegend). The following day, sections were washed and incubated with biotinylated goat anti-rabbit IgG antibody (Vector Laboratories) or rabbit anti-rat IgG antibody (Vector Laboratories) for 30 min at room temperature. The antigen–antibody complex was visualized using VECTASTAIN R.T.U. Kit (Vector Laboratories) and 3-diaminobenzidine chromogen (DakoCytomation). Sections were counterstained with hematoxylin, dehydrated, and coverslipped. Analysis of apoptosis in control and uterine GR KO uteri was carried out by TUNEL assay using the ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit according to the manufacturer’s instructions (EMD Millipore). Sections were counterstained with hematoxylin, dehydrated, and coverslipped. For immunofluorescence studies, harvested uteri were fixed in 4% paraformaldehyde overnight and frozen sectioned. Antibodies to GR and Hoechst 33342 were used to visualize nuclear translocation. Images were taken using the Zeiss LSM710 and Zen 2012 software.
Real-time RT-PCR.
Total RNA was extracted from mouse tissue using the Qiagen RNeasy Mini Kit (Qiagen) with DNase treatment performed on-column using a Ribonuclease-Free DNase Kit (Qiagen) according to the manufacturer’s instruction. The abundance of mRNAs was determined from at least three biological replicates using real-time probes and primers (Applied Biosystems) on a 7900HT sequence detection system (Applied Biosystems).
Immunoblotting.
Tissues from control and uterine GR KO mice were lysed in RIPA buffer. Membranes with equivalent amounts of protein were incubated with polyclonal anti-GR antibodies (1:1,000), ERα (HC-20) antibodies (1:1,000; Santa Cruz Biotechnology), PR (C-19) antibodies (1:1,000; Santa Cruz Biotechnology), or monoclonal anti–β-actin antibodies (1:10,000; EMD Millipore). Quantification of immunoreactivity was determined by incubation with a mixture of goat anti-rabbit Alexa Fluorophore 680 conjugated (Molecular Probes) and goat anti-mouse IRDye 800 conjugated secondary (Rockland Immunochemicals) antibodies for 1 h at room temperature and visualization using the Odyssey LI-COR imaging system.
Microarray analysis.
Gene expression analysis was performed on RNA from uteri of control and uterine GR KO mice using the Agilent Whole Mouse Genome oligonucleotide arrays (014868; Agilent Technologies). Significant changes in gene expression were defined on the basis of P value (P < 0.01). Specifically, an error-weighted ANOVA and Benjamini–Hochberg multiple test correction were performed using Rosetta Resolver system (Rosetta Biosoftware). The lists of significant probe sets by treatment were visually sorted by Venn diagram and further analyzed by IPA version 6.5 (Ingenuity Systems). Gene enrichment P values (P < 0.05) for biological functions were determined by IPA using Fisher’s exact test. Significantly regulated genes were also analyzed by Gene Ontology using GATHER (32). Gene Ontology GATHER analysis used a Bayes factor cutoff of 5.0.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1508056112/-/DCSupplemental.
References
- 1.Cha J, Sun X, Dey SK. Mechanisms of implantation: Strategies for successful pregnancy. Nat Med. 2012;18(12):1754–1767. doi: 10.1038/nm.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whirledge S, Cidlowski JA. A role for glucocorticoids in stress-impaired reproduction: Beyond the hypothalamus and pituitary. Endocrinology. 2013;154(12):4450–4468. doi: 10.1210/en.2013-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busillo JM, Cidlowski JA. The five Rs of glucocorticoid action during inflammation: Ready, reinforce, repress, resolve, and restore. Trends Endocrinol Metab. 2013;24(3):109–119. doi: 10.1016/j.tem.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matteri RL, Watson JG, Moberg GP. Stress or acute adrenocorticotrophin treatment suppresses LHRH-induced LH release in the ram. J Reprod Fertil. 1984;72(2):385–393. doi: 10.1530/jrf.0.0720385. [DOI] [PubMed] [Google Scholar]
- 5.Gore AC, Attardi B, DeFranco DB. Glucocorticoid repression of the reproductive axis: Effects on GnRH and gonadotropin subunit mRNA levels. Mol Cell Endocrinol. 2006;256(1-2):40–48. doi: 10.1016/j.mce.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Cole TJ, et al. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995;9(13):1608–1621. doi: 10.1101/gad.9.13.1608. [DOI] [PubMed] [Google Scholar]
- 7.Soyal SM, et al. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41(2):58–66. doi: 10.1002/gene.20098. [DOI] [PubMed] [Google Scholar]
- 8.Pawar S, Hantak AM, Bagchi IC, Bagchi MK. Minireview: Steroid-regulated paracrine mechanisms controlling implantation. Mol Endocrinol. 2014;28(9):1408–1422. doi: 10.1210/me.2014-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu G, Dong Y, Wang Z, Cao J, Chen Y. Restraint stress alters immune parameters and induces oxidative stress in the mouse uterus during embryo implantation. Stress. 2014;17(6):494–503. doi: 10.3109/10253890.2014.966263. [DOI] [PubMed] [Google Scholar]
- 10.Lee KY, et al. Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol. 2007;27(15):5468–5478. doi: 10.1128/MCB.00342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franco HL, et al. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J. 2011;25(4):1176–1187. doi: 10.1096/fj.10-175349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma WG, Song H, Das SK, Paria BC, Dey SK. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci USA. 2003;100(5):2963–2968. doi: 10.1073/pnas.0530162100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basak S, Dhar R, Das C. Steroids modulate the expression of alpha4 integrin in mouse blastocysts and uterus during implantation. Biol Reprod. 2002;66(6):1784–1789. doi: 10.1095/biolreprod66.6.1784. [DOI] [PubMed] [Google Scholar]
- 14.Rhen T, Grissom S, Afshari C, Cidlowski JA. Dexamethasone blocks the rapid biological effects of 17beta-estradiol in the rat uterus without antagonizing its global genomic actions. FASEB J. 2003;17(13):1849–1870. doi: 10.1096/fj.02-1099com. [DOI] [PubMed] [Google Scholar]
- 15.Rhen T, Cidlowski JA. Estrogens and glucocorticoids have opposing effects on the amount and latent activity of complement proteins in the rat uterus. Biol Reprod. 2006;74(2):265–274. doi: 10.1095/biolreprod.105.045336. [DOI] [PubMed] [Google Scholar]
- 16.Jones HW, Jr, et al. What is a pregnancy? A question for programs of in vitro fertilization. Fertil Steril. 1983;40(6):728–733. doi: 10.1016/s0015-0282(16)47471-6. [DOI] [PubMed] [Google Scholar]
- 17.Edmonds DK, Lindsay KS, Miller JF, Williamson E, Wood PJ. Early embryonic mortality in women. Fertil Steril. 1982;38(4):447–453. [PubMed] [Google Scholar]
- 18.Gaspar-Elsas MI, et al. Evidence for a regulatory role of alpha 4-integrins in the maturation of eosinophils generated from the bone marrow in the presence of dexamethasone. Clin Exp Allergy. 2009;39(8):1187–1198. doi: 10.1111/j.1365-2222.2009.03289.x. [DOI] [PubMed] [Google Scholar]
- 19.Salleh N. Diverse roles of prostaglandins in blastocyst implantation. ScientificWorldJournal. 2014;2014:968141. doi: 10.1155/2014/968141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Racicot K, Kwon JY, Aldo P, Silasi M, Mor G. Understanding the complexity of the immune system during pregnancy. Am J Reprod Immunol. 2014;72(2):107–116. doi: 10.1111/aji.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plaks V, et al. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J Clin Invest. 2008;118(12):3954–3965. doi: 10.1172/JCI36682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy SP, Fast LD, Hanna NN, Sharma S. Uterine NK cells mediate inflammation-induced fetal demise in IL-10-null mice. J Immunol. 2005;175(6):4084–4090. doi: 10.4049/jimmunol.175.6.4084. [DOI] [PubMed] [Google Scholar]
- 23.Ain R, Trinh ML, Soares MJ. Interleukin-11 signaling is required for the differentiation of natural killer cells at the maternal-fetal interface. Dev Dyn. 2004;231(4):700–708. doi: 10.1002/dvdy.20183. [DOI] [PubMed] [Google Scholar]
- 24.Brown MB, von Chamier M, Allam AB, Reyes L. M1/M2 macrophage polarity in normal and complicated pregnancy. Front Immunol. 2014;5:606. doi: 10.3389/fimmu.2014.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jasper MJ, et al. Macrophage-derived LIF and IL1B regulate alpha(1,2)fucosyltransferase 2 (Fut2) expression in mouse uterine epithelial cells during early pregnancy. Biol Reprod. 2011;84(1):179–188. doi: 10.1095/biolreprod.110.085399. [DOI] [PubMed] [Google Scholar]
- 26.Care AS, et al. Macrophages regulate corpus luteum development during embryo implantation in mice. J Clin Invest. 2013;123(8):3472–3487. doi: 10.1172/JCI60561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dey SK, et al. Molecular cues to implantation. Endocr Rev. 2004;25(3):341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- 28.Chrousos GP, Torpy DJ, Gold PW. Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: Clinical implications. Ann Intern Med. 1998;129(3):229–240. doi: 10.7326/0003-4819-129-3-199808010-00012. [DOI] [PubMed] [Google Scholar]
- 29.Oakley RH, et al. Essential role of stress hormone signaling in cardiomyocytes for the prevention of heart disease. Proc Natl Acad Sci USA. 2013;110(42):17035–17040. doi: 10.1073/pnas.1302546110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finn CA, Martin L. Endocrine control of the timing of endometrial sensitivity to a decidual stimulus. Biol Reprod. 1972;7(1):82–86. doi: 10.1093/biolreprod/7.1.82. [DOI] [PubMed] [Google Scholar]
- 31.Cidlowski JA, Bellingham DL, Powell-Oliver FE, Lubahn DB, Sar M. Novel antipeptide antibodies to the human glucocorticoid receptor: Recognition of multiple receptor forms in vitro and distinct localization of cytoplasmic and nuclear receptors. Mol Endocrinol. 1990;4(10):1427–1437. doi: 10.1210/mend-4-10-1427. [DOI] [PubMed] [Google Scholar]
- 32.Chang JT, Nevins JR. GATHER: A systems approach to interpreting genomic signatures. Bioinformatics. 2006;22(23):2926–2933. doi: 10.1093/bioinformatics/btl483. [DOI] [PubMed] [Google Scholar]