Pericentromeres are essential structural components of the chromosome that function to ensure proper sister chromatid segregation during cell division. These chromosomal domains are composed of major satellite DNA repeats whose constitutively heterochromatic structure facilitates kinetochore formation (1). Characteristically demarcated by the silencing histone mark trimethyl-histone-3-lysine-9, pericentromeres had long been thought to be transcriptionally silent. However, recent reports have demonstrated that transcription from pericentromeric satellite repeats is required for proper chromosome formation during differentiation (2, 3). Aberrant transcription from these regions has also been observed in certain cancers (4, 5). Thus far, the biological relevance of the resulting RNA products has remained a mystery. In PNAS, Bersani et al. (6) and Tanne et al. (7) have now ascribed distinct functional properties to a specific class of noncoding RNAs (ncRNAs) that are produced from pericentromeric satellite repeats in cancer cells. The findings from these complementary studies have broad implications for the role of repeat-derived ncRNAs in cancer development and prognosis.
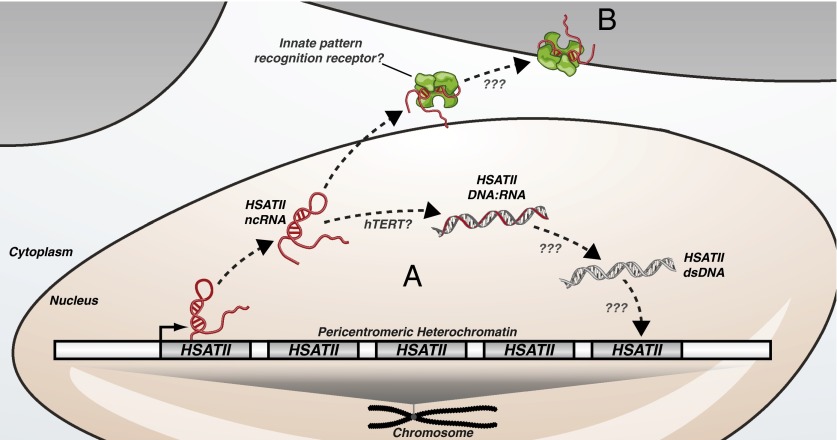
In Bersani et al. (6), the authors build on their previous observations that specific subclasses of satellite repeats are overexpressed in a variety of cancers (5). In the process of establishing model systems to study this phenomenon they discovered that one particular subclass of pericentromeric repeat, HSATII, is highly expressed when cancer cell lines are cultured as 3D tumor spheres. Intriguingly, the authors found that HSATII transcripts are partially susceptible to DNase I digestion and propose that HSATII RNA exists in the cell as a DNase-sensitive DNA:RNA heteroduplex (Fig. 1A).
Fig. 1.
Transcription of pericentromeric HSATII repeats in tumor cells gives rise to ncRNA transcripts that are (A) reverse-transcribed and reintegrated back into the genome resulting in HSATII repeat expansions, or (B) recognized by innate pattern recognition receptors and induce a proinflammatory immune response.
Bersani et al. (6) explore the possibility that HSATII transcripts are reverse-transcribed into DNA:RNA intermediates using DNA:RNA hybrid immunoprecipitation coupled with quantitative PCR. This method takes advantage of the S9.6 monoclonal antibody that has specificity toward DNA:RNA hybrids. The authors observed that introduction of in vitro-transcribed HSATII RNA into 293T cells, which do not express these transcripts endogenously, resulted in the detection of significant levels of HSATII DNA:RNA hybrids. Moreover, HSATII-expressing 3D tumor spheres treated with general reverse transcriptase inhibitors display a significant reduction in endogenous HSATII DNA:RNA hybrids. Altogether, these results provide compelling evidence that HSATII transcripts are indeed reverse-transcribed. The authors provide suggestive evidence that telomerase reverse transcriptase (hTERT) may be involved in the reverse transcription of HSATII RNAs. This is surprising because hTERT typically complexes with a well-characterized RNA subunit that serves as a short RNA template and the ability of the enzyme to reverse-transcribe long sequences of nonspecific RNA has not been previously described. Future studies will be required to confirm the exact role of hTERT and/or alternative reverse transcriptases in this process.
Reverse transcriptase activity is a hallmark property of retroelements that amplify in the genome through RNA intermediates. Using a combination of quantitative and qualitative assays, Bersani et al. (6) find that HSATII RNA expression is correlated with HSATII DNA copy number gains. These gains are restricted to pericentromeric domains and result in the expansion of existing HSATII loci (Fig. 1A). Blocking reverse transcription of HSATII RNA with reverse transcriptase inhibitors in an in vivo tumor xenograft model resulted in decreased HSATII DNA copy number gains, further supporting the role of HSATII RNA in HSATII repeat expansions. These findings raise a number of important questions. How are HSATII DNA:RNA hybrids converted into double-stranded DNA for integration? What is the enzyme that mediates HSATII integration? Although the authors implicate hTERT in the reverse transcription of HSATII RNA, this enzyme does not have DNA-dependent DNA-polymerase or integrase functionality. The mechanism for RNA-mediated HSATII expansion is likely complex and will be an exciting area for future research.
To evaluate potential physiological consequences of these HSATII expansions Bersani et al. (6) used data from The Cancer Genome Atlas (TCGA) project (8). TCGA is a publically available repository housing the genetic profiles for large panels of matched tumor and normal tissues from patients along with clinical data. The authors found that HSATII expansions were present in a variety of cancer types. Moreover, HSATII expansions are associated with poor prognosis as patients with HSATII copy gain tumors exhibit reduced survival rates. These observations raise the possibility that HSATII RNA may provide a previously unappreciated target for anticancer therapeutics. In support of this concept, the authors demonstrate that cancer cells treated with an antisense locked nucleic acid (LNA) targeting HSATII transcripts have a reduced ability to form 3D tumor spheres. These results are encouraging as LNA-based compounds are already showing promise in clinical trials for a range of diseases including cancer.
Interestingly, expansion of HSATII repeats within pericentromeres is not the only consequence of HSATII transcription. A study from Tanne et al. (7) has uncovered a role for HSATII transcripts in immune system stimulation. In this study the authors performed a systematic analysis of transcriptome-wide motif use in normal versus cancer cells. They found that motif use in ncRNA transcripts expressed from repetitive elements in cancer cells differs significantly from that of a healthy cell’s transcriptome. Whereas CpG and UpA dinucleotides are normally underrepresented, these motifs are abundant in ncRNAs produced from cancer-expressed repeats. Among the transcripts with the strongest CpG and UpA dinucleotide use were those produced from HSATII repeats.
With an atypical dinucleotide composition that resembles that of known viruses, Tanne et al. (7) hypothesized that HSATII transcripts may have immunostimulatory properties. To explore this possibility they introduced in vitro-transcribed HSATII RNA into human monocyte-derived dendritic cells (moDCs) and observed significant production of proinflammatory cytokines IL-6, IL-12, and TNF alpha. The authors suggest that HSATII repeats, which are typically transcriptionally silent in normal cells, are not under selective pressures to avoid immunostimulatory nucleotide motifs. They propose a model whereby ncRNA transcripts expressed from repetitive elements in tumor cells elicit an immune response that recruits and sequesters macrophages in the
The combined work of Bersani et al. and Tanne et al. has illuminated a previously unappreciated role for repeat-derived ncRNAs in carcinogenesis.
tumor microenvironment, a phenomenon that has been associated with increased tumorigenesis (9) (Fig. 1B).
In support of their proposed mechanism, Tanne et al. (7) also identify a class of murine satellite repeats, GSAT, that are highly expressed in cancer and have an atypical dinucleotide use profile similar to that of HSATII. Moreover, introduction of in vitro-transcribed GSAT RNA into immortalized murine bone marrow-derived macrophages (imBMs) resulted in significant production of TNF alpha. The immunostimulatory properties of cancer-expressed repeats seem to be species-specific because GSAT RNA had little effect in human moDCs and HSATII RNA had no effect in imBMs. How these transcripts, which the authors refer to as immunostimulatory ncRNAs, are detected within tumor cells and presented to effector cells of the immune system remains an open question. Given that major satellite repeats are also expressed during certain stages of normal differentiation and development, it will be interesting to explore how their immunostimulatory properties affect these processes as well (10, 11).
The combined work of Bersani et al. (6) and Tanne et al. (7) has illuminated a previously unappreciated role for repeat-derived ncRNAs in carcinogenesis. Although these studies have focused on ncRNAs that are produced from major satellite repeats within pericentromeres, the properties of these RNAs have striking overlap with those of retroviruses. This raises the question as to whether endogenous retroelements may also have functional roles, either positive or negative, in cancer development. Supporting this possibility, independent studies have shown that ncRNAs up-regulated in response to DNA damage are enriched with specific classes of retroelements (12). Although much work remains to be done, it is becoming more and more evident that repetitive DNA elements are not the “junk DNA” they were once considered.
Footnotes
References
- 1.Vos LJ, Famulski JK, Chan GKT. How to build a centromere: From centromeric and pericentromeric chromatin to kinetochore assembly. Biochem Cell Biol. 2006;84(4):619–639. doi: 10.1139/o06-078. [DOI] [PubMed] [Google Scholar]
- 2.Casanova M, et al. Heterochromatin reorganization during early mouse development requires a single-stranded noncoding transcript. Cell Reports. 2013;4(6):1156–1167. doi: 10.1016/j.celrep.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Probst AV, et al. A strand-specific burst in transcription of pericentric satellites is required for chromocenter formation and early mouse development. Dev Cell. 2010;19(4):625–638. doi: 10.1016/j.devcel.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Saksouk N, Simboeck E, Déjardin J. Constitutive heterochromatin formation and transcription in mammals. Epigenetics Chromatin. 2015;8:3. doi: 10.1186/1756-8935-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ting DT, et al. Aberrant overexpression of satellite repeats in pancreatic and other epithelial cancers. Science. 2011;331(6017):593–596. doi: 10.1126/science.1200801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bersani F, et al. Pericentromeric satellite repeat expansions through RNA-derived DNA intermediates in cancer. Proc Natl Acad Sci USA. 2015;112:15148–15153. doi: 10.1073/pnas.1518008112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanne A, et al. Distinguishing the immunostimulatory properties of noncoding RNAs expressed in cancer cells. Proc Natl Acad Sci USA. 2015;112:15154–15159. doi: 10.1073/pnas.1517584112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinstein JN, et al. Cancer Genome Atlas Research Network The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013;45(10):1113–1120. doi: 10.1038/ng.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noy R, Pollard JW. Tumor-associated macrophages: From mechanisms to therapy. Immunity. 2014;41(1):49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enukashvily NI, Malashicheva AB, Waisertreiger IS-R. Satellite DNA spatial localization and transcriptional activity in mouse embryonic E-14 and IOUD2 stem cells. Cytogenet Genome Res. 2009;124(3-4):277–287. doi: 10.1159/000218132. [DOI] [PubMed] [Google Scholar]
- 11.Terranova R, Sauer S, Merkenschlager M, Fisher AG. The reorganisation of constitutive heterochromatin in differentiating muscle requires HDAC activity. Exp Cell Res. 2005;310(2):344–356. doi: 10.1016/j.yexcr.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 12.Younger ST, Kenzelmann-Broz D, Jung H, Attardi LD, Rinn JL. Integrative genomic analysis reveals widespread enhancer regulation by p53 in response to DNA damage. Nucleic Acids Res. 2015;43(9):4447–4462. doi: 10.1093/nar/gkv284. [DOI] [PMC free article] [PubMed] [Google Scholar]