Significance
This study reports the first use, to our knowledge, of triboelectric extraction of protein from parchment. The method is noninvasive and requires no specialist equipment or storage. Samples can be collected without the need to transport the artifacts; instead, researchers can sample when and where possible and analyze when required. The level of access we have achieved highlights the importance of this technique. For this study, we have extracted proteins from 513 parchment samples, used to resolve the long-standing question of the origin of “uterine vellum.” We find no evidence of unexpected species, such as rabbit or squirrel. We suggest that uterine vellum was often an achievement of technological production using available resources, and would not have demanded unsustainable agricultural practices.
Keywords: pocket Bible, parchment, vellum, collagen, mass spectrometry
Abstract
Tissue-thin parchment made it possible to produce the first pocket Bibles: Thousands were made in the 13th century. The source of this parchment, often called “uterine vellum,” has been a long-standing controversy in codicology. Use of the Latin term abortivum in many sources has led some scholars to suggest that the skin of fetal calves or sheep was used. Others have argued that it would not be possible to sustain herds if so many pocket Bibles were produced from fetal skins, arguing instead for unexpected alternatives, such as rabbit. Here, we report a simple and objective technique using standard conservation treatments to identify the animal origin of parchment. The noninvasive method is a variant on zooarchaeology by mass spectrometry (ZooMS) peptide mass fingerprinting but extracts protein from the parchment surface by using an electrostatic charge generated by gentle rubbing of a PVC eraser on the membrane surface. Using this method, we analyzed 72 pocket Bibles originating in France, England, and Italy and 293 additional parchment samples that bracket this period. We found no evidence for the use of unexpected animals; however, we did identify the use of more than one mammal species in a single manuscript, consistent with the local availability of hides. These results suggest that ultrafine vellum does not necessarily derive from the use of abortive or newborn animals with ultrathin hides, but could equally well reflect a production process that allowed the skins of maturing animals of several species to be rendered into vellum of equal quality and fineness.
Hamlet: Is not parchment made of sheepskins?
Horatio: Ay, my lord, and of calfskins too.
Hamlet, V.i
One of the outstanding controversies in the field of codicology concerns the origin and production of so-called “uterine vellum.” Researchers in the field of manuscript studies have long disputed the origin of this ultrafine writing material. Some older scholarship suggested that uterine vellum probably derived from the hides of smaller, more thin-skinned mammals, such as rabbits or squirrels (1, 2). However, most paleographers continue to view the notion of medieval uterine vellum as a myth, on the grounds that its production on the scale implied by extant manuscripts would have entailed an untenably high number of aborted fetuses (3). Other proposed solutions to the derivation of this material have involved specific production processes, such as the splitting of skins (4). The importance of analyzing parchment carefully to determine its origin species and, more specifically, the origin of uterine vellum is a priority research question for several disciplines. Such analysis might not only settle the long-standing debate over the source of supposedly “aborted” hides but would also provide valuable data about the localization and distribution of hides, and thus of membrane books and documents (5). The scholarly disputes notwithstanding, uterine vellum must represent either (i) the selection of specific animals whose skin was uniquely fine or (ii) the development of craft skills to work a wide range of skins into ultrafine sheets, or, of course, both at once.
The most frequently cited examples of uterine vellum are the 13th-century Paris Bibles (1, 2, 4, 6, 7). These books were single-volume Bibles (pandects) with a consistent organization, which meant that they were easily searchable, making them the ideal reference guide for study and sermon preparation (8). One of the most important subgroups of 13th-century Paris Bibles is the pocket or portable Bible, volumes sufficiently small to be easily transported. Ruzzier (6) suggests that the total output of portable Bibles in the 13th century could have exceeded 20,000 copies. The majority of surviving copies (∼54%) were made in France, most in Paris (6), although Bibles of this style were also produced in England at the same time and slightly later in both Italy (notably in the Veneto) and Spain.
To explore the question of uterine vellum, we examined the animal composition of ultrafine parchment (which ranged in thickness from 0.03–0.28 mm in our selected samples), sampled primarily from pocket Bibles, and compared these samples with other parchments that book-end their main period of production. If the skins of small animals (rabbits and squirrels) were used, their presence would be revealed by zooarchaeology by mass spectrometry (ZooMS). If the production of uterine vellum represented instead a specialized craft skill, then the selection of animals would be similar to the selection of animals evident in other coarser membranes from the same geographic region.
Species Identification of Parchment
To assess the origin of uterine vellum, the principal line of evidence is the identification of the skin. Skins have previously been identified by overall size, thickness, color, levels of grease, and patterns of follicles (9). A notable proponent of follicle pattern analysis was Ryder (10), who used the technique to identify animal species; however, not all parchments had discernible follicle patterns, not every pattern could be identified, and paleographers have often been overconfident in their ability to discern species origin from such patterns. Protein (11, 12) and DNA-based methods (13–17) potentially offer absolute determination of species but, until now, have had other limitations. Toniolo et al. (11) analyzed 5 mg of parchment from the 13th-century “Marco Polo Bible” at the Biblioteca Medicea Laurenziana, and used peptide sequences to identify a single leaf as calfskin, although if more leaves had been analyzed the results may have revealed different species as was the case with the family's archival documents (Table S1). Kirby et al. (12) also used destructive sampling techniques to analyze a Koran and other objects. Teasdale et al. (13) have recently reported genomic data from postmedieval parchment using, on average, 50 mg of parchment. In addition to the destructive nature of sampling, a further hidden cost of molecular analysis is adequate storage of extracted samples. It is usually necessary to freeze DNA and protein extracts to preserve sample integrity, which adds a further cost burden for libraries and archives in either shipping or storage.
Table S1.
Sample details for three of Marco Polo’s family archival documents
| Sample no. | Library | Date | Type of document | Location of production | Species |
| VVK 26 | Biblioteca Marciana | August 5, 1280 | Testament of Marco Polo “il vecchio” (Marco Polo’s uncle) | Italy | Sheep |
| VVK 29 | Biblioteca Marciana | August 31, 1300 | Testament of Matteo Polo (Marco Polo’s step-brother) | Italy | Sheep |
| VVK 31 | Biblioteca Marciana | January 9, 1324 | Testament of Marco Polo “il viaggiatore” | Italy | Sheep |
Triboelectric Extraction
Here, we describe a novel noninvasive molecular identification method to identify the origin of parchment, using electrostatic molecular extraction onto a solid-phase PVC polymer. PVC polymer erasers are widely accepted by the conservation community as a noninvasive measure for removing dirt. Indeed, every archive and conservation studio has access to and experience of the use of PVC erasers. A further advantage of the extraction method is that protein is preserved on the PVC polymer waste at room temperature with no further storage requirements (apparently indefinitely). It can therefore be retained at the point of collection without special preparation or storage until the researcher deems it appropriate to analyze a larger set of samples. The method is easily scalable and places the sampling in the hands of curators, codicologists, and conservators. Using this approach, we have sent kits (consisting of PVC erasers, nitrile gloves, and sampling tubes) to 14 archives and 40 libraries in Europe and North America. They have returned samples from 79 13th-century pandect Bibles for analysis, including 72 pocket Bibles.
The aim of this study was to identify the animal origin of the tissue paper-thin “uterine” vellum, used in 13th-century pocket Bibles, through the use of a novel noninvasive triboelectric extraction of skin collagen, subsequently analyzed by conventional peptide mass fingerprinting.
Results
Validation of Triboelectric Extraction.
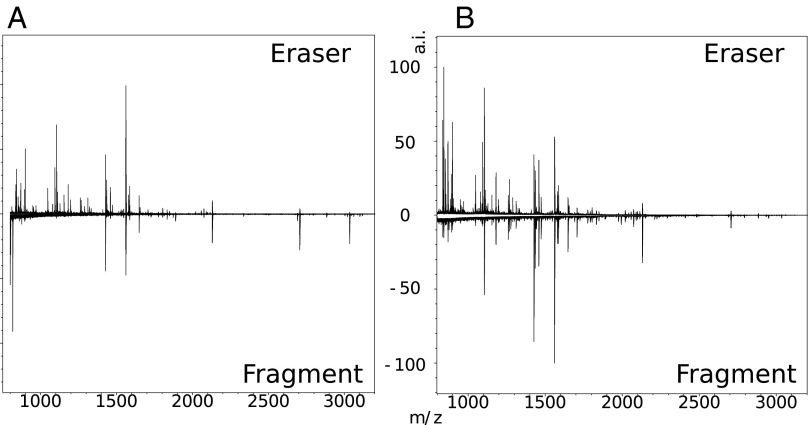
To assess the quality of our results, we performed a comparative analysis of the same sample using conventional ZooMS techniques that require destructive sampling (12), as well as nondestructive electrostatic ZooMS (eZooMS). For the purpose of this experiment, we used two different documents: (i) a 16th-century manorial court roll (Fig. 1A) and (ii) an 18th-century seal tag (Fig. 1B). In case i, a fragment of parchment of ∼0.5 cm × 0.5 cm was used, and in case ii, a fragment of ∼0.1 cm × 0.3 cm was used. In both cases, an eZooMS sample was taken from the main document.
Fig. 1.
Comparison of peptide mass fingerprint from samples A and B using destructive sampling (lower spectra) and noninvasive eZooMS sampling (top spectra). (A) For one sample, the resulting peptide mass fingerprint detected fewer peaks than in its equivalent eZooMS sample. (B) In the other sample, the results obtained were very similar for both methods. eZooMS samples extracted and analyzed 12 mo apart, having been stored at room temperature, revealed no loss of signal.
The optimization of our eZooMS methodology has allowed us to obtain equal, if not better, results from PVC sampling than when using an actual fragment of parchment (Fig. 1). The observed difference in quality is probably due to an excessive amount of collagen being extracted during destructive analyses (Fig. 2). However, the eraser technique itself may also contribute to a cleaner signal by the eraser retaining contaminating molecules that interfere with the subsequent analysis on the PVC.
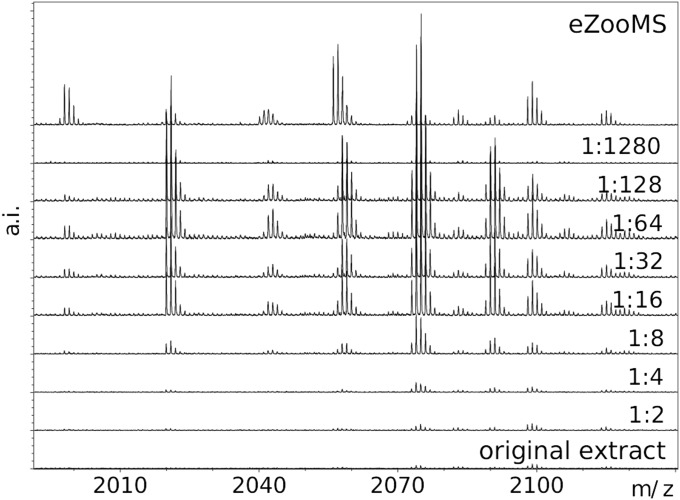
Fig. 2.
Comparison of peptide mass fingerprint from serial dilution of destructive sample and noninvasive eZooMS sampling. A destructive sample from historic calf parchment (0.5 cm × 0.5 cm) was extracted and digested using a serial dilution ranging from 1:2–1:1,280 and compared with a noninvasive eZooMS extraction. A cleaner signal is seen between the 1:16 and 1:128 dilutions, likely due to the dilution of excessive collagen molecules or contaminants that interfere with the subsequent analysis.
Species Variation.
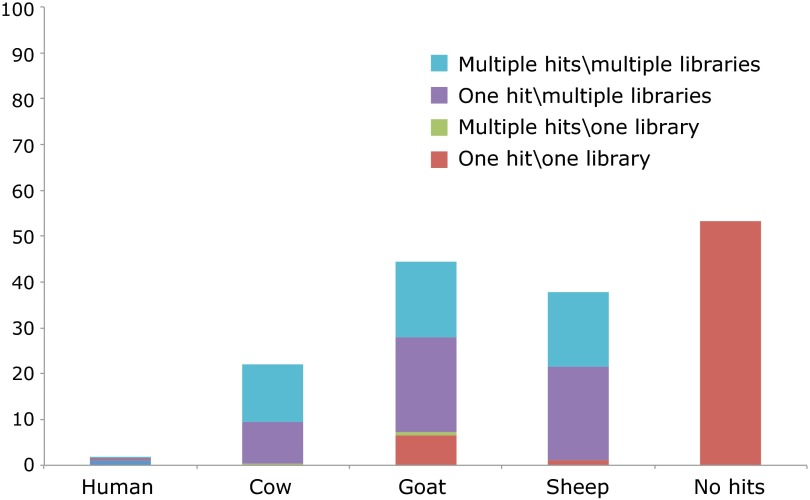
Species identifications of all of the samples analyzed are included in Tables S2 and S3. A total of 220 folios were analyzed from the 72 pocket Bibles. Of these folios, 68% were calf, 26% were goat, and 6% were sheep. We found no evidence for the rabbit skin duodecimos suggested by Pollard (1). Of the 72 Bibles we sampled, 62 were sampled on multiple leaves. In most cases, the identification was consistent for each of the folios of one manuscript, including the 18 sampled leaves of the Hornby Bible (OSU.MS.MR.Frag.74). However, at least five Bibles were composed of parchment from multiple species. To confirm the presence of multiple animals, we were able to analyze 20 folios from Cambridge University Library Ee.6.26, six of which were identified as calf and 14 as sheep. The species distinction is mirrored by stylistic differences within the Bible, which suggest that this manuscript may, in fact, be a composite of two different Bibles. The first part of the Bible (fol. 1–108), which contained five folios identified as calf parchment, resembles a “proto-‘Paris’ Bible” model produced ca. 1200–1230, whereas the second part (fol. 109–459), containing the 14 folios made of sheepskin (and one of calfskin) parchment, is a far better fit for the “mature ‘Paris’ Bible” blueprint of ca. 1230–1280 as described by Light (8, 18–20). It is also worth noting that the attributed English provenance derives from inscriptions present in the second part of the Bible; no provenance indicators are recorded for the first part, so it is possible that the Bible’s first 108 folios were produced in France.
Table S2.
Sample details for 72 13th century pocket Bibles
| Sample no. | Shelf-mark | Library | Date | Size, mm | No. of leaves | Thickness, mm | No. of pages sampled | Location of production | Species |
| CCC 31–32 | CCCC MS 246 | Corpus Christi College Cambridge | 1200–1300 | 156 × 102 | 410 | 0.05–0.08 | 2 | England | Calf |
| CL 001 | Gg.6.15 | Cambridge University Library | 1225–1250 | 184 × 130 | 504 | 0.06–0.09 | 1 | France | Calf |
| CL 002 | Gg.6.45 | Cambridge University Library | 1225–1250 | 136 × 92 | 532 | 0.07–0.1 | 1 | France | Calf |
| CL 003–005 | Add.1848 | Cambridge University Library | 1225–1250 | 156 × 109 | 407 | 0.07–0.2 | 3 | France | Goat |
| CL 006 | Add.6159 | Cambridge University Library | 1225–1250 | 137 × 88 | 594 | 0.04–0.06 | 1 | France | Calf |
| CL 057–059 | Pembroke 268 | Cambridge University Library | 1200–1300 | 198 × 128 | 480 | 0.06–0.2 | 3 | France | Calf |
| CL 064–067 | Add.8438 | Cambridge University Library | 1200–1300 | 185 × 130 | 424 | 0.07–0.13 | 4 | France | Calf |
| CL 082–086 | Hh.1.3 | Cambridge University Library | 1250–1275 | 198 × 195 | 367 | 0.06–0.15 | 5 | England | Calf |
| CL 087–091 | Ii.6.22 | Cambridge University Library | 1250–1300 | 142 × 102 | 299 | 0.06–0.12 | 5 | Italy | Goat |
| CL 092–094 | Ii.6.12 | Cambridge University Library | 1200–1300 | 165 × 104 | 469 | 0.06–0.14 | 3 | France | Calf |
| CL 100–101 | Dd.5.52 | Cambridge University Library | 1200–1300 | 210 × 140 | 333 | 0.1–0.18 | 2 | England | Calf and goat |
| CL 102–104 | Gg.1.27 | Cambridge University Library | 1225–1250 | 224 × 153 | 406 | 0.07–0.22 | 3 | England | Calf and goat |
| CL 105–106 | Dd.10.29 | Cambridge University Library | 1250–1275 | 235 × 160 | 439 | 0.08–0.15 | 2 | England | Calf |
| CL 107–108 | Ee.1.16 | Cambridge University Library | 1200–1300 | 208 × 150 | 349 | 0.08–0.13 | 2 | Northern France or England | Calf |
| CL 109–110 | Ii.4.10 | Cambridge University Library | 1225–1250 | 242 × 170 | 346 | 0.1–0.2 | 2 | England | Calf |
| CL 111–112 | Ee.1.9 | Cambridge University Library | 1200–1300 | 210 × 150 | 285 | 0.08–0.18 | 4 | England | Calf |
| CL 118–119 | |||||||||
| CL 113–114 | Mm.1.2 | Cambridge University Library | 1200–1300 | 245 × 163 | 610 | 0.06–0.1 | 2 | Northern France or England | Goat |
| CL 120–121 | Gg.1.3 | Cambridge University Library | 1225–1275 | 211 × 140 | 375 | 0.08–0.11 | 2 | England | Calf |
| CL 122–123 | Ff.6.19 | Cambridge University Library | 1200–1300 | 146 × 97 | 567 | 0.04–0.09 | 2 | — | Calf |
| CL 124–125 | Ff.6.20 | Cambridge University Library | 1200–1300 | 146 × 98 | 453 | 0.03–0.06 | 2 | — | Goat |
| CL 126–127 | Dd.15.35 | Cambridge University Library | 1200–1300 | 148 × 94 | 476 | 0.06–0.1 | 2 | — | Goat |
| CL 128–129 | Dd.12.47 | Cambridge University Library | 1200–1300 | 150 × 106 | 502 | 0.04–0.08 | 2 | Northern France or England | Calf |
| CL 130–131CL 179–196 | Ee.6.26 | Cambridge University Library | 1220–1230 | 198 × 138 | 459 | 0.07–0.28 | 20 | England | Calf and sheep |
| CL 132–136 | Ff.6.45 | Cambridge University Library | 1225–1275 | 144 × 103 | 478 | 0.05–0.09 | 5 | England | Goat |
| DCL 05–06 | A.IV.37 | Durham Cathedral Library | 1200–1300 | 235 × 147 | 376 | — | 2 | England? | Calf |
| DCL 07–10 | A.IV.30 | Durham Cathedral Library | 1225–1275 | 159 × 106 | 576 | — | 4 | France? | Calf |
| DUL 01–03 | DUL MS Cosin V.V.17 | Durham University Library | 1200–1300 | 127 × 92 | 322 | 0.08–0.08 | 3 | Southern France or Northern Italy | Goat |
| DUL 04–06 | DUL MS Cosin V.V.18 | Durham University Library | 1200–1300 | 132 × 87 | 420 | 0.05–0.05 | 3 | France | Calf |
| FLP 01–03 | Lewis E 029 | Free Library of Philadelphia | 1230–1240 | 182 × 113 | 160 | — | 3 | England | Calf |
| FLP 04–06 | Lewis E 028 | Free Library of Philadelphia | 1270–1270 | 159 × 105 | 659 | — | 3 | France | Calf |
| FLP 07–09 | Lewis E 030 | Free Library of Philadelphia | 1225–1250 | 142 × 98 | 539 | — | 3 | England | Goat |
| FLP 10–12 | Lewis E 033 | Free Library of Philadelphia | 1250–1250 | 150 × 104 | 341 | — | 3 | France | Calf |
| FLP 13–15 | Lewis E 036 | Free Library of Philadelphia | 1260–1280 | 166 × 123 | 370 | — | 3 | Italy | Goat |
| FLP 16–18 | Lewis E 039 | Free Library of Philadelphia | 1250–1250 | 186 × 126 | 403 | — | 3 | Italy | Goat |
| JRL 158–159 | MSM0163 | Morgan Library | 1229–1229 | 220 × 150 | 454 | 0.09–0.15 | 2 | France | Goat |
| KRPB | Ms. 56 | University of St. Louis | 1200–1300 | 150 × 100 | 236 | 0.11–0.18 | 1 | France | Calf |
| MAGGS 02 | 14739 | Maggs Bros. Ltd. | 1200–1300 | — | — | — | 1 | — | Goat |
| MAGGS 03 | 14754 | Maggs Bros. Ltd. | 1200–1300 | — | — | — | 1 | — | Goat |
| MAGGS 05 | 12307 | Maggs Bros. Ltd. | 1200–1300 | — | — | — | 1 | — | Goat |
| NRO W 14 | PARIS BIBLE | Private collection | 1225–1275 | 158 × 112 | 395 | 0.12–0.12 | 1 | France | Calf |
| OSU 01–18 | OSU.MS.MR.Frag.74 | Ohio State University | 1220–1220 | 220 × 145 | 440 | 0.1–0.2 | 18 | France | Calf |
| RLB 01–03 | Ms 2053 | Royal Library of Belgium | 1200–1300 | 223 × 157 | 443 | — | 3 | France (north) | Goat |
| RLB 04–06 | Ms 4911 | Royal Library of Belgium | 1200–1300 | 130 × 93 | 180 | 0.093–0.149 | 3 | France (north), Low Countries, or England | Calf |
| RLB 07–09 | Ms 5627 | Royal Library of Belgium | 1200–1300 | 140 × 94 | 588 | 0.061–0.078 | 3 | France (north) | Calf |
| RLB 10–12 | Ms 8428 | Royal Library of Belgium | 1200–1300 | 208 × 159 | 412 | 0.082–0.174 | 3 | England | Calf |
| RLB 13–15 | Ms 8882 | Royal Library of Belgium | 1200–1300 | 140 × 96 | 486 | 0.067–0.088 | 3 | France (north) | Calf |
| RLB 16–18 | Ms 10517 | Royal Library of Belgium | 1200–1300 | 150 × 95 | 664 | — | 3 | France (north) | Calf |
| RLB 19–21 | Ms 10518 | Royal Library of Belgium | 1200–1300 | 140 × 90 | 642 | — | 3 | France (north) | Calf |
| RLB 22–24 | Ms 10520 | Royal Library of Belgium | 1200–1300 | 140 × 85 | 790 | — | 3 | France (north) | Calf |
| RLB 25–27 | Ms 10521 | Royal Library of Belgium | 1200–1300 | 154 × 97 | 592 | — | 3 | France (north) | Calf |
| RLB 28–30 | Ms 10522 | Royal Library of Belgium | 1200–1300 | 178 × 122 | 481 | 0.074–0.126 | 3 | France or Italy | Calf and goat |
| RLB 31–33 | Ms 10523 | Royal Library of Belgium | 1200–1300 | 178 × 116 | 359 | 0.101–0.172 | 3 | France (north) | Calf |
| RLB 34–36 | Ms 10524 | Royal Library of Belgium | 1200–1300 | 169 × 117 | 418 | 0.087–0.147 | 3 | France (north), Low Countries, or England | Calf |
| RLB 37–39 | Ms 10545 | Royal Library of Belgium | 1200–1300 | 221 × 149 | 462 | 0.031–0.105 | 3 | France (north) | Calf |
| RLB 40–42 | Ms 10753 | Royal Library of Belgium | 1200–1300 | 211 × 147 | 550 | 0.06–0.083 | 3 | France (north) | Calf |
| RLB 43–45 | Ms 10860 | Royal Library of Belgium | 1200–1300 | 180 × 124 | 604 | 0.056–0.104 | 3 | France (north) or Low Countries or England | Calf and goat |
| RLB 46–48 | Ms 14680 | Royal Library of Belgium | 1200–1300 | 143 × 95 | 545 | 0.06–0.083 | 3 | France (north) | Calf |
| RLB 49–51 | Ms 21700 | Royal Library of Belgium | 1200–1300 | 125 × 91 | 583 | 0.05–0.084 | 3 | France (north) | Calf |
| RLB 52–54 | Ms 2663–64 | Royal Library of Belgium | 1200–1300 | 193 × 133 | 456 | 0.087–0.124 | 3 | France (north) | Calf |
| RLB 55–57 | Ms IV 290 | Royal Library of Belgium | 1200–1300 | 244 × 175 | 478 | 0.135–0.211 | 3 | Spain? | Goat |
| UPenn 02 | Ms. Codex 236 | Schoenberg Institute, University of Pennsylvania | 1235–1240 | 218 × 148 | 465 | — | 1 | France | Calf |
| UPenn 03 | Ms. Codex 1065 | Schoenberg Institute, University of Pennsylvania | 1240–1250 | 178 × 117 | 356 | — | 4 | England? | Goat |
| DNA UPenn 04–06 | |||||||||
| UMLB 07–08 | Bible Vulgata RARES 091 B47C | University of Melbourne | 1250–1300 | 130 × 90 | 595 | — | 2 | France or England | Calf |
| UVA 15 | M.MS.N | Albert and Shirley Small Special Collections Library, University of Virginia | 1200–1300 | 158 × 95 | 603 | 0.07–0.07 | 1 | France | Calf |
| VV 17–18 | Holkham Hall MS 10 | Holkham Hall | 1240–1260 | 226 × 152 | — | — | 2 | France | Calf |
| VV 19–20 | Holkham Hall MS 11 | Holkham Hall | 1200–1300 | 178 × 134 | — | — | 2 | France | Calf |
| VV 21–22 | Holkham Hall MS 12 | Holkham Hall | 1200–1300 | 185 × 123 | — | — | 2 | France | Goat |
| VV 23–25 | Holkham Hall MS 13 | Holkham Hall | 1230–1250 | 225 × 144 | — | — | 3 | France | Goat |
| VV 26–27 | Holkham Hall MS 1 | Holkham Hall | 1300–1400 | 163 × 108 | — | — | 2 | England | Calf |
| YML 04–06 | XVI.N.6 | York Minster Library | 1225–1275 | 203 × 143 | 333 | 0.1–0.14 | 3 | England | Calf |
| YML 13–15 | XVI.N.5 | York Minster Library | 1200–1250 | 200 × 140 | 420 | 0.07–0.11 | 3 | France | Calf |
| YML 16–18 | XVI.N.10 | York Minster Library | 1225–1275 | 151 × 99 | 665 | 0.06–0.1 | 3 | France? | Calf |
Table S3.
Sample details for seven 13th century nonpocket pandect Bibles
| Sample no. | Shelf-mark | Library | Date | Size, mm | No. of leaves | Thickness, mm | No. of pages sampled | Location of production | Species |
| CL 07–08 | Add.7801 | Cambridge University Library | 1225–1275 | 243 × 164 | 515 | 0.08–0.12 | 2 | France | Calf |
| DCL 01–02 | C.III.22 | Durham Cathedral Library | 1200–1300 | 342 × 233 | 438 | — | 2 | England | Sheep |
| DCL 03–04 | A.II.3 | Durham Cathedral Library | 1200–1300 | 415 × 265 | 415 | — | 2 | Northern France | Calf |
| RLB 58–60 | Ms 9111–9114 | Royal Library of Belgium | 1286–1286 | 421 × 314 | 972 | 0.125–0.28 | 3 | Germany | Calf |
| RLB 61–63 | Ms II 2523 | Royal Library of Belgium | 1200–1300 | 495 × 358 | 588 | 0.186–0.261 | 3 | Low Countries | Calf |
| U Penn 04 | Ms. Codex 724 | Schoenberg Institute, University of Pennsylvania | 1275–1299 | 375 × 246 | 330 | — | 1 | France | Calf |
| YML 01–03 | XVI.D.13 | York Minster Library | 1200–1250 | 277 × 195 | 326 | 0.09–0.22 | 3 | England | Sheep |
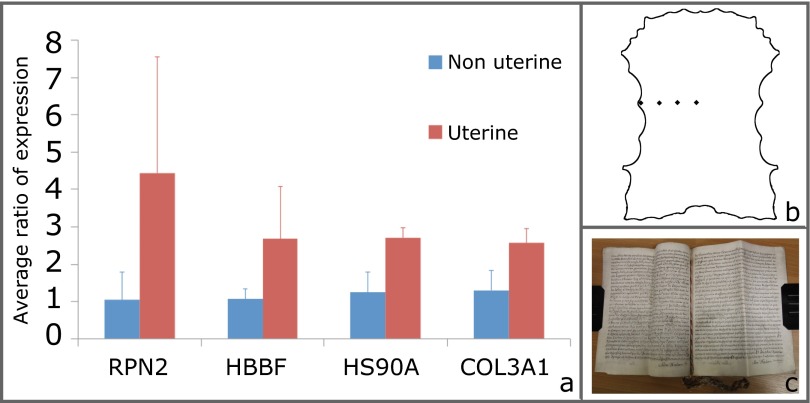
In addition, we explored the possibility of using liquid chromatography-tandem MS to determine if differential biomarkers for uterine skin could be identified (SI Materials and Methods). Modern uterine samples showed elevated expression of four proteins (Fig. S1); however, none of the peptide markers observed in eZooMS mapped to any of these proteins, so it is not possible to report the presence of these proteins in our Bible samples.
Fig. S1.
iTRAQ experiment. (A) Comparison of protein expression of uterine and nonuterine parchment samples. Uterine samples show higher expression levels for four proteins, including dolichyl-diphosphooligosaccharide-protein glycosyltransferase subunit 2 (RPN2), hemoglobin fetal subunit beta (HBBF), heat shock protein 90-alpha (HSP90A), and collagen alpha-1(III) chain (COL3A1). An error rate of 2 SDs is assumed. (B) Uterine calfskin indicating the four sampling points. (C) Historical legal document dated to 1776 used as one of the nonuterine samples.
Geographic Distribution.
Previous authors have identified geographic variation in the animals used for making parchment. Forbes (21), citing Wattenbach, notes that hides used for parchment were predominantly calf in the north of Europe and sheep and goat in the south. Ruzzier (6) agrees, and suggests that fine parchment north of the Alps was likely made from calfskins. Clarkson (4), noting the “warm, creamy tint” and “flexibility” of late-medieval Italian skins, attributes these qualities to the use of goatskin and possibly alternative methods of preparation. De Hamel (3) observes that Italian parchment is often prepared from goatskin, which contrasts with the poor representation of goatskin in England (22–24).
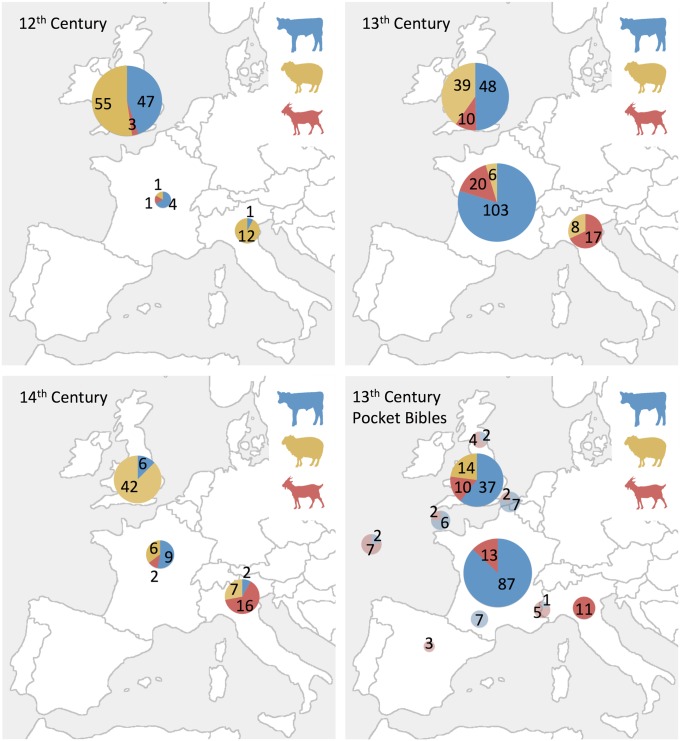
Our results are in agreement with most of these assessments. We see a predominance of calfskin being used in France, a pronounced use of goatskin in Italy, and a more mixed pattern emerging from England (Fig. 3).
Fig. 3.
Relative proportions of animals used to make parchment in each of the three regions studied (France, England, and Italy) during the 12th to 14th centuries. The size of the circle indicates the number of samples. Except for the figure describing exclusively pocket Bibles, data were obtained from all sources of parchment, including legal documents, secular codices, and Bibles, using the eZooMS method. Circles shown in paler colors indicate inconclusive provenance with respect to location.
To examine whether the geographic variation observed for ultrafine parchment differed from coarser membranes, we surveyed an additional 293 parchment objects from the 12th to 14th centuries. The selection of skins appears to reflect available livestock, and therefore a city’s or a region’s preferences: Sheepskin is most abundant in England, calfskin in France, and goatskin in Italy. In Italy, there is an absence of goatskin in the 12th century (contra 25), which possibly reflects regional variation. Six (of 12) 12th-century Italian sheep samples were sourced from Sicily, where a sheep-based dairy economy was practiced. Although there are differences between the two centuries spanning the period of interest, a more comprehensive investigation is required to study any long-term changes.
The pattern of selection of ultrafine parchment for Bible production seems to be broadly in line with the pattern of selection found in other parchment analyzed (Fig. 3). The English Bibles seem to be more varied in their composition, including multiple species in the same volume. Of the five Bibles that were composed of a mixture of animals (calf and goat or calf and sheep), three of them are of English provenance, one of possibly English provenance, and the fifth of either French or Italian provenance. Of the seven larger Paris Bibles (i.e., classified as nonpocket pandect Bibles), the three described as being of French production, as well as the two Bibles from the Low Countries and Germany, were all identified as calf; however, the two larger Paris Bibles of English origin were found to be made from sheep. All these nonpocket Bibles also seem to follow general regional preferences.
The scant use of sheepskin parchment for pocket Bibles is provocative and merits further investigation. A survey of English archival documents from the Borthwick Archive (York), using the same methods described in this paper (Table S4), reveal that all are written on sheepskin parchment. This selection of sheepskin parchment for legal documents may not merely reflect cost. The Dialogus de Scaccario (26) argues for the use of sheepskins as the medium for English legal documents due to the difficulty of erasure of text as a result of the propensity of sheepskin parchment to delaminate. This characteristic also means that sheepskin is the easiest skin to split, apparently making it ideally suitable for the thin parchment used in pocket Bibles. However, apart from the two larger Paris Bibles of English origin, we have found only one example of a pocket Bible made of sheepskin: This pocket Bible is of supposed English provenance (Cambridge University Library Ee.6.26).
Table S4.
Sample details of English archival documents held at the Borthwick Archive, York
| Sample no. | Shelf-mark | Date | Country | Type of document | Species |
| BA 61 | YM/D/CAMP 1 | 1322–1322 | England | Legal deed | Sheep |
| BA 26 | MOR 2 | 1326–1326 | England | Legal deed | Sheep |
| BA 81 | YM/D/PO | 1330–1330 | England | Legal deed | Sheep |
| BA 41 | YM/D/ASK 1 | 1332–1332 | England | Legal deed | Sheep |
| BA 141 | YM/D/HAT 1 | 1376–1376 | England | Legal deed | Sheep |
| BA 06 | YM/D/BAL a. 1 | 1377–1377 | England | Legal deed | Sheep |
| BA 27 | MOR 3 | 1380–1380 | England | Legal deed | Sheep |
| BA 01 | YM/D/BAL(M) 1 | 1382–1382 | England | Legal deed | Sheep |
| BA 28 | MOR 4 | 1392–1392 | England | Legal deed | Sheep |
| BA 21 | BED 1 | 1393–1393 | England | Legal deed | Sheep |
| BA 42 | YM/D/ASK 2 | 1404–1404 | England | Legal deed | Sheep |
| BA 29 | MOR 6 | 1415–1415 | England | Legal deed | Sheep |
| BA 62 | YM/D/CAMP 2 | 1427–1427 | England | Legal deed | Sheep |
| BA 30 | MOR 7 | 1443–1443 | England | Legal deed | Sheep |
| BA 82 | YM/D/PO | 1447–1447 | England | Legal deed | Sheep |
| BA 31 | MOR 8 | 1448–1448 | England | Legal deed | Sheep |
| BA 32 | MOR 10 | 1455–1455 | England | Legal deed | Sheep |
| BA 63 | YM/D/CAMP 3 | 1456–1457 | England | Legal deed | Sheep |
| BA 136 | YM/D/YOR 1 | 1466–1466 | England | Legal deed | Sheep |
| BA 33 | MOR 11 | 1481–1481 | England | Legal deed | Sheep |
| BA 142 | YM/D/HAT 2 | 1484–1484 | England | Legal deed | Sheep |
| BA 43 | YM/D/ASK 4 | 1492–1492 | England | Legal deed | Sheep |
| BA 44 | YM/D/ASK 7 | 1494–1495 | England | Legal deed | Sheep |
| BA 83 | YM/D/PO | 1503–1503 | England | Legal deed | Sheep |
| BA 64 | YM/D/CAMP 4 | 1507–1507 | England | Legal deed | Sheep |
| BA 34 | MOR 12a | 1511–1511 | England | Legal deed | Sheep |
| BA 09 | YM/D/BAL a. 5 | 1513–1513 | England | Legal deed | Sheep |
| BA 161 | YM/D/OUS 1 | 1518–1518 | England | Legal deed | Sheep |
| BA 137 | YM/D/YOR 2 | 1520–1520 | England | Legal deed | Sheep |
| BA 10 | YM/D/BAL a. 6 | 1525–1525 | England | Legal deed | Sheep |
| BA 22 | BED 2 | 1529–1530 | England | Legal deed | Sheep |
| BA 35 | MOR 13 | 1534–1534 | England | Legal deed | Sheep |
| BA 139 | YM/D/YOR 6 | 1537–1537 | England | Legal deed | Sheep |
| BA 65 | YM/D/CAMP 5 (b) | 1540–1540 | England | Legal deed | Sheep |
| BA 101 | YM/D/LANC 1 | 1542–1542 | England | Legal deed | Sheep |
| BA 102 | YM/D/LANC 2 | 1546–1546 | England | Legal deed | Sheep |
| BA 66 | YM/D/CAMP 10 | 1547–1547 | England | Legal deed | Sheep |
| BA 144 | YM/D/HAT 5 | 1549–1550 | England | Legal deed | Sheep |
| BA 121 | YM/D/KIRK 1 | 1552–1552 | England | Legal deed | Sheep |
| BA 67 | YM/D/CAMP 13 | 1554–1554 | England | Legal deed | Sheep |
| BA 46 | YM/D/ASK 11 | 1556–1556 | England | Legal deed | Sheep |
| BA 84 | YM/D/PO | 1557–1557 | England | Legal deed | Sheep |
| BA 85 | YM/D/PO | 1558–1558 | England | Legal deed | Sheep |
| BA 103 | YM/D/LANC 3 | 1561–1561 | England | Legal deed | Sheep |
| BA 68 | YM/D/CAMP 16 | 1563–1564 | England | Legal deed | Sheep |
| BA 02 | YM/D/BAL(M) 4 | 1565–1565 | England | Legal deed | Sheep |
| BA 11 | YM/D/BAL a. 9 | 1567–1568 | England | Legal deed | Sheep |
| BA 47 | YM/D/ASK 13 | 1571–1571 | England | Legal deed | Sheep |
| BA 70 | YM/D/CAMP 21 | 1573–1573 | England | Legal deed | Sheep |
| BA 107 | YM/D/LANC 9 | 1574–1575 | England | Legal deed | Sheep |
| BA 03 | YM/D/BAL(M) 10 | 1576–1576 | England | Legal deed | Sheep |
| BA 124 | YM/D/KIRK 4 | 1577–1577 | England | Legal deed | Sheep |
| BA 109 | YM/D/LANC 14 | 1579–1580 | England | Legal deed | Sheep |
| BA 127 | YM/D/KIRK 8 | 1580–1581 | England | Legal deed | Sheep |
| BA 48 | YM/D/ASK 16 | 1581–1581 | England | Legal deed | Sheep |
| BA 87 | YM/D/PO | 1582–1582 | England | Legal deed | Sheep |
| BA 169 | YM/D/OUS 9 | 1586–1587 | England | Legal deed | Sheep |
| BA 170 | YM/D/OUS 10 | 1589–1589 | England | Legal deed | Sheep |
| BA 14 | YM/D/BAL a. 18 | 1591–1591 | England | Legal deed | Sheep |
| BA 130 | YM/D/KIRK 12 | 1592–1593 | England | Legal deed | Sheep |
| BA 153 | YM/D/HEN 5 | 1595–1596 | England | Legal deed | Sheep |
| BA 154 | YM/D/HEN 6 | 1596–1597 | England | Legal deed | Sheep |
| BA 115 | YM/D/LANC 24 | 1598–1598 | England | Legal deed | Sheep |
| BA 52 | YM/D/ASK 21 | 1599–1599 | England | Legal deed | Sheep |
| BA 116 | YM/D/LANC 25 | 1600–1600 | England | Legal deed | Sheep |
| BA 05 | YM/D/BAL(M) 21 | 1601–1601 | England | Legal deed | Sheep |
| BA 131 | YM/D/KIRK 13 | 1603–1604 | England | Legal deed | Sheep |
| BA 15 | YM/D/BAL a. 28 | 1605–1605 | England | Legal deed | Sheep |
| BA 16 | YM/D/BAL a. 33 | 1607–1607 | England | Legal deed | Sheep |
| BA 73 | YM/D/CAMP 44 | 1608–1608 | England | Legal deed | Sheep |
| BA 117 | YM/D/LANC 27 | 1610–1610 | England | Legal deed | Sheep |
| BA 74 | YM/D/CAMP 46 | 1611–1611 | England | Legal deed | Sheep |
| BA 118 | YM/D/LANC 28 | 1611–1612 | England | Legal deed | Sheep |
| BA 133 | YM/D/KIRK 16 | 1614–1615 | England | Legal deed | Sheep |
| BA 18 | YM/D/BAL a. 44 | 1615–1615 | England | Legal deed | Sheep |
| BA 75 | YM/D/CAMP 50 | 1619–1619 | England | Legal deed | Sheep |
| BA 20 | YM/D/BAL a. 49 | 1621–1622 | England | Legal deed | Sheep |
| BA 93 | YM/D/PO | 1624–1624 | England | Legal deed | Sheep |
| BA 58 | YM/D/ASK 40 | 1629–1630 | England | Legal deed | Sheep |
| BA 77 | YM/D/CAMP 56 | 1632–1632 | England | Legal deed | Sheep |
| BA 59 | YM/D/ASK 42 | 1633–1633 | England | Legal deed | Sheep |
| BA 134 | YM/D/KIRK 20 | 1635–1636 | England | Legal deed | Sheep |
| BA 78 | YM/D/CAMP 58 | 1650–1650 | England | Legal deed | Sheep |
| BA 90 | YM/D/PO | 1661–1661 | England | Legal deed | Sheep |
| BA 96 | YM/D/PO | 1669–1669 | England | Legal deed | Sheep |
| BA 37 | MOR 45 | 1677–1677 | England | Legal deed | Sheep |
| BA 135 | YM/D/KIRK 22 | 1678–1678 | England | Legal deed | Sheep |
| BA 25 | BED 14 | 1691–1691 | England | Legal deed | Sheep |
| BA 147 | YM/D/HAT 10(b) | 1699–1699 | England | Legal deed | Sheep |
| BA 148 | YM/D/HAT 11 | 1717–1717 | England | Legal deed | Sheep |
| BA 97 | YM/D/PO | 1726–1726 | England | Legal deed | Sheep |
| BA 39 | MOR 48 | 1744–1744 | England | Legal deed | Sheep |
| BA 98 | YM/D/PO | 1754–1754 | England | Legal deed | Sheep |
| BA 79 | YM/D/CAMP 59 (a) | 1765–1765 | England | Legal deed | Sheep |
| BA 80 | YM/D/CAMP 60 | 1767–1767 | England | Legal deed | Sheep |
| BA 99 | YM/D/PO | 1775–1775 | England | Legal deed | Sheep |
| BA 100 | YM/D/PO | 1790–1790 | England | Legal deed | Sheep |
| BA 60 | YM/D/ASK 44 | 1794–1794 | England | Legal deed | Sheep |
| BA 149 | YM/D/HAT 12 | 1818–1818 | England | Legal deed | Sheep |
| BA 40 | MOR 49 | 1819–1819 | England | Legal deed | Sheep |
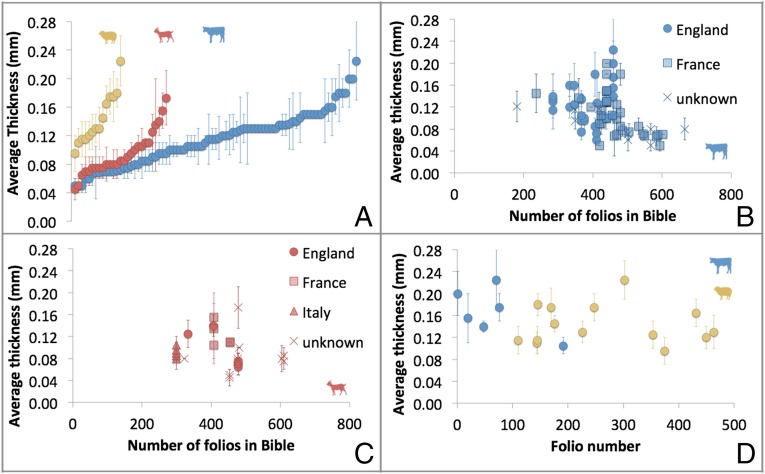
Our results have shown that pocket Bibles, some of the first examples of commercial book production (8), were written on all three species used widely in parchment production. In our survey of 220 folios spanning three centuries, no unexpected species were recorded. The use of sheepskin in only one of 72 pocket Bibles indicates that it was not favored for these very thin membranes despite the facts that sheepskin delaminates and that the range of thicknesses measured for calf and sheep parchment are similar (0.09–0.28 mm for calf and 0.07–0.26 mm for sheep) (Fig. 4D). The presence of goatskin and sheepskin parchment in the sample set would seem to indicate that uterine calfskin was not necessarily used to produce very fine membranes. Indeed, similar thickness measurements can be achieved for all three species (Fig. 4A).
Fig. 4.
Thickness of folios. (A) Thickness of all pocket Bible folios by species. (B) Thickness of calf pocket Bible folios by total number of folios in Bible. (C) Thickness of goat pocket Bible folios by total number of folios in Bible. (D) Thickness of folios from pocket Bible Ee.6.26.
SI Materials and Methods
Four samples of uterine calfskin, each measuring 0.5 cm × 0.25 cm, were prepared, as well as four control “nonuterine” calfskins, also measuring 0.5 cm × 0.25 cm. Samples were incubated in 0.5 M triethylammoniumbicarbonate (TEAB) (pH 8.5) at 65 °C for 1 h to solubilize the collagen. The supernatant was quantified using a Qubit (Thermo Fisher Scientific). One hundred fifty micrograms of protein from each sample was digested overnight with trypsin at 37 °C at a ratio of 1:75 (trypsin to protein). The tryptic digest was transferred over C18 resin to desalt and concentrate peptides by washing with 0.1% TFA. Peptides were eluted in a final volume of 50 μL of 50% acetonitrile (ACN)/0.1% TFA (vol/vol). Digested samples were labeled with the iTRAQ reagents following the protocol provided by the vendor (Applied Biosystems). Briefly, one vial of iTRAQ labeling reagent was used for every 100 μg of protein. Ethanol was used to solubilize the iTRAQ reagent and then added to the peptide sample, ensuring a final organic concentration of at least 60% (vol/vol). The labeling reaction was performed by 2 h of incubation at room temperature. Samples were cleaned up before LC-MS/MS analysis using a cation-exchange cartridge system (SCIEX) and desalted using a C18 cartridge.
Dried peptides were resuspended in 2% (vol/vol) ACN and 0.1% TFA and analyzed by nano–LC-MS/MS as described previously (52). In brief, samples were separated using nano-ultraperformance liquid chromatography (Easy spray C18 column with a 75 μm × 500 mm, 2.1-μm particle size; Thermo Fisher Scientific) coupled to a Q Exactive tandem mass spectrometer (Thermo Scientific). MS data were acquired with a resolution of 70,000 at an m/z of 200 and by selecting the top 15 precursor ions. The ion target in MS1 mode was 3 × 106, and it was 5 × 105 in MS2 mode. Ions with an m/z between 380 and 1,800 were accumulated for up to 100 ms in MS1 and for up to 128 ms in MS2. We used a stepped normalized collision energy at 31%, 34%, and 37%; a precursor isolation window of 1.6 m/z with an offset of 0.3 m/z; a fixed first mass of 100 m/z; and a resolution of 35,000 (profile) for MS2 spectrum acquisition. The samples were loaded in 0.1% TFA in 1% CH3CN and eluted with a gradient of 3–35% (vol/vol/vol) CH3CN in 5% DMSO and 0.1% formic acid for 60 min at a flow rate of 250 nL⋅min−1.
Identification/quantitation of iTRAQ signals was processed with PEAKS 7 (Bioinformatics Solutions, Inc.). Raw files were refined to correct precursor mass, and MS2 spectra were searched against the UniProt reference proteome of Bos taurus (April 7, 2014). Search parameters include trypsin with up to two missed cleavage sites, 10 ppm mass tolerance for the precursor, and 0.05 Da mass tolerance for fragment masses. Carbamidomethylation was a fixed modification, whereas we used the posttranslational modifications (PTM) search module to identify otherwise modified peptides. For quantitation of the eight-plex iTRAQ reporter signals, we used the default setting of the quantitation module.
DNA Analysis.
DNA extraction.
DNA was extracted from a 0.001-g cut piece of parchment UPenn 03 (Ms. Codex 1065), following a protocol based on the technique of Yang et al. (53), in a dedicated ancient DNA facility at Trinity College Dublin. Briefly, the parchment sample was incubated at 55 °C for 24 h in 0.5 mL of lysis buffer [1 M Tris⋅Cl (pH 7.4), proteinase K, 30% (vol/vol) sarkosyl NL, 0.5 M EDTA]. Following this incubation, the sample was centrifuged at 16,249 × g for 5 min to precipitate any undigested material; the supernatant was then removed, and DNA was recovered using the Qiagen Minelute kit. The first DNA binding step of this protocol (Qiagen Minelute) was repeated four times due to the large volume of starting material, and a final elution volume of 20 μL was used.
Library preparation and sequencing.
An Illumina sequencing library was produced for UPenn 03 from the 20 μL of extracted DNA following the protocol of Meyer and Kircher (54) with the previously described modifications of Gamba et al. (55). The 50-bp single-end sequencing of the constructed library was performed on the Illumina MiSeq platform at the Trinity Genome Sequencing Laboratory (TrinSeq), Trinity College Dublin.
Bioinformatic analysis.
Raw sequencing reads were trimmed of contaminating adapter sequences using Cutadapt (56). Identification of the source species of the parchment was performed from the trimmed reads using FastQ Screen (www.bioinformatics.babraham.ac.uk/projects/fastq_screen//) with default settings (13).
Numbers of Membranes.
Ruzzier (6) provides (conservative) estimates of the total volume of pocket Bibles produced during this period. If we assume that 20,000 Bibles (a number dependent upon estimates of survival rate) were produced, of which 54% were produced in France, and if we assume that the vast majority of these Bibles produced in France were produced in Paris (10,000), 44% of French production (∼4,700 Bibles) occurred in middle of the 13th century, which, if correct, would average more than 150 Bibles per year.
Using Ruzzier’s (6) data, we can estimate a maximum production of ∼200 Bibles per year. Assuming an average of 474 leaves (237 bifolia) per Bible, with an average height + width of 310 mm, the production of 200 Bibles would be the equivalent of 55,000 rabbits (assuming one rabbit per bifolia), ∼27,500 stillborn sheep/goats (slink), 18,000 stillborn calves (slunk) or young (20-d-old) kids/lambs, or 4,500 young calves or full-grown caprines.
After the early 11th century, when the population of Paris was estimated to be only a few thousand inhabitants (57), a period of rapid population growth occurred linked to the status of capital city conferred on Paris under the reign of Philippe Auguste (1180–1223). This population expansion continued until the early 14th century, when it reached 200,000–300,000 capita (58), falling following the plague (August 1348 through the winter of 1349/1350) (59). Le Menagier de Paris was written a century after the height of Bible production (1393), and the author estimates 306 veal calves were consumed per week (60) (15,000 per year). In modern-day herds, ∼2–5% of calves die at birth and a further 3–6% die before their first month of age (61). Therefore, even given alternative uses for calfskin parchment, our best estimates do not suggest that demand and supply are wildly at odds. As for sheep, Postan (62) estimates from taxation records that there were four to five sheep for every head of cattle in England in the 13th century, and perinatal mortalities are higher in sheep and goats than in cattle [10–12% in modern flocks (63)]. Because at the height of British production, only 9,000 stillborn lambskins would be required, and even fewer if parchment was being obtained from Italy (although this volume would be beyond the 10,000 skins required to satisfy annual Italian production at its height), once again, nothing indicates unsustainable herd management practices.
Discussion
Aside from local availability of livestock and preferences in meat consumption, parchment production in medieval Europe was limited by logistical challenges. Parchment production was presumably located close to the point of slaughter, because skins deteriorate within days, resulting in poor quality and spotty membranes (2). Hides can be preserved by salting, but this process would be prohibitively expensive in Northern Europe: The salt price in England rose from a base above 0.1 g⋅Ag⋅kg−1 during this period.
In addition, the prestige and value of particular types of skin played an important role. Fourteenth-century accounts from Beaulieu Abbey (27) show that calfskin was more highly valued than sheepskin. The importance of the hide as a source of revenue is repeatedly evidenced by the abundance of skinning marks on animal remains, the range of decrees and bylaws restricting the flaying of animals that had died of diseases, and the lengths the authorities went to prevent these skins being used (28). The intact skeleton of a cow from a 14th-century burial at Téteghem Carlines 3 (Northern France) that died of a dystocic calving (29) gives a further insight into the importance (or otherwise) of uterine hides. Although the cow’s hide had been removed, the calf remained trapped and unflayed in the birth canal, despite the fact that it could have been easily released and flayed to obtain uterine calfskin for parchment.
However, most of the geographic variations can be explained by the local livestock availability and preferences in meat consumption in the different regions.
France.
The relatively low use of goat parchment in Parisian pocket Bibles may be explained, in part, by the patterns of consumption. In Le Viandier de Taillevent (a recipe book first produced in the early 14th century), of 140 recipes, veal appears in seven (5%). Kid and lamb are listed in only one recipe and are considered interchangeable depending on availability. Evidence from the 15th century suggests that sheep and goats were much more common in Southern France (30). Excavations in multiple sites from the Parisian area revealed 3,102 bone fragments dating to the 13th century, the majority of which were caprines (sheep/goat, 38.2%) followed by similar levels of ∼30% cattle and pig (31).
Although the species of animal used would reflect local livestock availability, the age of the animals was limited by the craft production. La Lande’s book Art de faire le parchemin, written in 1762, indicates that calfskins suitable for making parchment should not be taken from an animal older than 6 wk of age (32). The skins of young calves, although suitable for parchment production, are already several times thicker than the skin of adult goats or sheep. Although adult goats and sheep can be used for the production of certain types of parchment, adult cow skins are too thick for parchment production and are instead used to produce heavy, durable leather (33). However, Clavel (31) notes that calf bones are rarely found in 13th and 14th century sample collections and comprise only 2–5% of all cattle bones from excavated sites, appearing with greater frequency in later periods. Veal calves were typically slaughtered at around 6 mo of age, but the bones of very young cattle have rarely been found in French medieval archaeological sites (34).
England.
The presence of goatskin in the English pocket Bibles is intriguing, an assertion that is further supported for one sample with DNA evidence (Fig. S2). Goatskin folios produced in England are thinner than their Italian or French counterparts (Fig. 4C). This evidence would seem to indicate that goatskin was not routinely available and may have been acquired specifically for the production of pocket Bibles. However, goat horn-core accumulations are not unusual, particularly in the eastern part of the country and in urban sites, possibly the product of an international skin trade, because horns were routinely left within the transported skins (22). In both early and later medieval England, sheep are overwhelmingly more common than goats; when identifiable, the latter invariably represent less than 10% of the total sheep/goat bone and tooth fragments, and this proportion is often close to 1%. Goat bones occur on ∼50% of sites, but invariably in small frequency. In the late-medieval period, both documentary (35) and archaeological (34) sources attest to a further decline in the use of goat.
Fig. S2.
FastQ Screen analysis of DNA extracted from UPenn 03 (Ms. Codex 1065) following Teasdale et al. (13). A species identification of goat is confirmed, both by the alignment to the goat genome being the maxima for all species considered and by the most uniquely aligning sequences (red) belonging to goat. Cross-alignment of other reads is expected owing to the high homology, especially of repeated elements, among ruminant genomes.
Cattle were predominantly slaughtered as adults in medieval England, which reflects their major role as tractors. The gradual replacement of working cattle with horses toward the end of the period, at least in some regions, provided the opportunity for an increase in early culling. From the 15th century onward, several sites, mainly urban, have produced relatively high proportions of calf bones, probably a consequence of an increased demand for veal and milk, as well as a greater production of calfskins (34, 36), but this evidence does not explain the predominant use of calfskin in English pocket Bibles in the 13th century.
Italy.
At Italian archaeological sites, sheep bones also tend to predominate during the medieval period, but far less so than in England, with a typical sheep/goat ratio ranging between 3:1 and 2:1 (37–39). Italian zooarchaeological reports, however, rarely provide specific identifications for caprines, perhaps due to the difficulty in separating sheep from goat morphologically. The predominance of goat horn-cores is not a phenomenon known for Italian sites.
The use of cattle is clearly variable, with emphasis on meat production or traction, depending on the site. The presence of very young animals is, however, reported at several urban (39) and high-status sites (40). However, the nature of the current evidence is insufficient to allow us to identify any clear chronological trends.
Conclusion
This study reveals the value of the triboelectric eZooMS approach to the analysis of parchment. It requires no specialist equipment or storage, samples can be collected when appropriate without the need to transport the artifacts, and the samples can then be analyzed when required. As we have shown (Fig. 1), triboelectric extraction is more efficient than physical sampling for four reasons. First, much less material is required. Second, the molecular extraction is bulked onto a large volume of eraser waste, which means it is easy to subsample. Third, molecules are stabilized on the surface of the eraser waste. Fourth, eZooMS acts as a prepurification step, as evidenced by the results from whole samples. Following extraction, the pigmented extracts remain on the eraser crumbs and only the macromolecules are extracted.
We have been able to provide the first significant molecular evidence, to our knowledge, to resolve the long-standing question of the origin of uterine vellum. We find no evidence of unexpected species, such as rabbit or squirrel. Although this type of parchment derived mostly from calfskin in France, in other places, different skins were used subject to local availability. The production of ultrathin parchment was the result of a technological production process using available resources and, as such, would not have demanded unsustainable agricultural practices. We have further shown that manuscript documents made from animal skins are a valuable additional resource for archaeologists exploring livestock economies.
Although the use of genuine uterine vellum cannot be discounted, our results suggest that its availability was not a defining factor in medieval parchment production. Instead, our findings would seem to emphasize dependence on a highly specialized craft technique rather than the supply of a particular raw material. A more likely explanation for the production of fine parchment is the use of relatively young animals and the deployment of specific finishing techniques that enabled the corium to be ground to the desired thickness. The density of collagen fibrils in calf and goat parchment, compared with a more open weave and higher fat content in sheep parchment, favors the former two species; nevertheless, it is evident that parchment makers had the skills to make the finest parchments from all three.
Materials and Methods
All codices sampled were classified in their catalog entries as 13th-century Bibles. The 79 Bibles were subdivided, depending on their dimensions, into two groups based on criteria used by Ruzzier (6): pocket Bibles (height + width < 385 cm) and nonpocket Bibles (height + width >385 cm). Details of all Bibles sampled can be found in Tables S2 and S3.
Sampling.
Samples were extracted in the participating archives and libraries using kits sent by one of the authors (S.F.) consisting of 1.5-mL microcentrifuge tubes, nitrile gloves, acid-free paper, and nonabrasive conservator’s erasers. Sampling was performed using a Staedtler “Mars Plastic” eraser, rubbing the eraser in one direction and collecting the resulting eraser waste fragments in individual 1.5-mL microcentrifuge tubes. For each sample, a new individual piece of eraser and acid-free paper was used; the eraser and paper were thrown away once sampling of the folio was completed to avoid cross-contamination. Nitrile gloves were worn throughout the sampling process to avoid keratin (human skin) contamination. Sample collection was undertaken on areas of the document that had no writing and presented structural integrity (absence of holes or tears in the parchment). All samples were stored at room temperature until required, usually by the partner. Details of each document sampled were entered onto an online spreadsheet shared between the partner and the laboratories in York.
eZooMS.
Initially, eraser crumb samples [sometimes known as “erdu” (41)] were spun down at maximum speed on a benchtop centrifuge for 1 min and 75 μL of 0.05 M NH3CO3 (AmBic) buffer (pH 8) was added to each sample. Samples were heated at 65 °C for 1 h. Once cooled, 1 μL of trypsin (0.4 μg/μL) was added and samples were incubated at 37 °C for 18 h. However, at a later stage, the method of collagen extraction was optimized by removing the heating step and condensing the process into one incubation step at 37 °C for 4 h with both AmBic and trypsin added simultaneously. After incubation with trypsin, digests were spun down at maximum speed on a benchtop centrifuge for 1 min and 1 μL of 5% (vol/vol) TFA was added. Samples were desalted and concentrated using C18 resin (Millipore), following the manufacturer’s instructions. Peptides were eluted in a final volume of 50 μL of 50% acetonitrile (ACN)/0.1% TFA (vol/vol). One microliter of eluted peptides was mixed on a ground steel plate with 1 μL of α-cyano-4-hydroxycinnamic acid matrix solution [1% in 50% ACN/0.1% TFA (vol/vol/vol)] and air-dried. All samples were spotted in triplicate. Samples were analyzed using a calibrated Ultraflex III (NLD1; Bruker Daltonics) MALDI-TOF instrument in reflector mode. Spectral analysis was performed using the open-source cross-platform software mMass (www.mmass.org) (42), and individual peptides were identified manually according to Buckley et al. (43, 44).
Acknowledgments
We thank Suzanne Reynolds (Fitzwilliam Museum) for donating samples and commenting on the manuscript and Katharine C. Chandler (Free Library of Philadelphia), Tatiana Gersten (Royal Library of Belgium), Maria Fredericks (Morgan Library & Museum), Libby Melzer (University of Melbourne), Peter Young (York Minster Library), Richard Higgins (Durham University Library), Gabriel Sewell (Durham Cathedral Library), Eliza Gilligan (University of Virginia), the University of St. Louis, and Maggs Bros. Ltd. for donating samples to this project. We also thank Alison Fairburn, Catherine Dand, and Chris Webb (Borthwick Archive, University of York) for their role in developing the sampling technique; Adam Dowle (Technology Facility, University of York) for his help with isobaric tags for relative and absolute quantification; Jesse Meyer (Pergamena) for his expertise and for supplying parchments for analysis; and Suzanne Paul (Cambridge University Library) for her curatorial expertise. We acknowledge Science Foundation Ireland European Research Council (ERC) Support Award 12/ERC/B2227, Valeria Mattiangeli, and the Trinity Genome Sequencing Laboratory (TrinSeq) for MiSeq support. This work was supported by the Marie Curie International Fellowship PALIMPSEST FP7-PEOPLE-2011-IEF 299101, a University of Manchester Research Institute seedcorn grant, British Academy Postdoctoral Fellowship funding, and ERC Investigator Grant 295729-CodeX.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.G.C. is a guest editor invited by the Editorial Board.
Data deposition: All MS data files have been deposited in the Archaeological Data Service (ADS), and may be accessed through www.dx.doi.org/10.5284/1035166.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1512264112/-/DCSupplemental.
References
- 1.Pollard G. Notes on the size of the sheet. The Library: Transactions of the Bibliographical Society. 1941;4(2-3):105–137. [Google Scholar]
- 2.Thompson D. The Materials and Techniques of Medieval Painting. Dover; New York: 1956. [Google Scholar]
- 3.De Hamel C. Scribes and Illuminators. Univ of Toronto Press; Toronto: 1992. [Google Scholar]
- 4.Clarkson C. Rediscovering parchment: The nature of the beast. The Paper Conservator. 1992;16(1):5–26. [Google Scholar]
- 5.Clarke M. The archaeology of the book: Formulating analytical research questions. E-Conservation Journal. 2014;2:10–16. [Google Scholar]
- 6.Ruzzier C. The miniaturisation of Bible manuscripts in the thirteenth century. A comparative study. In: Light L, Poleg E, editors. Form and Function in the Late Medieval Bible. Brill; Leiden, The Netherlands and Boston: 2013. pp. 105–125. [Google Scholar]
- 7.Dove M. In: The Blackwell Companion to the Bible and Culture. Sawyer JFA, editor. Wiley; New York: 2012. [Google Scholar]
- 8.Light L. The Bible and the individual: The thirteenth century Paris Bible. In: Boynton S, Reilly DJ, editors. The Practice of the Bible in the Middle Ages: Production, Reception, and Performance in Western Christianity. Columbia University Press; New York: 2011. pp. 228–246. [Google Scholar]
- 9.Juchauld F, Bonnenberger P, Komenda A. 2010. Identification de l’espèce animale des cuirs de reliure et des parchemins. Matériaux Du Livre Médiéval, eds M. Zerdoun Bat-Yehouda C, Bourlet C (Brepols, Turnhout, Belgium), pp 13–28. French.
- 10.Ryder ML. Parchment—its history, manufacture and composition. J Soc Arch. 1960;2(9):391–399. [Google Scholar]
- 11.Toniolo L, D’Amato A, Saccenti R, Gulotta D, Righetti PG. The Silk Road, Marco Polo, a Bible and its proteome: A detective story. J Proteomics. 2012;75(11):3365–3373. doi: 10.1016/j.jprot.2012.03.051. [DOI] [PubMed] [Google Scholar]
- 12.Kirby DP, Buckley M, Promise E, Trauger SA, Holdcraft TR. Identification of collagen-based materials in cultural heritage. Analyst (Lond) 2013;138(17):4849–4858. doi: 10.1039/c3an00925d. [DOI] [PubMed] [Google Scholar]
- 13.Teasdale MD, et al. Paging through history: Parchment as a reservoir of ancient DNA for next generation sequencing. Philos Trans R Soc Lond B Biol Sci. 2015;370(1660):20130379. doi: 10.1098/rstb.2013.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stinson T. 2011. Counting sheep: Potential applications of DNA analysis to the study of medieval parchment production. Kodikologie Und Paläographie Im Digitalen Zeitalter 2 [Codiology and Palaeography in the Digital Age 2], Schriften des Instituts für Dokumentologie und Editorik, Band 3, eds Franz F, Fritze C, Vogeler G (Books on Demand, Norderstedt, Germany), pp 191–207.
- 15.Bower MA, Campana MG, Checkley‐Scott C, Knight B, Howe CJ. The potential for extraction and exploitation of DNA from parchment: A review of the opportunities and hurdles. Journal of the American Institute for Conservation. 2010;33(1):1–11. [Google Scholar]
- 16.Campana MG, et al. A flock of sheep, goats and cattle: Ancient DNA analysis reveals complexities of historical parchment manufacture. J Archaeol Sci. 2010;37(6):1317–1325. [Google Scholar]
- 17.Burger J, Hummel S, Herrmann B. Palaeogenetics and cultural heritage. Species determination and STR-genotyping from ancient DNA in art and artefacts. Thermochim Acta. 2000;365(1–2):141–146. [Google Scholar]
- 18.Light L. 1984. Versions et révisions du texte biblique. Le Moyen Age et la Bible. Bible de tous les temps 4, eds. Riche P, Lobrichon G (Beauchesne, Paris), pp 55–93. French. [Google Scholar]
- 19.Light L. In: The Early Medieval Bible. Its Production, Decoration, and Use. Gameson R, editor. Cambridge Univ Press; Cambridge, UK: 1994. [Google Scholar]
- 20.Light L, Marsden R, Matter EA. The thirteenth century and the Paris Bible. In: Marsden R, Matter EA, editors. The New Cambridge History of the Bible. Cambridge Univ Press; Cambridge, UK: 2012. pp. 380–391. [Google Scholar]
- 21.Forbes RJ. Fibres and Fabrics of Antiquity: Washing, Bleaching, Fulling and Felting; Dyes and Dyeing; Spinning, Sewing, Basketry and Weaving; Weaving and Looms; Fabrics and Weavers. Brill, Leiden; The Netherlands: 1955. [Google Scholar]
- 22.Albarella U. Tanners, tawyers, horn working and the mystery of the missing goat. In: Murphy P, Wiltshire PEJ, editors. The Environmental Archaeology of Industry. Oxbow Books; Oxford: 2003. pp. 71–86. [Google Scholar]
- 23.Pascua E. From forest to farm and town: Domestic animals from ca. 1000 to ca. 1450. In: Resl B, editor. A Cultural History of Animals in the Medieval Age. Berg; Oxford: 2009. pp. 81–102. [Google Scholar]
- 24.Magrini S. Production and use of Latin Bible manuscripts in Italy during the thirteenth and fourteenth centuries. Manuscripta. 2007;51(2):209–257. [Google Scholar]
- 25.Bianchi F, et al. 1993. Facteurs de variations de l’épaisseur du parchemin italien du VIII Eau XV Esiècle. Ancient and Medieval Book Materials and Techniques (Erice, September 18-25, 1992), Studi e Testi, eds Maniaci M, Paola Munafò PF (Biblioteca apostolica vaticana, Vatican City, Italy), pp 95–184. Italian.
- 26.Henderson EF. 1896. Select Historical Documents of the Middle Ages (George Bell and Sons, London), pp 20–134. [Google Scholar]
- 27.Hockey SF. 1975. The Account-Book of Beaulieu Abbey, Camden Fourth Series (London Offices of the Royal Historical Society, London)
- 28.Vallat F. 2009 Les Bøeufs Malades de la Peste, la Peste Bovine en France et en Europe, XVIIIe-XIXe Siècle (Presses universitaires de Rennes, Rennes, France)
- 29.Binois A. Excavating the history of ancient veterinary practices. Vet Rec. 2015;176(22):564–569. doi: 10.1136/vr.h991. [DOI] [PubMed] [Google Scholar]
- 30.Banegas López RA. Consumption of meat in Western European cities during the late middle ages: A comparative study. Food and History. 2010;8(1):63–86. [Google Scholar]
- 31.Clavel B. L’animal dans l’alimentation médiévale et moderne en France du Nord (XIIIe-XVIIe siècles) Rev Med Picardie. 2001;19(1):9–204. French. [Google Scholar]
- 32. La Lande J de (1762) Art de faire le parchemin par M. de la Lande (De l’imprimerie de H. L. Guerin et L. F. Delatour, Paris). French.
- 33.Johansen K, Petersen ML. Making a Replica of the Treaty of Kiel. The Royal Library of Copenhagen and The Norwegian Parliament Archives; Copenhagen, Denmark, and Oslo, Norway: 2015. [Google Scholar]
- 34.Albarella U. Size, power, wool and veal: Zooarchaeological evidence for late medieval innovations. In: De Boe G, Verhaeghe F, editors. Environment and Subsistence in Medieval Europe. Institute for the Archaeological Heritage of Flanders; Bruges, Belgium: 1997. pp. 19–30. [Google Scholar]
- 35.Dyer C. Alternative agriculture: Goats in medieval England. In: Hoyle RW, Thirsk J, editors. People, Landscape and Alternative Agriculture. British Agricultural History Society; Exeter, UK: 2004. pp. 20–38. [Google Scholar]
- 36.Albarella U. Meat production and consumption in town and country. In: Giles K, Dyer C, editors. Town and Country in the Middle Ages: Contrasts, Contacts, and Interactions 1100–1500. Maney; Leeds, UK: 2005. pp. 131–148. [Google Scholar]
- 37.Colonnelli G, De Grossi Mazzorin J. 2000. Nuovi dati sll’alimentazione a Farnese (VT) nei secoli XV e XVI. Atti Del Secondo Convegno Nazionale Di Archeozoologia (Abaco, Forli, Italy), pp 369–376. Italian.
- 38.Minniti C. Economia e alimentazione nel Lazio medievale: nuovi dati dalle evidenze archeozoologiche. Archeologia Medievale. 2009;36:275–283. Italian. [Google Scholar]
- 39.Corbino C. 2009. “Dall’allevamento alle mense” la Toscana tra il XIII e la prima metà del XV secolo. Analisi archeozoologiche. PhD dissertation (Universita degli Studi di Siena, Siena, Italy). Italian.
- 40.Baker P, Clark G. Archaeozoological evidence for medieval Italy: A critical review of the present state of research. Archeologia Medievale. 1993;20:45–78. [Google Scholar]
- 41.Hall R. Sniglets (snig’lit): Any Word That Doesn’t Appear in the Dictionary, But Should. Macmillan; New York: 1984. [Google Scholar]
- 42.Strohalm M, Kavan D, Novák P, Volný M, Havlícek V. mMass 3: A cross-platform software environment for precise analysis of mass spectrometric data. Anal Chem. 2010;82(11):4648–4651. doi: 10.1021/ac100818g. [DOI] [PubMed] [Google Scholar]
- 43.Buckley M, Collins M, Thomas-Oates J, Wilson JC. Species identification by analysis of bone collagen using matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2009;23(23):3843–3854. doi: 10.1002/rcm.4316. [DOI] [PubMed] [Google Scholar]
- 44.Buckley M, et al. Distinguishing between archaeological sheep and goat bones using a single collagen peptide. J Archaeol Sci. 2010;37(1):13–20. [Google Scholar]
- 45.Možir A, Cigić IK, Marinšek M, Strlič M. Material properties of historic parchment: A reference collection survey. Stud Conserv. 2014;59(3):136–149. [Google Scholar]
- 46.Horman W. Vulgaria Viri Doctissimi Guil. Hormani. Richard Pynson; London: 1519. [Google Scholar]
- 47.Mays PK, Bishop JE, Laurent GJ. Age-related changes in the proportion of types I and III collagen. Mech Ageing Dev. 1988;45(3):203–212. doi: 10.1016/0047-6374(88)90002-4. [DOI] [PubMed] [Google Scholar]
- 48.Kitchen H, Brett I. Embryonic and fetal hemoglobin in animals. Ann N Y Acad Sci. 1974;241(0):653–671. doi: 10.1111/j.1749-6632.1974.tb21921.x. [DOI] [PubMed] [Google Scholar]
- 49.Brandt LØ, et al. Species identification of archaeological skin objects from Danish bogs: Comparison between mass spectrometry-based peptide sequencing and microscopy-based methods. PLoS One. 2014;9(9):e106875. doi: 10.1371/journal.pone.0106875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grimes RM, Duncan CW, Lassiter CA. Bovine fetal hemoglobin. I. Postnatal persistence and relation to adult hemoglobins. J Dairy Sci. 1958;41(11):1527–1533. [Google Scholar]
- 51.Breathnach CS. Foetal and neonatal haemoglobins in sheep and goats. Q J Exp Physiol Cogn Med Sci. 1964;49(3):277–289. doi: 10.1113/expphysiol.1964.sp001732. [DOI] [PubMed] [Google Scholar]
- 52.Fischer R, Kessler BM. Gel-aided sample preparation (GASP)--a simplified method for gel-assisted proteomic sample generation from protein extracts and intact cells. Proteomics. 2015;15(7):1224–1229. doi: 10.1002/pmic.201400436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang DY, Eng B, Waye JS, Dudar JC, Saunders SR. Technical note: Improved DNA extraction from ancient bones using silica-based spin columns. Am J Phys Anthropol. 1998;105(4):539–543. doi: 10.1002/(SICI)1096-8644(199804)105:4<539::AID-AJPA10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 54.Meyer M, Kircher M. Illumina sequencing library preparation for highly multiplexed target capture and sequencing. Cold Spring Harb Protoc. 2010;2010(6):db.prot5448. doi: 10.1101/pdb.prot5448. [DOI] [PubMed] [Google Scholar]
- 55.Gamba C, et al. Genome flux and stasis in a five millennium transect of European prehistory. Nat Commun. 2014;5:5257. doi: 10.1038/ncomms6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal. 2011;17(1):10–12. [Google Scholar]
- 57.Favier J. 1997. Paris: deux mille ans d’histoire (Fayard, Paris). French.
- 58.Cazelles R. La population de Paris avant la peste noire. Comptes rendus des séances de l’Académie des Inscriptions et Belles-Lettres. 1966;110(4):539–550. French. [Google Scholar]
- 59.Mollat M. Notes sur la mortalité à Paris au temps de la Peste Noite d'après les comptes de l'oeuvre de Saint-Germain-l'Auxerrois. In: Mollat M, editor. Études Sur L'économie et La Société de l'Occident Médiéval XIIe-XIVe Siècle. Variorum Reprints; London: 1977. pp. 505–527. [Google Scholar]
- 60.Laurioux B. 1997. Le règne de Taillevent: livres et pratiques culinaires à la fin du Moyen age (Publications de la Sorbonne, Paris). French.
- 61.Dardillat C, Vallet A. 1984. Mesures d’hygiene et de menagement a la periode perinatale pour prevenir les maladies neonatales infectieuses des veaux. 14. Journees Du Grenier de Theix. Physiologie et Pathologie Perinatales Chez Les Animaux de Ferme, December 15–17, 1982, Ceyrat, France (Institut National de la Recherche Agronomique, Paris). French.
- 62.Postan MM. Essays on Medieval Agriculture and General Problems of the Medieval Economy. Cambridge Univ Press; Cambridge, UK: 2008. [Google Scholar]
- 63.Villette Y, et al. 1984. Viabilite de l’agneau et du chevreau nouveau-nes. Poids a la naissance et types genetiques [mortalite]. 14. Journees Du Grenier de Theix. Physiologie et Pathologie Perinatales Chez Les Animaux de Ferme. December 15–17, 1982, Ceyrat France. (Institut National de la Recherche Agronomique, Paris, France) French.