Significance
The agriculturally important symbiosis between nitrogen-fixing bacteria (rhizobia) and their legume hosts occurs within root nodules. This partnership requires a molecular dialogue that ensures specificity and directs the codevelopment of the two organisms during nodule formation. This paper characterizes a protein, host range restriction peptidase (HrrP), which plays a role in this dialogue. Rhizobial strains that express HrrP tend to exhibit more parasitic properties, such as failing to provide fixed nitrogen for their hosts and proliferating more abundantly within nodule tissue. HrrP likely exhibits these properties by actively degrading plant-derived chemical signals that normally stimulate symbiotic cooperation.
Keywords: symbiosis, nitrogen fixation, metallopeptidase, NCR peptides
Abstract
Legume–rhizobium pairs are often observed that produce symbiotic root nodules but fail to fix nitrogen. Using the Sinorhizobium meliloti and Medicago truncatula symbiotic system, we previously described several naturally occurring accessory plasmids capable of disrupting the late stages of nodule development while enhancing bacterial proliferation within the nodule. We report here that host range restriction peptidase (hrrP), a gene found on one of these plasmids, is capable of conferring both these properties. hrrP encodes an M16A family metallopeptidase whose catalytic activity is required for these symbiotic effects. The ability of hrrP to suppress nitrogen fixation is conditioned upon the genotypes of both the host plant and the hrrP-expressing rhizobial strain, suggesting its involvement in symbiotic communication. Purified HrrP protein is capable of degrading a range of nodule-specific cysteine-rich (NCR) peptides encoded by M. truncatula. NCR peptides are crucial signals used by M. truncatula for inducing and maintaining rhizobial differentiation within nodules, as demonstrated in the accompanying article [Horváth B, et al. (2015) Proc Natl Acad Sci USA, 10.1073/pnas.1500777112]. The expression pattern of hrrP and its effects on rhizobial morphology are consistent with the NCR peptide cleavage model. This work points to a symbiotic dialogue involving a complex ensemble of host-derived signaling peptides and bacterial modifier enzymes capable of adjusting signal strength, sometimes with exploitative outcomes.
Plants have a fundamental dependence on nitrogen as a macronutrient, but the vast supply of diatomic nitrogen in the atmosphere is not directly available to plants. Various species of nitrogen-fixing bacteria (collectively known as “rhizobia”) form symbiotic associations with grain and forage legumes to produce biologically available nitrogen. Free-living rhizobia recognize potential legume nodulation partners through a highly specific chemical dialogue involving the secretion and perception of the small-molecule signaling compound known as “nodulation (Nod) factor.” Recognition of Nod factor by the plant host initiates nodule development, starting with the proliferation of root cortical cells to produce nodule tissue and the formation of microscopic tubules (infection threads) through which trapped rhizobia grow into the expanding nodule tissue. A large fraction of the nodule cells becomes competent to take up the incoming bacteria via endocytosis. Internalized rhizobia (termed “bacteroids”) occupy host cells at high density while remaining encased in host-derived symbiosome membranes. Nascent symbiosomes with enclosed bacteroids then undergo a precisely controlled developmental program, becoming large organelle-like structures that are capable of nitrogen fixation (1).
In a subset of legumes, bacteroid development involves an arrest in cell division, genomic endoreduplication, and a 5- to10-fold increase in bacteroid size, resulting in swelled bacteroids that fix copious amounts of nitrogen and are terminally differentiated (2). This is the case for the well-characterized symbiosis between Sinorhizobium meliloti and its legume host Medicago truncatula. Legumes that induce bacteroid swelling and terminal differentiation also encode complex assortments of nodule-specific cysteine-rich (NCR) peptides that are specifically expressed in nodules and are delivered to the bacteroid-containing symbiosomes, suggesting a peptide-based mechanism for bacteroid development that is under strict host control (3–5). M. truncatula encodes ∼600 distinct NCR peptides that are expressed in at least two waves during nodule development (6–8). These peptides are generally 30–60 amino acids in length and share little sequence similarity with one another, except for the presence of four to six cysteine residues that are thought to form intramolecular disulfide bridges (9). When applied to free-living rhizobia in vitro, certain synthetic NCR peptides are sufficient to cause many of the metabolic and morphological changes observed for bacteroids in planta (5, 10). As the accompanying article (11) details the first strong genetic evidence, to our knowledge, a specific NCR peptide (NCR169) plays a decisive role in bacteroid development in M. truncatula nodules. It currently is supposed that the in vivo deployment of NCR peptides to bacteroid-containing symbiosomes is optimized to bring about cell division arrest, genomic endoreduplication, cell enlargement, and gentle membrane permeabilization while maintaining the robust metabolic activity required for nitrogen fixation and nutrient exchange with the host.
We previously described several naturally occurring Sinorhizobium accessory plasmids (pHRs) capable of restricting nodule development and nitrogen fixation (12). This effect is host dependent, with pHR plasmids causing dramatic symbiotic defects on certain Medicago host species but having no effect on others. We report here that a pHR-encoded metallopeptidase modulates symbiotic outcomes at a late developmental stage by triggering the premature degeneration of differentiated bacteroids, concomitant with nodule senescence in some cases. This enzyme is capable of NCR peptide cleavage in vitro, and its expression pattern is consistent with a role in modifying NCR peptide pools in nodule tissue.
Results
Identification of Host Range Restriction Peptidase as a Nitrogen-Fixation Blocking Factor.
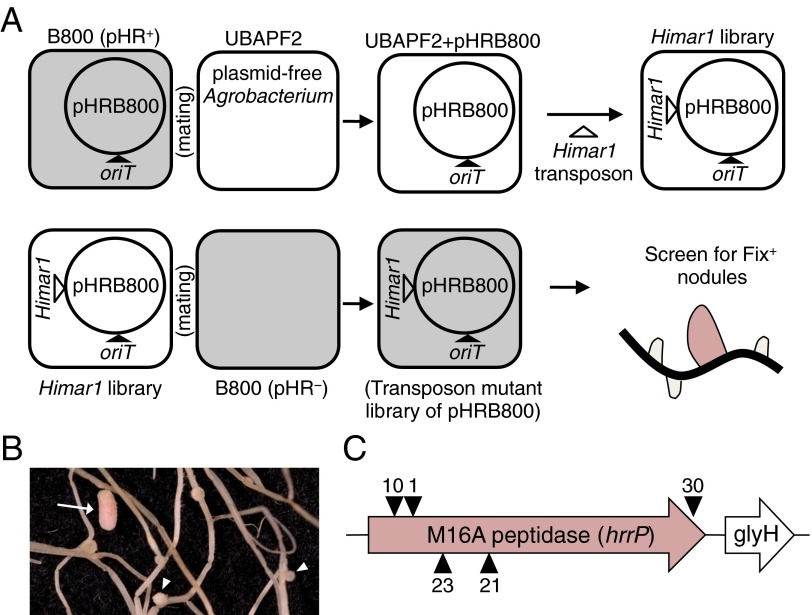
We have previously shown that S. meliloti strain B800 (a streptomycin-resistant derivative of USDA1963) is able to fix nitrogen (Fix+) on M. truncatula accession A17 but not on accession A20 (Fix−) and that nitrogen fixation is restored upon spontaneous curing of its accessory plasmid, pHRB800 (12). This large (199-kb) plasmid contains ∼290 protein-coding genes and is able to confer a Fix− phenotype when present in multiple S. meliloti strain backgrounds. To determine which part of this plasmid is responsible for the Fix− phenotype, a saturating plasmid-specific transposon mutagenesis screen was carried out on pHRB800, and this mutant library was screened for the inability to induce a Fix− phenotype on A20 plants (outlined in Fig. S1). Five pHRB800 transposon insertions that prevented the fixation-blocking phenotype all mapped to a single gene encoding an M16A family zinc-metallopeptidase, hereafter designated “host range restriction peptidase” (hrrP).
Fig. S1.
Plasmid-specific transposon mutagenesis strategy used to identify hrrP. (A) oriT and GmR (pPP101) were integrated into pHRB800, which then was mobilized into the plasmid-free Agrobacterium strain UBAPF2. The resulting strain, UBAPF2 pHRB800 (PP233), then was mutagenized with an Himar1 minitransposon (NmR) (26). (B) A library of ∼2 × 105 transposon mutants was mated with Sinorhizobium strain B800 pHR− (C403) to generate a plasmid-specific transposon mutant library, which was screened on M. truncatula accession A20 for Fix+ nodules (arrow) among predominately Fix− nodules (arrowheads). (C) All mapped transposon insertions were located in an M16A family metallopeptidase gene, designated hrrP.
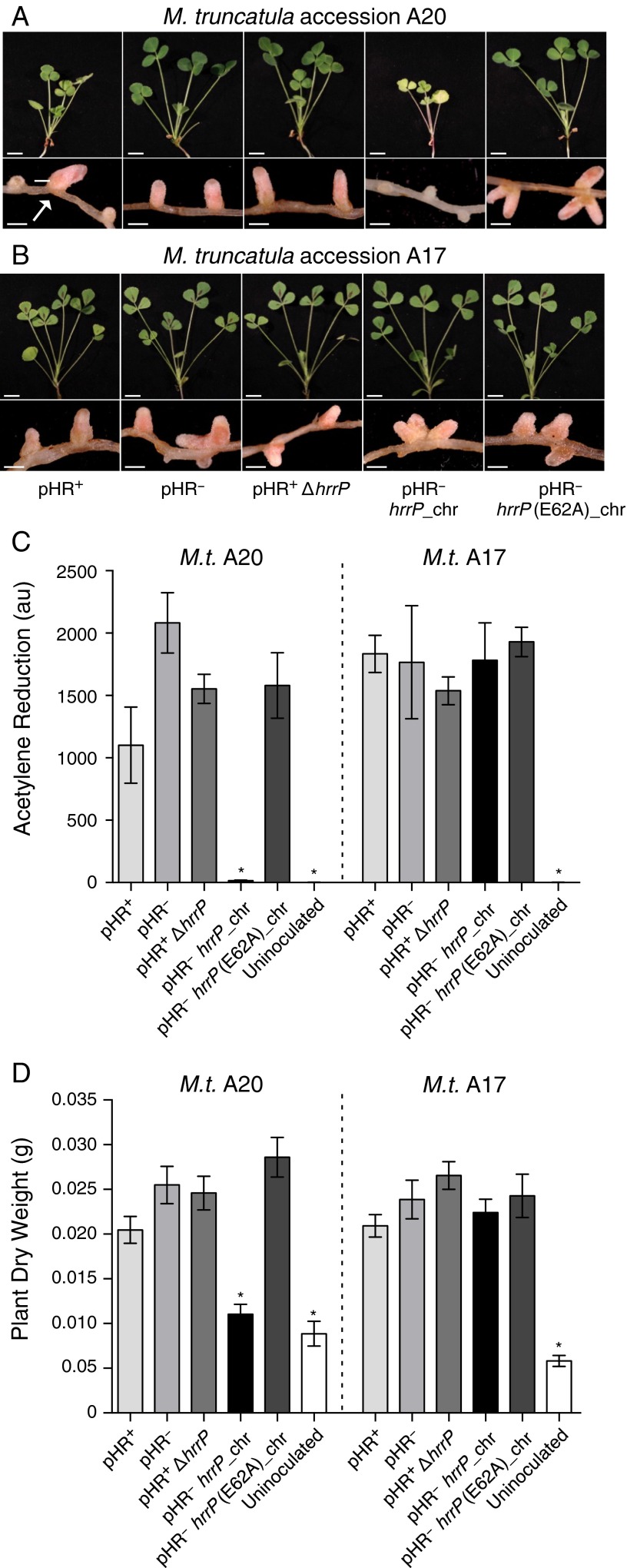
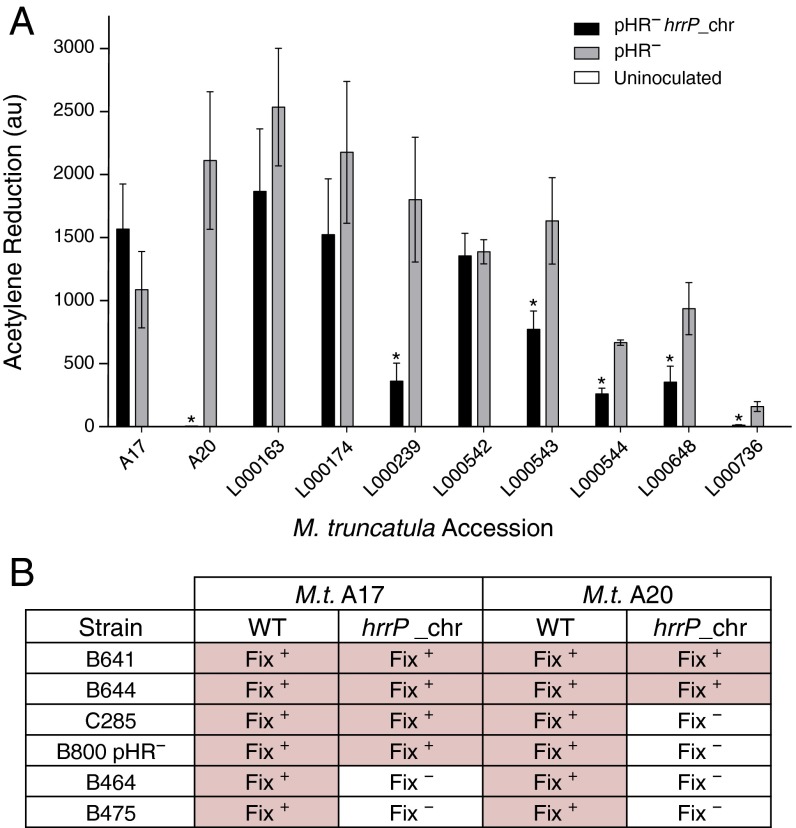
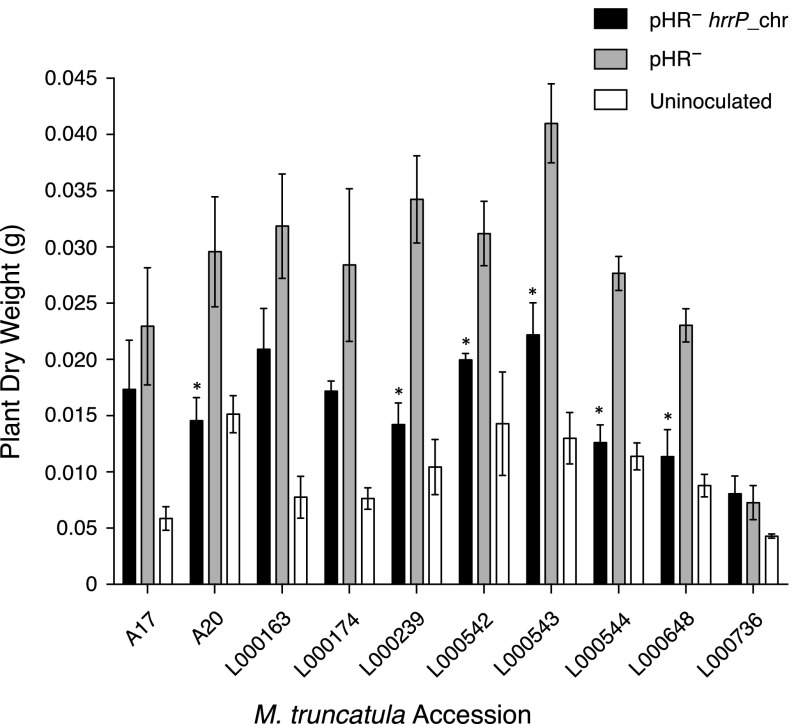
As expected, targeted disruptions of hrrP also yield a Fix+ phenotype on accession A20 (Fig. S2). When the hrrP gene is placed on a small plasmid vector and moved into a pHR-cured (pHR−) version of strain B800, the Fix− phenotype is restored as though pHRB800 were present. Placement of hrrP onto the chromosome (hrrP_chr) of B800 pHR− using a mini-Tn5 transposon vector also restores the fixation-blocking phenotype on A20, mimicking the behavior of pHRB800 (Fig. 1A). Indeed, chromosomal hrrP has a more complete fixation-blocking effect, presumably because the chromosome, unlike a plasmid, cannot be cured during symbiotic invasion of the nodule. Notably, hrrP does not appear to affect nitrogen fixation on accession A17 (Fig. 1B). Acetylene reduction assays, which directly measure nitrogenase activity, reveal that hrrP inhibits activity on M. truncatula A20 almost completely but has no effect on A17 (Fig. 1C and Figs. S2C and S3). The lack of nitrogen fixation is also evident in the reduced shoot dry weight and yellowing chlorotic leaves of plants grown under nitrogen-limiting conditions (Fig. 1 A and D). Together these results show that hrrP is necessary and sufficient for host-specific inhibition of nitrogen fixation.
Fig. S2.
hrrP is necessary and sufficient to inhibit nitrogen fixation on M. truncatula accession A20 but not A17. (A and B) Images of shoots and nodules at 28 dpi of M. truncatula A20 (A) and A17 (B) induced with strains B800 pHR+ (wild type), B800 pHR− (plasmid-cured derivative; C307), B800 with hrrP disrupted (PP236), B800 pHR− with a chromosomal copy of hrrP (PP317), or B800 pHR− with a chromosomal copy of hrrP(E62A) (PP316). The arrow indicates aberrant Fix+ nodules resulting from the spontaneous natural curing of pHRB800, as described by Crook et al. (12). (Scale bars: shoots, 1 cm; nodules, 0.1 cm.) (C and D) Acetylene reduction data at 21 dpi (n = 4) (C) and plantdry weight data at 28 dpi (n = 5) (D) for whole M. truncatula A20 and A17 plants inoculated with these same Sinorhizobium strains. Error bars indicate SEM. *P > 0.0001.
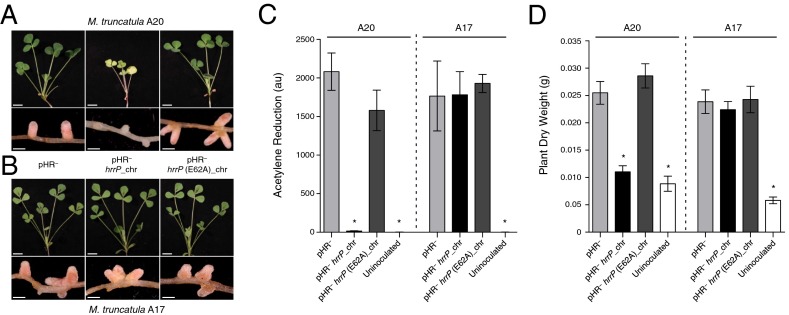
Fig. 1.
hrrP is necessary and sufficient to inhibit nitrogen fixation on M. truncatula accession A20 but not A17. (A and B) Images of shoots and nodules at 28 dpi of M. truncatula A20 (A) and A17 (B) induced with strains B800 pHR− (plasmid-cured derivative of B800; C307), B800 pHR− with a chromosomal copy of hrrP (PP317), or B800 pHR− with a chromosomal copy of hrrP(E62A) (PP316). (C and D) Acetylene reduction data at 21 dpi (n = 4) (C) and plant shoot dry weight data at 28 dpi (n = 5) (D) for M. truncatula A20 and A17 plants inoculated with these same Sinorhizobium strains. (Scale bars: shoots, 1 cm; nodules, 0.1 cm.) Error bars indicate SEM. *P < 0.0001 for Student’s t test vs. strain B800 pHR−.
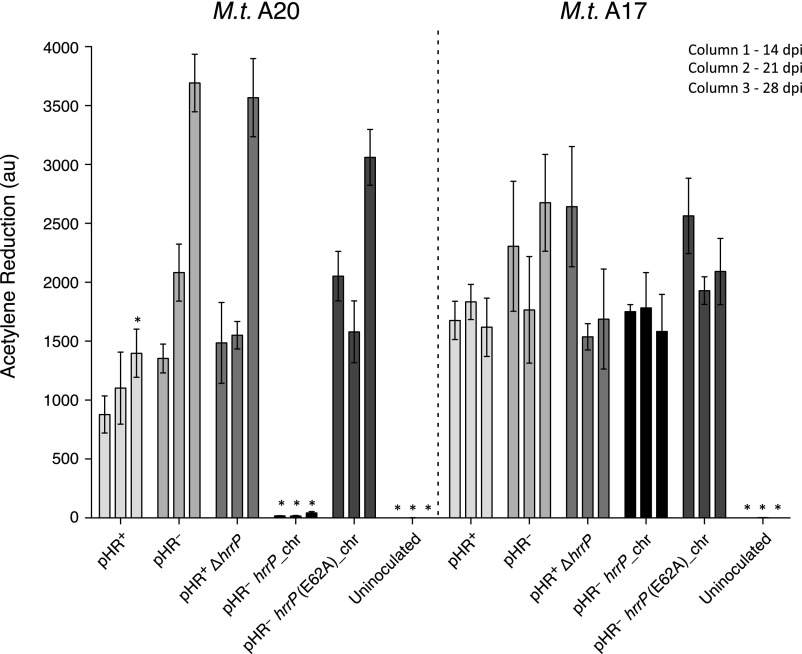
Fig. S3.
hrrP is necessary and sufficient to inhibit nitrogen fixation on M. truncatula accession A20 but not A17. A time series of acetylene reduction data at 14, 21, and 28 dpi (n = 4) for whole M. truncatula A20 and A17 plants inoculated with strains B800 pHR+ (wild type), B800 pHR− (plasmid-cured derivative; C307), B800 with hrrP disrupted (PP236), B800 pHR− with a chromosomal copy of hrrP (PP317), or B800 pHR− with a chromosomal copy of hrrP(E62A) (PP316). Error bars indicate SEM. *P > 0.01.
The M16A metallopeptidase family is characterized by a highly conserved HxxEH structural motif that coordinates the active-site Zn2+ ion. To determine whether the proteolytic function of HrrP is necessary for is its fixation-blocking phenotype, we introduced an E62A (HxxEH→HxxAH) alteration that is predicted to disable the peptidase catalytic motif. This variant no longer blocks nitrogen fixation (Fig. 1 A, C, and D), indicating that the peptidase activity of HrrP is responsible for the fixation-blocking phenotype.
The Symbiotic Effects of hrrP Are Conditioned by both Host and Strain Genetic Background.
It is clear that hrrP distinguishes M. truncatula accessions A20 and A17 when in the S. meliloti B800 strain background (Fig. 1). Fig. 2A shows that on a larger panel of M. truncatula accessions hrrP exhibits a broad spectrum of symbiotic effects (Fig. S4). These effects range from unimpeded nitrogenase activity (A17, L000163, L000174, and L000542) to nearly complete elimination of nitrogen fixation (A20 and L000239). Therefore, different closely related legume hosts, all of which form infected nodules, exhibit differing responses to rhizobially expressed hrrP.
Fig. 2.
The symbiotic effects of hrrP are conditioned by both host and strain genetic backgrounds. (A) Acetylene reduction data (n = 3) for a panel of wild M. truncatula accessions inoculated with B800 pHR− (C307), B800 pHR− with a chromosomal copy of hrrP (PP317), or dH2O (uninoculated). Error bars indicate SEM. *P < 0.05. (B) Phenotypic outcomes [Fix+ (pink shading) and Fix− (white shading)] for M. truncatula A17 and A20 inoculated with Sinorhizobium strains B641, B644, C285, B800 pHR− (C307), B464, and B475 with and without a chromosomal hrrP transgene (n = 8).
Fig. S4.
The symbiotic effects of hrrP are conditioned by host genetic background. Dry weight data (n = 4) for a panel of wild M. truncatula accessions inoculated with B800 pHR− (C307), B800 pHR− with a chromosomal copy of hrrP (PP317), or dH2O (uninoculated). Error bars indicate SEM. *P > 0.05.
hrrP also can have differing effects on host responses, depending on which rhizobial strain is expressing it. For example, hrrP expressed in S. meliloti strain B800 blocks nitrogen fixation on M. truncatula A20 but not on A17, whereas hrrP expressed in S. meliloti strain B464 blocks nitrogen fixation on both A20 and A17. Such variation in strain range can be observed for diverse Sinorhizobium isolates (Fig. 2B, Fig. S5, and Table S1). The strain-range effect demonstrates the existence of rhizobial genes that act as modifiers of the hrrP-induced fixation-blocking phenotype. In an attempt to identify the gene(s) that modulate strain range, we performed saturating transposon mutagenesis on B800 pHR− hrrP_chr, followed by a screen for Fix+ nodules on A20 (i.e., suppressors of the Fix− phenotype). This screen yielded eight Fix+ nodules; however, each of these strains had independent transposon insertions in hrrP itself. Our inability to uncover an hrrP-modifying function in this simple manner suggests that the unknown function either is essential for nitrogen fixation or is distributed over multiple overlapping functions.
Fig. S5.
The symbiotic effects of hrrP are conditioned by strain genetic background. Images of shoots and nodules at 28 dpi of M. truncatula A20 (A) and A17 (B) induced with strains B464, B464 with a chromosomal copy of hrrP (PP339), B470, B470 with a chromosomal copy of hrrP (PP340), B634, B634 with a chromosomal copy of hrrP (PP342), B641, or B641 with a chromosomal copy of hrrP (PP344). (Scale bars: shoots, 1 cm; nodules, 0.1 cm.)
Table S1.
Phenotypic outcomes for M. truncatula accessions A17 and A20 inoculated with various Sinorhizobium strains with and without a chromosomal hrrP transgene (n = 8)
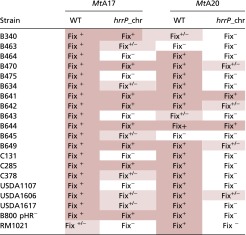 |
Pink shading is a visual representation of the Fix+ or Fix+/− phenotype.
hrrP Transcription Is Nodule Specific and Not Host Conditioned.
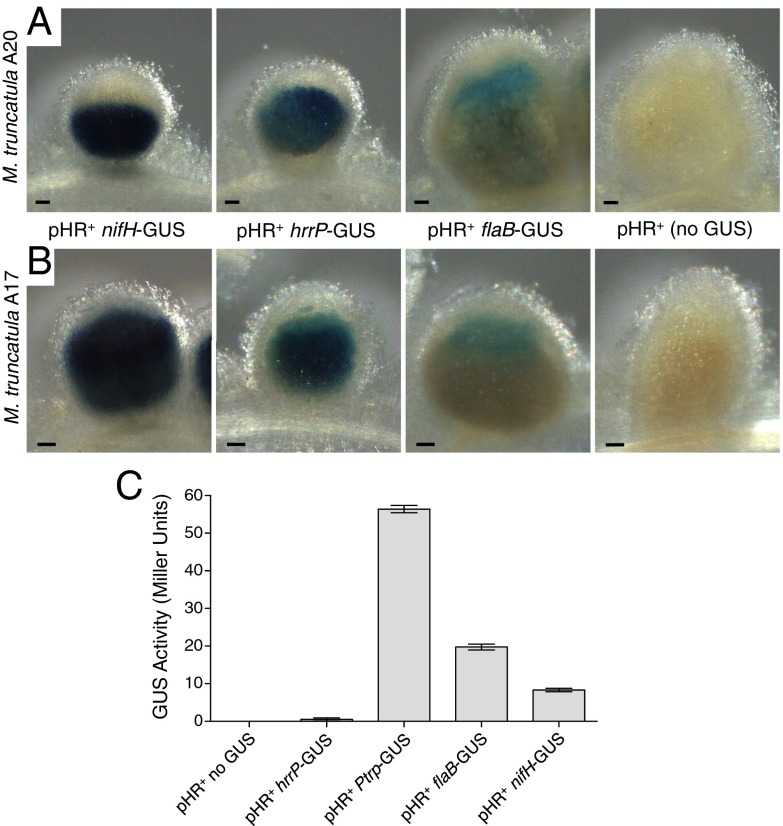
To evaluate hrrP gene expression in the nodule, transcriptional fusions to β-glucuronidase (GUS) were constructed in a manner that would not disrupt normal hrrP function. Similar fusions also were made to the nifHDK nitrogenase promoter (normally up-regulated in the nodule) and the flaB flagellin promoter [normally down-regulated in the nodule (13)]. All reporter strains carried pHRB800, and nodules were stained at 10 d postinoculation (dpi) to capture events before major morphological differences between Fix− and Fix+ nodules are evident. We observed strong hrrP-GUS staining in nodules in both A17 and A20 host plants (Fig. 3 A and B). The hrrP-GUS expression pattern overlaps with that of nifH-GUS, with staining evident throughout the zones of the nodule that ordinarily are occupied by developing and mature bacteroids. Remarkably, hrrP transcription in the nodule does not appear to interfere with nif gene expression, even in A20 where hrrP evokes a Fix− phenotype. Because nif gene expression requires a microaerophilic environment, this observation implies that the early stages of nodule development required to create such an environment are not perturbed by hrrP function. In free-living cells, hrrP-GUS expression is nearly undetectable compared with expression from the flaB promoter or the commonly used trp promoter from Salmonella, which is constitutive in S. meliloti (Fig. 3C).
Fig. 3.
GUS promoter fusion studies showing nodule-specific expression of hrrP. (A and B) Representative micrographs of 10-d-old M. truncatula accession A20 (A) and A17 (B) nodules stained with X-GLUC to reveal the location and relative expression of the GUS reporter fusions integrated downstream of the nifHDK, hrrP, and flaB promoters (strains PP404, PP389, and PP397, respectively). All samples were stained under identical conditions. Wild-type B800 (no GUS) was used as a negative control for GUS straining in planta. (C) GUS expression levels in free-living cells grown to midlog phase. The constitutive Salmonella-derived trp promoter was included as a positive control (PP471). (Scale bars: 100 μm.) Error bars indicate SEM.
HrrP Cleaves NCR Peptides in Vitro.
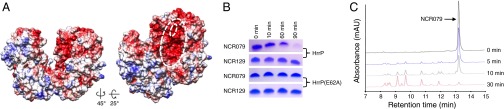
The observations described above are consistent with the HrrP peptidase acting on host-encoded NCR peptides in the nodule to modulate symbiotic development. Fig. 4A depicts a structural model for the HrrP enzyme based on crystallographically determined M16A metallopeptidase family members. The predicted clamshell structure of HrrP is reminiscent of previously solved enzymes, which are known to select peptide substrates based on their ability to be accommodated in the interior of the proteolytic chamber formed by the two roughly symmetrical domains. Substrate size and charge, rather than sequence, seems to be a key determinant of proteolytic specificity within this family (14). The peptide size range accommodated by M16A enzymes (30–70 residues) is remarkably similar to the range of sizes predicted for mature NCR peptides (30–60 residues), leading us to believe that HrrP may act on a large subset of NCR peptides despite their extensive sequence diversity.
Fig. 4.
Purified HrrP cleaves synthetic NCR peptides. (A) Electrostatic map of the structural model of HrrP derived using I-TASSER online (closest threading model was PDB ID code 1bccA). The outer white dotted line indicates the catalytic chamber, and the inner white dotted line indicates the Zn2+ coordinating residues. (B) Tris-Tricine SDS/PAGE gels showing the time course of NCR079 and NCR129 peptide cleavage by purified HrrP or HrrP (E62A). (C) HPLC analysis of NCR079 cleavage over time (0, 5, 10, and 30 min) by HrrP.
To test the NCR peptide cleavage model directly, HrrP and the catalytically inactivated variant HrrP(E62A) were purified to homogeneity, and these preparations were used to treat various purified NCR peptides. Evaluating degradation by gel electrophoresis (Fig. 4B) allows the direct observation of NCR079 degradation over a 90-min period in the presence of HrrP that is not observed in the presence of HrrP(E62A). The more limited degradation of NCR129 by HrrP over the same time period suggests some level of NCR peptide substrate selectivity. Time-course analysis of NCR079 degradation by HPLC shows the formation of discrete product peaks during the course of the reaction. Two minor peaks, eluting around 11.5 min and 12.1 min, accumulate during intermediate time points and then diminish in the final time point, suggesting that substrate peptides may be degraded in multiple steps (Fig. 4C). HrrP activity on 10 NCR peptides is quantified in Table 1. Degradation is detected for all NCR peptides tested, but activity varies by up to 15-fold. HrrP is modestly active in vitro against NCR169, a peptide reported in the accompanying article (11) as being required for bacteroid development.
Table 1.
Specific activity of HrrP against various synthetic NCR peptides
| Peptide | Length, aa | pI | HrrP-specific activity, nmol peptide⋅mg HrrP−1⋅min−1 |
| NCR035 | 37 | 9.44 | 57.13 ± 4.59 |
| NCR041 | 31 | 9.44 | 52.69 ± 1.27 |
| NCR079 | 33 | 6.77 | 70.16 ± 6.36 |
| NCR094 | 39 | 6.21 | 4.99 ± 1.16 |
| NCR124 | 30 | 6.92 | 14.81 ± 3.38 |
| NCR129 | 30 | 4.68 | 17.15 ± 0.62 |
| NCR142 | 29 | 4.01 | 4.61 ± 3.28 |
| NCR169 | 38 | 8.45 | 10.23 ± 0.67 |
| NCR211 | 34 | 5.38 | 15.17 ± 0.17 |
| NCR247 | 24 | 10.15 | 32.92 ± 0.58 |
hrrP Increases Sinorhizobium Fitness Within Nodules.
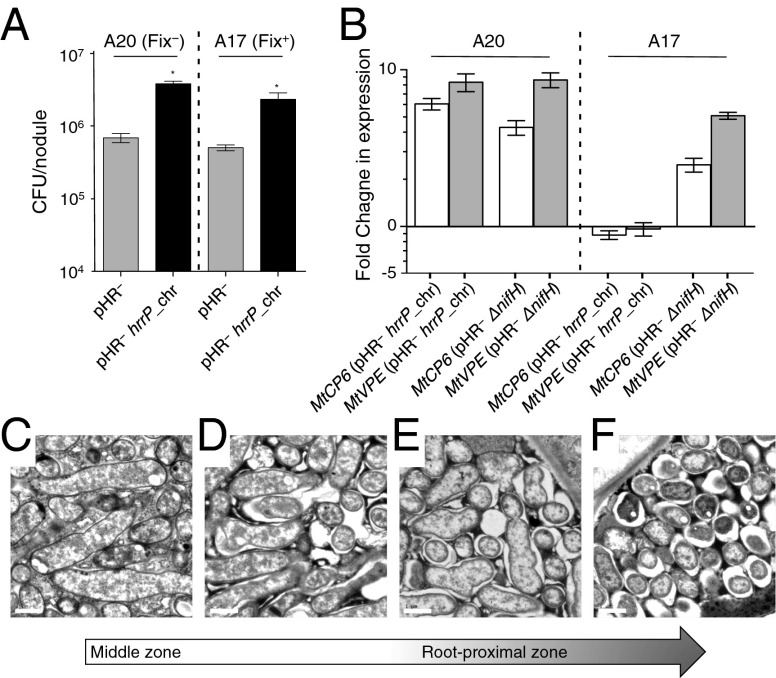
Previous work with pHR plasmids indicated that they can increase bacterial proliferation inside the nodule (12). To test whether hrrP can confer this effect and to evaluate whether this property also might be host conditioned, cfus were quantified from individual 28-d-old, surface-sterilized nodules. For A20, hrrP+ (Fix−) nodules yielded 5.5-fold more cfus than hrrP− (Fix+) nodules (Fig. 5A). This finding is striking, considering the small size of the nonfixing hrrP+ nodules compared with the well-developed hrrP− nodules (Fig. 1A). Given that the hrrP+ strain induces 5–10 times more nodules on A20 than the hrrP− strain, the effective gain in bacterial proliferation by the hrrP+ strain on a per-plant basis is ∼30-fold. Remarkably, hrrP confers a similar fitness benefit in association with accession A17 (a 4.5-fold increase in cfus per nodule), a host background in which hrrP does not interfere with nitrogen fixation (Fig. 5A).
Fig. 5.
The effects of hrrP on bacterial fitness, senescence, and bacteroid morphology in M. truncatula nodules. (A) Bacterial fitness for the strains B800 pHR− (C307) and B800 pHR− with a chromosomal copy of hrrP (PP317), as measured by cfus released from nodules 28 dpi for M. truncatula accessions A20 and A17 (n = 40 nodules per condition). *P < 0.05. (B) qRT-PCR analysis of the fold change in expression of the senescence markers MtCP6 and MtVPE for A20 and A17 nodules induced with B800 pHR− with a chromosomal copy of hrrP (PP317) or B800 pHR− ΔnifH (PP411) compared with B800 pHR− (C307). Error bars in A and B indicate SEM. (C–F) Spatial series of transmission electron microscopy images starting at the middle zone and ending at the root-proximal zone of a single M. truncatula A20 nodule induced with B800 pHR− with a chromosomal copy of hrrP (PP317). The nodule was harvested at 28 dpi. (Scale bars: 1 μm.)
We tested the idea that the hyperproliferation of hrrP+ strains in nodules may correspond to the induction of nodule senescence because a senescing nodule may support enhanced saprophytic growth of undifferentiated bacteria. Two expression markers associated with nodule senescence in M. truncatula have been evaluated recently: MtCP6, which encodes a papain-like proteolytic enzyme, and MtVPE, which encodes a caspase-like enzyme (15). At 21 dpi, we find that these markers are induced in A20 plants inoculated with hrrP+ rhizobia but not A17 plants inoculated with the same strain (Fig. 5B). Because both plant genotypes permit the hrrP-dependent hyperproliferation phenotype, but only A20 exhibits hrrP-dependent senescence, we conclude that senescence may not completely explain the proliferation phenomenon. As expected, A20 and A17 nodules exhibit senescence marker induction when nodulated with nitrogenase-null (ΔnifH) rhizobia.
Because the HrrP enzyme cleaves the very peptides that are known to drive bacteroid development, it is tempting to speculate that the enhanced bacterial viability observed in hrrP+ nodules is caused by a subset of bacteroids either failing to terminally differentiate or experiencing some sort of dedifferentiation. To begin to explore this hypothesis, we imaged hrrP+ bacteroids from A20 nodules by transmission electron microscopy. In M. truncatula, the temporal pattern of bacteroid development can be followed indirectly by moving spatially down a single nodule from its meristematic apex to the older, root-proximal zone. For the experiment shown in Fig. 5 C–F, an A20 nodule infected with B800 hrrP+ bacteria was allowed to develop for 28 d before sample preparation. In the middle region of the nodule (Fig. 5C) bacteroids are swollen and elongated, as is typical; in sections taken from the same nodule but closer to the root, bacteroids that normally would maintain their swollen, elongated structure appear to pinch and fragment, resulting in large spaces separating the symbiosome membrane from the bacteroid (Fig. 5 D–F). In the most root-proximal region of the nodule bacteroids are small and round (Fig. 5F). These degenerative events are not observed in nodules from A17 plants or in newer nodules from A20 plants. The cellular processes that account for this phenomenon are currently under investigation.
The Frequency and Sequence Diversity of hrrP Genes in Nature.
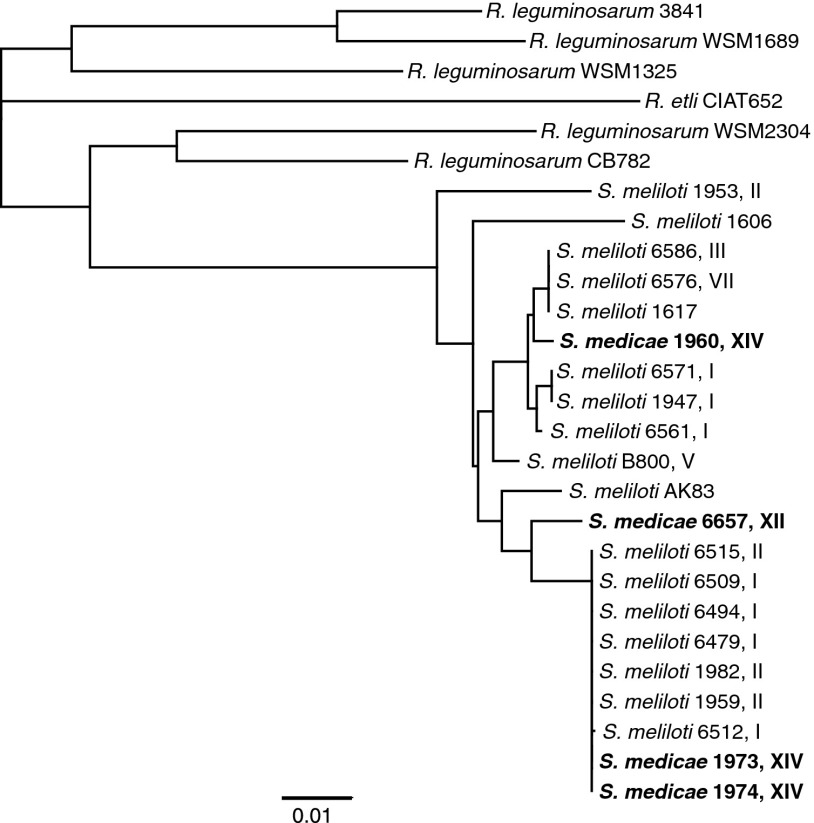
The marked increase in overall bacterial fitness of hrrP-containing strains and the plasmid-encoded nature of hrrP would suggest that hrrP-harboring strains may be somewhat abundant in the environment. A PCR-based screen of 150 Sinorhizobium isolates from our collection indicates that ∼20% of natural isolates harbor hrrP homologs, all of which appear to be encoded on large accessory plasmids. Analysis of a subset of these hrrP homologs reveals that they share over 96% identity at the DNA sequence level. Evaluation of an hrrP gene tree indicates that hrrP phylogeny is discordant with the chromosomal phylogeny of the Sinorhizobium strains that harbor them (Fig. 6 and Fig. S6). For example, in several cases, distinct Sinorhizobium species harbor nearly identical hrrP sequences, but very closely related Sinorhizobium strains often harbor relatively divergent hrrP alleles.
Fig. 6.
Sequence diversity of natural hrrP alleles. Neighbor-joining phylogenetic tree constructed using DNA sequences for a subset of sequenced hrrP homologs identified in the USDA Sinorhizobium collection and GenBank for the genera Rhizobium and Sinorhizobium. To help visualize the lack of concordance between the hrrP phylogeny and Sinorhizobium phylogeny, we assigned Sinorhizobium clades (I–XVI) based on the chromosomal phylogeny reported by Van Berkum et al. (24).
Fig. S6.
(Continued)
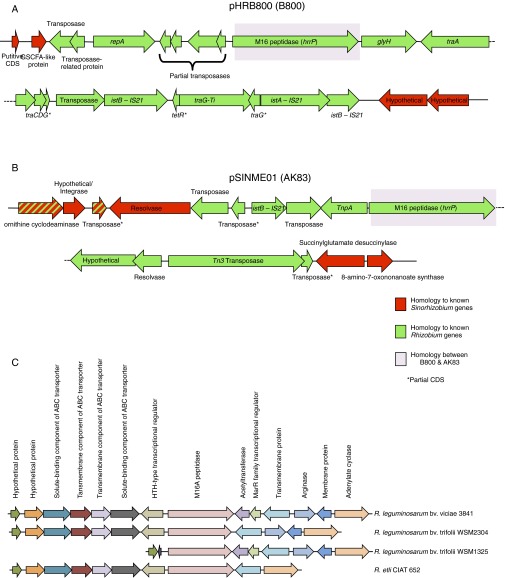
hrrP may have been introduced into Sinorhizobium accessory plasmids relatively recently. The genetic landscape adjacent to hrrP has been determined for only two plasmids: the pHR from strain B800 and an accessory plasmid (pSINME01) from S. meliloti strain AK83, which is noted for its ineffective nitrogen fixation (16). Despite 97.4% nucleotide sequence identity between these hrrP homologs, there is no synteny surrounding the hrrP coding region; even the promoter regions are highly divergent (Fig. S7 A and B). Moreover, both homologs are surrounded by multiple transposable elements that most closely resemble sequences found in the genus Rhizobium. Indeed, hrrP homologs also are found in various Rhizobium species, but in this genus hrrP is chromosomally encoded and maintains synteny across different species and strains (Fig. S7C). We therefore speculate that Sinorhizobium likely acquired hrrP from Rhizobium via horizontal gene transfer and that hrrP has spread among different Sinorhizobium genotypes via conjugative- and transposon-mediated processes.
Fig. S7.
Lack of synteny between pHRB800 (B800) and pSINME01 (AK83) surrounding the hrrP locus. (A and B) The genetic region surrounding hrrP for pHRB800 (A) and pSINME01 (B). Genes with homologs that are more closely related to genes found in Rhizobium species are shown in green, and genes with homologs that are more closely related to genes found in Sinorhizobium species are shown in red. The homology between hrrP is highlighted in purple. (C) Synteny between the regions surrounding hrrP in Rhizobium. The genetic region surrounding hrrP for R. leguminosarum bv. viciae 3841, R. leguminosarum bv. trifolii WSM2304, R. leguminosarum bv. trifolii WSM1325, and R. elti CIAT 652. Homologous genes are shown in the same color.
Discussion
Symbiotic compatibility between rhizobia and legumes has been well characterized at the level of early, Nod factor-dependent stages of development (17). This signaling interaction has been appropriately referred to as a “molecular dialogue” (18), but there has been great difficulty in elucidating how that dialogue proceeds into later stages of the symbiosis that encompass the morphological and physiological differentiation of bacteroids. The data presented here are consistent with HrrP providing a means for rhizobia to modulate their own symbiotic developmental fate by directly altering host-derived NCR peptides. The number of NCR peptides thought to be expressed in M. truncatula nodules exceeds 590 (19), but the number that have major effects on symbiotic development may be somewhat smaller. The accompanying article (11) reporting the symbiotic essentiality of M. truncatula NCR peptide (NCR169) opens the possibility that a rather small ensemble of core NCR peptides may be necessary and sufficient for normal symbiotic development. Therefore a symbiotic modifier such as HrrP may not need to target very many peptides to have a major effect.
One of the more interesting properties of hrrP is the host and strain dependence of its phenotypic action. These observations imply that genes other than hrrP, in both microsymbiont and host, modulate symbiotic compatibility at late developmental stages. For example, hosts may vary in their deployment of NCR peptide arsenals, and rhizobial strains can have varying intrinsic sensitivities to NCR peptides. This idea is supported by the hypervariability of NCR gene sequences across M. truncatula accessions (7) and the clear demonstration that at least one Sinorhizobium gene, bacA, can modulate NCR peptide susceptibility (7, 10). Therefore, the ultimate symbiotic outcome for a given host–strain pair will depend on the interplay between NCR dosage and the bacterial response; hrrP may have the effect of shifting the outcome in favor of bacterial insensitivity to NCR peptides.
The symbiosis between rhizobia and legumes is traditionally thought of as a mutualism, but our characterization of HrrP shows that each partner is able to evolve traits that maximize its own benefit from the partnership. Recall that in the nodules of M. truncatula A17, hrrP does not inhibit nitrogen fixation significantly, but it still increases rhizobial fitness by 4.5-fold (Fig. 5A). In natural settings, hrrP probably provides an accessory function to Sinorhizobium strains that does not detract largely from the benefit they provide their hosts but that substantially increases the ability of bacterial cells to regain proliferative competency after symbiotic services have been rendered. In agricultural settings, however, the pairing of crop plants with genetically incongruous soil microbes may set the stage for synthetic symbiotic incompatibilities such as the one we have dissected here. Exploitative rhizobia in these settings tend to outcompete “superior” inoculant strains for nodule occupancy, as has been reported repeatedly (20, 21). Our data show how readily a single gene such as hrrP might contribute to the suppression of nitrogen fixation and how the movement of such a gene on a mobile plasmid could corrupt otherwise superior rhizobium inoculants.
Materials and Methods
Bacterial Strains, Plant Lines, and Culture Conditions.
Specific strains and plant lines are listed in Tables S2 and S3, respectively. Escherichia coli, S. meliloti, and Agrobacterium tumefaciens cultures were grown at 37 °C, 30 °C, and 30 °C, respectively, in LB or on LB agar plates with the appropriate antibiotics. For details, see Supporting Information.
Table S2.
Bacterial strains used in this study
| Strain | Genotype | Source/reference |
| NiCO21 (DE3) | E. coli NiCO21 (DE3) | New England Biolabs |
| B001 | E. coli DH5α + pRK600 conjugation plasmid, cmR | (25) |
| B100 | S. meliloti RM1021 | (25) |
| B800 | S. meliloti USDA1963 SmR | (12) |
| C237 | A. tumefaciens UBAPF2, RfR | (12) |
| C307 | S. meliloti B800 pHR− (pHRB800−), SmR | (12) |
| C320 | A. tumefaciens UBAPF2 + pHRB800KmR (pJG476), rifR, KmR | (12) |
| C403 | S. meliloti B800 pHR− + pJG505, SmR, TcR | (12) |
| PP233 | A. tumefaciens UBAPF2 + pHRB800GmR (pPP101), RfR, GmR | This study |
| PP236 | S. meliloti B800 + pHRB800 KmR::hrrP (pPP105), SmR, KmR | This study |
| PP316 | S. meliloti C307 + Tn5_hrrP (E62A) (insertion into SM_b21034), SmR, KmR | This study |
| PP317 | S. meliloti C307 + Tn5_hrrP (insertion into SMc03928), SmR, KmR | This study |
| PP337 | S. meliloti B340 (USDA1940 SmR) + Tn5_hrrP, SmR, KmR | This study |
| PP338 | S. meliloti B463 (USDA6572 SmR) + Tn5_hrrP, SmR, KmR | This study |
| PP339 | S. meliloti B464 (USDA6574 SmR) + Tn5_hrrP, SmR, KmR | This study |
| PP340 | S. meliloti B470 (USDA6635 SmR) + Tn5_hrrP, SmR, KmR | This study |
| PP341 | S. meliloti B475 (USDA6478 SmR) + Tn5_hrrP, SmR, KmR | This study |
| PP342 | S. meliloti B634 (USDA6582 SmR) + Tn5_hrrP, SmR, KmR | This study |
| PP344 | S. meliloti B641 (USDA6563 SmR) + Tn5_hrrP, SmR, KmR | This study |
| PP345 | S. meliloti B642 (USDA6592 SmR) + Tn5_hrrP, SmR, KmR | This study |
| PP346 | S. meliloti B643 (USDA6654 SmR) + Tn5_hrrP, SmR, KmR | This study |
| PP347 | S. meliloti B644 (USDA6546 SmR) + Tn5_hrrP, SmR, KmR | This study |
| PP348 | S. meliloti B645 (USDA6646 SmR) + Tn5_hrrP, SmR, KmR | This study |
| PP349 | S. meliloti B649 (USDA6655 SmR) + Tn5_hrrP, SmR, KmR | This study |
| PP350 | S. meliloti C131 (NRG23 SmR) + Tn5_hrrP, SmR, KmR | This study |
| PP351 | S. meliloti C285 (USDA6623 SmR) + Tn5_hrrP, SmR, KmR | This study |
| PP352 | S. meliloti C378 (USDA6649 pHRC377− SmR) + Tn5_hrrP, SmR, KmR | This study |
| PP353 | S. meliloti USDA1107 + Tn5_hrrP, SmR, KmR | This study |
| PP354 | S. meliloti USDA1606 + Tn5_hrrP, SmR, KmR | This study |
| PP355 | S. meliloti USDA1617 + Tn5_hrrP, SmR, KmR | This study |
| PP377 | S. meliloti B800GOC + pPP147, TcR, KmR | This study |
| PP389 | S. meliloti B800 + pPP132 (hrrP-GUS), SmR, KmR | This study |
| PP397 | S. meliloti B800 + pPP157 (flaB-GUS), SmR, KmR | This study |
| PP398 | S. meliloti RM1021 + Tn5_hrrP, SmR, KmR | This study |
| PP404 | S. meliloti B800 + pPP156 (nifHDK-GUS), SmR, KmR | This study |
| PP411 | S. meliloti B800 + pPP169 (nifH::KmR), SmR, KmR | This study |
| PP471 | S. meliloti B800 + pPP255 (Ptrp-GUS – rhaK locus), SmR, KmR | This study |
Table S3.
M. truncatula accessions used in this study
| M. truncatula line | Accession label | Source/reference |
| A17 | Jemalong A17 | Sharon Long laboratory* |
| A20 | Jemalong A20 | Sharon Long laboratory* |
| L000163 | SA22322 | INRA |
| L000174 | SA28064 | INRA |
| L000239 | SA26063 | INRA |
| L000542 | DZA233-4 | INRA |
| L000543 | DZA327-7 | INRA |
| L000544 | ESP105-L | INRA |
| L000648 | Salses 42B | INRA |
| L000736 | DZA045-6 | INRA |
INRA, National Institute for Agricultural Research.
Department of Biology, Stanford University, Stanford, CA.
Plasmid and Strain Construction.
Strains, plasmids, NCR peptides, and oligonucleotides are listed in Tables S2 and S4–S6, respectively. Plasmids were constructed using standard molecular techniques. For details, see Supporting Information. Plasmid mobilization between strains was mediated via triparental matings with the helper E. coli B001 (DH5α harboring pRK600) followed by selection with the appropriate antibiotics.
Table S4.
Plasmids used in this study
| Plasmid | Relevant features | Source/reference |
| pJG476 | pJG194 (25) carrying the acdS upstream region; KmR | (12) |
| pJG542 | lacI-Ptac expression vector | This study |
| pPP047 | Plasmid carrying a Himar1-based transposon system with T7 promotors oriented toward the chromosome; KmR | (26) |
| pPP101 | pJQ200 (27) derivative carrying the acdS upstream region but lacking the sacB gene; GmR | This study |
| pPP105 | pJG194 (25) carrying an hrrP internal fragment; KmR | This study |
| pPP119 | pJG542 carrying hrrP with a C-terminal His6X-tag | This study |
| pPP123 | pJG110 (mini-Tn5) (25) carrying hrrP with a E62A mutation with native promoter; ApR, KmR | This study |
| pPP124 | pJG110 (mini-Tn5) (25) carrying hrrP with native promoter; ApR, KmR | This study |
| pPP132 | pJG194 (25) carrying an hrrP promoter (474 bp)-GUS fusion; KmR | This study |
| pPP147 | pRF771 (28) carry hrrP driven by the trp promoter; TcR | This study |
| pPP156 | pJG194 (25) carrying an nifH promoter (523 bp)-GUS fusion; KmR | This study |
| pPP157 | pJG194 (25) carrying an flaB promoter (124 bp)-GUS fusion; KmR | This study |
| pPP169 | pJG392 (25) carrying an nifH internal fragment; TcR | This study |
| pPP196 | pJG542 carrying hrrP with a E62A mutation and a C-terminal His6X-tag | This study |
| pPP255 | pJG194 (25) carrying the Ptrp promoter from pRF771 (26)-GUS fusion and a rhaK locus integration sequence; KmR | This study |
Table S6.
Oligonucleotides used in this study
| Oligonucleotide | Sequence | Source/reference |
| oPP013 | CATCGCCTTCTATCGCCTTCTTGAC | This study |
| oPP014 | GAGTTCTTCTGAGCGGGACTCTGGGGT | This study |
| oPP017 | GATCGGATCCCTTCGATCGCGAACGCGGCGTCATT | This study |
| oPP020 | GATCTCTAGAATCTTCGCGCACCAGCACTTCATTG | This study |
| oPP027 | TGTATATCCTGGCAATCCATGGCCTT | This study |
| oPP028 | TGATGCTGATGCGCGAGACGGC | This study |
| oPP031 | GATCGGATCCTCGCCCGCTATCTTACCGTCGC | This study |
| oPP033 | GATCCCTAGGTTGTCGATTGACGCCTGAAAAGCCG | This study |
| oPP071 | GCATTTTCTCGCGCACATGGCCTT | This study |
| oPP072 | AAGGCCATGTGCGCGAGAAAATGC | This study |
| oPP073 | GTGGATCCCTAATGATGATGATGATGATGACCTCCTCTTACCGTCGCCGGCAG | This study |
| oPP074 | GATCCCTAGGCATGCAACCGACAGCAAGCGATC | This study |
| oPP093 | GATCTCTAGACAGCAGCATTTCCTCGACCATCC | This study |
| oPP094 | GGTTTCTACAGGACGGACCATCTGTCAGCTCCTGACGG | This study |
| oPP095 | CCGTCAGGAGCTGACAGATGGTCCGTCCTGTAGAAACC | This study |
| oPP096 | GATCGGATCCTTATTGTTTGCCTCCCTGCTGCG | This study |
| oPP107 | GGCTACCCGTGATATTGCTGAAGAG | This study |
| oPP108 | CTGACCGCTTCCTCGTGCTTTACG | This study |
| oPP109 | GATCGGATCCTCAGTTGCGTGCTGGAGACGG | This study |
| oPP110 | GATCCCTAGGGCAAGCCGCATTCGTGATGAAATC | This study |
| oPP111 | GATCTCTAGACAAGTACGCTCGAGCAGGCG | This study |
| oPP116 | GATCCCTAGGATTCCGTCAGGAGCTGACAGATGC | This study |
| oPP118 | GGTTTCTACAGGACGGACCATCTTGCTTCCTTTGTTGTTTAAGC | This study |
| oPP119 | GCTTAAACAACAAAGGAAGCAAGATGGTCCGTCCTGTAGAAACC | This study |
| oPP120 | GATCTCTAGAGCTCTCGGACTCGATCGACTCG | This study |
| oPP121 | GGTTTCTACAGGACGGACCATGGTTTAGTGCCCCTTGGTGG | This study |
| oPP122 | CCACCAAGGGGCACTAAACCATGGTCCGTCCTGTAGAAACC | This study |
| oPP152 | TCGGCGAAAAGATCGAATATGCTGC | This study |
| oPP153 | ATGGCATACCATAAGCCGTAATATCTCG | This study |
| oPP154 | TTGCCTTCGACCACCTGATCTTCAC | This study |
| oPP155 | GTCGGCGGTTCTAACACCTGTGC | This study |
| oPP156 | GAAGCGGAATTGTCGGTGAGAGTC | This study |
| oPP157 | CAGTTCGAATTGACGCCGTTTTTAAAGG | This study |
| oPP158 | TTGGCTGCCCTGAGTGTTACCTC | This study |
| oPP159 | ATGTACGCCCGTGGTATGACAGTG | This study |
| oPP160 | GCTTGGAAATGAGTGAAGGCCAACAAG | This study |
| oPP161 | GGTTCGACGTCTTTATGGCTCGTG | This study |
| oPP162 | CAGATGCGCGGATAGTAGAACAAAGC | This study |
| oPP163 | GATAGGTAGCTCAGACGGCCCTA | This study |
| oPP164 | GAGTCTGCCTCGGATCTCATGGAATG | This study |
| oPP165 | GAAGATAGAACCGAACACCACCCTGAG | This study |
| oPP172 | GAAGTTGATCGAGGTGATAACGCCG | This study |
| oPP173 | GGCAACGAGCTTGGCCTTGATTTC | This study |
| oPP194 | GATCGGATCCCTCATCCTGAACGCAAAGGCACAG | This study |
| oPP195 | GATCGGATCCGCTCTGCGTGCTGAACGATATTGTC | This study |
| oPP199 | GCGAATTCGTAACCGGTCCCTAC | This study |
| oPP278 | GATCCCCGGGTGCTCGCCAACAGGCTGAACAG | This study |
| oPP279 | GATCGTCGACGGATCTACTTGCCGTTCAGTATCGAAGC | This study |
| oPP280 | GATCTAGAGGAGCTGACAGATGGTCCGTCCTGTAGAAACC | This study |
| oPP281 | GATCGAGCTCTTATTGTTTGCCTCCCTGCTGCG | This study |
| ARB1A | GCCACGCGTCGACTAGTACNNNNNNNNNNACGCC | (25) |
| ARB2 | GCCACGCGTCGACTAGTAC | (25) |
| oJG115 | TTCCTCGTGCTTTACGGTATCG | This study |
| oJG1127 | CGCGGATCCAGTCGACGAGGACAGCCTG | (12) |
| oJG1128 | CGCTCTAGAAGATCGTCGATGCCACCTC | (12) |
| oSM65c | GAAAACCTGTGGCTGCGTTC | This study |
| MtCP6-qPCR-forward | CCTGCTGCTACTATTGCTGGATATG | (15) |
| MtCP6-qPCR-reverse | CACTCGCATCAATGGCTACGG | (15) |
| MtVPE-qPCR-forward | CCAGGGGTTCTTGGTATGCCCG | (15) |
| MtVPE-qPCR-reverse | ACTGCCAGATTCACATGCCTCCA | (15) |
| MtC27-qPCR-forward | TGAGGGAGCAACCAAATACC | (15) |
| MtC27-qPCR-reverse | GCGAAAACCAAGCTACCATC | (15) |
| MtA39-qPCR-forward | CGGCAACTAGATCTTTGACA | (15) |
| MtA39-qPCR-reverse | CATTTGCTGTTGGTGTGTACG | (15) |
Table S5.
NCR peptides used in this study
| NCR peptide | Sequence | Mt4.0v1 gene ID |
| NCR035 | NSSFLGTFISSCKRDKDCPKLYGANFRCRKGTCVPPI | contig_121179_1 |
| NCR041 | QIIFSECKTDKDCPKYQRANIRCRKGQCVRI | Medtr0753s0010.1 |
| NCR079 | AFVKCETDDDCPKYNGFRKYECVNNWCRLTGLH | Medtr6g452900.1 |
| NCR094 | FQPCVTTADCMKTLKTDENIWYECINDFCIPFPIPKGRK | Medtr5g055370.1 |
| NCR124 | AVHKECKTDVDCRQIWFVTKCINHECQPIL | Medtr1g073510.1 |
| NCR129 | TIPCTFIDDCPKMPLVVKCIDNFCNYFEIK | Medtr4g055680.1 |
| NCR142 | CISDDDCPEALSPQFPKCIHNVCVYFVEE | contig_69672_2 |
| NCR169 | EDIGHIKYCGIVDDCYKSKKPLFKIWKCVENVCVLWYK | Medtr7g029760.1 |
| NCR211 | DRECDTDTECQKKFPGVNAHHLWCDNGNCVSYPK | Medtr4g035705.1 |
| NCR247 | RNGCIVDPRCPYQQCRRPLYCRRR | Medtr5g056815.1 |
Construction and Screening of the Plasmid-Specific Transposon Mutant Library.
The plasmid-specific transposon library was generated using a mating-out procedure as described in Fig. S1. Transposon insertion sites were mapped onto pHRB800 using arbitrary-PCR for mutant strains that yielded a Fix+ phenotype and maintained pHRB800. More specific details of the protocol are included in Supporting Information.
Plant Growth and Nodulation.
M. truncatula plants were grown in a 4:1 Turface:Vermiculite mixture (Turface Athletics; Thermo-O-Rock West Inc.). Seedlings were inoculated with Sinorhizobium 4 d after planting and then were grown under nitrogen-limiting conditions as indicated for individual experiments. For details, see Supporting Information. For plant dry weights, samples were placed in an oven at 60 °C for 48 h and then were weighed on an analytical balance. To determine cfus per nodule, nodules were surface sterilized, crushed, serially diluted, and plated on LB agar. The Student’s t test was used to compare differences between samples.
Acetylene Reduction Assays.
Plants were harvested at 21 dpi. Three whole plants were placed in sealed test tubes containing a 10% total atmosphere of acetylene. Plants were incubated for ∼12 h, and relative ethylene levels were measured using an Agilent 6890N gas chromatograph (Agilent Technologies, Inc.) For details, see Supporting Information.
GUS Staining.
5-Bromo-4-chloro-1H-indol-3-yl β-d-glucopyranosiduronic acid (X-GLUC) was used for in planta visualization of GUS expression in 10-d-old M. truncatula A20 and A17 nodules. p-Nitrophenyl-β-d-glucuronide (PNPG) was used to measure in vitro GUS expression in free-living cells. For details, see Supporting Information.
HrrP Modeling, Purification, and Proteolysis Assays.
HrrP structural model predictions were generated using iTASSER online (22), and electrostatic modeling predictions were generated using University of California, San Francisco (UCSF) Chimera (23). Overexpressed His6x-tagged HrrP was purified from NiCO21 (DE3) E. coli cells (New England Biolabs). NCR peptides were purchased from GenScript or were produced using solid-phase peptide synthesis. HrrP-specific activity was determined by the decrease in the amount of residual substrate compared with HrrP (E62A) matched controls as analyzed via HPLC. HrrP activity also was visualized on Tris-Tricine SDS/PAGE gels. For details, see Supporting Information.
RNA Extraction and Quantitative Real-Time PCR Analysis.
Total RNA was extracted from M. truncatula A17 and A20 nodules, and cDNA pools were synthesized using standard techniques. Quantitative real-time PCR (qRT-PCR) analyses for MtCP6 and MtVPE were performed as described by Pierre et al. (15) using the constitutively expressed MtC27 and MtA39 genes as endogenous controls.
Transmission Electron Microscopy.
At 28 dpi, whole nodules were excised, fixed, stained, and embedded in Spurr’s resin. An ultramicrotome was used to generate the 80-nm sections that then were imaged using a Tecnai G2 T-12 transmission electron microscope. For details, see Supporting Information.
Identification of hrrP-Harboring Strains and Phylogenic Tree Assembly.
hrrP homologs were identified and sequenced from the US Department of Agriculture (USDA) Sinorhizobium strain collection or were retrieved from GenBank. For details, see Supporting Information. The hrrP ORF was used to generate a neighbor-joining phylogenic tree. Clades I–XVI were assigned based on the chromosomal phylogeny reported by Van Berkum et al. (24) at a linkage distance of 0.35.
SI Results
Screen for Gene(s) Responsible for the Nitrogen-Blocking Phenotype of pHRB800.
A plasmid-specific transposon library was generated as described in Fig. S1. A library consisting of ∼6,000 plasmid-specific transposon insertion mutants were inoculated onto 10 M. truncatula A20 plants in pools of ∼300 mutants. At 28 dpi, large pink nodules (Fix+) then were surface sterilized, crushed, and plated, and individual isolated colonies were used to verify the Fix+ phenotype on a second round of M. truncatula A20 plants. The transposon insertion sites were mapped onto pHRB800 using aPCR for strains that maintained pHRB800 and yielded a Fix+ phenotype. All transposon insertions mapped to peptidase gene (Protein_ID: AJT61688.1, accession no CP011000).
Phenotypes of Wild-Type B800 and Mutants with Targeted Disruptions in hrrP.
When inoculated onto M. truncatula accession A20, S. meliloti strain B800 predominately forms Fix− nodules with relatively few aberrant Fix+ nodules. As reported by Crook et al. (12), these aberrant Fix+ nodules are the result of the spontaneous curing of a single large accessory plasmid, pHRB800, as observed in the left panel of Fig. S2A (arrow). As expected, designed disruptions of hrrP also yield a Fix+ phenotype on accession A20. The relatively small number of aberrant Fix+ nodules found on A20 plants results in moderate levels of nitrogen fixation as seen in the acetylene reduction assays and the plant dry weight data (Fig. S2 C and D).
Time Course of Nitrogen Fixation.
Host legumes require increasing amounts of fixed nitrogen to maintain plant growth over time. Plants are able to acquire increasingly higher levels of nitrogen as individual nodules expand and increase their nitrogen-fixing capacity and as the number of root nodules available for nitrogen fixation expands. To see the effect that HrrP has on nitrogen fixation over time, we performed a time series of acetylene reduction assays on M. truncatula accessions A20 and A17. Similar to the single time point reported in the main text, hrrP almost completely inhibits nitrogen fixation on accession A20 at all time points examined but appears to have no effect on A17 (Fig. S3). These data indicate that HrrP activity leads to an early block in nitrogen fixation, which is maintained at later time points.
Plant Dry Weight for Different Accessions.
It is clear that hrrP is responsible for differentiating between M. truncatula accessions A20 and A17 when in the S. meliloti B800 strain background. Fig. S4 further shows that in a larger panel of M. truncatula accessions, hrrP exhibits a broad spectrum of symbiotic effects. These effects range from little to no effect on plant dry weight or plant health (A17, L000163, and L000174) to nearly complete reduction in plant dry weight to the same levels as uninoculated plants (A20 and L000239) (Fig. S4). Intriguingly, accession L000542 did not exhibit an overall loss in nitrogen-fixation capacity between strains with and without hrrP as measured using acetylene reduction data, but the hrrP+ strain showed a significant loss in plant dry weight compared with the hrrP− strain. Upon examining the root systems of these plants, we noted that the hrrP+ strains had ∼50% more nodules (25–30 nodules per plant) than the hrrP− strain (15–20 nodules per plant). These data imply that, although the nodules are producing the same overall levels of fixed nitrogen, the plant must allocate significantly more resources to construct additional nodules to maintain nitrogen production, thus reducing the resources available for shoot growth and expansion. Therefore, different closely related legume hosts, all of which form infected nodules, exhibit differing responses to rhizobially expressed hrrP.
Effects of hrrP Are Dependent on the Strain Genetic Background.
As noted in the main text, hrrP can have differing effects on host responses depending on which strain is expressing it. For example, hrrP in S. meliloti strain B800 blocks nitrogen fixation on M. truncatula A20 but not A17, whereas hrrP in S. meliloti strain B464 blocks nitrogen fixation on both A20 and A17. This kind of variation in strain range can be observed in diverse Sinorhizobium isolates (Fig. S5 and Table S1). Table S1 shows the phenotypic data for M. truncatula A20 and A17 inoculated with various strains with and without hrrP, and Fig. S5 shows representative images of shoots and nodules from these experiments. This strain-range effect demonstrates that additional rhizobial genes can modify the effects that hrrP has on nitrogen fixation.
Synteny Surrounding hrrP Homologs in Sinorhizobium and Rhizobium.
The genomic landscape surrounding hrrP is known for only two plasmids, pHRB800 and pSINME01, the latter being an accessory plasmid from S. meliloti strain AK83. Although these hrrP homologs have 97.4% nucleotide sequence identity, there is no synteny in the genetic loci surrounding hrrP including its own promoter region (Fig. S7 A and B). Moreover, both hrrP homologs are surrounded by multiple complete or partial transposable elements that most closely resemble sequences found in the genus Rhizobium, as shown in green in Fig. S7. Indeed, hrrP homologs are also found in various Rhizobium species, but in this genus, hrrP is chromosomally encoded and maintains synteny across different species and strains, suggesting that it might perform more of a housekeeping function in this genus (Fig. S7C). We therefore speculate that Sinorhizobium likely acquired hrrP from Rhizobium via horizontal gene transfer and that hrrP has spread between different Sinorhizobium genotypes via conjugative- and transposon-based transfer mechanisms. Fig. S6 shows a protein aliment for known HrrP sequences.
SI Materials and Methods
Plant Growth and Nodulation.
Scarified and surface-sterilized seeds were allowed to germinate in Petri plates, and 2-d-old seedlings were planted in a 4:1 mixture of sterile Turface clay particles and Vermiculite (Turface Athletics; Thermo-O-Rock West, Inc.). Seedlings were grown for 4 d prior to inoculation with 0.5 mL of S. meliloti cells suspended in dH2O to an OD600 of ∼0.1. Nodulation was allowed to proceed as indicated for individual experiments. Plants were watered with standard nodulation medium [1 mM KH2PO4, 0.5 mM MgSO4, 0.5 mM CaCl2·2H2O, and 2 mL/L of a minor salts solution (500× minor salts/L = 9.5 g Na2-EDTA·2H2O, 7 g FeSO4·7H2O, 1 g H3BO3, 250 mg MnSO4·H2O, 50 mg ZnSO4·7H2O, 50 mg Na2MoO4·2H2O, 50 mg CuSO4, and 10 mg CoCl2)]. Sterilized stock solutions of each component were prepared (KH2PO4 was adjusted to 7.0 with 2N KOH) and added to sterile dH2O just before watering. Plants were maintained at ∼25 °C under fluorescent lights (2.7-klx intensity, 16-h day length). To determine dry weight of plants, samples were placed in an oven at 60 °C for 48 h and then were weighed on an analytical balance. To determine cfus per nodule, nodules were sterilized (5 s in 75% ethanol, 5 s in 25% bleach, three washes in ddH2O), crushed in 100 μL LB, serially diluted in LB, and plated on LB agar.
Plasmid and Strain Construction.
Bacterial strains, plants, plasmids, peptides, and oligonucleotides used in this study are listed in Tables S2–S6. Plasmids were constructed using standard molecular techniques with enzymes obtained from New England Biolabs and oligonucleotides from Life Technologies. Briefly, pPP101 was constructed by inserting the PCR product from oJG1127 and oJG1128 into pJQ200. pPP105 was constructed by inserting the PCR product from oPP017 and oPP020 into pJG194, and the correct loop-in was verified using oPP027 and oPP028. pPP119 was constructed by inserting the PCR product from oPP073 and oPP074 into pJG542, a lac-inducible expression vector. pPP123 was constructed by inserting the splicing-by-overlap-extension (SOE) PCR product from oPP031, oPP071, oPP72, and oPP031 into pJG110, and the Tn5 insertion site was mapped using arbitrary PCR (aPCR) with oPP107 and oPP108. pPP124 was constructed by inserting the PCR product from oPP031 and oPP031 into pJG110, and the Tn5 insertion site was mapped using aPCR with oPP107 and oPP108. pPP132 was constructed by inserting the SOE PCR product from oPP093, oPP094, oPP095, and oPP096 into pJG194, and the correct loop-in was verified using oPP072 and oPP108. pPP147 was constructed by inserting the PCR product from oPP116 and oPP109 into pRF771. pPP156 was constructed by inserting the SOE PCR product from oPP111, oPP118, oPP119, and oPP096 into pJG194, and the correct loop-in was verified using oPP172 and oPP108. pPP157 was constructed by inserting the SOE PCR product from oPP120, oPP121, oPP122, and oPP096 into pJG194, and the correct loop-in was verified using oPP173 and oPP108. pPP169 was constructed by inserting the PCR product from oPP194 and oPP195 into pJG392, and the correct loop-in was verified using oMC86 and oPP199. pPP196 was constructed by inserting the SOE PCR product from oPP71, oPP72, oPP073, and oPP074 into pJG542, a lac-inducible expression vector. pPP255 was constructed by inserting the PCR products from oPP278, oPP279, oPP280, and oPP281 and the Ptrp promoter from pRF771 into pJG194; the correct loop-in downstream of the rhaK locus was confirmed with primers oJG115 and oSM65c. All plasmid sequences were verified by Sanger sequencing. Plasmid mobilization between strains was mediated via triparental matings with the helper E. coli B001 (DN5α harboring pRK600) followed by selection with the appropriate antibiotics and streptomycin (S. meliloti) or rifampicin (A. tumefaciens) counter selection. The following antibiotics were used as needed: 2.5 μg/mL tetracycline, 3 μg/mL gentamicin for E. coli, 15 μg/mL gentamicin for S. meliloti/A. tumefaciens, 30 μg/mL kanamycin, 30 μg/mL chloramphenicol, 50 μg/mL rifampicin, 100 μg/mL neomycin, 100 μg/mL ampicillin, and 200 μg/mL streptomycin.
aPCR.
aPCR was performed as described previously (25) using primers oPP013 (first round), oPP014 (second round), ARB1A, and ARB2 to determine himar1 insertion sites, and oPP107 and oPP108 to determine the Tn5 hrrP and hrrP(E62A) complementation insertion sites.
Sequencing and Annotation of pHRB800.
For pyrosequencing of pHRB800, the plasmid was marked with an oriT/neo cassette (pJG476) and conjugated into plasmid-free A. tumefaciens UBAPF2 to yield strain C320. C320 was grown overnight in 50 mL of LB, pelleted, and resuspended in 5 mL of Qiagen P1 buffer. Five milliliters of Qiagen P2 buffer was added to the cells and incubated at room temperature for 10 min before the addition of 7.5 mL of ice-cold Qiagen N3 buffer. Lysates were incubated for 30 min on ice and centrifuged twice at 10,000 × g for 30 min to remove cellular debris. DNA was precipitated with isopropanol, washed with 70% ethanol, dried, and resuspended in Tris-EDTA (TE). Samples were treated with 200 μg of proteinase K and incubated at 42°C for 2 h, followed by chloroform extraction, isopropanol/sodium acetate precipitation, and resuspension in TE. The 454 library preparation was performed according to the rapid library preparation protocol, followed by sequencing on the 454 Genome Analyzer FLX (Roche). Assembly into contigs was performed using Newbler (version 2.5.3), and contigs corresponding to A. tumefaciens were removed. Contig edge ambiguities were resolved by PCR using oPP152–oPP165. The sequence corresponding to oJG476 then was removed from the assembly to reconstitute the native pHRB800 sequence. Reads were remapped to this assembly using Geneious (version 5.3.4) to confirm the sequence. The final assembly was annotated using DNA Master, using Glimmer (version 3.02) to predict ORFs and BLASTx to assign putative functions. This sequence can be accessed in GenBank (accession number CP011000).
Construction and Screening of the Plasmid-Specific Transposon Mutant Library.
The plasmid-specific transposon library was generated as described in Fig. S1. Briefly, a mobilizable version of pHRB800 (pHRB800GmR) was conjugated into A. tumefaciens strain UBAPF2 to yield PP233, which then was mutagenized using a Himar1-based minitransposon system (pPP47) (26). Approximately 200,000 transposon-insertion mutants were mated en masse with a tetracycline-resistant strain of S. meliloti B800 pHR− (C403). S. meliloti C403 mutants with pHRB800-specific transposon insertions were selected on LB agar containing tetracycline, streptomycin, and neomycin. The resulting library consisted of ∼6,000 plasmid-specific transposon insertion mutants. The stable transfer and random transposition of pHRB800GmR was confirmed for 10 mutants using Eckhardt gel electrophoresis and aPCR. Pools of ∼300 plasmid-specific transposon insertion mutants were inoculated onto 10 M. truncatula A20 plants. At 28 dpi, roots were examined for large pink nodules (Fix+), which then were surface sterilized, crushed, and plated. Individual isolated colonies were used to verify the Fix+ phenotype on a second round of M. truncatula A20 plants. The transposon insertion sites were mapped onto pHRB800 using aPCR for strains that maintained pHRB800 and yielded a Fix+ phenotype.
Modified Eckhardt Gel Electrophoresis.
Modified Eckhardt gels were performed as previously described (12). Briefly, bacteria were grown to an OD600 of 0.6 in LB, and 150 μL of culture was added to 500 μL of chilled 0.3% sarkosyl in SBE (10 mN NaOH, 1 mM Na2EDTA·2H2O, pH adjusted to 8.0 with boric acid). Each sample was pelleted, resuspended in 20 μL of lysis solution (SBE, 1% sucrose, 100 mg/mL of lysozyme, 20 mg/mL RNase A), and loaded immediately into an SDS–SBE agarose gel (SBE, 0.8% agarose, 0.5% SDS). Each sample remained in the well for 5 min, followed by electrophoresis at 23 V for 10 min and then at 96 V for 90 min. The gel was stained for 15 min in 0.4 μg/mL of ethidium bromide and was destained for 10 min in dH2O before imaging.
Acetylene Reduction Assays.
Plants were harvested at 14, 21, or 28 dpi. Three plants were placed in 25-mL test tubes containing 0.5 mL of dH2O.The test tubes were sealed with rubber stoppers, and 10% of the total atmosphere (2.5 mL) was replaced with 100% acetylene. Plants then were incubated for ∼12 h, and 0.1 mL of air was removed with a gas-tight syringe to measure relative ethylene levels using an Agilent 6890N gas chromatograph (Agilent Technologies, Inc.).
GUS Staining/Quantification.
GUS reporter strains were generated by integrating reporter plasmids (pPP132, pPP156, and pPP157) into their respective native loci. PNPG was used to measure in vitro GUS activity of S. meliloti (PP389, PP397, and PP404) cultures grown to midlog in LB broth. Reactions were performed in a GUS reaction buffer (15 mM Na2HPO4, 10 mM NaH2PO, 5 mM KCl, 1 mg/mL PNPG, 0.01% Triton X-100, and 0.5% β-Mercaptoethanol, pH 7.0) at 37 °C and were stopped using an equal volume of stop buffer (1 M Na2CO3). OD410 readings were used to calculate Miller units [(1000*OD410)/(OD600*volume in milliliters*time in minutes)]. X-GLUC was used to measure GUS expression in 7-d-old M. truncatula line A20 nodules. Briefly, nodules were fixed (1.25% glutaraldehyde, 0.1 M sodium citrate) on ice for 30 min, washed three times with 50 mM sodium citrate, stained (0.5 mg/mL X-GLUC, 50 mM sodium citrate, 1 mM potassium ferrocyanide, 1 mM potassium ferricyanide, 0.1% Triton-X, 10% methanol, vacuum infiltration) for 8 h at 37 °C in the dark, washed with ddH2O, bleached for 3 min, washed with ddH2O, and imaged under a Leica EZ4D dissecting microscope (Leica Microsystems, Inc.).
Transmission Electron Microscopy.
At 10 and 28 dpi, whole nodules were excised, fixed with 2% glutaraldehyde in 50 mM cacodylate (pH 7.2) for 2 h, washed with 50 mM cacodylate, postfixed in 1% osmium tetroxide for 2 h, and washed again with H2O. Nodules then were stained in 0.5% uranyl acetate overnight, followed by dehydration in an acetone series. Samples were embedded in Spurr’s resin and cured for 48 h at 70 °C. Next, 80-nm sections were generated using an ultramicrotome, stained in Reynold’s lead citrate for 10 min, and then washed with H2O. Sections were imaged using a Tecnai G2 T-12 transmission electron microscope.
RNA Extraction and qRT-PCR Analysis.
A TRIzol-based procedure was used to extract total RNA from M. truncatula A17 and A20 nodules frozen in liquid nitrogen as recommended by the manufacturer (Life Technologies). cDNA pools were synthesized from 500 ng of RNA using the Bio-Rad iScript Reverse Transcription Supermix (Bio-Rad) following the manufacturer’s instructions. qRT-PCR was performed using a StepOne Real-Time PCR System (Applied Biosystems) with SsoAdvanced Universal SYBR Green Supermix (Bio-Rad) following the manufacturer’s instructions. Each reaction was performed with 4 μL of 100-fold diluted cDNA template and 500 nM of each specific primer set (Table S6) as described by Pierre et al. (15). The following reaction conditions were used: 10 min at 95 °C for initial denaturation and 40 cycles of 15 s at 95 °C and 1 min at 60 °C. The amplification quality was checked by the presence of a single dissociation peak in a melting curve performed in each well at the end of the PCR. Technical reactions were performed in triplicate for two biological replicates, and the results were averaged. The expression levels were calculated and normalized using the 2-ΔΔCT method. Constitutively expressed MtC27 and MtA39 were used as endogenous controls (15).
Sequencing of hrrP Homologs in Sinorhizobium.
A total of 150 USDA isolates were screened for the presence of hrrP homologs using the oligonucleotides oPP17 and oPP20. hrrP homologs were PCR amplified using either oPP033 or oPP110 for the region 200 bp upstream of the start codon and oPP109 for 50 bp downstream of the stop codon. Oligonucleotides oPP017, oPP027, and oPP78 were used to generate the internal hrrP sequence. Sequence data were generated for the following USDA stains: USDA 1606, USDA 1617, USDA 1947, USDA 1953, USDA 1959, USDA 1960, USDA 1973, USDA 1974, USDA 1982, USDA 6479, USDA 6494, USDA 6509, USDA 6512, USDA 6561, USDA 6576, USDA 6571, USDA 6586, USDA 6515, and USDA 6657. We were unable to amplify a usable sequence from the strains USDA 6632, USDA 6643, USDA 6511, USDA 6491, or three natural isolates in our collection. The hrrP ORF was used to generate a neighbor-joining phylogenic tree using Geneious v5.3.4 (Biomatters Ltd).
Protein Alignment of HrrP Homologs.
Predicted HrrP protein sequences for S. meliloti strains B800, AK83, USDA 1606, USDA 1617, USDA 1947, USDA 1953, USDA 1959, USDA 1960, USDA 1973, USDA 1974, USDA 1982, USDA 6479, USDA 6494, USDA 6509, USDA 6512, USDA 6561, USDA 6576, USDA 6571, USDA 6586, USDA 6515, and USDA 6657, R. leguminosarum bv. viciae 3841, R. leguminosarum bv. trifolii WSM2304, R. leguminosarum bv. trifolii WSM1325, R. leguminosarum bv. trifolii WSM1689 and Rhizobium elti CIAT 652 were aligned using Geneious v5.3.4 (Biomatters Ltd.) and visualized using BOXSHADE v3.21 (available at: www.ch.embnet.org/software/BOX_form.html).
HrrP Purification.
A C-terminal His6x-tagged version of HrrP (or the E62A variant) was overexpressed in NiCO21 (DE3) E. coli cells (New England Biolabs) at room temperature for 18 h. E. coli cells were collected by centrifugation at 8,000 × g, 4 °C for 5 min, suspended in lysis buffer [10 mM KCl, 20 mM imidazole, 300 mM NaCl, 50 mM Hepes (pH 7.8), and Sigma P8465 Protease Inhibitor Mixture], and mechanically disrupted (MicroFluidics). Soluble fractions obtained by centrifugation at 23,000 × g, 4 °C for 30 min were applied to a column containing Ni-NTA Agarose (Qiagen) and were incubated at 4 °C for 3 h. His6x-tagged proteins were eluted in 10 mM KCl, 270 mM imidazole, 300 mM NaCl, 50 mM Hepes (pH 7.8), and 1 mM 2-Mercaptoethanol (BME). The eluate then was applied to a column containing Chitin Resin (New England Biolabs), and the flow-through containing the enzyme was exchanged in a 50-mM phosphate buffer (pH 7.2) using Bio-Gel P6-DG Medium (Bio-Rad). Purified protein was stored at −80 °C in 20% glycerol. Protein concentrations were measured using Coomassie Protein Assay Reagent (Thermo Scientific) using known BSA standards.
Synthesis of NCR Peptides.
NCR peptides 124, 142, 211, and 247 were synthesized using standard Fmoc solid-phase peptide synthesis procedures, using preloaded resin for coupling of the first amino acid. Briefly, the crude peptide was dissolved in 75 mM Hepes, 6 M GduHCl, pH 8.2, to a final concentration of 10 mg/mL and was incubated with 10 mM Tris(2-carboxyethyl)phosphine (TCEP) for 20 min. After 20 min, the reaction was quenched by the addition of 6% vol/vol TFA in Milli-Q water to give 2% vol/vol TFA. The quenched reaction was centrifuged (1,100 × g for 20 min), and the supernatant was filtered (0.2-μm filter). The filtrate was purified by preparative reverse-phase HPLC (RP-HPLC), and the desired reduced peptide was isolated using a gradient of 10–60% solvent B (60% acetonitrile and 0.1% TFA) over 30 min (10 mL/min).
HrrP-Specific Peptidase Activity.
The standard condition was as follows: the NCR peptides substrate (0.1 mg/mL peptide) in 50 mM potassium phosphate (pH 7.2), 100 nM zinc sulfate, and 1 mM DTT was incubated with ∼2.5 μg enzyme at 30 °C for 10 or 30 min depending on the time needed to degrade 10–40% of the peptide substrate. The reaction was stopped by adding two volumes of 1% TFA, and the reaction products were analyzed by RP-HPLC using an octadecyl silica column [Luna C18 (2), 4.6 mm × 250 mm; Phenomenex], which previously had been equilibrated with 0.05% TFA. Peptides were eluted with a linear gradient of acetonitrile (0–60%) at a flow rate of 1 mL/min. The amount of residual substrate was estimated from the peak area detected at 220 nm through HPLC analysis (Agilent 1200). Enzymatic activity was determined by the decrease in the amount of residual substrate compared with matched assays using HrrP (E62A). Specific activity was defined as enzymatic activity per milligram of HrrP per minute at 30 °C. For visualization of cleavage reactions (0.25 mg/mL peptide), the reaction products were resolved on a 19% Tris-Tricine gel and stained with Coomassie Brilliant Blue R250.
Acknowledgments
We thank Michael Standing for technical assistance with the electron microscopy, P. Van Berkum [US Department of Agriculture (USDA) Agricultural Research Service] for providing many of the strains used in this study, and the Biological Resources Centre for Medicago truncatula for providing the M. truncatula accessions used in this study. Structural graphics and analyses were performed with the University of California, San Francisco (UCSF) Chimera package developed by the Resource for Biocomputing, Visualization, and Informatics at UCSF (supported by National Institute of General Medical Sciences Grant P41-GM103311). This work was funded by National Science Foundation Grant IOS-1054980 and USDA/National Institute of Food and Agriculture Grant 2015-67013-22915 (to J.S.G.). This work was supported in part by National Institutes of Health Grant GM31010 (to G.C.W.); G.C.W. is an American Cancer Society Professor. M.S. is supported by Human Frontier Science Program LT000852/2012.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. CP011000).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1417797112/-/DCSupplemental.
References
- 1.Jones KM, Kobayashi H, Davies BW, Taga ME, Walker GC. How rhizobial symbionts invade plants: The Sinorhizobium-Medicago model. Nat Rev Microbiol. 2007;5(8):619–633. doi: 10.1038/nrmicro1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mergaert P, et al. Eukaryotic control on bacterial cell cycle and differentiation in the Rhizobium-legume symbiosis. Proc Natl Acad Sci USA. 2006;103(13):5230–5235. doi: 10.1073/pnas.0600912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mergaert P, et al. A novel family in Medicago truncatula consisting of more than 300 nodule-specific genes coding for small, secreted polypeptides with conserved cysteine motifs. Plant Physiol. 2003;132(1):161–173. doi: 10.1104/pp.102.018192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, et al. A nodule-specific protein secretory pathway required for nitrogen-fixing symbiosis. Science. 2010;327(5969):1126–1129. doi: 10.1126/science.1184096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van de Velde W, et al. Plant peptides govern terminal differentiation of bacteria in symbiosis. Science. 2010;327(5969):1122–1126. doi: 10.1126/science.1184057. [DOI] [PubMed] [Google Scholar]
- 6.Maunoury N, et al. Differentiation of symbiotic cells and endosymbionts in Medicago truncatula nodulation are coupled to two transcriptome-switches. PLoS ONE. 2010;5(3):e9519. doi: 10.1371/journal.pone.0009519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nallu S, Silverstein KAT, Zhou P, Young ND, VandenBosch KA. 2014. Patterns of divergence of a large family of nodule cysteine-rich peptides in accessions of Medicago truncatula. Plant J 78(4):697–705. [DOI] [PMC free article] [PubMed]
- 8.Penterman J, et al. Host plant peptides elicit a transcriptional response to control the Sinorhizobium meliloti cell cycle during symbiosis. Proc Natl Acad Sci USA. 2014;111(9):3561–3566. doi: 10.1073/pnas.1400450111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kondorosi E, Mergaert P, Kereszt A. A paradigm for endosymbiotic life: Cell differentiation of Rhizobium bacteria provoked by host plant factors. Annu Rev Microbiol. 2013;67:611–628. doi: 10.1146/annurev-micro-092412-155630. [DOI] [PubMed] [Google Scholar]
- 10.Haag AF, et al. Protection of Sinorhizobium against host cysteine-rich antimicrobial peptides is critical for symbiosis. PLoS Biol. 2011;9(10):e1001169. doi: 10.1371/journal.pbio.1001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horváth B, et al. 112:15232–15237. doi: 10.1073/pnas.1500777112. (2015) Loss of the nodule-specific cysteine rich peptide, NCR169 abolishes symbiotic nitrogen fixation in the Medicago truncatula dnf7 mutant. Proc Natl Acad Sci USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crook MB, et al. Rhizobial plasmids that cause impaired symbiotic nitrogen fixation and enhanced host invasion. Mol Plant Microbe Interact. 2012;25(8):1026–1033. doi: 10.1094/MPMI-02-12-0052-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capela D, Filipe C, Bobik C, Batut J, Bruand C. Sinorhizobium meliloti differentiation during symbiosis with alfalfa: A transcriptomic dissection. Mol Plant Microbe Interact. 2006;19(4):363–372. doi: 10.1094/MPMI-19-0363. [DOI] [PubMed] [Google Scholar]
- 14.Shen Y, Joachimiak A, Rosner MR, Tang W-J. Structures of human insulin-degrading enzyme reveal a new substrate recognition mechanism. Nature. 2006;443(7113):870–874. doi: 10.1038/nature05143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierre O, et al. Involvement of papain and legumain proteinase in the senescence process of Medicago truncatula nodules. New Phytol. 2014;202(3):849–863. doi: 10.1111/nph.12717. [DOI] [PubMed] [Google Scholar]
- 16.Galardini M, et al. Exploring the symbiotic pangenome of the nitrogen-fixing bacterium Sinorhizobium meliloti. BMC Genomics. 2011;12:235–249. doi: 10.1186/1471-2164-12-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long SR. Rhizobium symbiosis: Nod factors in perspective. Plant Cell. 1996;8(10):1885–1898. doi: 10.1105/tpc.8.10.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denarie J, Debelle F, Truchet G, Prome J. Rhizobium and legume nodulation: A molecular dialogue. New Horizons in Nitrogen Fixation 1993 , Current Plant Science and Biotechnology in Agriculture, eds Palacios R, Mora J, Newton WE (Springer, Dordrecht, The Netherlands), Vol 17, pp 19–30. [Google Scholar]
- 19.Young ND, et al. The Medicago genome provides insight into the evolution of rhizobial symbioses. Nature. 2011;480(7378):520–524. doi: 10.1038/nature10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Streeter J. Failure of inoculant rhizobia to overcome the dominance of indigenous strains for nodule formation. Can J Microbiol. 1994;40(7):513–522. [Google Scholar]
- 21.Vlassak K, Vanderleyden J, Graham P. Factors influencing nodule occupancy by inoculant rhizobia. Crit Rev Plant Sci. 1997;16:163–229. [Google Scholar]
- 22.Yang J, et al. The I-TASSER Suite: Protein structure and function prediction. Nat Methods. 2015;12(1):7–8. doi: 10.1038/nmeth.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pettersen EF, et al. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 24.van Berkum P, Elia P, Eardly BD. Multilocus sequence typing as an approach for population analysis of Medicago-nodulating rhizobia. J Bacteriol. 2006;188(15):5570–5577. doi: 10.1128/JB.00335-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffitts JS, Long SR. A symbiotic mutant of Sinorhizobium meliloti reveals a novel genetic pathway involving succinoglycan biosynthetic functions. Mol Microbiol. 2008;67(6):1292–1306. doi: 10.1111/j.1365-2958.2008.06123.x. [DOI] [PubMed] [Google Scholar]
- 26.Price PA, Jin J, Goldman WE. Pulmonary infection by Yersinia pestis rapidly establishes a permissive environment for microbial proliferation. Proc Natl Acad Sci USA. 2012;109(8):3083–3088. doi: 10.1073/pnas.1112729109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quandt J, Hynes MF. Versatile suicide vectors which allow direct selection for gene replacement in gram-negative bacteria. Gene. 1993;127(1):15–21. doi: 10.1016/0378-1119(93)90611-6. [DOI] [PubMed] [Google Scholar]
- 28.Wells DH, Long SR. The Sinorhizobium meliloti stringent response affects multiple aspects of symbiosis. Mol Microbiol. 2002;43(5):1115–1127. doi: 10.1046/j.1365-2958.2002.02826.x. [DOI] [PubMed] [Google Scholar]