A review of the published data on the role of travel burden influencing four items was performed. The results showed that increasing travel requirements are associated with more advanced disease at diagnosis, inappropriate treatment, a worse prognosis, and a worse quality of life, suggesting that clinical oncologists should remember the specific travel burden of cancer patients, who often need health care services every week or month for many years.
Keywords: Travel burden, Distance from hospitals, Cancer patients, Cancer diagnosis and treatment, Outcome, Quality of life
Abstract
The burden of travel from a patient’s residence to health care providers is an important issue that can influence access to diagnosis and treatment of cancer. Although several studies have shown that the travel burden can result in delays in diagnosis and treatment of many common cancers, its role appears underestimated in the treatment of patients in clinical practice. Therefore, we performed a review of the published data on the role of travel burden influencing four items: delay of diagnosis, adequate treatment of cancer, outcome, and quality of life of cancer patients. Forty-seven studies published up to December 2014 were initially identified. Twenty studies were excluded because they did not regard specifically the four items of our review. Twenty-seven studies formed the basis of our study and involved 716,153 patients. The associations between travel burden and (a) cancer stage at diagnosis (12 studies), (b) appropriate treatment (8 studies), (c) outcome (4 studies), and (d) quality of life (1 study) are reported. In addition, in two studies, the relation between travel burden and compliance with treatment was examined. The results of our review show that increasing travel requirements are associated with more advanced disease at diagnosis, inappropriate treatment, a worse prognosis, and a worse quality of life. These results suggest that clinical oncologists should remember the specific travel burden problem for cancer patients, who often need health care services every week or every month for many years.
Implications for Practice:
The influence of travel burden on cancer patients has been previously studied, but this is the first comprehensive review of the available literature. This review shows that travel burden negatively influences stage at diagnosis, appropriate treatment, outcome, and quality of life in cancer patients. The results demonstrate that clinical oncologists should keep in mind the specific travel burden problem for cancer patients who often need health care services every week or every month for many years.
Introduction
It is well known that patients with cancer must overcome many psychological, social, economic, and family barriers to obtain the diagnosis and treatment needed. In addition, the burden of travel from a patient’s residence to his or her health care provider can be an important issue that can influence access to diagnosis and treatment services for cancer needs [1]. The necessity for repeated visits for cancer diagnosis and treatment on an outpatient or an inpatient basis makes distance an important issue with which the patient with cancer must manage during the disease course [2].
Oncologists agree that the selection of the treatment regimen for a cancer patient depends on numerous factors, including the type of malignancy, stage, histologic features, tumor genetics, and previous therapies [3]. Other factors are patient-related, such as functional status, burden of comorbidities, and status of social support [4].
Several studies have documented that the travel burden (measured as the travel distance or travel time) can result in delays in diagnosis and can influence the choice of treatment of a variety of common cancers [5–9]. However, these factors might not necessarily be considered consistently in the daily management of cancer, such as in the selection of the treatment regimen and the selection of treatment dosing [3, 4].
The most relevant objectives of health policies are to improve the quality, safety, patient satisfaction, and health care efficiency, as reported in a recent review [10]. It must be emphasized, however, that consideration of the role played by the travel burden in cancer patient management might be insufficient [3, 4, 10]. It is therefore with special emphasis on patient-centered care that we provide a review of the available data on the association between travel burden and the cancer stage at diagnosis, appropriate treatment, outcome, and quality of life. In particular, the objective of our review was to examine whether the travel burden has a negative influence on cancer patients regarding these four aspects: (a) cancer stage at diagnosis, (b) appropriate treatment (treatment performed or omitted), (c) outcome, and (d) quality of life (QoL).
Methods
We provide a narrative review of the published data.
Inclusion and Exclusion Criteria
Studies that evaluated the association between travel burden and the four aspects considered relevant in the treatment of cancer patients: (a) cancer stage at diagnosis, (b) appropriate treatment (treatment performed or omitted), (c) outcome, and (d) quality of life were included in the present review, which was centered on both curable and incurable cancer patients. Only studies exploring the role of travel burden itself were considered for the review. Consequently, studies that had reported other aspects, such as sociodemographic factors, race, ethnicity, or insurance, rather than travel burden (or in association), were excluded. Studies investigating other aspects of oncological treatments, such as palliative radiotherapy for bone pain or adherence to screening programs, even if related to travel burden, were also excluded if they were not related to one of the four items already summarized (cancer stage at diagnosis, appropriate treatment, treatment performed or omitted, outcome, and quality of life).
Study Identification
A computerized literature search through PubMed, CANCERLIT, Embase, and Cochrane Library was performed applying the words travel burden; distance from hospitals; time to therapy; time to medical services; time to hospitals; cancer patients; cancer diagnosis and treatment; outcome; survival; and quality of life. Reports and abstracts were also identified by back-referencing from the original and relevant studies. Only studies published in English before December 2014 were selected for the present review. Studies published only in abstract form were excluded. Four of us (M.A., C.B., F.F., and L.C.) evaluated the titles and abstracts and then the full text of the studies considered potentially eligible for the present review. If in doubt, a fifth author (C.D.G.) was consulted to reach an agreement.
Data Extraction and Analysis
Three of us (M.A., C.B., and L.C.) independently extracted qualitative and numerical data from the included studies, resolved differences through consensus, and analyzed the data qualitatively.
Results
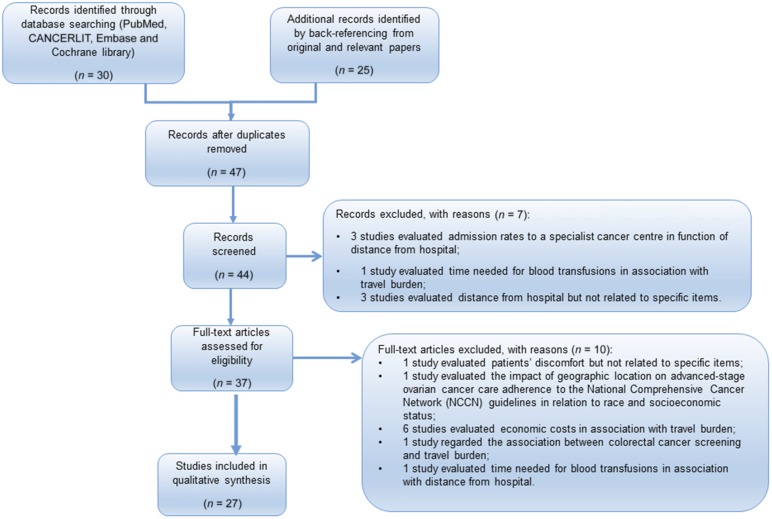
The total number of studies identified in our search was 47; 20 studies were excluded because they had not specifically considered the objects of our review [11, 12] (Fig. 1). Reviewing the existing data, we found that the distance from the treating hospitals can influence the four different aspects: stage at diagnosis, appropriate treatment, outcome, and QoL.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.
Travel Burden and Stage at Diagnosis
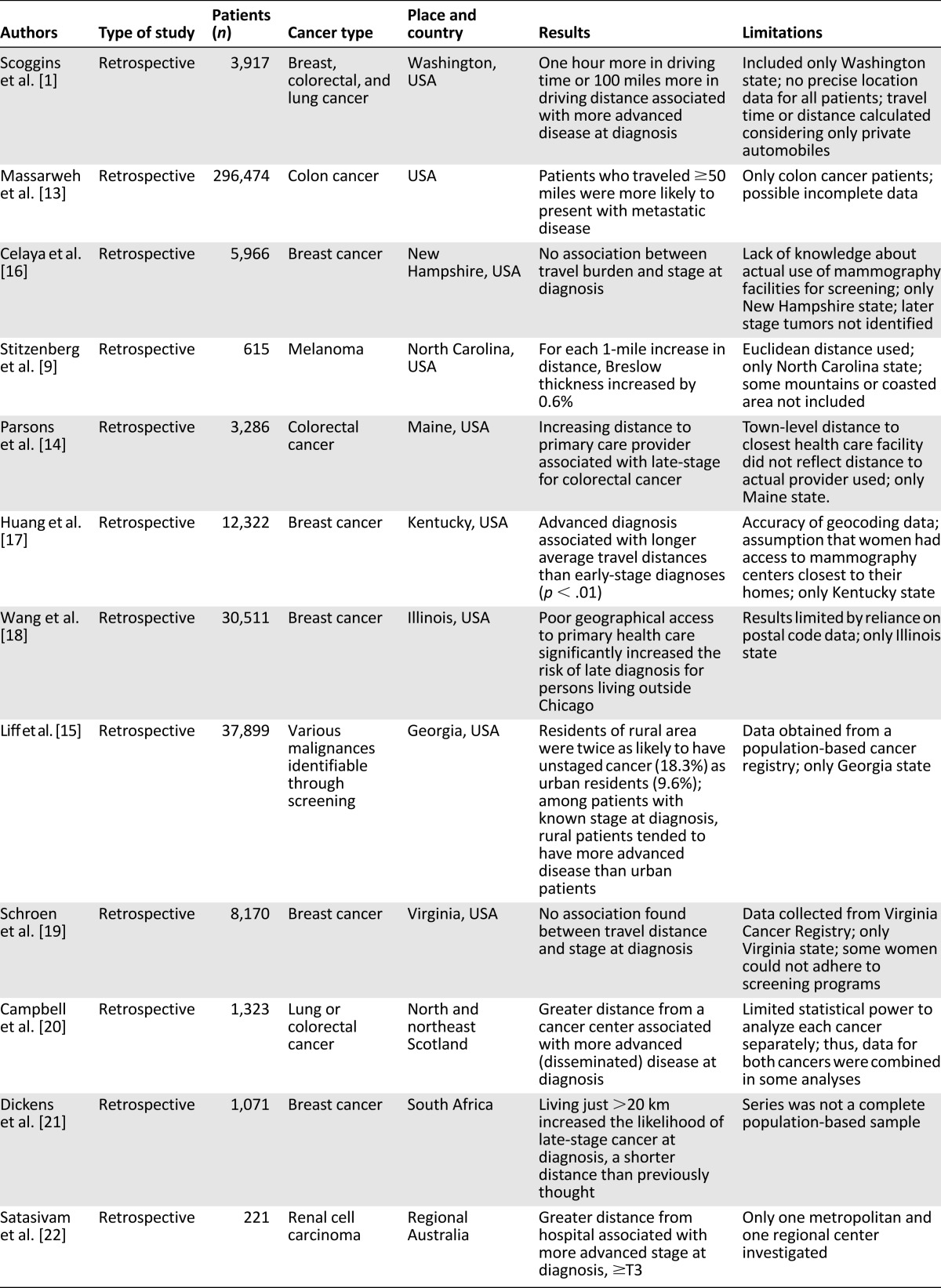
The role of travel burden influencing cancer stage at diagnosis was analyzed in 12 retrospective studies [1, 9, 13–22], which involved 401,775 patients (Table 1). All but three of these studies were performed in the United States. One study was done in the northeast of Scotland, one in Australia, and one in South Africa. Most of these studies included patients with breast, colorectal, lung, or kidney cancer or melanoma.
Table 1.
Travel burden and stage at diagnosis
In 10 of these 12 studies, analyzing 387,639 patients, the travel burden conditioned the stage at diagnosis [1, 9, 13–15, 17, 18, 20–22]. Patients who traveled 50 miles or 1 hour or more in driving time were associated with a more advanced disease at diagnosis.
The same behavior was present in patients with poor geographical access or in patients of the rural area, who were twice as likely to have unstaged cancer (18.3%) than were urban residents (9.6%). Also, among patients with a known stage at diagnosis, rural patients tended to have more advanced disease than that of urban patients.
In only two studies, which exclusively involved women with breast cancer, was the travel burden not associated with an advanced stage at diagnosis [16, 19]. In patients with melanoma, for each 1-mile increase in distance, the Breslow thickness had increased by 0.6% [9].
It must be emphasized that these studies had some limitations. The main limitations (Table 1) included the methods used to calculate the travel time or distance; that in some studies, only 1 state in the United States was considered; the reliance on postal code data; that the data were obtained from a population-based cancer registry; and the inclusion of retrospective studies only.
Travel Burden and Appropriate Treatment
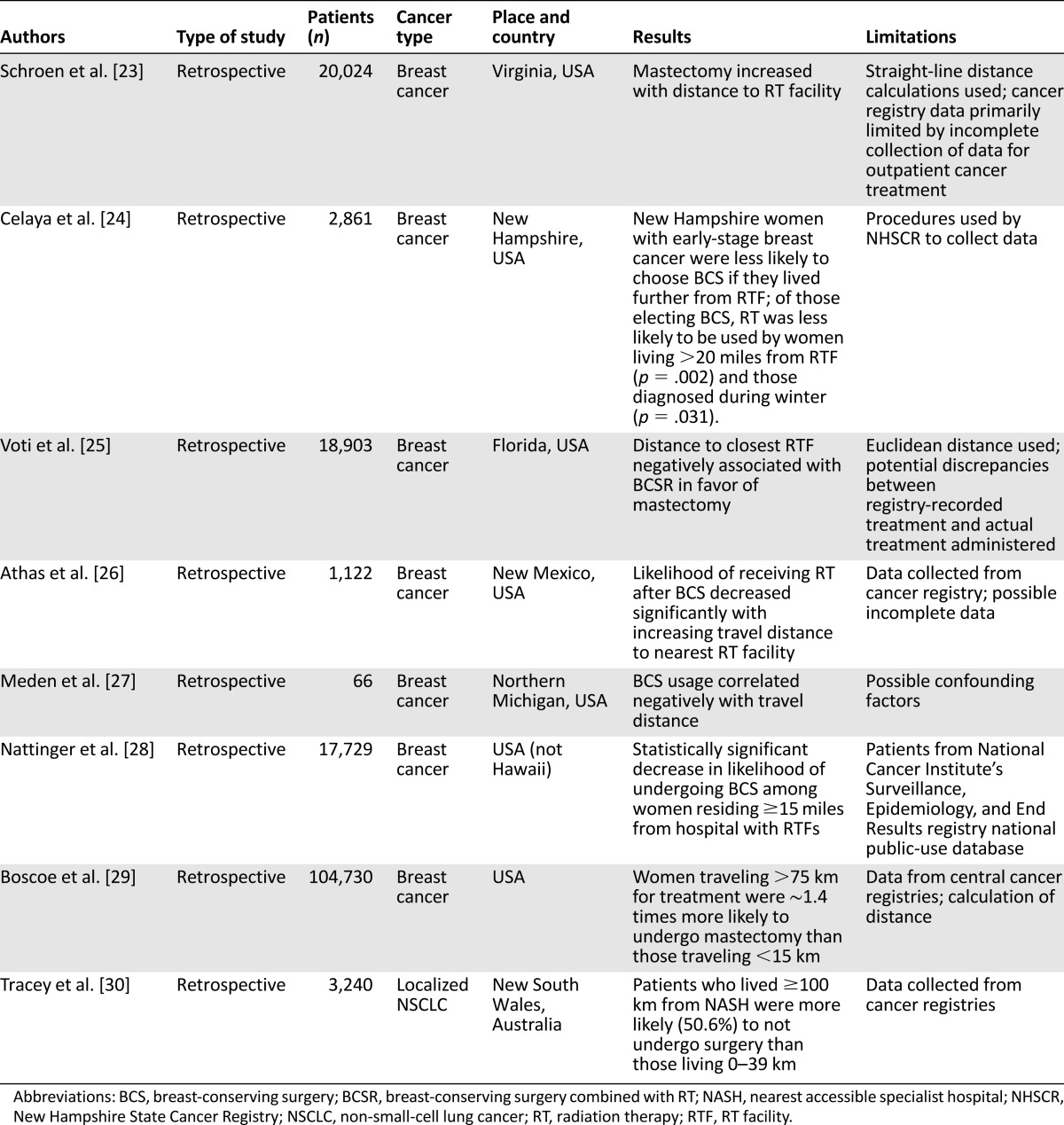
Eight retrospective studies examined the travel burden and appropriate treatment (Table 2). All but one of these studies included female patients with breast cancer and were performed in the United States. The association between the travel distance to radiation therapy services and breast-conserving surgery (BCS), when indicated, compared with mastectomy for early breast cancer was analyzed. All these studies were performed in the United States and included 165,435 patients. All seven studies [23–29] demonstrated a statistically significant decrease in the likelihood of undergoing BCS among women with early-stage breast cancer living 15–20 miles or more from a hospital with radiation therapy services and a significant decrease in the likelihood of receiving radiotherapy after BCS. Thus, the distance was an important factor in women’s decision-making in favor of mastectomy in situations in which breast-conserving surgery and radiotherapy could also have been a reasonable alternative.
Table 2.
Travel burden and appropriate treatment
The Australian study [30] included 3,240 patients with localized non-small-cell lung cancer (NSCLC). Patients who lived 100 km or more from the nearest accessible specialist hospital were more likely to have not undergone surgery (50.6%) than those living 0–39 km away. The main limitations of these studies (Table 2) included the use of a cancer registry and the consequent risk of incomplete data and the inclusion of retrospective studies only.
Travel Burden and Patient Outcome
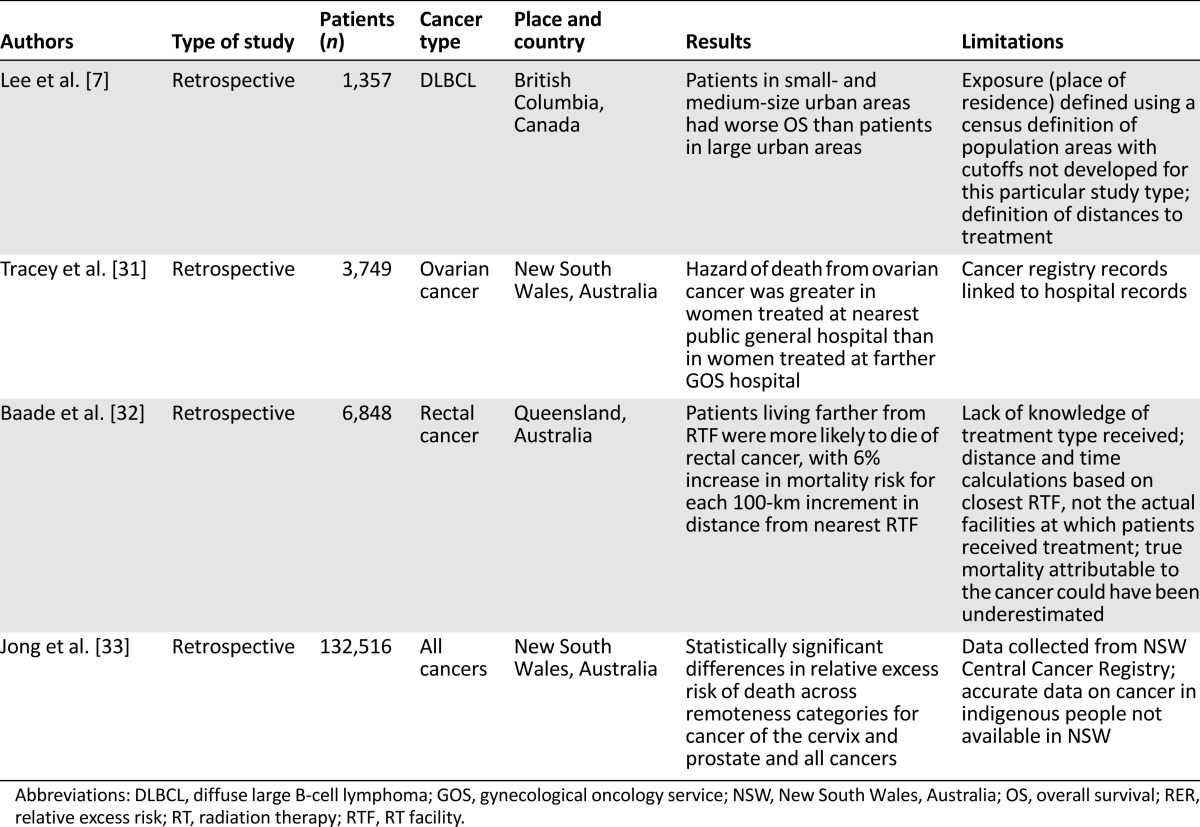
The relationship between travel burden and outcome was examined in four retrospective studies, including 144,470 patients (Table 3). Patients with diffuse large B-cell lymphoma (DLBCL) (British Columbia study) or with ovarian, rectal, or other cancers (Australian studies) were analyzed [7, 31–33]. These studies demonstrated that patients with lymphomas living in small and medium urban areas had worse overall survival (OS) than that of patients living in large urban areas [7].
Table 3.
Travel burden and patient outcome
The hazard of death from ovarian cancer was greater in women treated at a public general hospital than in women treated at a gynecological oncology service (GOS) [31]. The women were 19 times more likely to be treated only at a general hospital when they lived 180 km or more from a public GOS hospital than women who lived within 5 km of one. Patients living farther from a radiotherapy service were more likely to die of rectal cancer, with 6% increase in mortality risk found for each 100-km increase in distance from the nearest radiotherapy facility [32]. In addition, statistically significant differences were found in the relative excess risk of death across remoteness categories for cancer of the cervix and prostate and for all cancers [33]. The main limitations of these studies (Table 3) included the exact definition of the place of residence; the lack of knowledge of the type of treatment received by patients; that the availability of accurate data on cancer in indigenous people was lacking; and the inclusion of retrospective studies only.
Travel Burden and QoL
Only one prospective study was found (performed in Ireland and including 496 colorectal survivors) that had analyzed the association between travel burden and quality of life [6]. Patients living farther from treatment were associated with lower physical functioning and role functioning for women, who had also experienced more trouble with daily activities than did men. Remoteness also had a significant negative relationship with global health status for men. The limitations of that study were the low response rate to the survey (39%) and the younger age of responders compared with nonresponders.
Treatment Compliance
In addition, the travel burden influences treatment compliance, as reported in two studies. A retrospective Indian study evaluated 144 patients affected by locally advanced cancer of the cervix in a rural medical college hospital. Of the 144 patients, 88 could not complete the treatment, and 63.89% were not able to travel more than 100 km from home to hospital for their treatment [34]. A prospective study performed in Texas analyzed 593 patients affected by breast, colon, cervical, or prostate cancer or lymphoma to determine the correlation between the distance and mode of transportation to radiotherapy and chemotherapy and the perceptions of transportation as a barrier to care [2]. They found that some patients might forgo necessary treatment because of problems with transportation, which was perceived as an issue more for minority patients (blacks and Hispanics) than for white patients [2].
Discussion
In the 21st century, oncologists are ready to deliver personalized medicine using the molecular profile of patients’ cancer genome to optimize disease management. Also, as recently reported in the position paper by the European Society for Medical Oncology [35], at the center is the patient, with personalized medicine offering the promise of delivering safe and efficacious target cancer treatment.
However, oncology care must also aim at improving the quality of life of cancer patients and giving patients and their families the possibility to realize their full potential, whether the cancer is curable or not. In this context, oncologists cannot ignore the problems related to the travel burden of cancer patients.
In the present review, we found that the distance from the hospital, or the travel burden, had a negative impact on patients affected by cancer, in terms of the stage at diagnosis, appropriate treatment received, prognosis, and QoL. In almost all the studies analyzed, patients who lived far from hospitals and had to travel more than 50 miles had a more advanced stage at diagnosis, lower adherence to encoded treatments, a worse prognosis, and a worse QoL. These four aspects are all very important for patients and for health care policies and costs.
In almost all the studies analyzed, patients who lived far from hospitals and had to travel more than 50 miles had a more advanced stage at diagnosis, lower adherence to encoded treatments, a worse prognosis, and a worse QoL.
We realize that a publication bias could be present, because the studies reported in our review were almost all positive. However, we performed a narrative review of the published data, and we did not find other studies with negative findings, except those by Celaya et al. [16] and Schroen and Lohr [19].
The cancer stage at presentation significantly influences treatment planning, as well as the short- and long-term prognosis. A diagnosis at an earlier stage can allow for less-invasive, more efficacious, and less costly management [13]. The travel time also can be considered a direct cost of cancer treatment that is usually borne solely by the patients and their families. As such, the time costs associated with travel are an important component of the full economic burden of cancer. Travel can be of particular importance for socioeconomically disadvantaged persons, because the time costs associated with care can strain limited resources. Also, lower provider accessibility or transportation barriers can result in longer travel times for low-income individuals [1]. It is also reasonable to surmise that differences in the stage at diagnosis, for example, in the Breslow thickness for melanoma patients, could translate into differences in overall survival, even if these studies had not provided survival data [9].
The distance from the hospital (i.e., travel burden) also influences the choice of appropriate treatment by cancer patients. Some studies found that patients living farther from a radiation treatment facility more often underwent mastectomy instead of BCS [23–25, 27–29] or did not undergo radiotherapy after BCS [22, 24]. The results reported by Schroen et al. [23] suggest that a marked change in geographic access to radiotherapy by opening new facilities might correlate with an increase in the proportion of patients undergoing breast conservation therapy. Tracey et al. [30] found that patients with localized NSCLC were most likely to not undergo potentially curative surgery if they lived far from a specialist hospital and only attended a general hospital for their care.
In addition, the distance of the residence from GOS hospitals in Australia was an important determinant of access to GOS hospitals, and treatment in a public or private GOS hospital and undergoing surgery were the strongest predictors of survival from epithelial ovarian cancer [31]. In the population-based cohort of patients with DLBCL reported by Lee et al. [7], the place of residence at diagnosis also significantly affected the health outcomes. Rural patients experienced OS and disease-specific survival similar to that of those in urban areas, in particular, large urban areas. However, patients living in small- and medium-size urban areas experienced worse outcomes.
Regarding the association between travel burden and QoL, the results of the study by Thomas et al. [6] suggest that remote colorectal cancer survivors, women in particular, have more trouble with daily activities, which was evidenced by their lower physical functioning scores. The lower role functioning scores for remote female colorectal cancer survivors suggest that they might feel unable to work or that they are limited in their ability to work.
The worse prognosis for patients living farther from treating hospitals could have been because the compliance with treatment or the follow-up program was suboptimal [34]. In addition, transportation to the health care provider can be perceived as a barrier to care and can limit patients’ compliance with treatment [2].
The worse prognosis for patients living farther from treating hospitals could have been because the compliance with treatment or the follow-up program was suboptimal.
It is well known that participation in randomized controlled trials is associated with improved cancer survival; however, many trials require frequent examinations. Thus, the travel burden can exclude patients from trials owing to the distance of their residence from the trial center. However, published data are lacking in this area, and further studies are required to explore this issue [36].
The review of the published data has shown that the more remote the area in which a person lives, the greater is his or her chance of dying from cancer. The worst survival figures were for areas where the proportion of indigenous persons is highest. The survival rates were particularly poor for those with cervical, prostate, head and neck, or colorectal cancer or melanoma [36].
Investigators have sought solutions to this problem. In Australia, Sabesan and Piliouras tried to find a solution to this problem with telemedicine [36]. Using telemedicine facilities, rural patients can have immediate access to specialist services without having to travel long distances. Chemotherapy can also be supervised with the use of this technology. The Townsville Hospital medical oncologists provide consultation to patients in the town of Mount Isa (a mining town approximately 800 km from Townsville) using videoconferencing. This allows cancer patients in Mount Isa to avoid the 10-hour drive or 2-hour flight to consult a specialist medical oncologist. The telemedicine clinics are conducted weekly; thus, urgent consultations can be arranged and treatment started within 1 week.
This method of service delivery also saves specialists many hours of travel time to examine 6–7 patients. Mount Isa doctors and nurses also receive one-on-one support and education from the medical oncologists by telemedicine, and, most importantly, patients can be treated in their home town. A preliminary analysis by the Department of Medical Oncology at Townsville Hospital showed that most patients are satisfied with this service and that it allows the safe delivery of chemotherapy. However, one of the criticisms of telemedicine is the potential for missed information, leading to errors, because their study lacked information on the safety aspects of remote supervision of chemotherapy delivery. Audits are underway to address this concern [37].
In our region, we have developed a provincial oncological network. Thus, rural oncologic patients have ready access to oncological services, travelling a maximum of 30 km, at a unit of internal medicine and can be treated under the supervision of a medical oncologist who travels to the peripheral units. This network hosts a centralized unit for the preparation of anticancer drugs with a computerized system. A preliminary report has shown that oncologic patients can be treated with a minimal travel burden, achieving a cost-containment policy for the delivery of cancer care [38].
In conclusion, the results of the present review suggest that the travel burden is an important factor affecting access to appropriate and current cancer diagnosis and treatment and that it can worsen the achievement of universal high-quality care for cancer patients. The implications of an increasing travel distance should not be ignored. It has been reported that even a small increase in distance can result in a substantial barrier for this subset of the population [39].
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgment
We thank Dr. Sandro Barni for the suggestions, Charles Mackay for help with the English, and AMOP for technical and administrative support.
Author Contributions
Conception/Design: Fabio Fornari, Luigi Cavanna
Provision of study material or patients: Cinzia Del Giovane
Collection and/or assembly of data: Massimo Ambroggi, Claudia Biasini, Cinzia Del Giovane
Data analysis and interpretation: Massimo Ambroggi, Luigi Cavanna
Manuscript writing: Massimo Ambroggi, Claudia Biasini, Cinzia Del Giovane, Luigi Cavanna
Final approval of manuscript: Luigi Cavanna, Cinzia Del Giovane
Disclosures
The authors indicated no financial relationships.
References
- 1.Scoggins JF, Fedorenko CR, Donahue SMA, et al. Is distance to provider a barrier to care for Medicaid patients with breast, colorectal, or lung cancer? J Rural Health. 2012;28:54–62. doi: 10.1111/j.1748-0361.2011.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guidry JJ, Aday LA, Zhang D, et al. Transportation as a barrier to cancer treatment. Cancer Pract. 1997;5:361–366. [PubMed] [Google Scholar]
- 3.Walko CM, McLeod HL. Personalizing medicine in geriatric oncology. J Clin Oncol. 2014;32:2581–2586. doi: 10.1200/JCO.2014.55.9047. [DOI] [PubMed] [Google Scholar]
- 4.McCleary NJ, Dotan E, Browner I. Refining the chemotherapy approach for older patients with colon cancer. J Clin Oncol. 2014;32:2570–2580. doi: 10.1200/JCO.2014.55.1960. [DOI] [PubMed] [Google Scholar]
- 5.Bosanac EM, Parkinson RC, Hall DS. Geographic access to hospital care: A 30-minute travel time standard. Med Care. 1976;14:616–624. doi: 10.1097/00005650-197607000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Thomas AA, Gallagher P, O’Céilleachair A, et al. Distance from treating hospital and colorectal cancer survivors’ quality of life: A gendered analysis. Support Care Cancer. 2015;23:741–751. doi: 10.1007/s00520-014-2407-9. [DOI] [PubMed] [Google Scholar]
- 7.Lee B, Goktepe O, Hay K, et al. Effect of place of residence and treatment on survival outcomes in patients with diffuse large B-cell lymphoma in British Columbia. The Oncologist. 2014;19:283–290. doi: 10.1634/theoncologist.2013-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birkmeyer JD, Siewers AE, Marth NJ, et al. Regionalization of high-risk surgery and implications for patient travel times. JAMA. 2003;290:2703–2708. doi: 10.1001/jama.290.20.2703. [DOI] [PubMed] [Google Scholar]
- 9.Stitzenberg KB, Thomas NE, Dalton K, et al. Distance to diagnosing provider as a measure of access for patients with melanoma. Arch Dermatol. 2007;143:991–998. doi: 10.1001/archderm.143.8.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breugom AJ, Boelens PG, van den Broek CBM, et al. Quality assurance in the treatment of colorectal cancer: The EURECCA initiative. Ann Oncol. 2014;25:1485–1492. doi: 10.1093/annonc/mdu039. [DOI] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Murad MH, Montori VM, Ioannidis JP, et al. How to read a systematic review and meta-analysis and apply the results to patient care: Users’ guides to the medical literature. JAMA. 2014;312:171–179. doi: 10.1001/jama.2014.5559. [DOI] [PubMed] [Google Scholar]
- 13.Massarweh NN, Chiang Y-J, Xing Y, et al. Association between travel distance and metastatic disease at diagnosis among patients with colon cancer. J Clin Oncol. 2014;32:942–948. doi: 10.1200/JCO.2013.52.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parsons MA, Askland KD. Cancer of the colorectum in Maine, 1995-1998: Determinants of stage at diagnosis in a rural state. J Rural Health. 2007;23:25–32. doi: 10.1111/j.1748-0361.2006.00064.x. [DOI] [PubMed] [Google Scholar]
- 15.Liff JM, Chow WH, Greenberg RS. Rural-urban differences in stage at diagnosis. Possible relationship to cancer screening. Cancer. 1991;67:1454–1459. doi: 10.1002/1097-0142(19910301)67:5<1454::aid-cncr2820670533>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 16.Celaya MO, Berke EM, Onega TL, et al. Breast cancer stage at diagnosis and geographic access to mammography screening (New Hampshire, 1998-2004) Rural Remote Health. 2010;10:1361–1372. [PMC free article] [PubMed] [Google Scholar]
- 17.Huang B, Dignan M, Han D, et al. Does distance matter? Distance to mammography facilities and stage at diagnosis of breast cancer in Kentucky. J Rural Health. 2009;25:366–371. doi: 10.1111/j.1748-0361.2009.00245.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang F, McLafferty S, Escamilla V, et al. Late-stage breast cancer diagnosis and health care access in Illinois. Prof Geogr. 2008;60:54–69. doi: 10.1080/00330120701724087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schroen AT, Lohr ME. Travel distance to mammography and the early detection of breast cancer. Breast J. 2009;15:216–217. doi: 10.1111/j.1524-4741.2009.00706.x. [DOI] [PubMed] [Google Scholar]
- 20.Campbell NC, Elliott AM, Sharp L, et al. Rural and urban differences in stage at diagnosis of colorectal and lung cancers. Br J Cancer. 2001;84:910–914. doi: 10.1054/bjoc.2000.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dickens C, Joffe M, Jacobson J, et al. Stage at breast cancer diagnosis and distance from diagnostic hospital in a periurban setting: A South African public hospital case series of over 1,000 women. Int J Cancer. 2014;135:2173–2182. doi: 10.1002/ijc.28861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Satasivam P, O’Neill S, Sivarajah G, et al. The dilemma of distance: Patients with kidney cancer from regional Australia present at a more advanced stage. BJU Int. 2014;113(Suppl 2):57–63. doi: 10.1111/bju.12459. [DOI] [PubMed] [Google Scholar]
- 23.Schroen AT, Brenin DR, Kelly MD, et al. Impact of patient distance to radiation therapy on mastectomy use in early-stage breast cancer patients. J Clin Oncol. 2005;23:7074–7080. doi: 10.1200/JCO.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 24.Celaya MO, Rees JR, Gibson JJ, et al. Travel distance and season of diagnosis affect treatment choices for women with early-stage breast cancer in a predominantly rural population (United States) Cancer Causes Control. 2006;17:851–856. doi: 10.1007/s10552-006-0025-7. [DOI] [PubMed] [Google Scholar]
- 25.Voti L, Richardson LC, Reis IM, et al. Treatment of local breast carcinoma in Florida: The role of the distance to radiation therapy facilities. Cancer. 2006;106:201–207. doi: 10.1002/cncr.21557. [DOI] [PubMed] [Google Scholar]
- 26.Athas WF, Adams-Cameron M, Hunt WC, et al. Travel distance to radiation therapy and receipt of radiotherapy following breast-conserving surgery. J Natl Cancer Inst. 2000;92:269–271. doi: 10.1093/jnci/92.3.269. [DOI] [PubMed] [Google Scholar]
- 27.Meden T, St John-Larkin C, Hermes D, et al. MSJAMA. Relationship between travel distance and utilization of breast cancer treatment in rural northern Michigan. JAMA. 2002;287:111. [PubMed] [Google Scholar]
- 28.Nattinger AB, Kneusel RT, Hoffmann RG, et al. Relationship of distance from a radiotherapy facility and initial breast cancer treatment. J Natl Cancer Inst. 2001;93:1344–1346. doi: 10.1093/jnci/93.17.1344. [DOI] [PubMed] [Google Scholar]
- 29.Boscoe FP, Johnson CJ, Henry KA, et al. Geographic proximity to treatment for early stage breast cancer and likelihood of mastectomy. Breast. 2011;20:324–328. doi: 10.1016/j.breast.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 30.Tracey E, McCaughan B, Badgery-Parker T, et al. Patients with localized non-small cell lung cancer miss out on curative surgery with distance from specialist care. ANZ J Surg. 2015;85:658–663. doi: 10.1111/ans.12855. [DOI] [PubMed] [Google Scholar]
- 31.Tracey E, Hacker NF, Young J, et al. Effects of access to and treatment in specialist facilities on survival from epithelial ovarian cancer in Australian women: A data linkage study. Int J Gynecol Cancer. 2014;24:1232–1240. doi: 10.1097/IGC.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 32.Baade PD, Dasgupta P, Aitken JF, et al. Distance to the closest radiotherapy facility and survival after a diagnosis of rectal cancer in Queensland. Med J Aust. 2011;195:350–354. doi: 10.5694/mja10.11204. [DOI] [PubMed] [Google Scholar]
- 33.Jong KE, Smith DP, Yu XQ, et al. Remoteness of residence and survival from cancer in New South Wales. Med J Aust. 2004;180:618–622. doi: 10.5694/j.1326-5377.2004.tb06123.x. [DOI] [PubMed] [Google Scholar]
- 34.Dutta S, Biswas N, Muhkherjee G. Evaluation of socio-demographic factors for non-compliance to treatment in locally advanced cases of cancer cervix in a rural medical college hospital in India. Indian J Palliat Care. 2013;19:158–165. doi: 10.4103/0973-1075.121530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciardiello F, Arnold D, Casali PG, et al. Delivering precision medicine in oncology today and in future—The promise and challenges of personalised cancer medicine: A position paper by the European Society for Medical Oncology (ESMO) Ann Oncol. 2014;25:1673–1678. doi: 10.1093/annonc/mdu217. [DOI] [PubMed] [Google Scholar]
- 36.Sabesan S, Piliouras P. Disparity in cancer survival between urban and rural patients—How can clinicians help reduce it? Rural Remote Health. 2009;9:1146–1150. [PubMed] [Google Scholar]
- 37.Sabesan S, Larkins S, Evans R, et al. Telemedicine for rural cancer care in North Queensland: Bringing cancer care home. Aust J Rural Health. 2012;20:259–264. doi: 10.1111/j.1440-1584.2012.01299.x. [DOI] [PubMed] [Google Scholar]
- 38.Mordenti P, Vecchia S, Damonti E, et al. An anticancer drug unit for the whole provincial oncologic network of Piacenza: Improving safety and savings. Med Oncol. 2015;32:457. doi: 10.1007/s12032-014-0457-y. [DOI] [PubMed] [Google Scholar]
- 39.Stitzenberg KB, Sigurdson ER, Egleston BL, et al. Centralization of cancer surgery: Implications for patient access to optimal care. J Clin Oncol. 2009;27:4671–4678. doi: 10.1200/JCO.2008.20.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]