Significance
G protein-coupled receptors (GPCRs) regulate the activity of virtually all cell types including pancreatic β-cells. β-Cell M3 muscarinic receptors (M3Rs) play an essential role in maintaining proper whole-body glucose homeostasis. Activity of the M3R, like that of other GPCRs, is modulated by phosphorylation by various kinases, including GRKs and casein kinase 2 (CK2). The potential physiological relevance of M3R phosphorylation (or of GPCRs in general) by CK2 remains unknown. We here show that CK2-dependent phosphorylation of β-cell M3Rs significantly impairs M3R-mediated increases in insulin release in vitro and in vivo. Our data demonstrate, for the first time to our knowledge, the physiological relevance of CK2 phosphorylation of a GPCR and suggest the novel concept that kinases acting on β-cell GPCRs may represent therapeutic targets.
Keywords: G protein-coupled receptors, β-cell function, mouse models, glucose homeostasis, GPCR regulation
Abstract
G protein-coupled receptors (GPCRs) regulate virtually all physiological functions including the release of insulin from pancreatic β-cells. β-Cell M3 muscarinic receptors (M3Rs) are known to play an essential role in facilitating insulin release and maintaining proper whole-body glucose homeostasis. As is the case with other GPCRs, M3R activity is regulated by phosphorylation by various kinases, including GPCR kinases and casein kinase 2 (CK2). At present, it remains unknown which of these various kinases are physiologically relevant for the regulation of β-cell activity. In the present study, we demonstrate that inhibition of CK2 in pancreatic β-cells, knockdown of CK2α expression, or genetic deletion of CK2α in β-cells of mutant mice selectively augmented M3R-stimulated insulin release in vitro and in vivo. In vitro studies showed that this effect was associated with an M3R-mediated increase in intracellular calcium levels. Treatment of mouse pancreatic islets with CX4945, a highly selective CK2 inhibitor, greatly reduced agonist-induced phosphorylation of β-cell M3Rs, indicative of CK2-mediated M3R phosphorylation. We also showed that inhibition of CK2 greatly enhanced M3R-stimulated insulin secretion in human islets. Finally, CX4945 treatment protected mice against diet-induced hyperglycemia and glucose intolerance in an M3R-dependent fashion. Our data demonstrate, for the first time to our knowledge, the physiological relevance of CK2 phosphorylation of a GPCR and suggest the novel concept that kinases acting on β-cell GPCRs may represent novel therapeutic targets.
G protein-coupled receptors (GPCRs) are known to play central roles in regulating the function of pancreatic β-cells, in particular the release of insulin (1, 2). Consistent with this notion, several β-cell GPCRs are considered attractive targets to enhance β-cell function for the treatment of type 2 diabetes (T2D) (1, 2).
The neurotransmitter, acetylcholine, following its release from pancreatic parasympathetic nerve terminals, is highly efficacious in facilitating insulin secretion (3, 4). We (5) and others (4, 6) previously demonstrated that this effect is mediated via activation of β-cell M3 muscarinic receptors (M3Rs). Importantly, mutant mice lacking M3Rs selectively in their pancreatic β-cells display impaired glucose tolerance and significantly reduced insulin release (7). Transgenic mice overexpressing M3Rs in their pancreatic β-cells show the opposite phenotype characterized by greatly improved glucose tolerance and enhanced insulin release under different experimental conditions (7). Moreover, these transgenic mice, similar to mutant mice that express a constitutively active mutant M3R in their β-cells (8), are resistant to diet-induced glucose intolerance and hyperglycemia (7). These results clearly indicate that the development of strategies aimed at facilitating signaling through β-cell M3Rs may prove useful to facilitate insulin secretion for therapeutic purposes.
As has been observed with most other GPCRs (9), M3R signaling is regulated by the activity of various kinases that directly phosphorylate the M3R protein. In the case of the M3R, such kinases include different GPCR kinases (GRKs) such as GRK2 (10, 11), GRK3 (11, 12), and GRK6 (11–13), as well as CK1α (14) and casein kinase 2 (CK2) (15). We therefore speculated that inhibition of kinases that act on β-cell M3Rs might prove an effective approach to regulating M3R signaling in this cell type.
An initial siRNA screen demonstrated that knocking down the expression of CK2α, one of the two catalytic subunits of CK2, greatly augmented M3R-mediated calcium responses in cultured β-cells (MIN6 cells). Protein kinase CK2 is a serine/threonine protein kinase that is expressed in most cell types (16). The CK2 holoenzyme consists of a hetero-tetrameric complex containing one of the two catalytic isoforms (CK2α or CK2α′) and two regulatory β-subunits in various combinations. CK2 is known to regulate a large number of cellular functions including cell growth and survival (16). However, the potential role of CK2 in modulating the function of pancreatic β-cells has not been investigated so far.
In the present study, we demonstrate that CK2α (CK2) is a potent and selective negative regulator of M3R signaling in pancreatic β-cells in vitro and in vivo. Notably, this study provides, to our knowledge, the first piece of evidence indicating that CK2 phosphorylation of a GPCR is of physiological relevance. Our findings may stimulate the development of novel classes of drugs useful for the treatment of T2D.
Results
Knockdown of the Expression of the α-Subunit of CK2 (CK2α) Enhances M3R Function in Cultured β-Cells.
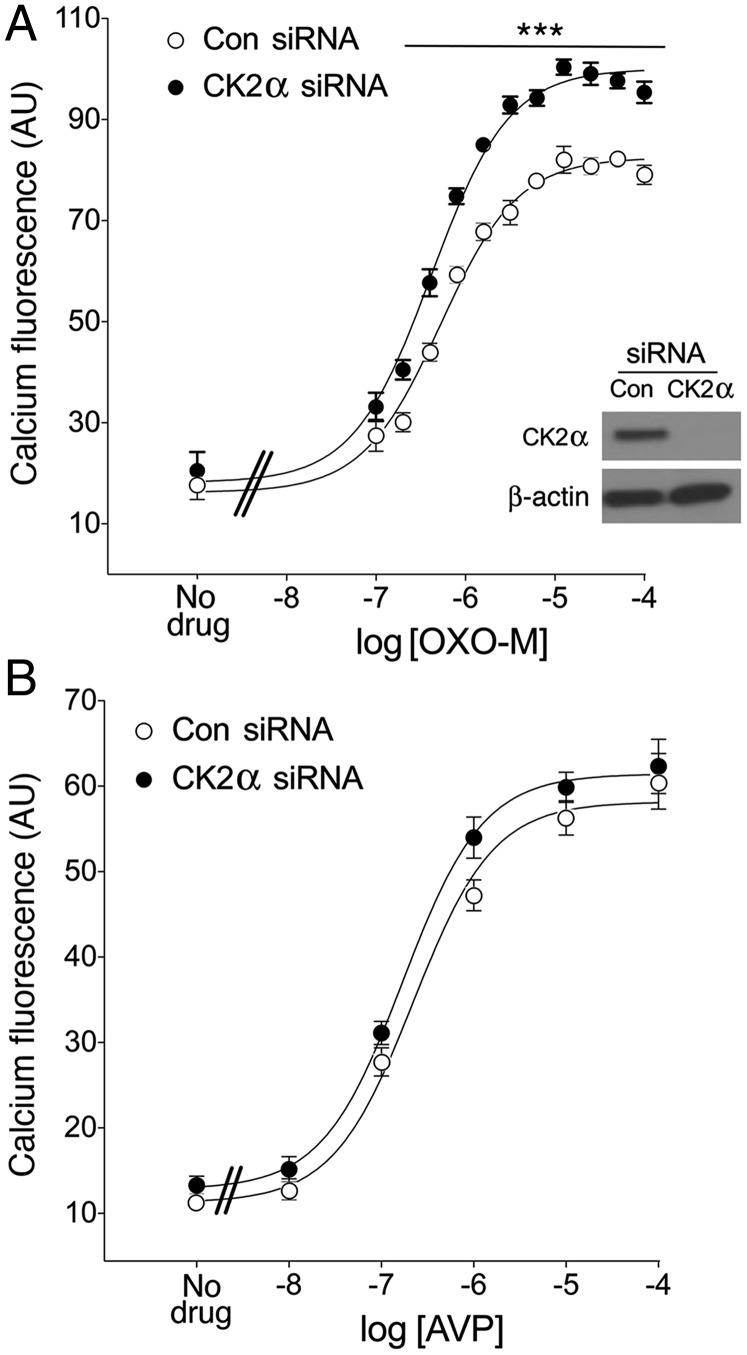
We first screened for kinases that might interfere with M3R function in cultured β-cells (MIN6 cells). In a previous study, we demonstrated that the M3R is the only muscarinic receptor subtype expressed in MIN6 cells (17). Specifically, we determined M3R-mediated increases in intracellular calcium levels ([Ca2+]i) following siRNA-mediated knockdown of kinases known to phosphorylate the M3R (GRK2, GRK3, GRK6, CK1α, CK2α, and CK2α′) (10–15). This screen identified a single kinase, CK2α, the knockdown of which led to a significant augmentation of M3R-mediated increases in [Ca2+]i, as assessed via Fluorescent Imaging Plate Reader (FLIPR) technology. Fig. 1A shows that the muscarinic agonist, OXO-M, triggered greatly enhanced M3R-dependent calcium responses following treatment of MIN6 cells with CK2α siRNA, compared with scrambled control siRNA.
Fig. 1.
Knockdown of CK2α expression selectively augments M3R-mediated increases in [Ca2+]i in MIN6 cells. (A and B) MIN6 cells were electroporated with CK2α siRNA or scrambled control siRNA. The insert in A shows a representative Western blot indicating that the use of CK2α siRNA led to a very efficient knockdown of CK2α expression. Cells were then incubated with increasing concentrations of the muscarinic agonist, OXO-M (A), which acts on endogenous M3Rs or AVP (B), which stimulates endogenous V1B vasopressin receptors, respectively. Agonist-induced increases in [Ca2+]i were determined via FLIPR. Data are expressed as means ± SEM of three independent experiments, each carried out in quadruplicate. AU, arbitrary units. ***P < 0.001, compared with the corresponding control value.
Knockdown of CK2α Expression Does Not Affect V1B Vasopressin Receptor-Mediated Calcium Responses in Cultured β-Cells.
In addition to the M3R, pancreatic β-cells express other GPCRs that can mediate increases in [Ca2+]i via coupling to G proteins of the Gq family, including the V1B vasopressin receptor (18). Although siRNA-mediated knockdown of CK2α greatly enhanced M3R-mediated increases in [Ca2+]i in MIN6 cells (Fig. 1A), this effect was not observed following stimulation of V1B vasopressin receptors by arginine vasopressin (AVP) (Fig. 1B). This observation suggests that knockdown of CK2α does not augment M3R-mediated calcium responses by modulating the activity of signaling molecules downstream of Gq activation.
Knockdown of CK2α Expression Does Not Affect Agonist-Induced M3R Internalization and Total M3R Density in Cultured β-Cells.
To investigate whether CK2α plays a role in terminating M3R signaling by promoting agonist-induced receptor internalization, we carried out an M3R internalization assay. After treatment of MIN6 cells with CK2α siRNA or scrambled control siRNA, cells were incubated with OXO-M (100 μM) for 60 min at 37 °C in FLIPR buffer. After this step, cells were incubated for 2 h at 4 °C in binding buffer with a saturating concentration (2 nM) of [3H]NMS, a membrane-impermeable muscarinic radioligand. We found that knockdown of CK2α expression had no significant effect on the magnitude of OXO-M–induced M3R internalization (SI Appendix, Fig. S1A). Moreover, radioligand-binding studies with [3H]-QNB, a lipophilic, membrane-permeable muscarinic radioligand, showed that the total number of M3R-binding sites remained unaffected after CK2α knockdown (SI Appendix, Fig. S1B).
Knockdown of CK2α Expression Specifically Augments M3R-Mediated Insulin Secretion in Cultured β-Cells.
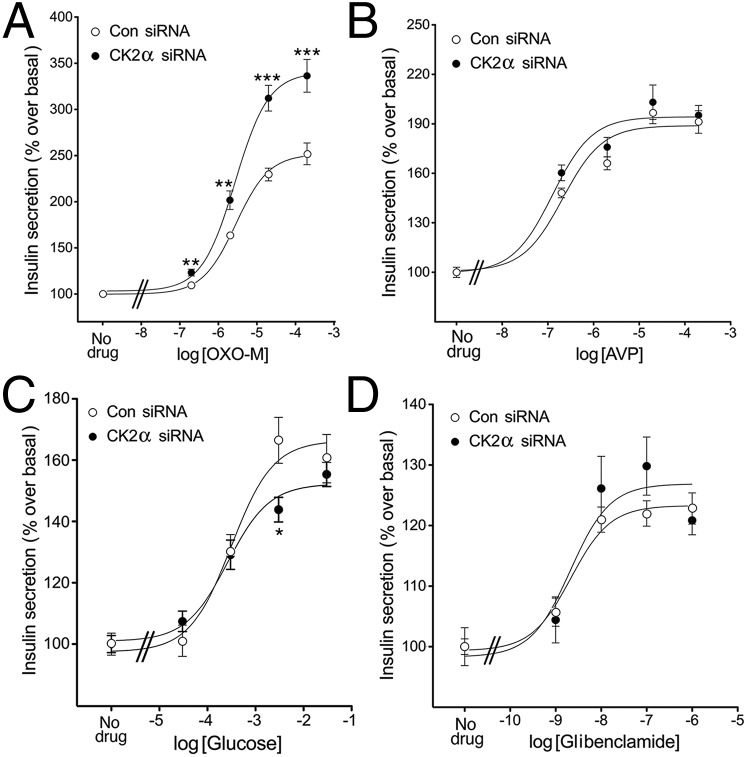
We next examined whether the augmentation of M3R-mediated calcium responses observed with MIN6 cells following knockdown of CK2α expression also led to changes in M3R-mediated insulin secretion. Consistent with the calcium mobilization data, OXO-M triggered greatly enhanced insulin secretion in MIN6 cells that had been treated with CK2α siRNA, compared with cells treated with control siRNA (Fig. 2A). We also studied whether CK2α knockdown affected insulin release in response to other insulin secretagogues, including AVP, glucose, and glibenclamide, an inhibitor of ATP-sensitive K+ channels. This analysis demonstrated that CK2α knockdown had no significant effect on the magnitude of AVP-, glucose-, and glibenclamide-induced insulin responses (Fig. 2 B–D).
Fig. 2.
Knockdown of CK2α specifically augments M3R-mediated insulin secretion in MIN6 cells. Insulin release assays were carried out with MIN6 cells that had been treated with CK2α siRNA or scrambled control siRNA. (A–D) Cells were incubated with increasing concentrations of OXO-M (acting on endogenous M3Rs) (A), AVP (acting on endogenous V1B receptors) (B), glucose (C), or glibenclamide (D), an inhibitor of ATP-sensitive K+ channels. CK2α knockdown greatly enhanced M3R-mediated insulin release but had little or no effect on AVP-, glucose-, or glibenclamide-induced insulin secretion. Note that basal insulin secretion was slightly increased (P < 0.05) in cells treated with CK2α siRNA (insulin in ng/mL; control siRNA vs. CK2α siRNA: (A) 23.2 ± 1.5 vs. 28.1 ± 1.2; (B) 23.2 ± 1.6 vs. 28.2 ± 0.8; (C) 16.6 ± 0.6 vs. 22.0 ± 0.8; (D) 27.3 ± 0.6 vs. 31.0 ± 1.0. Data are expressed as the percentage increase in insulin release above basal levels and represent means ± SEM from three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, compared with the corresponding control value.
CX4945 Enhances M3R-Stimulated Insulin Release in Mouse Pancreatic Islets in a CK2α-Dependent Fashion.
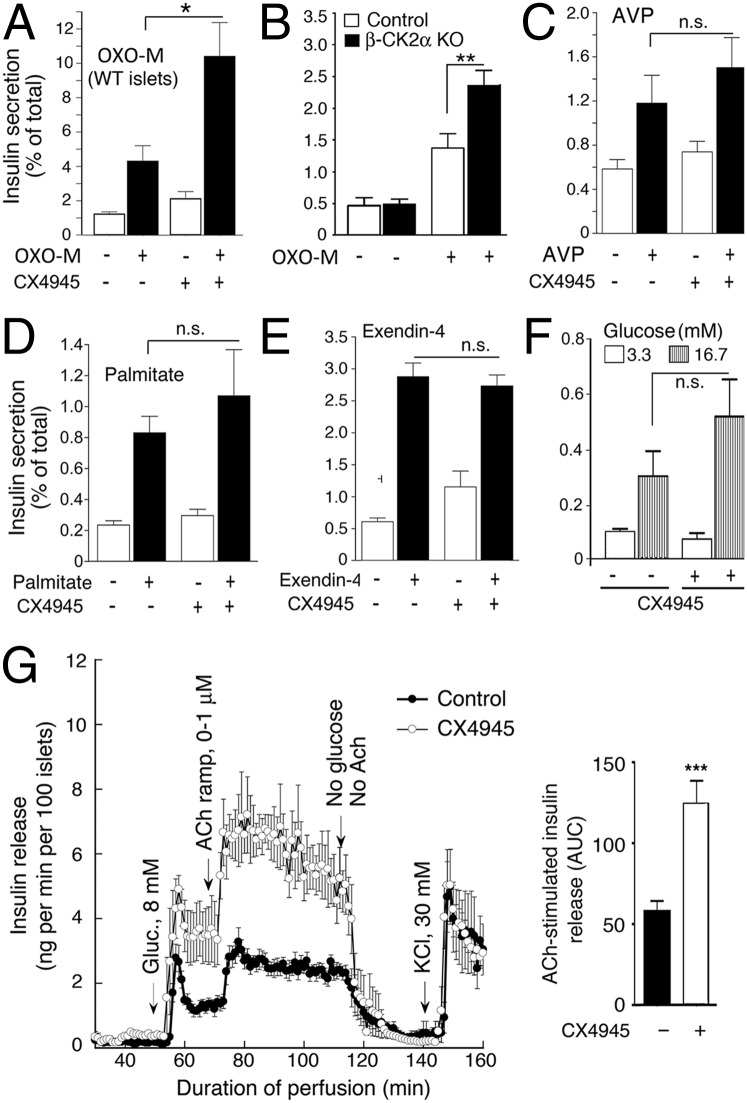
To study whether CK2α regulates the function of β-cell M3Rs in a more physiological setting, we prepared pancreatic islets from WT mice (∼15-wk-old males) and determined OXO-M–induced insulin secretion. In this set of experiments, we blocked CK2α activity by treatment of islets with the highly selective and potent CK2 inhibitor CX4945 (19). Because M3R-mediated augmentation of insulin release in mouse islets requires a stimulatory concentration of glucose (4, 7), all insulin secretion studies were carried out in the presence of 16.7 mM glucose. We found that CX4945 (10 μM) treatment of mouse pancreatic islets led to a striking increase in muscarinic agonist-mediated (OXO-M, 100 μM) augmentation of glucose-stimulated insulin secretion, compared with control islets that had not been exposed to the CK2 inhibitor (Fig. 3A). Incubation of islets with CX4945 had no significant effect on islet insulin content (ng insulin/12 islets: control islets, 818 ± 94; CX4945-treated islets, 838 ± 124; n = 12).
Fig. 3.
CK2 inhibition or CK2α deletion selectively increases M3R-mediated insulin secretion from pancreatic islets. (A and C–E) Isolated pancreatic islets from adult WT mice were incubated for 1 h in Krebs solution containing 16.7 mM glucose in either the absence or the presence of the selective CK2 inhibitor CX4945 (10 μM) and the muscarinic agonist OXO-M (100 μM) (A), AVP (100 nM) (C), palmitate (0.5 mM) (D), or exendin-4 (10 nM) (E). (B) Isolated pancreatic islets prepared from adult β-CK2α-KO mice and control littermates were incubated with OXO-M, as described above. Note that deletion of CK2α in mouse β-cells leads to a similar enhancement in M3R-stimulated insulin release as observed after CX4945 treatment of WT islets. (F) Glucose-induced insulin secretion in WT mouse islets in the absence or presence of CX4945 (10 μM). In A–F, the amount of insulin secreted into the medium during the 1-h incubation period was normalized to the total insulin content of each well (islets plus medium). Data are expressed as means ± SEM of three independent experiments, each carried out in triplicate. *P < 0.05 and **P < 0.01, compared with the corresponding control value. n.s., no statistically significant difference. (G) Insulin perifusion studies carried out with human islets. Perifused human islets were stimulated with increasing concentration of ACh in the presence of a stimulatory concentration of glucose (8 mM; the initial glucose concentration was 3.3 mM). Experiments were carried out in the absence (control) or the presence of CX4945 (10 μM). (Right) ACh-induced augmentation of insulin release at 8 mM glucose expressed as area under the curve (AUC). For experimental details, see SI Appendix, Methods. Each curve represents the mean ± SEM of four independent perfusion experiments (180 human islets per group and perifusion; ***P < 0.001).
To study the role of CK2α as a modulator of M3R-stimulated insulin secretion in a more direct fashion, we generated β-CK2α-KO mice in which we deleted the CK2α gene selectively in pancreatic β-cells of adult mice (in a tamoxifen-dependent fashion). The generation of β-CK2α-KO mice is described in detail in SI Appendix, Methods (for a representative Western blot, see SI Appendix, Fig. S2). Strikingly, OXO-M treatment of islets prepared from β-CK2α-KO mice resulted in a similar augmentation of M3R-stimulated insulin release as CX4945 treatment of WT islets (Fig. 3B). This finding strongly supports the concept that CK2 acts as a potent negative regulator of M3R-stimulated insulin secretion.
We also examined whether CX4945 affected insulin release from WT islets stimulated with other GPCR agonists. Fig. 3 C–E shows that incubation of WT islets with AVP (100 nM), palmitate (0.5 mM), or exendin-4 (10 nM) triggered enhanced insulin release, as expected (1, 18). AVP and palmitate act on Gq-coupled β-cell V1 vasopressin and FFA1 (GPR40) receptors, respectively, whereas exendin-4, a GLP-1 analog, stimulates Gs-coupled β-cell GLP-1 receptors (1, 18). In contrast to M3R-mediated insulin release (see above), CX4945 treatment of WT islets had no significant effect on the insulin responses caused by AVP, palmitate, or exendin-4 (Fig. 3 C–E), indicating that inhibition of CK2 in pancreatic β-cells selectively augments M3R-stimulated insulin release.
In WT mouse pancreatic islets, CX4945 treatment had no significant effect on basal insulin release (3.3 mM glucose) but caused a clear trend toward enhanced insulin secretion in the presence of a stimulatory glucose concentration (16.7 mM) (Fig. 3F). However, this latter effect failed to reach statistical significance (Fig. 3F).
CK2 Inhibition Enhances M3R-Stimulated Insulin Secretion also in Human Pancreatic Islets.
We next studied whether the results described above were also relevant to human islets/β-cells. To address this question, we stimulated perifused human islets with increasing concentrations of acetylcholine (ACh), the endogenous activator of β-cell M3Rs (Fig. 3G). ACh was added in the presence of a stimulatory concentration of glucose (8 mM; the initial glucose concentration was 3.3 mM). As observed with mouse islets, CX4945 (10 μM) treatment of human islets led to a pronounced augmentation of muscarinic agonist (ACh)-stimulated insulin release (Fig. 3G). We also noted that glucose-induced insulin secretion was elevated even in the absence of ACh (Fig. 3G), perhaps due to inhibition of other CK2 substrates.
Acute Inhibition of CK2 Augments M3R-Mediated Insulin Secretion in Vivo.
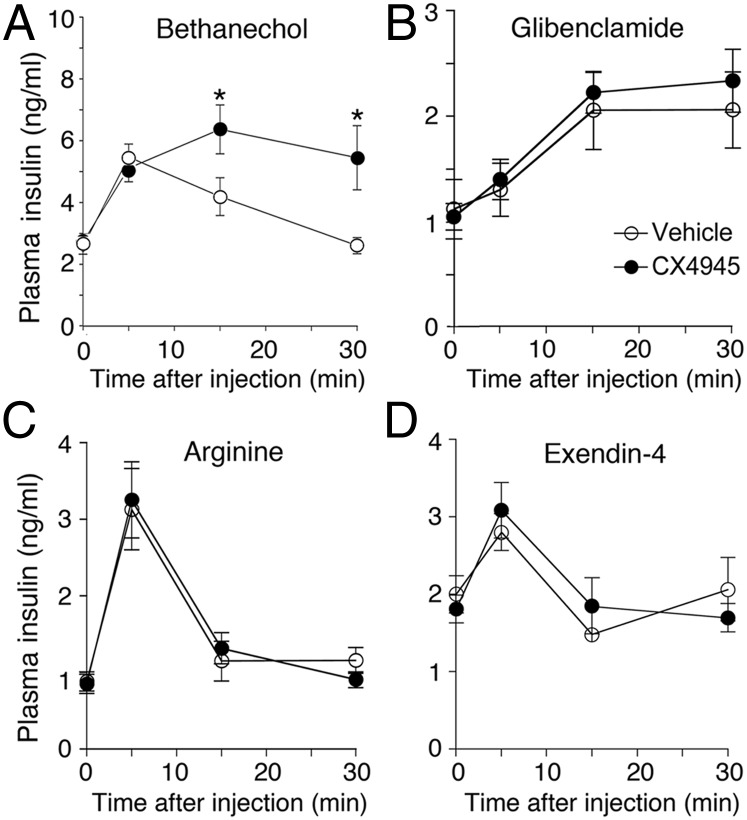
Previous studies have shown that treatment of WT mice with bethanechol, a peripherally acting muscarinic agonist, triggers pronounced increases in plasma insulin levels due to activation of β-cell M3Rs (17, 20). Thus, to explore whether a CK2 inhibitor was also able to affect M3R-mediated insulin release in vivo, we measured bethanechol (2 mg/kg, s.c.)-induced plasma insulin levels in WT mice (age: ∼8 wk) following pretreatment of mice with either CX4945 (25 mg/kg, i.p.) or vehicle. We found that bethanechol-stimulated insulin secretion was significantly more pronounced and longer-lasting in CX4945-pretreated mice, compared with vehicle-pretreated control littermates (Fig. 4A), indicating that CK2 also functions as a potent negative regulator of M3R-mediated insulin release in vivo.
Fig. 4.
Acute inhibition of CK2 selectively augments bethanechol-induced insulin secretion in vivo. WT mice (age: ∼8–12 wk) received a single dose of CX4945 (25 mg/kg, i.p.), followed by a 4-h fast. Mice were then injected with the muscarinic agonist bethanechol (2 mg/kg, s.c.) (A), glibenclamide (5 mg/kg i.p.) (B), arginine (1 g/kg i.p.) (C), or exendin-4 (12 nmol/kg i.p.) (D). Before CX4945 treatment, mice were either fed ad libitum (A, B, and D) or fasted for 5 h (C). Under these experimental conditions, bethanechol stimulates insulin release in WT mice via activation of β-cell M3Rs (17). Plasma insulin levels were measured at the indicated time points. Values are given as means ± SEM (n = 7 or 8 per group). *P < 0.05, compared with the corresponding control value.
In a series of control experiments, we coinjected WT mice with CX4945 (25 mg/kg, i.p.) and other pharmacological agents known to promote insulin release in vivo, including glibenclamide (5 mg/kg i.p.), arginine (1 g/kg i.p.), and the GLP-1 analog exendin-4 (12 nmol/kg i.p.) (Fig. 4 B–D). CX4945 treatment had no significant effect on the ability of these three agents to raise plasma insulin levels (Fig. 4 B–D), indicating that CX4945 does not act as a nonspecific insulin release-promoting agent in vivo.
Inhibition of CK2 in Vivo Prevents Diet-Induced Hyperglycemia and Glucose Intolerance.
To study the effect of prolonged CK2 blockade on blood glucose homeostasis, we injected WT mice (∼7-wk-old males or females) maintained on regular mouse chow twice a day for 6 d with CX4945 (25 mg/kg, i.p.) or vehicle (control littermates). At the end of this 6-d injection period, we carried out an i.p. glucose tolerance test (IGTT). We noted that CX4945-treated mice showed a slight improvement in glucose tolerance (SI Appendix, Fig. S3). Total pancreatic insulin content remained unaffected by CX4945 treatment (SI Appendix, Fig. S4A). Likewise, peripheral insulin sensitivity was similar in CX4945- and vehicle-treated mice (SI Appendix, Fig. S5B).
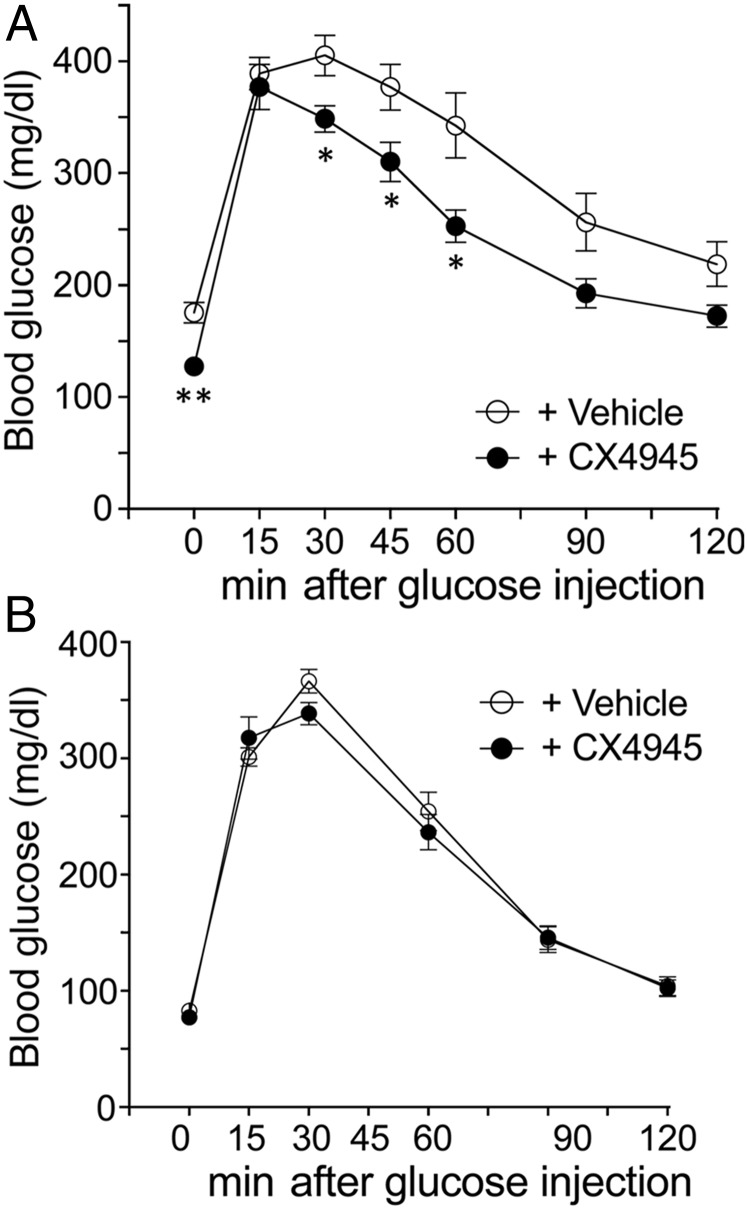
We next used the same experimental protocol to study glucose homeostasis in WT mice (15-wk-old males) that had been maintained on a high-fat diet (HFD) for 9 wk (diet D12492; 60% kcal% fat; Research Diets Inc.). As expected, the vehicle-treated mice showed fasting hyperglycemia and impaired glucose tolerance (Fig. 5A). Strikingly, the CX4945-treated mice displayed significantly reduced fasting blood glucose levels and greatly improved glucose tolerance (Fig. 5A). Total pancreatic insulin content was not significantly different between the two groups of mice (SI Appendix, Fig. S4B). Likewise, peripheral insulin sensitivity (SI Appendix, Fig. S5C) and the plasma levels of GLP-1, the most important incretin hormone, were not affected by CX4945 treatment (SI Appendix, Fig. S6). Strikingly, under the same experimental conditions, CX4945 treatment had no effect on glucose tolerance in M3R-deficient mice (Fig. 5B), indicating that the beneficial metabolic effects of the CK2 inhibitor are dependent on the presence of M3Rs.
Fig. 5.
Inhibition of CK2 prevents diet-induced hyperglycemia and glucose intolerance in an M3R-dependent fashion. (A) WT or (B) M3R-deficient (M3R KO) mice (males) that had been maintained on a HFD for 9–10 wk were injected twice a day for 6 d with CX4945 (25 mg/kg, i.p.) or vehicle (DMSO). At the end of the 6-d injection period, an IGTT was carried out as described in SI Appendix, Methods. Data are given as means ± SEM (A: vehicle, n = 7; CX4945, n = 6; B: vehicle, n = 4; CX4945, n = 6). *P < 0.05, **P < 0.01, compared with the corresponding control value.
CX4945 Inhibits the Phosphorylation of a Canonical CK2 Target in Mouse Pancreatic Islets.
CK2 has been shown to selectively phosphorylate Akt1 at S129 (21). To confirm that CX4945 inhibits this phosphorylation event in mouse pancreatic islets under our experimental conditions, we prepared islet lysates from WT mice that had been treated with CX4945 (25 mg/kg i.p.) either acutely or chronically (for 6 consecutive days). Western blotting studies demonstrated that acute or chronic CX4945 administration caused an ∼40–50% reduction of Akt1 phosphorylation at S129 (SI Appendix, Fig. S7), indicating that the CX4945 dose used in our in vivo experiments strongly inhibits the phosphorylation of a canonical CK2 target in mouse pancreatic islets.
CK2 Phosphorylates the WT M3R Expressed in Cultured Cells.
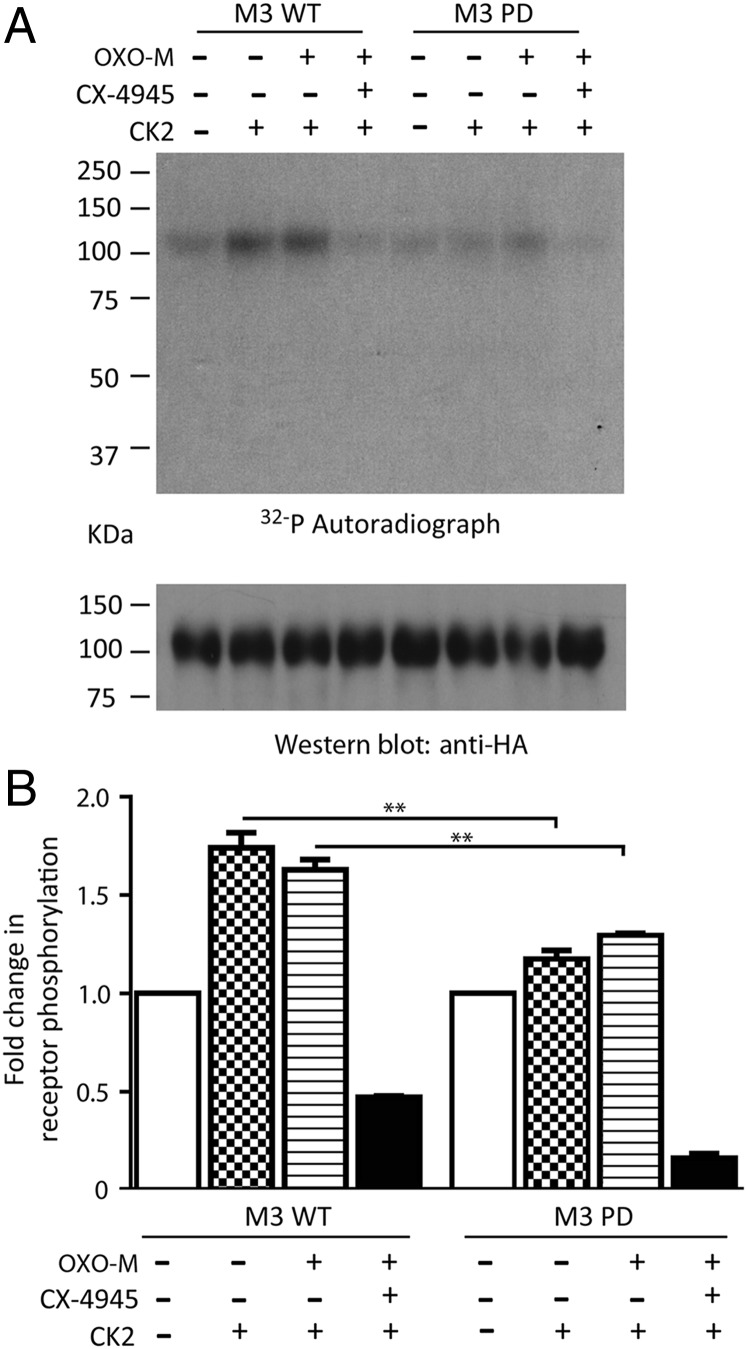
In agreement with previously published data (15), we demonstrated that CK2 is able to phosphorylate the WT mouse M3R in transfected HEK-293 cells (Fig. 6). Interestingly, M3R phosphorylation by CK2 was observed in both the absence and the presence of OXO-M (100 μM). As expected, CK2 phosphorylation of the WT M3R was abolished by pretreatment of receptor-expressing membranes with CX4945 (10 μM; Fig. 6 A and B).
Fig. 6.
CK2 phosphorylates the M3R in vitro in a CX4945-sensitive fashion. (A) CK2 phosphorylation assays. HEK-293 cells were transiently transfected with HA-tagged versions of the WT mouse M3R or a PD mutant M3R (see text for details). Phosphorylation assays were carried out by incubating receptor-expressing membranes with 500 units of CK2 in the absence or the presence of OXO-M (100 μM) and/or CX4945 (10 μM) (for experimental details, see SI Appendix, Methods). (A) A representative autoradiograph and a corresponding Western blot demonstrating that equal amounts of receptor protein were loaded. (B) A summary of three independent phosphorylation experiments (means ± SEM). **P < 0.01, compared with the corresponding WT M3R value.
We also carried out phosphorylation assays with a phosphorylation-deficient (PD) mutant mouse M3R (PD-M3R) that contained 15 serine-to-alanine point mutations within the third intracellular loop (i3 loop) (22). As expected, CK2 treatment of membranes expressing the PD-M3R mutant receptor resulted in drastically reduced receptor phosphorylation (Fig. 6 A and B).
Phosphorylation Studies with β-Cell M3Rs Using Transgenic Mice.
Because M3Rs are expressed only at very low levels by mouse pancreatic islets/β-cells (<2 fmol/100 islets) (23), we were unable to study the phosphorylation status of M3Rs endogenously expressed by mouse pancreatic islets. To circumvent this problem, we generated a mutant mouse line that overexpresses WT M3Rs containing an N-terminal HA tag in β-cells. Using standard transgenic techniques (7, 24), we eventually obtained mutant mice that selectively overexpressed M3Rs in β-cells (β-M3R Tg mice; SI Appendix, Fig. S8).
To quantitate M3R densities in pancreatic islets/β-cells of β-M3R Tg and WT mice, we incubated membranes prepared from pancreatic islets with a saturating concentration (2 nM) of [3H]NMS. This analysis showed that β-M3R Tg mice expressed ∼10-fold more β-cell M3Rs than WT mice (WT: 1.6 ± 0.2 fmol/100 islets, n = 8; β-M3R Tg: 18.6 ± 3.9 fmol/100 islets, n = 3).
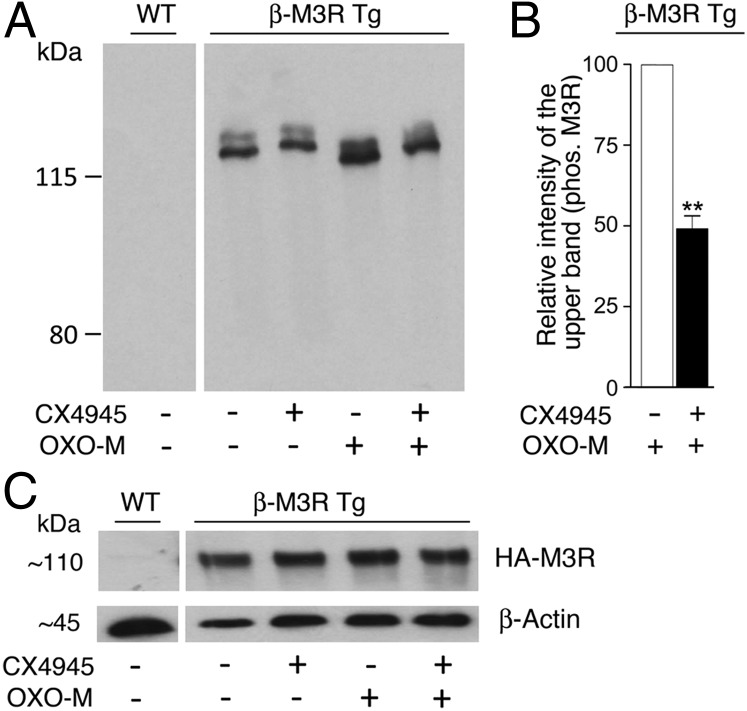
To demonstrate that β-cell M3Rs are subject to CK2-mediated phosphorylation, we used Phos-tag technology, which slows the mobility of phosphorylated proteins on polyacrylamide gels containing a dinuclear metal complex (25, 26). Specifically, we prepared lysates from pancreatic islets of β-M3R Tg mice and WT control mice that had been incubated with or without CX4945 (10 μM) in either the absence or the presence of OXO-M (100 μM). Cell lysates were then subjected to Zn2+-Phos-tag 5.5% (wt/vol) SDS/PAGE (26). Blots were probed with an anti-HA antibody, which led to the detection of two distinct HA-M3R species (Fig. 7A). Because the Zn-Phos-tag slows the migration of phosphorylated proteins, the upper band is predicted to represent a phosphorylated (or hyper-phosphorylated) form of the HA-M3R. OXO-M (100 μM) treatment of transgenic islets significantly enhanced the intensity of this upper band (Fig. 7A). The magnitude of this effect was significantly reduced in the presence of CX4945 (10 μM) (Fig. 7 A and B), supporting the notion that activated β-cell M3Rs serve as a CK2 substrate. When aliquots of the same islet lysates were subjected to regular 5.5% SDS/PAGE, the anti-HA antibody recognized only a single HA-M3R band (Fig. 7C), consistent with the concept that the higher molecular mass HA-M3R bands in Fig. 7A correspond to phosphorylated forms of the receptor.
Fig. 7.
CX4945-sensitive phosphorylation of mouse β-cell M3Rs. Lysates were prepared from pancreatic islets of WT or β-M3R Tg mice (note that the transgenic mice overexpress an HA-tagged version of the WT M3R selectively in β-cells). (A) Immunoblotting studies using Phos-tag technology. Proteins were separated via Zn-Phos-tag 5.5% SDS/PAGE (∼5 μg islet protein per lane; for details, see SI Appendix, Methods) and probed with a monoclonal anti-HA antibody to detect HA-tagged β-cell M3Rs. Note that two distinct HA-M3R bands can be detected in islet lysates from β-M3R Tg mice. Because the Zn-Phos-tag slows the migration of phosphorylated proteins, the upper band is predicted to represent a phosphorylated (or hyper-phosphorylated) form of the receptor. OXO-M (100 μM) treatment of transgenic islets enhanced the intensity of this upper band. This effect was significantly reduced in the presence of CX4945 (10 μM). (B) Quantification of the OXO-M data shown in A. In each individual experiment, the intensity of the higher molecular mass band observed with the OXO-M–treated transgenic islet was set equal to 100. The data shown are means ± SEM of three independent experiments. **P < 0.01, compared with control samples. (C) Aliquots of islet lysates corresponding to samples run in A were also subjected to regular 5.5% SDS/PAGE, and blots were probed with anti-HA and anti–β-actin antibodies. Note that only a single HA-M3R band was detectable under these conditions. The blots shown are representative of three independent experiments.
Acute Inhibition of CK2α Fails to Enhance Calcium Responses in Cells Expressing the PD-M3R Mutant Receptor.
To further explore the concept that CK2-mediated phosphorylation of the M3R interferes with M3R signaling, we carried out studies with COS-7 cells expressing the WT M3R or the phosphorylation-deficient PD-M3R mutant receptor. As observed with M3Rs endogenously expressed by MIN6 cells (Fig. 1A), OXO-M treatment of WT M3R-expressing COS-7 cells led to concentration-dependent increases in [Ca2+]i (SI Appendix, Fig. S9A). A similar pattern was observed with PD-M3R–expressing COS-7 cells, although maximum calcium responses were somewhat reduced (SI Appendix, Fig. S9B). One possible explanation for this latter phenomenon is that the PD-M3R mutant receptor was expressed at lower levels than the WT M3R (number of [3H]NMS-binding sites in fmol/mg protein: PD-M3R, 212 ± 21; WT M3R, 514 ± 134; n = 3).
Consistent with the outcome of the CK2 knockdown/inhibition studies carried out with M3Rs endogenously expressed by MIN6 cells or mouse pancreatic islets, treatment of WT M3R-expressing COS-7 cells with CX4945 (10 μM) led to a significant augmentation of OXO-M–induced increases in [Ca2+]i (SI Appendix, Fig. S9A). In striking contrast, CX4945 (10 μM) treatment had no significant effect on OXO-M–induced calcium responses in PD-M3R–expressing cells (SI Appendix, Fig. S9B). Taken together, these data strongly support the concept that the inhibitory effect of CK2 on M3R signaling is due to CK2-mediated phosphorylation of the M3R protein.
Discussion
Previous studies have shown that the M3R is phosphorylated by various kinases including GRKs and CK2 and that the pattern of M3R phosphorylation differs among tissues (10–15, 27). These observations led to the proposal that the pattern of M3R phosphorylation (or GPCR phosphorylation in general) represents a “bar code” that may lead to different signaling outcomes in different tissues (27, 28). However, the physiological relevance of these various phosphorylation events remains poorly understood. In the present study, we provide in vitro and in vivo evidence that CK2 phosphorylation of β-cell M3Rs plays a critical role in modulating the activity of this receptor subtype in pancreatic β-cells. Thus, to the best of our knowledge, the present study represents the first report that links CK2 phosphorylation of a specific GPCR to distinct physiological changes.
CK2α knockdown studies in cultured β-cells (Fig. 2A), the use of a chemical CK2 inhibitor (CX4945) in vitro and in vivo (Figs. 3A and 4A), and studies with pancreatic islets lacking CK2α selectively in β-cells (Fig. 3B) strongly suggest that reduced CK2-mediated phosphorylation of β-cell M3Rs promotes M3R-stimulated insulin release in various experimental settings. Importantly, treatment of human islets with the CK2 inhibitor also greatly enhanced M3R-mediated insulin secretion (Fig. 3G), suggesting that CK2 modulation of β-cell M3R signaling is also of potential clinical relevance. In agreement with these functional data, Western blotting/receptor phosphorylation studies demonstrated that both M3Rs expressed in cultured cells (Fig. 6) as well as M3Rs expressed by mouse islets/β-cells (Fig. 7) are a CK2 substrate.
We also demonstrated that inhibition of CK2 ameliorated the metabolic deficits associated with the consumption of a HFD, including hyperglycemia and glucose intolerance (Fig. 5A). Studies with M3R-deficient mice demonstrated that this effect was dependent on the presence of M3Rs (Fig. 5B). These observations suggest that strategies aimed at inhibiting CK2 phosphorylation of β-cell M3Rs may prove useful in the treatment of metabolic disorders such as T2D. Many studies have shown that structurally different agonists acting on a particular GPCR can induce different active receptor conformations that can feature distinct signaling profiles (“biased ligands”) (29, 30). Thus, it may be possible to develop M3R agonists or allosteric M3R modulators that can stabilize distinct M3R confirmations that are capable of activating Gq-type G proteins but are less likely to be phosphorylated by CK2.
GRK-mediated phosphorylation of GPCRs usually triggers receptor internalization and arrestin-dependent signaling (31, 32). Studies with CK2α-deficient β-cells (MIN6 cells) suggested that M3R phosphorylation by CK2α does not interfere with M3R internalization (SI Appendix, Fig. S1A). It remains to be explored whether CK2 phosphorylation of the M3R or other GPCRs causes the recruitment of other regulatory/signaling proteins including arrestins.
In addition to the M3R, pancreatic β-cells express several other Gq-coupled receptors including the V1b and FFA1 receptors (endogenous agonists: AVP and long-chain fatty acids, respectively) (1, 18). This class of β-cell GPCRs is known to promote insulin release via multiple mechanisms including the increase of [Ca2+]i and the activation of different isoforms of PKC (3, 4). Whereas CK2α knockdown, β-cell–selective CK2α deletion, or CK2 inhibition greatly augmented M3R-stimulated insulin secretion in cultured β-cells or isolated pancreatic islets, V1b and FFA1 receptor-mediated increases in insulin release remained unaffected by these manipulations (Fig. 3). This observation strongly suggests that the ability of CK2α knockdown/deletion or CK2 inhibition to augment M3R-dependent insulin release is most likely a direct consequence of M3R phosphorylation.
It should be noted that treatment of mouse or human islets with CX4945 alone (in the absence of GPCR agonists) caused a modest increase in insulin secretion in the presence of a stimulatory concentration of glucose (Fig. 3 F and G). The cause underlying this phenomenon remains unclear at present. One possibility is that this receptor-independent augmentation of insulin release is due to inhibition of other β-cell CK2 substrates. It is also possible that constitutive M3R signaling contributes to this effect.
We previously showed that phosphorylation of β-cell M3R promotes coupling of the M3R to arrestin/PKD1-dependent signaling pathways that augment glucose-stimulated insulin secretion (22), indicating that M3R-stimulated insulin release is mediated by both Gq (Ca2+)- and arrestin-dependent signaling pathways (22; also see ref. 33). In the present study, we demonstrated that CK2-mediated M3R phosphorylation interferes with M3R-stimulated insulin release. Taken together, these findings strongly support the concept that specific GPCR phosphorylation events can lead to different physiological outcomes.
Interestingly, analysis of human microarray data revealed a significant increase in CK2α (CSNK2A1) expression (by ∼1.2-fold; P = 0.0079) in human β-cells isolated from T2D subjects, compared with β-cells from nondiabetic donors (Gene Expression Omnibus database no. GSE20966) (34). However, it remains to be explored whether this rather small change contributes to impaired β-cell function in T2D.
To the best of our knowledge, this is the first study demonstrating that CK2 (CK2α) can regulate a key function of the endocrine pancreas (i.e., insulin secretion from β-cells). Importantly, our data suggest that CK2 inhibitors may prove useful as therapeutic agents for the treatment of T2D. It should also be noted that CX-4945, also known as silmitasertib, has shown great potential as an anticancer agent in several clinical trials (35). For these reasons, the data reported here should be of considerable clinical interest.
Methods
All animal experiments were conducted according to US National Institutes of Health Guidelines for Animal Research and were approved by the National Institute of Diabetes and Digestive and Kidney Diseases Institutional Animal Care and Use Committee. Details of materials and methods can be found in SI Appendix, Methods. These describe in vitro and in vivo insulin release studies using MIN6 cells as well as mouse and human pancreatic islets, the generation of mutant mice overexpressing M3Rs or lacking CK2α selectively in pancreatic β-cells, calcium measurements, radioligand-binding studies, various in vivo metabolic tests, Western blotting studies, and CK2 phosphorylation assays.
Supplementary Material
Acknowledgments
We thank Dr. Weiping Chen [National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Genomics Core] and Dr. Hans Luecke (NIDDK Advanced Mass Spectrometry Core) for their help with the analysis of human β-cell microarray data and the use of Phos-tag technology, respectively. This work was supported by the Intramural Research Program, NIDDK, NIH, Department of Health and Human Services (M.R., I.R.d.A., L.F.B., W.S., L.Z., Y.C., and J.W.); the Medical Research Council Toxicology Unit (A.B.T. and A.J.B.); American Diabetes Association Grant 7-11-BS-34 (to N.M.D.); Diabetes Center Grant NIH DK-19525 (to N.M.D. and F.M.M.); and a Rapid Response Grant by the Michael J. Fox Foundation (to H.R.). W.S. was the recipient of a stipend from the NIH–Japan Society for Promotion of Science Research Fellowship Program. L.F.B. received a stipend through the NIH-Brazilian National Council for Scientific and Technological Development Visiting Fellows Program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1519430112/-/DCSupplemental.
References
- 1.Ahrén B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov. 2009;8(5):369–385. doi: 10.1038/nrd2782. [DOI] [PubMed] [Google Scholar]
- 2.Amisten S, Salehi A, Rorsman P, Jones PM, Persaud SJ. An atlas and functional analysis of G-protein coupled receptors in human islets of Langerhans. Pharmacol Ther. 2013;139(3):359–391. doi: 10.1016/j.pharmthera.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Ahrén B. Autonomic regulation of islet hormone secretion: Implications for health and disease. Diabetologia. 2000;43(4):393–410. doi: 10.1007/s001250051322. [DOI] [PubMed] [Google Scholar]
- 4.Gilon P, Henquin JC. Mechanisms and physiological significance of the cholinergic control of pancreatic β-cell function. Endocr Rev. 2001;22(5):565–604. doi: 10.1210/edrv.22.5.0440. [DOI] [PubMed] [Google Scholar]
- 5.Duttaroy A, et al. Muscarinic stimulation of pancreatic insulin and glucagon release is abolished in m3 muscarinic acetylcholine receptor-deficient mice. Diabetes. 2004;53(7):1714–1720. doi: 10.2337/diabetes.53.7.1714. [DOI] [PubMed] [Google Scholar]
- 6.Zawalich WS, et al. Effects of muscarinic receptor type 3 knockout on mouse islet secretory responses. Biochem Biophys Res Commun. 2004;315(4):872–876. doi: 10.1016/j.bbrc.2004.01.139. [DOI] [PubMed] [Google Scholar]
- 7.Gautam D, et al. A critical role for β cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab. 2006;3(6):449–461. doi: 10.1016/j.cmet.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Gautam D, et al. Beneficial metabolic effects caused by persistent activation of beta-cell M3 muscarinic acetylcholine receptors in transgenic mice. Endocrinology. 2010;151(11):5185–5194. doi: 10.1210/en.2010-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3(9):639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 10.Willets JM, Nahorski SR, Challiss RA. Roles of phosphorylation-dependent and -independent mechanisms in the regulation of M1 muscarinic acetylcholine receptors by G protein-coupled receptor kinase 2 in hippocampal neurons. J Biol Chem. 2005;280(19):18950–18958. doi: 10.1074/jbc.M412682200. [DOI] [PubMed] [Google Scholar]
- 11.Luo J, Busillo JM, Benovic JL. M3 muscarinic acetylcholine receptor-mediated signaling is regulated by distinct mechanisms. Mol Pharmacol. 2008;74(2):338–347. doi: 10.1124/mol.107.044750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willets JM, Challiss RA, Kelly E, Nahorski SR. G protein-coupled receptor kinases 3 and 6 use different pathways to desensitize the endogenous M3 muscarinic acetylcholine receptor in human SH-SY5Y cells. Mol Pharmacol. 2001;60(2):321–330. doi: 10.1124/mol.60.2.321. [DOI] [PubMed] [Google Scholar]
- 13.Willets JM, Mistry R, Nahorski SR, Challiss RA. Specificity of g protein-coupled receptor kinase 6-mediated phosphorylation and regulation of single-cell m3 muscarinic acetylcholine receptor signaling. Mol Pharmacol. 2003;64(5):1059–1068. doi: 10.1124/mol.64.5.1059. [DOI] [PubMed] [Google Scholar]
- 14.Budd DC, McDonald JE, Tobin AB. Phosphorylation and regulation of a Gq/11-coupled receptor by casein kinase 1α. J Biol Chem. 2000;275(26):19667–19675. doi: 10.1074/jbc.M000492200. [DOI] [PubMed] [Google Scholar]
- 15.Torrecilla I, et al. Phosphorylation and regulation of a G protein-coupled receptor by protein kinase CK2. J Cell Biol. 2007;177(1):127–137. doi: 10.1083/jcb.200610018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.St-Denis NA, Litchfield DW. Protein kinase CK2 in health and disease: From birth to death: The role of protein kinase CK2 in the regulation of cell proliferation and survival. Cell Mol Life Sci. 2009;66(11-12):1817–1829. doi: 10.1007/s00018-009-9150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruiz de Azua I, et al. RGS4 is a negative regulator of insulin release from pancreatic beta-cells in vitro and in vivo. Proc Natl Acad Sci USA. 2010;107(17):7999–8004. doi: 10.1073/pnas.1003655107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oshikawa S, Tanoue A, Koshimizu TA, Kitagawa Y, Tsujimoto G. Vasopressin stimulates insulin release from islet cells through V1b receptors: A combined pharmacological/knockout approach. Mol Pharmacol. 2004;65(3):623–629. doi: 10.1124/mol.65.3.623. [DOI] [PubMed] [Google Scholar]
- 19.Siddiqui-Jain A, et al. CX-4945, an orally bioavailable selective inhibitor of protein kinase CK2, inhibits prosurvival and angiogenic signaling and exhibits antitumor efficacy. Cancer Res. 2010;70(24):10288–10298. doi: 10.1158/0008-5472.CAN-10-1893. [DOI] [PubMed] [Google Scholar]
- 20.Fukudo S, et al. Muscarinic stimulation and antagonism and glucoregulation in nondiabetic and obese hyperglycemic mice. Diabetes. 1989;38(11):1433–1438. doi: 10.2337/diab.38.11.1433. [DOI] [PubMed] [Google Scholar]
- 21.Di Maira G, et al. Protein kinase CK2 phosphorylates and upregulates Akt/PKB. Cell Death Differ. 2005;12(6):668–677. doi: 10.1038/sj.cdd.4401604. [DOI] [PubMed] [Google Scholar]
- 22.Kong KC, et al. M3-muscarinic receptor promotes insulin release via receptor phosphorylation/arrestin-dependent activation of protein kinase D1. Proc Natl Acad Sci USA. 2010;107(49):21181–21186. doi: 10.1073/pnas.1011651107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahrén B, Sauerberg P, Thomsen C. Increased insulin secretion and normalization of glucose tolerance by cholinergic agonism in high fat-fed mice. Am J Physiol. 1999;277(1 Pt 1):E93–E102. doi: 10.1152/ajpendo.1999.277.1.E93. [DOI] [PubMed] [Google Scholar]
- 24.Vasavada RC, et al. Overexpression of parathyroid hormone-related protein in the pancreatic islets of transgenic mice causes islet hyperplasia, hyperinsulinemia, and hypoglycemia. J Biol Chem. 1996;271(2):1200–1208. doi: 10.1074/jbc.271.2.1200. [DOI] [PubMed] [Google Scholar]
- 25.Kinoshita E, Kinoshita-Kikuta E, Koike T. Separation and detection of large phosphoproteins using Phos-tag SDS-PAGE. Nat Protoc. 2009;4(10):1513–1521. doi: 10.1038/nprot.2009.154. [DOI] [PubMed] [Google Scholar]
- 26.Kinoshita E, Kinoshita-Kikuta E. Improved Phos-tag SDS-PAGE under neutral pH conditions for advanced protein phosphorylation profiling. Proteomics. 2011;11(2):319–323. doi: 10.1002/pmic.201000472. [DOI] [PubMed] [Google Scholar]
- 27.Tobin AB, Butcher AJ, Kong KC. Location, location, location...site-specific GPCR phosphorylation offers a mechanism for cell-type-specific signalling. Trends Pharmacol Sci. 2008;29(8):413–420. doi: 10.1016/j.tips.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nobles KN, et al. Distinct phosphorylation sites on the β(2)-adrenergic receptor establish a barcode that encodes differential functions of β-arrestin. Sci Signal. 2011;4(185):ra51. doi: 10.1126/scisignal.2001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kenakin T, Christopoulos A. Signalling bias in new drug discovery: Detection, quantification and therapeutic impact. Nat Rev Drug Discov. 2013;12(3):205–216. doi: 10.1038/nrd3954. [DOI] [PubMed] [Google Scholar]
- 30.Wisler JW, Xiao K, Thomsen AR, Lefkowitz RJ. Recent developments in biased agonism. Curr Opin Cell Biol. 2014;27:18–24. doi: 10.1016/j.ceb.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luttrell LM, Gesty-Palmer D. Beyond desensitization: Physiological relevance of arrestin-dependent signaling. Pharmacol Rev. 2010;62(2):305–330. doi: 10.1124/pr.109.002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shenoy SK, Lefkowitz RJ. β-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci. 2011;32(9):521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakajima K, Wess J. Design and functional characterization of a novel, arrestin-biased designer G protein-coupled receptor. Mol Pharmacol. 2012;82(4):575–582. doi: 10.1124/mol.112.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marselli L, et al. Gene expression profiles of beta-cell enriched tissue obtained by laser capture microdissection from subjects with type 2 diabetes. PLoS One. 2010;5(7):e11499. doi: 10.1371/journal.pone.0011499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chon HJ, Bae KJ, Lee Y, Kim J. The casein kinase 2 inhibitor, CX-4945, as an anti-cancer drug in treatment of human hematological malignancies. Front Pharmacol. 2015;6:70. doi: 10.3389/fphar.2015.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.