This review summarizes the relevant clinical trial experience with epidermal growth factor receptor inhibitors for treatment of squamous cell cancers of the head and neck, with attention to efficacy, toxicity, and methods of selecting patients most likely to benefit from therapy.
Keywords: Carcinoma, Squamous cell of head and neck, EGFR inhibitors
Abstract
The epidermal growth factor receptor (EGFR) is overexpressed in more than 80% of squamous cell cancers of the head and neck (SCCHN). An evolving understanding of the role of EGFR in tumorigenesis has made the receptor an important therapeutic target in SCCHN. Several EGFR inhibitors (EGFRIs) are active in SCCHN, and their use is associated with improvement in progression-free survival and overall survival in various treatment settings. Nevertheless, EGFR inhibition is associated with significant mucocutaneous toxicity that must be balanced against its anticipated efficacy. This review summarizes the relevant clinical trial experience with EGFRIs, with attention to efficacy, toxicity, and methods of selecting patients most likely to benefit from therapy.
Implications for Practice:
Cetuximab and other inhibitors of the epidermal growth factor receptor (EGFR) have entered the medical oncologist’s arsenal against squamous cell carcinoma of the head and neck (SCCHN). They are modestly active as single agents and in combination with chemotherapy and radiotherapy. Despite their efficacy across multiple treatment settings, cetuximab and other EGFR inhibitors (EGFRIs) have not supplanted platinum-based therapies, which remain a standard of care for SCCHN. The modest benefits of EGFRI therapy must take into consideration patient, disease, and treatment characteristics and must be balanced against potential treatment toxicity.
Introduction
Each year more than 500,000 patients globally are diagnosed with squamous cell carcinoma (SCC) of the head and neck (SCCHN), and more than 300,000 deaths are caused by the disease [1]. The majority of patients present with stage III/IV locoregionally advanced disease and are treated with combined modality therapy often incorporating surgery, radiotherapy (RT), and chemotherapy for patients with the most advanced locoregional disease [2]. Despite curative intent, approximately 70% of patients with locoregionally advanced disease relapse, underscoring the importance of primary locoregional control and of systemic therapies in recurrent or metastatic (R/M) disease [3]. Platinum compounds (cisplatin and carboplatin), 5-fluorouracil (5-FU), taxanes (paclitaxel and docetaxel), and methotrexate are among the most active chemotherapeutics in SCCHN [3]. Despite the activity associated with combination regimens, the absolute benefit of cytotoxic therapy in the curative and R/M settings is marginal [2, 3]. Moreover, the treatment of SCCHN is often complicated by patients’ poor performance status and by medical comorbidities [4]. Consequently, efforts are ongoing to identify compounds with more favorable side effect profiles that maintain efficacy.
The epidermal growth factor receptor (EGFR) is a 170-kDa receptor tyrosine kinase expressed in epidermal tissues [5]. Ligand binding to the extracellular domain of EGFR promotes dimerization and autophosphorylation, activating the downstream mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK), phosphatidylinositol-3-kinase (PI3K), the serine/threonine kinase AKT, and Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathways, which are associated with DNA synthesis, cell proliferation, and survival [6].
If mutated or overexpressed, EGFR can promote tumorigenesis across multiple tumor types. Whereas activating mutations in the gene encoding EGFR are seen in a subset of patients with non-small cell lung cancer (NSCLC) [7], such mutations are only very rarely seen in SCCHN [8]. Instead, some 80%–100% of SCCHNs are associated with EGFR protein overexpression and pathway activation, rendering EGFR a potential target in this disease [8]. EGFR-directed therapy is principally achieved with monoclonal antibodies (mAbs) or small molecule tyrosine kinase inhibitors (TKIs) [9]. Existing anti-EGFR mAbs target domain III of the EGFR and competitively inhibit the extracellular ligand-binding domain of the molecule, disrupting the EGFR pathway and promoting antibody-dependent cellular cytotoxicity (ADCC) [10]. The small molecule TKIs act on the intracellular portion of EGFR, impairing downstream signaling through inhibition of EGFR’s intrinsic kinase domain without effecting ADCC [10].
The first and only molecularly targeted therapy approved for the treatment of SCCHN is cetuximab, a mAb directed against EGFR [11]. Since cetuximab’s initial U.S. Food and Drug Administration approval in 2006, several other EGFR inhibitors (EGFRIs) in early phases of development have shown activity in SCCHN; these include panitumumab, zalutumumab, matuzumab, nimotuzumab, erlotinib, gefitinib, lapatinib, afatinib, and dacomitinib [10, 12]. The incorporation of these and other EGFRIs into the head and neck oncologist’s armamentarium may be broadly considered in terms of three treatment settings: (a) locoregionally advanced disease for which surgery is the primary modality of therapy, with adjuvant chemoradiotherapy (CRT) offered to those with high-risk resected disease; (b) locally and regionally advanced disease in patients unfit or inappropriate for surgery whose therapy depends on definitive CRT; and (c) patients with R/M disease not amenable to salvage strategies, in whom systemic chemotherapy is the mainstay of therapy. CRT with high-dose cisplatin is the standard of care for high-risk resected disease and for definitive treatment of unresectable disease [13].
We reviewed the relevant published experience with EGFR inhibition in SCCHN, with attention to efficacy, toxicity, and methods of selecting patients most likely to benefit from therapy.
Materials and Methods
The PubMed, Embase, Cochrane Collaboration, and ClinicalTrials.gov databases and conference proceedings of the American Society of Clinical Oncology and the Multidisciplinary Head and Neck Cancer Symposium were queried. Search terms were carcinoma, squamous cell, head and neck, epidermal growth factor receptor, and antagonist or inhibitor. Results were limited to prospective clinical trials published as of January 2014. An additional search was performed in October 2014 with the same search terms. Publications were limited to those in English and involving human subjects. Both papers and abstracts were considered.
Evidence of Benefit
Adjuvant Therapy in Resected Disease
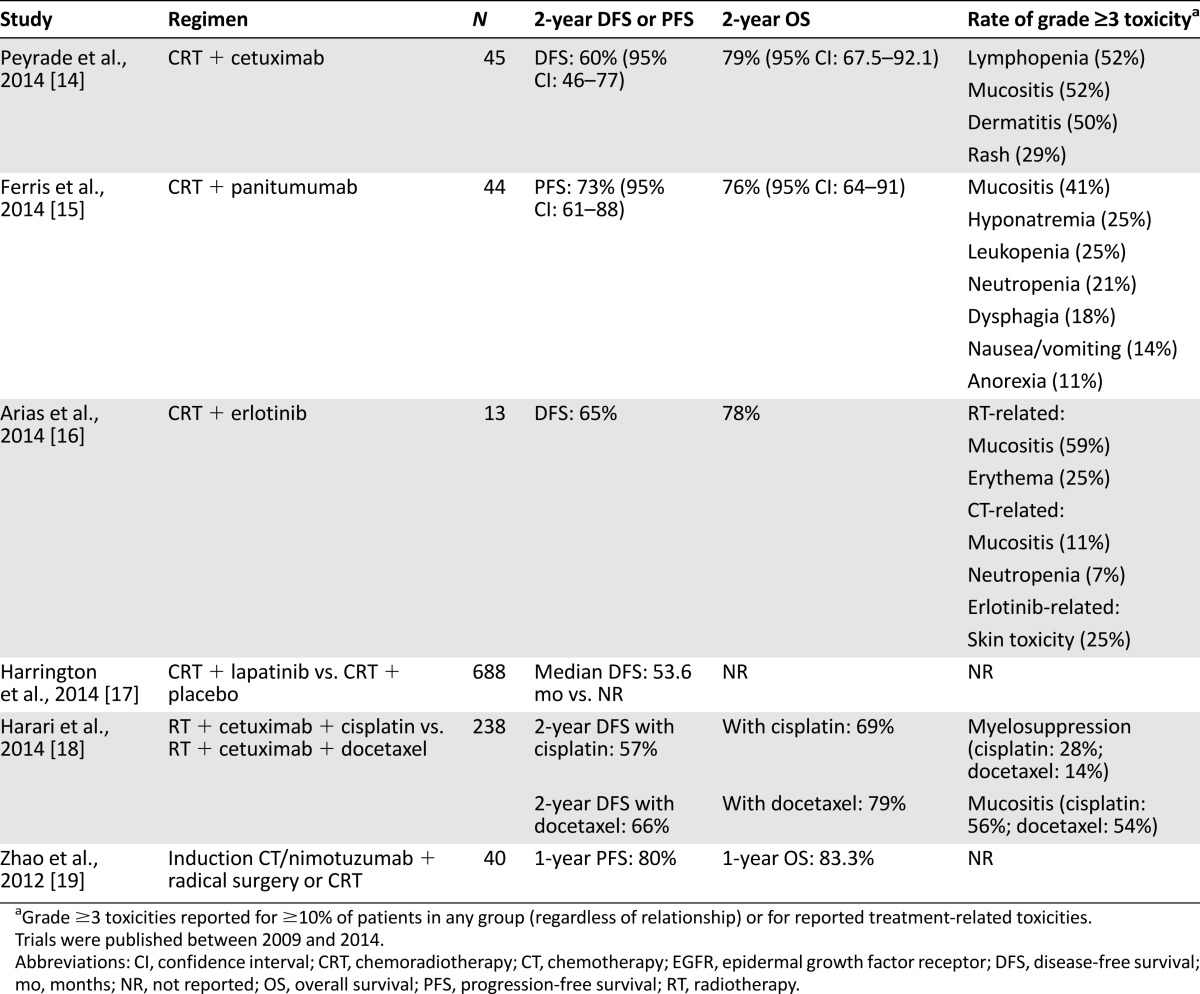
Table 1 summarizes relevant trials of EGFRIs in the adjuvant treatment of high-risk resected disease. Although such trials are under way, there are presently no published head-to-head comparisons of RT plus an EGFRI compared with conventional CRT in the adjuvant setting. The published experience of anti-EGFR therapy in the adjuvant setting is limited to single-arm studies of an EGFRI plus conventional CRT and to comparative studies of EGFRIs added to various CRT backbones.
Table 1.
Selected phase II and III clinical trials of adjuvant CRT with EGFR inhibitors
Single-arm studies have demonstrated the feasibility and tolerability of RT/cisplatin in combination with cetuximab [14], panitumumab [15], matuzumab [20], and erlotinib [16, 21] in the adjuvant setting. Concurrent lapatinib plus RT/cisplatin has also been studied, but this combination failed to confer a disease-free survival benefit over RT/cisplatin in a placebo-controlled phase III study [17]. The randomized phase II trial RTOG 0234 demonstrated numerically superior 2-year overall survival (OS) with RT/docetaxel/cetuximab compared with RT/cisplatin/cetuximab (79% vs. 69%), driven principally by a reduction in distant recurrence (13% vs. 25%) [18]. In this latter trial, it is unclear whether and to what degree cetuximab contributed to the observed OS difference because the mAb was included in both treatment arms.
Future studies will focus on the optimal combination of EGFRIs and a CRT backbone. The RT/docetaxel/cetuximab combination used in RTOG 0234, for example, is being evaluated in the phase II/III RTOG 1216 trial, in which patients with SCCHN negative for human papillomavirus (HPV) undergo surgery and are randomized to adjuvant RT/cisplatin, RT/docetaxel, or RT/docetaxel/cetuximab [22]. In addition, a phase III study is under way investigating 18 months of adjuvant afatinib compared with placebo, following CRT [23]. A phase III comparison of CRT in combination with nimotuzumab or placebo in the adjuvant setting is also under way (ClinicalTrials.gov identifier NCT00957086).
Definitive Therapy for Unresectable Disease
For patients with locoregionally advanced, unresectable SCCHN, the mainstay of therapy is concurrent platinum-based CRT with curative intent [24]. EGFRIs have been combined with RT as single agents or with cytotoxic chemotherapy in this setting.
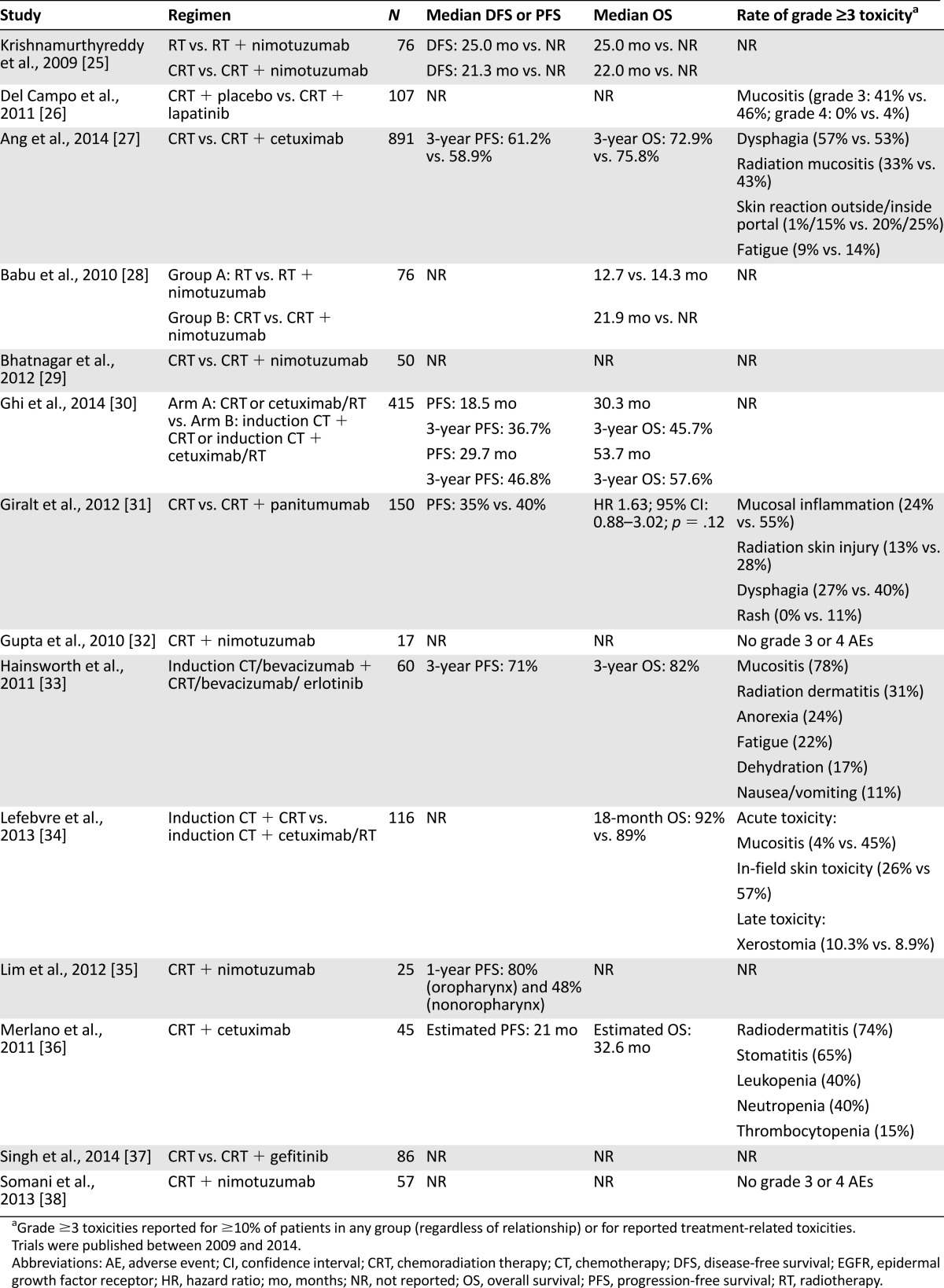
Table 2 summarizes the relevant published experience with RT plus an EGFRI for the definitive treatment of locoregionally advanced, unresectable SCCHN. The most influential study was the phase III trial by Bonner et al. that demonstrated an OS benefit with concurrent RT/cetuximab compared with RT alone (49 vs. 29 months), with a durable OS benefit at 5 years (45.6% vs. 36.4%) [39, 40].
Table 2.
Selected phase II and III clinical trials of definitive CRT with EGFR inhibitors
Combination RT/EGFRI has been compared with RT/platinum in several contexts within the definitive setting. The phase II/III study by Ghi et al. randomized patients to induction docetaxel, cisplatin, and 5-FU or no induction chemotherapy, with a second randomization to RT/cetuximab or RT/cisplatin [30]. At a median follow-up of 41.3 months, induction chemotherapy was associated with improved complete response (CR) rates (43.5% vs. 28%), median progression-free survival (PFS; 29.7 vs. 18.5 months), and median OS (53.7 vs. 30.3 months). These benefits were independent of the agent administered with concomitant RT. Similarly, the phase II TREMPLIN study treated patients with induction chemotherapy followed by randomization to either RT/cetuximab or RT/cisplatin [34]. Although there were numerically fewer local recurrences in the RT/cisplatin arm, there were no significant differences in 3-month larynx preservation rates (93% vs. 95%), larynx function preservation rates (82% vs. 87%), or 18-month OS (89% vs. 92%). At first glance, these data suggest that induction therapy may abrogate the need for more intensive CRT regimens and that RT/cetuximab may be sufficient among patients treated first with induction chemotherapy; however, caution is advised in extrapolating these observations into routine treatment decisions because these data are preliminary. Moreover, because these studies did not include an RT-alone comparator, it is not known whether RT/cetuximab confers an advantage over RT alone in this setting.
Trials of other EGFRIs in the definitive setting have been limited largely to early phase studies. Nimotuzumab, an anti-EGFR mAb available in Asia, Africa, and South America, has been shown in a phase II study to be active in combination with cisplatin/5-FU induction chemotherapy, yielding an objective response rate (ORR) of 87% with a pathologic CR rate of 15% [19]. In addition, nimotuzumab demonstrated its safety and tolerability in combination with RT [41]. A retrospective study of 835 patients with advanced carcinomas of diverse tissue types suggested that nimotuzumab was well tolerated in combination with CRT, without potentiating the toxicities of concurrent therapy [42]. Randomized phase II data demonstrated that nimotuzumab plus CRT improved median OS compared with RT alone [25, 28]. Similarly, sequential lapatinib and RT yielded improvements in ORR compared with placebo/RT (70% vs. 53%) in the definitive setting [26]. In contrast, concurrent RT/gefitinib was poorly tolerated and resulted in unexpectedly low median PFS and OS (6.7 and 8.5 months, respectively) [43].
Direct comparisons of RT/EGFRI to RT/platinum are scarce. The randomized phase II ARTFORCE trial will attempt to address this by randomizing unresectable SCCHN patients to RT plus either cetuximab or cisplatin [44]. A caveat with this forthcoming trial is that its comparator is low-dose cisplatin delivered weekly rather than conventional high-dose cisplatin administered every 21 days.
In lieu of published data comparing RT/EGFRI with RT/platinum, insight has been gained from studies of platinum-based CRT plus EGFRI. Early phase studies of EGFRIs given concurrently with RT/platinum have demonstrated feasibility and an attendant increase in both expected and unexpected toxicities [45]. A single-arm phase II study of RT/cisplatin/cetuximab, for example, demonstrated encouraging 3-year OS of 76% and 3-year PFS of 56%, but rates of adverse events (AEs) including myocardial infarction were unacceptably high [46]. When studied in a prospective comparative fashion, as occurred in the RTOG 0522 study, the addition of cetuximab to RT/cisplatin increased toxicity without improving PFS or OS [27]. Similarly, no significant improvements were seen when RT/platinum was combined with panitumumab [31], erlotinib [47], lapatinib [17], or gefitinib [37]. Analyses of outcomes among patients with HPV- and non-HPV-associated disease treated with RT/platinum/EGFRI have been mixed [17, 27]. Of interest, the addition of nimotuzumab to conventional RT/cisplatin appears to improve the CR rate (96% vs. 72%) [29] and 30-month OS (70% vs. 22%) [25] but does not appear to potentiate the toxicity of CRT compared with RT/cisplatin alone [25, 29, 32, 35, 38].
EGFR inhibition in combination with RT and two or more cytotoxic agents has also been studied with carboplatin/paclitaxel, carboplatin/5-FU, and cisplatin/5FU all as chemotherapy backbones. Regimens that include cetuximab [33, 36, 48–50] and erlotinib [33] have shown ORRs in excess of 70%, with a manageable increase in toxicities. The phase II REACH study is currently investigating RT/carboplatin/5-FU plus cetuximab [49], with preliminary results indicating a promising ORR of 92.9% and 2-year PFS of 63.3% [50].
A randomized phase II study demonstrated that concurrent RT/cetuximab followed by 12 weeks of cetuximab improved event-free survival compared with RT/cetuximab alone (23.7 vs. 18.4 months), although an OS benefit has not been demonstrated [51]. Maintenance EGFRI has not been compared with a nonmaintenance strategy in which EGFR-directed therapy is reinstituted at the time of relapse, and thus an OS benefit likely will not be observed. The benefit of maintenance cetuximab, however, appears to lose its significance after 2 years [51], suggesting that EGFR inhibition controls but cannot eradicate microscopic residual disease.
Chemotherapy in Recurrent or Metastatic Disease
The majority of studies of single-agent EGFRIs in R/M SCCHN are phase I and nonrandomized phase II studies. Selected randomized phase II and III trials of anti-EGFR therapy are provided in Table 3.
Table 3.
Selected phase II and III clinical trials for EGFR inhibitors in recurrent/metastatic disease
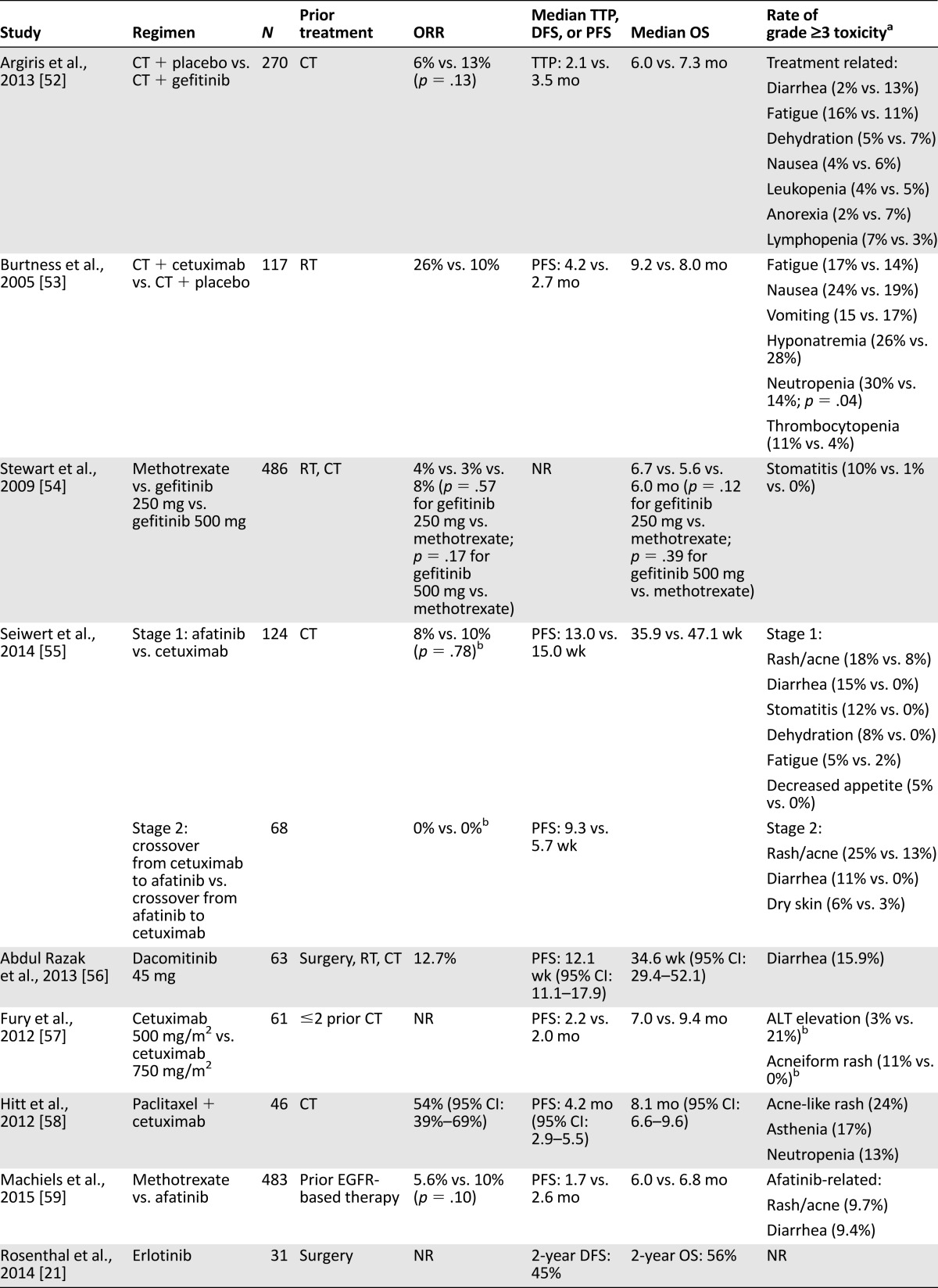
Single-agent studies included a phase II study of weekly cetuximab in patients with R/M disease that was associated with an ORR of 13%, a disease control rate (DCR) of 46%, and median OS of 25.4 weeks among patients with platinum-refractory disease [62]. Similarly, the efficacy of dacomitinib 45 mg/day is based on a phase II study that demonstrated a DCR of 69.8%, with median PFS and OS of 12.1 and 34.6 weeks, respectively [56]. Single-agent erlotinib and gefitinib were associated with numerically lower ORR (1.6%–10.6%) and DCR (34.0%–53%) in phase II studies [63–66].
Few trials have compared one EGFRI to another or to single-agent cytotoxic therapy. One of the most robust studies compared gefitinib with conventional methotrexate delivered intravenously on a weekly schedule [54]. Patients received gefitinib 250 mg/day, gefitinib 500 mg/day, or methotrexate 40 mg/m2 i.v. weekly. There was no statistically significant difference in ORR (2.7%, 7.6%, and 3.9%, respectively) or median OS (5.6, 6.0, and 6.7 months, respectively). A randomized phase II comparison of afatinib 50 mg/day versus standard-dose cetuximab demonstrated a similar DCR (50% vs. 57%), with an increase in grade ≥3 AEs with afatinib, including diarrhea (15% vs. 0%), rash (18% vs. 8%), and mucositis (12% vs. 0%) [55]. This study’s design allowed crossover, and tumor shrinkage was observed in 40% and 31% of patients who crossed over to afatinib or cetuximab, respectively. Although this observation raises the possibility that EGFR mAbs and TKIs do not promote cross-resistance, caution is warranted in overinterpreting these data because only 2 objective responses (shrinkage by ≥30%) were seen in 56% of patients who crossed over. Regarding safety, tolerability of afatinib among SCCHN patients was lower than that with cetuximab, reflected in the high rates of treatment discontinuation in this trial (23% vs. 5%). Due in part to tolerability concerns at the 50-mg/day dose, a phase III study comparing afatinib with methotrexate used a reduced afatinib dose [59]. In this study, LUX-Head & Neck 1, 483 patients with platinum-resistant R/M SCCHN were randomized to afatinib 40 mg/day or methotrexate 40 mg/m2 i.v. weekly and followed for the primary and secondary endpoints of PFS and OS, respectively. At a median follow-up of 6.7 months, median PFS modestly favored afatinib (2.6 vs. 1.7 months; p = .030) without a statistically significant impact on median OS (6.8 vs. 6.0 months; p = .70). A trend toward improved ORR was seen with afatinib (10% vs. 5.6%; p = .10), although the observed ORR for methotrexate was quite low compared with historic patient cohorts treated with single-agent methotrexate (average approximately 30%) [67]. This may relate to patient selection factors because the study included only patients with platinum-refractory disease. Compared with the 50-mg/day dose, afatinib 40 mg/day was better tolerated; the most common grade 3/4 AEs included rash (9.7%) and diarrhea (9.4%) [59].
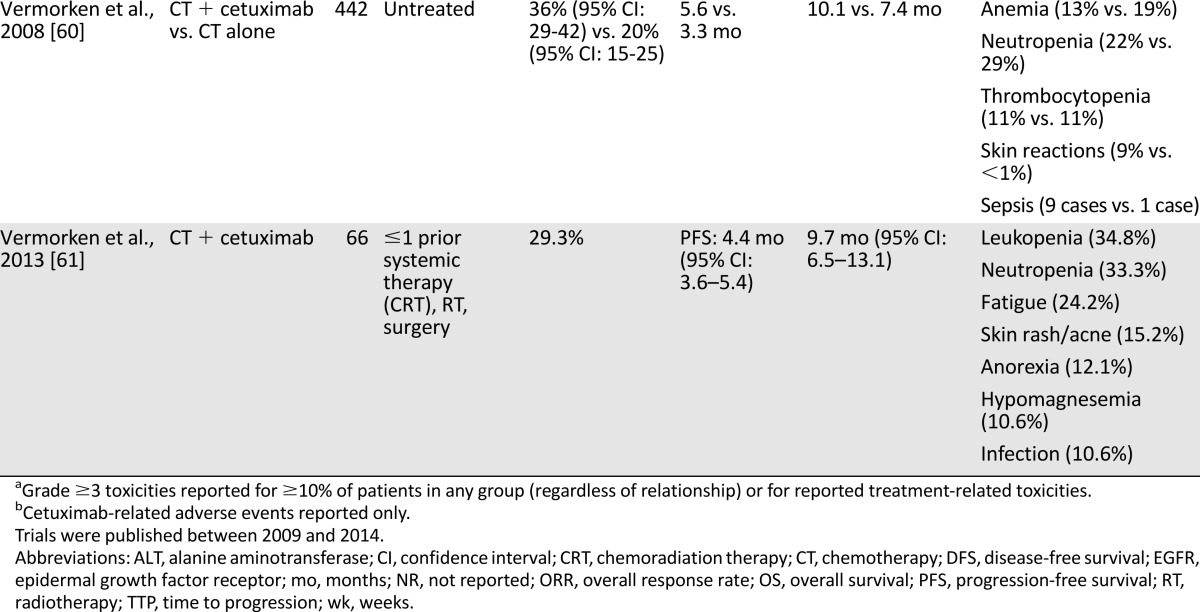
The combination of EGFRIs with platinum-based therapy has been studied extensively. In a placebo-controlled phase III study, the addition of cetuximab to conventional cisplatin increased ORR compared with cisplatin alone (26% vs. 10%), but this did not translate into an OS benefit (9.2 vs. 8.0 months) [53]. Although this study was underpowered to demonstrate a survival benefit, its hazard ratio for OS was comparable to more intensive regimens, such as platinum/5-FU/cetuximab [60, 67]. A single-arm phase II study demonstrated that among patients with R/M disease refractory to either cisplatin/paclitaxel or cisplatin/5-FU, the combination of cetuximab/cisplatin was associated with an ORR of 20% [68].
More intensive therapy with cetuximab in combination with platinum/5-FU, as studied in the EXTREME trial, has been associated with improvements in median PFS (5.6 vs. 3.3 months) as well as OS (10.1 vs. 7.4 months) compared with platinum/5-FU alone [60]. The EXTREME trial deserves particular attention because it remains the only robust trial of chemotherapy in R/M SCCHN to demonstrate an OS benefit. Importantly, the protocol allowed for either cisplatin- or carboplatin-based therapy for a maximum of six cycles. Patients in the cetuximab arm whose disease remained controlled—defined as CR, partial response (PR), or stable disease (SD)—received maintenance therapy with cetuximab until disease progression or unacceptable toxicity. Because of the trial’s design, it is impossible to know whether the benefit seen in the cetuximab arm was related to upfront cetuximab therapy or whether maintenance cetuximab therapy conferred the majority of the benefit. A subgroup analysis demonstrated improved PFS with cetuximab/cisplatin/5-FU (5.8 vs. 3.8 months) and cetuximab/carboplatin/5-FU regimens (5.3 vs. 3.2 months), yet only the cisplatin-containing regimen was associated with improved OS (10.6 vs. 7.3 months). These data suggest that in fit patients with R/M SCCHN, cisplatin should be the preferred platinum agent when combined with cetuximab/5-FU. Cumulatively, these results have been used to support platinum/cetuximab-based therapy in the first-line treatment of R/M SCCHN in the U.S. and Europe, although some have found the combination too expensive to justify the modest improvement in OS. A formal cost-effectiveness analysis in Canada, for example, suggested that the addition of cetuximab exceeded CAD$100,000 per quality-adjusted life-year gained and thus was felt to be not cost-effective despite the observed OS benefit [69, 70].
The combination of EGFRI plus a taxane has been similarly studied. A phase III study of combination gefitinib/docetaxel demonstrated that the doublet was well tolerated but did not improve median OS compared with docetaxel alone (7.3 vs. 6.0 months) [52]. A post hoc subgroup analysis suggested improved survival among younger patients treated with gefitinib, but its unplanned nature renders this analysis little more than hypothesis generating. In a phase II study of 46 patients deemed unlikely to derive benefit from cisplatin, combination cetuximab/paclitaxel was associated with a DCR of 80% and a CR rate of 22%, with acceptable rates of toxicity [58]. Randomized studies are necessary, but cetuximab/paclitaxel appears to be a reasonable option for patients who are not candidates for cisplatin-containing therapy.
Safety and Tolerability
The distinct mechanisms of action and largely nonoverlapping toxicities of EGFRIs and conventional cytotoxic chemotherapy have permitted the safe incorporation of EGFRIs into conventional chemotherapy regimens for SCCHN. Nevertheless, the immunogenicity of the mAbs and the widespread distribution of EGFR in epithelial tissues can lead to serious infusion-related reactions and mucocutaneous toxicities, rates of which vary by patient demographics, EGFRI used, concomitant cytotoxic agent administered, and concurrent use of RT.
Infusion reactions (IRs) complicate approximately 4% and 12%–19% of panitumumab and cetuximab administrations, respectively [71]. Their severity varies but is frequently grade 3–4, increasing resource utilization and cost of treatment [72]. Cetuximab-related IRs appear to be mediated by IgE directed against the oligosaccharide galactose-α-1,3-galactose [73], and levels of pretreatment anti-galactose-α-1,3-galactose IgE predict the occurrence of cetuximab-related IRs with high sensitivity and specificity [74]. Additional clinical risk factors include European ancestry and history of atopy [75]; geographic distribution also may play a role [73, 76], although this has not been found consistently [75]. Corticosteroids and antihistamines may limit the risk of EGFR mAb-associated IRs [71, 75], but proactive risk-benefit analysis and vigilant monitoring after treatment are prudent in regions with high incidence of IRs [76].
Data from dermatology referral clinics suggest that underdetection and underreporting of EGFR-related skin-specific AEs is an ongoing problem.
The mucocutaneous toxicities of EGFRIs have been amply described and include acneiform rash, augmentation of radiation mucositis and dermatitis, and diarrhea. Randomized controlled trials suggest that the addition of an EGFRI to standard therapy affects AE rates only modestly. In the definitive setting, for example, the addition of cetuximab to RT has been associated with a 5% absolute risk increase in the rate of grade ≥3 radiation dermatitis and a 16% absolute risk increase in the rate of grade ≥3 acneiform rash compared with RT alone; in contrast, the addition of cetuximab to RT only modestly increases grade ≥3 mucositis rates compared with RT alone (56% vs. 52%) [39, 40]. Rates of grade ≥3 mucosal toxicity were increased across trials comparing CRT plus EGFRI with CRT alone, including those that evaluated lapatinib (46% vs. 41%) [26], cetuximab (43% vs. 33%) [27], and panitumumab (55% vs. 24%) [31]. In the R/M setting, single-agent EGFRI causes grade ≥3 rash in up to 25% of patients [56, 54, 55, 57, 59], with fewer events among cetuximab-treated patients compared with those treated with afatinib [55]. Although in most cases the increased toxicity of EGFRIs was modest, it is worth noting that for some patients, including those with Asian ancestry, this toxicity can be quite severe [78].
Despite the modest increase in AE rates, clinical trial experience suggests that the addition of EGFRIs to conventional chemotherapy or CRT does not adversely affect quality of life [42, 79, 80]. Nevertheless, these reported observations are at odds with the experience of many medical oncologists and their patients [81], suggesting that patient-directed questionnaires used in early studies of EGFRIs may have been unable to detect subtle but meaningful effects on patients’ quality of life. Data from dermatology referral clinics suggest that underdetection and underreporting of EGFR-related skin-specific AEs is an ongoing problem [82]. Together, these observations underscore the importance of a multidisciplinary approach toward the management of treatment-related AEs. Efforts are under way to improve upon the detection and reporting of skin-related and other AEs associated with EGFR inhibition [83].
Discussion
The array of biologic and cytotoxic agents available for the treatment of SCCHN has increased dramatically in the past decade. Among them, EGFRIs have shown encouraging but limited efficacy. Despite countless trials of myriad agents, few adequately powered comparative studies exist; therefore, it is difficult to make evidence-based decisions concerning choices of agents from the published literature.
We continue to favor RT/platinum in the adjuvant treatment of fit patients with high-risk resected disease and in the definitive treatment of nonsurgical candidates. Combination RT/EGFRI is certainly active, but randomized phase III studies have not yet demonstrated that RT/EGFRI is superior or at least noninferior to RT/platinum in these settings. In the R/M setting, randomized data regarding the benefit of EGFRI monotherapy are conflicting. Cetuximab, erlotinib, gefitinib, and dacomitinib yield modest response rates that are significantly inferior to those of single-agent chemotherapeutic agents. Compared with the ORR of single-agent chemotherapy (20%–30%) [67], the poor single-agent activity of EGFRIs raises the question of whether EGFRI monotherapy should be incorporated into the standard care of patients with R/M disease [67]. Afatinib may be an exception, but the modest 4-week PFS benefit compared with methotrexate is not compelling [59].
Precisely why one anti-EGFR agent may have superior activity over another is unknown, although clues are available in different agents’ mechanisms of action. The mAbs may dually inhibit tumorigenesis through their disruption of EGFR signal transduction and via ADCC [84], whereas the small molecule TKIs inhibit the EGFR pathway but do not directly engage the immune system. Among mAbs, the robustness of ADCC may be an important component of therapeutic activity in SCCHN. IgG1 mAbs have greater ADCC potential compared with IgG2 agents; therefore, cetuximab may have greater activity than panitumumab [85]. Enhanced ADCC and improvements in clinical efficacy have been observed in patients with polymorphisms of the fragment C (Fc) receptor of immune cells that enhances their affinity for the Fc region [86]. Small molecule TKIs may be better suited to the rare patient whose tumor harbors the EGFRvIII truncation mutant; the tyrosine kinase domain of the EGFRvIII mutant is constitutively activated such that mAbs should have no activity [87]. Compared with mAbs, EGFR inhibition by currently available TKIs may be incomplete such that, at clinically achievable concentrations of TKI, sufficient EGFR activity persists to allow ongoing tumorigenesis [88]. Irreversible inhibition by second-generation EGFRIs such as afatinib theoretically may confer enhanced activity against EGFR compared with first-generation reversible inhibitors including erlotinib and gefitinib. Although the above discussion documents modestly improved activity with afatinib, this comes at the expense of increased toxicity compared with first-generation agents. Consequently, it remains to be seen whether irreversible TKIs should supplant the reversible inhibitors.
Compared with mAbs, EGFR inhibition by currently available TKIs may be incomplete such that, at clinically achievable concentrations of TKI, sufficient EGFR activity persists to allow ongoing tumorigenesis.
Across treatment settings, HPV status has emerged as an important prognostic factor among patients with SCCHN. Until recently, HPV status had not been routinely assayed or incorporated into clinical trial eligibility criteria; therefore, the preponderance of published data includes patients with and without HPV-associated disease. Patients with HPV-associated disease, as assessed by HPV in situ hybridization or P16 immunohistochemistry, are generally younger and present with more advanced nodal disease [89]. Deep sequencing of HPV-associated tumors has identified distinct molecular signatures: HPV-associated tumors rarely contain TP53 mutations or EGFR overexpression and instead are more commonly associated with activating mutations in PIK3CA [90]. In addition, clinical outcomes among HPV-associated SCCHN patients compare favorably with those of patients with non-HPV-associated disease [91]. Taken together, these clinicopathologic features suggest that HPV-associated SCCHN is a separate disease entity altogether, complicating the development, evaluation, and incorporation of EGFRIs into the treatment of SCCHN.
The favorable prognosis among patients with HPV-associated oropharyngeal SCC has prompted the practice of therapy de-escalation in this setting [92], which warrants further study. Several ongoing trials in patients with HPV-associated oropharyngeal SCC are comparing EGFRI-based concurrent chemoradiotherapy with standard platinum-based chemoradiotherapy. In RTOG 1016 (NCT01302834), for example, patients with low- and intermediate-risk disease (prior smokers allowed, T1–2 N2a–3 M0 or T3–4 N1–3 M0) receive accelerated RT (70 Gy over 6 weeks, with 6 fractions per week during weeks 2–6) with either conventional high-dose cisplatin (100 mg/m2 on days 1 and 22) or eight weekly cetuximab doses. Separately, in the Trans-Tasman Radiation Oncology Group 12.01 study (NCT01855451), patients with low-risk disease (for nonsmokers: T3 N0–1 M0 or T1–3 N2 M0; for smokers: T1-3 N0–2a M0) receive conventionally fractionated RT (70 Gy in 35 fractions over 7 weeks) with either low-dose weekly cisplatin (40 mg/m2 for 7 weeks) or weekly cetuximab during RT. In the ongoing British DE-ESCALATE HPV trial (NCT01874171), patients with low-risk disease (for nonsmokers: T3–4 N0 M0 or T1–4 N1–3 M0; for smokers: T1–4 N1–2a) receive conventionally fractionated RT and either high-dose cisplatin (100 mg/m2 on days 1, 22, and 43) or weekly cetuximab during RT. Finally, because RT alone may be sufficient in the lowest risk population, the National Cancer Institute-sponsored NRG-HN002 study (NCT02254278) enrolls patients with low-risk disease (T1–2 N1–2b M0 or T3 N0–2b M0) and randomizes to either accelerated RT alone (60 Gy over 5 weeks with 6 fractions per week) or to conventional RT (60 Gy over 6 weeks) plus weekly cisplatin (40 mg/m2 for 6 weeks). Until results from these studies are available, one cannot conclude that either the substitution of cetuximab for cisplatin or a reduction in radiation dose is appropriate. We presently recommend against the routine de-escalation of therapy for patients with HPV-associated oropharyngeal SCC in the definitive and adjuvant settings.
Performance status remains a major factor in considering appropriate therapy. Retrospective series have demonstrated inferior survival in patients with SCCHN and poor performance status [93]. Whether patients with advanced age or poor performance status gain any benefit from cytotoxic therapy or EGFRIs remains unknown because patients with poor performance have historically been excluded from clinical trials in SCCHN. The Elderly Head and Neck Cancer (ELAN) group of trials is currently enrolling elderly patients into a series of three trials based on treatment setting and level of fitness determined by a pragmatic geriatric evaluation [94]. In the curative setting, unfit patients will be enrolled in the phase III ELAN-RT study of conventional RT versus hypofractionated split-course RT (NCT01864850); in the R/M setting, patients will be enrolled based on performance status into either the phase II ELAN-FIT trial, in which patients receive therapy similar to that in the EXTREME trial (NCT01864772), or the phase III ELAN-UNFIT trial comparing single-agent cetuximab with methotrexate (NCT01884623).
Despite the emergence of a substantial body of data regarding EGFRIs in the treatment of SCCHN in the past decade, the principles of management remain unchanged. EGFRIs have not displaced cisplatin as the preferred agent to be administered concurrently with RT; single-agent EGFRIs have not established themselves as preferred agents in the R/M setting; and the clinical benefit from the addition of cetuximab to a doublet conventional chemotherapeutic backbone brings only a modest clinically relevant benefit to these patients, along with substantial toxicity. Several ongoing trials (e.g., RTOG 1016 in the HPV-associated definitive setting) may change this, but data to date do not suggest that EGFRIs will provide a major leap forward.
Acknowledgments
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors. The authors received no direct compensation related to the development of the manuscript. Editorial support in the form of table assembly, copyediting, data checking, and formatting assistance was provided by Melissa Brunckhorst of MedErgy, which was contracted and funded by Boehringer Ingelheim Pharmaceuticals, Inc (BIPI). BIPI was given the opportunity to review the manuscript for medical scientific accuracy and for intellectual property considerations, although this review did not result in modification of the manuscript.
Author Contributions
Conception/Design: David J. Iberri, A. Dimitrios Colevas
Collection and/or assembly of data: David J. Iberri, A. Dimitrios Colevas
Data analysis and interpretation: David J. Iberri, A. Dimitrios Colevas
Manuscript writing: David J. Iberri, A. Dimitrios Colevas
Final approval of manuscript: David J. Iberri, A. Dimitrios Colevas
Disclosures
A. Dimitrios Colevas: Novartis, Bayer, PX Biosolutions (C/A), Exelixis, Bayer, Bristol-Myers Squibb, National Institutes of Health, Genentech, Curis, Onconova (RF), Stanford University (E), Gilead (OI). The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Ferlay J, Soerjomataram I, Ervik M et al. GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC CancerBase no. 11. Lyon, France: International Agency for Research on Cancer, 2013. Available at http://globocan.iarc.fr. Accessed December 22, 2014.
- 2.Choong N, Vokes E. Expanding role of the medical oncologist in the management of head and neck cancer. CA Cancer J Clin. 2008;58:32–53. doi: 10.3322/CA.2007.0004. [DOI] [PubMed] [Google Scholar]
- 3.Argiris A, Karamouzis MV, Raben D, et al. Head and neck cancer. Lancet. 2008;371:1695–1709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stell PM, Morton RP, Campbell IT, et al. Survival after “palliative” cytotoxic chemotherapy for head-and-neck cancer. Lancet. 1983;2:1205. doi: 10.1016/s0140-6736(83)91259-x. [DOI] [PubMed] [Google Scholar]
- 5.Thompson DM, Gill GN. The EGF receptor: Structure, regulation and potential role in malignancy. Cancer Surv. 1985;4:767–788. [PubMed] [Google Scholar]
- 6.Olayioye MA, Neve RM, Lane HA, et al. The ErbB signaling network: Receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung BM, Tom E, Zutshi N, et al. Nexus of signaling and endocytosis in oncogenesis driven by non-small cell lung cancer-associated epidermal growth factor receptor mutants. World J Clin Oncol. 2014;5:806–823. doi: 10.5306/wjco.v5.i5.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharafinski ME, Ferris RL, Ferrone S, et al. Epidermal growth factor receptor targeted therapy of squamous cell carcinoma of the head and neck. Head Neck. 2010;32:1412–1421. doi: 10.1002/hed.21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160–1174. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 10.Markovic A, Chung CH. Current role of EGF receptor monoclonal antibodies and tyrosine kinase inhibitors in the management of head and neck squamous cell carcinoma. Expert Rev Anticancer Ther. 2012;12:1149–1159. doi: 10.1586/era.12.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erbitux (cetuximab) injection, for intravenous infusion [package insert]. Branchburg, NJ: ImClone LLC; 2013.
- 12.Agulnik M. New approaches to EGFR inhibition for locally advanced or metastatic squamous cell carcinoma of the head and neck (SCCHN) Med Oncol. 2012;29:2481–2491. doi: 10.1007/s12032-012-0159-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NCCN clinical practice guidelines in oncology (NCCN Guidelines®). Head and neck cancers, version 2.2014. Available at http://www.nccn.org/professionals/physician_gls/PDF/head-and-neck.pdf. Accessed September 22, 2014.
- 14.Peyrade F, Righini C, Gal J, et al. Adjuvant radiotherapy (RDT) plus cisplatinum (Cis) and cetuximab (Cet) in resected head and neck squamous cell carcinoma. J Clin Oncol. 2014;32:6042a. [Google Scholar]
- 15.Ferris RL, Schmitt NC, Heron DE, et al. Phase II trial of radiotherapy (RT) with concurrent cisplatin (C) plus panitumumab (pmAb) for patients (pts) with high-risk, resected head and neck cancer (HNC) J Clin Oncol. 2014;32(suppl):6090a. doi: 10.1093/annonc/mdw428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arias F, Herruzo I, Contreras J, et al. Postsurgical erlotinib and cisplatin concurrent chemoradiotherapy (CRT) promotes favorable outcomes in high-risk locally advanced head and neck squamous-cell cancer (LAHNSCC): A GICOR Working Group trial. J Clin Oncol. 2014;32(suppl):6067a. [Google Scholar]
- 17.Harrington KJ, Temam S, D’Cruz A, et al. Final analysis: A randomized, blinded, placebo (P)-controlled phase III study of adjuvant postoperative lapatinib (L) with concurrent chemotherapy and radiation therapy (CH-RT) in high-risk patients with squamous cell carcinoma of the head and neck (SCCHN) J Clin Oncol. 2014;32(suppl):6005a. [Google Scholar]
- 18.Harari PM, Harris J, Kies MS, et al. Postoperative chemoradiotherapy and cetuximab for high-risk squamous cell carcinoma of the head and neck: Radiation Therapy Oncology Group RTOG-0234. J Clin Oncol. 2014;32:2486–2495. doi: 10.1200/JCO.2013.53.9163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao X, Guo Y, Zhu Y, et al. A prospective phase II study of induction chemotherapy and nimotuzumab for resectable locally advanced head and neck squamous cell carcinoma. J Clin Oncol. 2012;30(suppl):e16039a. [Google Scholar]
- 20.Bier H, Hoffmann T, Hauser U, et al. Clinical trial with escalating doses of the antiepidermal growth factor receptor humanized monoclonal antibody EMD 72 000 in patients with advanced squamous cell carcinoma of the larynx and hypopharynx. Cancer Chemother Pharmacol. 2001;47:519–524. doi: 10.1007/s002800000270. [DOI] [PubMed] [Google Scholar]
- 21.Rosenthal EL, Chung TK, Carroll WR, et al. Assessment of erlotinib as adjuvant chemoprevention in high-risk head and neck cancer patients. Ann Surg Oncol. 2014;21:4263–4269. doi: 10.1245/s10434-014-3878-0. [DOI] [PubMed] [Google Scholar]
- 22.Rosenthal DI, Zhang Q, Kies MS, et al. Seamless phase II/III trial design with survival and PRO endpoints for treatment selection: Case study of RTOG 1216. J Clin Oncol. 2013;31(suppl):TPS6099a. [Google Scholar]
- 23.Burtness B, Bourhis J, Vermorken JB, et al. LUX head and neck 2: A randomized, double-blind, placebo-controlled, phase III study of afatinib as adjuvant therapy after chemoradiation in primarily unresected, clinically high-risk, head and neck cancer patients. J Clin Oncol. 2012;30(suppl):TPS5599a. doi: 10.1186/1745-6215-15-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 25.Krishnamurthyreddy B, Vidyasagar MS, Koteshwar R, et al. A phase IIb 4-arm open-label randomized study to assess the safety and efficacy of h-R3 monoclonal antibody against EGFR in combination with chemoradiation therapy or radiation therapy in patients with advanced (stage III or IVA) inoperable head and neck cancer. J Clin Oncol. 2009;27(suppl):6041a. [Google Scholar]
- 26.Del Campo JM, Hitt R, Sebastian P, et al. Effects of lapatinib monotherapy: Results of a randomised phase II study in therapy-naive patients with locally advanced squamous cell carcinoma of the head and neck. Br J Cancer. 2011;105:618–627. doi: 10.1038/bjc.2011.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ang KK, Zhang Q, Rosenthal DI, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32:2940–2950. doi: 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Babu KG, Viswanath L, Reddy BK, et al. An open-label, randomized study of h-R3mAb (nimotuzumab) in patients with advanced (stage III or IVa) squamous cell carcinoma of head and neck (SCCHN): Four-year survival results from a phase IIb study. J Clin Oncol. 2010;28(suppl):5530a. [Google Scholar]
- 29.Bhatnagar AR, Singh DP, Sharma R, et al. A comparative study of monoclonal antibody against EGFR (nimotuzumab) used in combination with chemoradiation versus chemoradiation alone in the treatment of locally advanced inoperable squamous cell carcinoma of the head and neck. J Clin Oncol. 2012;30(suppl):e16012a. [Google Scholar]
- 30.Ghi MG, Paccagnella A, Ferrari D, et al. Concomitant chemoradiation (CRT) or cetuximab/RT (CET/RT) versus induction docetaxel/cisplatin/5-fluorouracil (TPF) followed by CRT or CET/RT in patients with locally advanced squamous cell carcinoma of head and neck (LASCCHN). A randomized phase III factorial study ( NCT01086826) J Clin Oncol. 2014;32(suppl):6004a. [Google Scholar]
- 31.Giralt J, Fortin A, Mesia R, et al. A phase II, randomized trial (CONCERT-1) of chemoradiotherapy (CRT) with or without panitumumab (pmab) in patients (pts) with unresected, locally advanced squamous cell carcinoma of the head and neck (LASCCHN) J Clin Oncol. 2012;30(suppl):5502a. [Google Scholar]
- 32.Gupta M, Madholia V, Gupta N, et al. Results from a pilot study of nimotuzumab with concurrent chemoradiation in patients with locally advanced squamous cell carcinoma of head and neck. J Clin Oncol. 2010;28(suppl):5565a. [Google Scholar]
- 33.Hainsworth JD, Spigel DR, Greco FA, et al. Combined modality treatment with chemotherapy, radiation therapy, bevacizumab, and erlotinib in patients with locally advanced squamous carcinoma of the head and neck: A phase II trial of the Sarah Cannon oncology research consortium. Cancer J. 2011;17:267–272. doi: 10.1097/PPO.0b013e3182329791. [DOI] [PubMed] [Google Scholar]
- 34.Lefebvre JL, Pointreau Y, Rolland F, et al. Induction chemotherapy followed by either chemoradiotherapy or bioradiotherapy for larynx preservation: The TREMPLIN randomized phase II study. J Clin Oncol. 2013;31:853–859. doi: 10.1200/JCO.2012.42.3988. [DOI] [PubMed] [Google Scholar]
- 35.Lim W-T, Ang M-K, Ng QS, et al. A phase II study of nimotuzumab and CDDP concurrent with radiation in locally advanced squamous cell carcinoma of the head and neck (SCCHN) J Clin Oncol. 2012;30(suppl):e16024a. [Google Scholar]
- 36.Merlano M, Russi E, Benasso M, et al. Cisplatin-based chemoradiation plus cetuximab in locally advanced head and neck cancer: A phase II clinical study. Ann Oncol. 2011;22:712–717. doi: 10.1093/annonc/mdq412. [DOI] [PubMed] [Google Scholar]
- 37.Singh C. Gefitinib with concurrent chemoradiation in locally advanced head and neck cancers. J Clin Oncol. 2014;32(suppl):6054a. [Google Scholar]
- 38.Somani N, Karandikar SM, Bokil K, et al. Nimotuzumab with concurrent chemoradiotherapy in patients with locally advanced head and neck cancer (LASCCHN) J Clin Oncol. 2013;31(suppl):6084a. [Google Scholar]
- 39.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 40.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-Year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 41.Crombet T, Osorio M, Cruz T, et al. Use of the humanized anti-epidermal growth factor receptor monoclonal antibody h-R3 in combination with radiotherapy in the treatment of locally advanced head and neck cancer patients. J Clin Oncol. 2004;22:1646–1654. doi: 10.1200/JCO.2004.03.089. [DOI] [PubMed] [Google Scholar]
- 42.Chen L, Chen H, Zhang X, et al. Evaluation of the adverse event of nimotuzumab combined therapy in patients with advanced carcinoma. J Clin Oncol. 2011;29(suppl):e13081a. [Google Scholar]
- 43.Caponigro F, Romano C, Milano A, et al. A phase I/II trial of gefitinib and radiotherapy in patients with locally advanced inoperable squamous cell carcinoma of the head and neck. Anticancer Drugs. 2008;19:739–744. doi: 10.1097/CAD.0b013e32830676a8. [DOI] [PubMed] [Google Scholar]
- 44.Heukelom J, Hamming O, Bartelink H, et al. Adaptive and innovative Radiation Treatment FOR improving Cancer treatment outcomE (ARTFORCE); a randomized controlled phase II trial for individualized treatment of head and neck cancer. BMC Cancer. 2013;13:84. doi: 10.1186/1471-2407-13-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langer CJ, Li JW, Patel UA, et al. Preliminary analysis of ECOG 3303: Concurrent radiation (RT), cisplatin (DDP) and cetuximab (C) in unresectable, locally advanced (LA) squamous cell carcinoma of the head and neck (SCCHN) J Clin Oncol. 2008;26(suppl):6006a. [Google Scholar]
- 46.Pfister DG, Su YB, Kraus DH, et al. Concurrent cetuximab, cisplatin, and concomitant boost radiotherapy for locoregionally advanced, squamous cell head and neck cancer: A pilot phase II study of a new combined-modality paradigm. J Clin Oncol. 2006;24:1072–1078. doi: 10.1200/JCO.2004.00.1792. [DOI] [PubMed] [Google Scholar]
- 47.Martins RG, Parvathaneni U, Bauman JE, et al. Cisplatin and radiotherapy with or without erlotinib in locally advanced squamous cell carcinoma of the head and neck: A randomized phase II trial. J Clin Oncol. 2013;31:1415–1421. doi: 10.1200/JCO.2012.46.3299. [DOI] [PubMed] [Google Scholar]
- 48.Suntharalingam M, Kwok Y, Goloubeva O, et al. Phase II study evaluating the addition of cetuximab to the concurrent delivery of weekly carboplatin, paclitaxel, and daily radiotherapy for patients with locally advanced squamous cell carcinomas of the head and neck. Int J Radiat Oncol Biol Phys. 2012;82:1845–1850. doi: 10.1016/j.ijrobp.2011.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Habl G, Jensen AD, Potthoff K, et al. Treatment of locally advanced carcinomas of head and neck with intensity-modulated radiation therapy (IMRT) in combination with cetuximab and chemotherapy: The REACH protocol. BMC Cancer. 2010;10:651. doi: 10.1186/1471-2407-10-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jensen AD, Krauss J, Potthoff K, et al. Radiochemoimmunotherapy with intensity-modulated concomitant boost: Interim analysis of the REACH trial. Radiat Oncol. 2012;7:57. doi: 10.1186/1748-717X-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mesía R, Rueda A, Vera R, et al. Adjuvant therapy with cetuximab for locally advanced squamous cell carcinoma of the oropharynx: Results from a randomized, phase II prospective trial. Ann Oncol. 2013;24:448–453. doi: 10.1093/annonc/mds291. [DOI] [PubMed] [Google Scholar]
- 52.Argiris A, Ghebremichael M, Gilbert J, et al. Phase III randomized, placebo-controlled trial of docetaxel with or without gefitinib in recurrent or metastatic head and neck cancer: An Eastern Cooperative Oncology Group trial. J Clin Oncol. 2013;31:1405–1414. doi: 10.1200/JCO.2012.45.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burtness B, Goldwasser MA, Flood W, et al. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: An Eastern Cooperative Oncology Group study. J Clin Oncol. 2005;23:8646–8654. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 54.Stewart JS, Cohen EE, Licitra L, et al. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27:1864–1871. doi: 10.1200/JCO.2008.17.0530. [corrected] [published correction appears in J Clin Oncol. 2009;27:3410] [DOI] [PubMed] [Google Scholar]
- 55.Seiwert TY, Fayette J, Cupissol D, et al. A randomized, phase II study of afatinib versus cetuximab in metastatic or recurrent squamous cell carcinoma of the head and neck. Ann Oncol. 2014;25:1813–1820. doi: 10.1093/annonc/mdu216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abdul Razak AR, Soulières D, Laurie SA, et al. A phase II trial of dacomitinib, an oral pan-human EGF receptor (HER) inhibitor, as first-line treatment in recurrent and/or metastatic squamous-cell carcinoma of the head and neck. Ann Oncol. 2013;24:761–769. doi: 10.1093/annonc/mds503. [DOI] [PubMed] [Google Scholar]
- 57.Fury MG, Sherman E, Lisa D, et al. A randomized phase II study of cetuximab every 2 weeks at either 500 or 750 mg/m2 for patients with recurrent or metastatic head and neck squamous cell cancer. J Natl Compr Canc Netw. 2012;10:1391–1398. doi: 10.6004/jnccn.2012.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hitt R, Irigoyen A, Cortes-Funes H, et al. Phase II study of the combination of cetuximab and weekly paclitaxel in the first-line treatment of patients with recurrent and/or metastatic squamous cell carcinoma of head and neck. Ann Oncol. 2012;23:1016–1022. doi: 10.1093/annonc/mdr367. [DOI] [PubMed] [Google Scholar]
- 59.Machiels J-PH, Haddad RI, Fayette J, et al. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): An open-label, randomised phase 3 trial. Lancet Oncol. 2015;16:583–594. doi: 10.1016/S1470-2045(15)70124-5. [DOI] [PubMed] [Google Scholar]
- 60.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 61.Vermorken JB, Licitra L, Stöhlmacher-Williams J, et al. Phase II study of pemetrexed in combination with cisplatin and cetuximab in recurrent or metastatic squamous cell carcinoma of the head and neck. Eur J Cancer. 2013;49:2877–2883. doi: 10.1016/j.ejca.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 62.Vermorken JB, Trigo J, Hitt R, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007;25:2171–2177. doi: 10.1200/JCO.2006.06.7447. [DOI] [PubMed] [Google Scholar]
- 63.Soulieres D, Senzer NN, Vokes EE, et al. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol. 2004;22:77–85. doi: 10.1200/JCO.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 64.Kirby AM, A’Hern RP, D’Ambrosio C, et al. Gefitinib (ZD1839, Iressa) as palliative treatment in recurrent or metastatic head and neck cancer. Br J Cancer. 2006;94:631–636. doi: 10.1038/sj.bjc.6602999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cohen EE, Kane MA, List MA, et al. Phase II trial of gefitinib 250 mg daily in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res. 2005;11:8418–8424. doi: 10.1158/1078-0432.CCR-05-1247. [DOI] [PubMed] [Google Scholar]
- 66.Cohen EE, Rosen F, Stadler WM, et al. Phase II trial of ZD1839 in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2003;21:1980–1987. doi: 10.1200/JCO.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 67.Colevas AD. Chemotherapy options for patients with metastatic or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol. 2006;24:2644–2652. doi: 10.1200/JCO.2005.05.3348. [DOI] [PubMed] [Google Scholar]
- 68.Herbst RS, Arquette M, Shin DM, et al. Phase II multicenter study of the epidermal growth factor receptor antibody cetuximab and cisplatin for recurrent and refractory squamous cell carcinoma of the head and neck. J Clin Oncol. 2005;23:5578–5587. doi: 10.1200/JCO.2005.07.120. [DOI] [PubMed] [Google Scholar]
- 69.Greenhalgh J, Bagust A, Boland A, et al. Cetuximab for the treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck. Health Technol Assess. 2009;13(suppl 3):49–54. doi: 10.3310/hta13suppl3/08. [DOI] [PubMed] [Google Scholar]
- 70.Hannouf MB, Sehgal C, Cao JQ, et al. Cost-effectiveness of adding cetuximab to platinum-based chemotherapy for first-line treatment of recurrent or metastatic head and neck cancer. PLoS One. 2012;7:e38557. doi: 10.1371/journal.pone.0038557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chung CH. Managing premedications and the risk for reactions to infusional monoclonal antibody therapy. The Oncologist. 2008;13:725–732. doi: 10.1634/theoncologist.2008-0012. [DOI] [PubMed] [Google Scholar]
- 72.Schwartzberg LS, Stepanski EJ, Walker MS, et al. Implications of IV monoclonal antibody infusion reaction for the patient, caregiver, and practice: Results of a multicenter study. Support Care Cancer. 2009;17:91–98. doi: 10.1007/s00520-008-0474-5. [DOI] [PubMed] [Google Scholar]
- 73.Chung CH, Mirakhur B, Chan E, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358:1109–1117. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mariotte D, Dupont B, Gervais R, et al. Anti-cetuximab IgE ELISA for identification of patients at a high risk of cetuximab-induced anaphylaxis. MAbs. 2011;3:396–401. doi: 10.4161/mabs.3.4.16293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Touma W, Koro SS, Ley J, et al. Risk factors for and pre-medications to prevent cetuximab-induced infusion reactions in patients with squamous cell carcinoma of the head and neck. Oral Oncol. 2014;50:895–900. doi: 10.1016/j.oraloncology.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.O’Neil BH, Allen R, Spigel DR, et al. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol. 2007;25:3644–3648. doi: 10.1200/JCO.2007.11.7812. [DOI] [PubMed] [Google Scholar]
- 77.Grandvuillemin A, Disson-Dautriche A, Miremont-Salamé G, et al. Cetuximab infusion reactions: French pharmacovigilance database analysis. J Oncol Pharm Pract. 2013;19:130–137. doi: 10.1177/1078155212457965. [DOI] [PubMed] [Google Scholar]
- 78.Pryor DI, Porceddu SV, Burmeister BH, et al. Enhanced toxicity with concurrent cetuximab and radiotherapy in head and neck cancer. Radiother Oncol. 2009;90:172–176. doi: 10.1016/j.radonc.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 79.Curran D, Giralt J, Harari PM, et al. Quality of life in head and neck cancer patients after treatment with high-dose radiotherapy alone or in combination with cetuximab. J Clin Oncol. 2007;25:2191–2197. doi: 10.1200/JCO.2006.08.8005. [DOI] [PubMed] [Google Scholar]
- 80.Mesía R, Rivera F, Kawecki A, et al. Quality of life of patients receiving platinum-based chemotherapy plus cetuximab first line for recurrent and/or metastatic squamous cell carcinoma of the head and neck. Ann Oncol. 2010;21:1967–1973. doi: 10.1093/annonc/mdq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Giro C, Berger B, Bölke E, et al. High rate of severe radiation dermatitis during radiation therapy with concurrent cetuximab in head and neck cancer: Results of a survey in EORTC institutes. Radiother Oncol. 2009;90:166–171. doi: 10.1016/j.radonc.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 82.Rosen AC, Case EC, Dusza SW, et al. Impact of dermatologic adverse events on quality of life in 283 cancer patients: A questionnaire study in a dermatology referral clinic. Am J Clin Dermatol. 2013;14:327–333. doi: 10.1007/s40257-013-0021-0. [DOI] [PubMed] [Google Scholar]
- 83.Lacouture ME, Maitland ML, Segaert S, et al. A proposed EGFR inhibitor dermatologic adverse event-specific grading scale from the MASCC skin toxicity study group. Support Care Cancer. 2010;18:509–522. doi: 10.1007/s00520-009-0744-x. [DOI] [PubMed] [Google Scholar]
- 84.López-Albaitero A, Ferris RL. Immune activation by epidermal growth factor receptor specific monoclonal antibody therapy for head and neck cancer. Arch Otolaryngol Head Neck Surg. 2007;133:1277–1281. doi: 10.1001/archotol.133.12.1277. [DOI] [PubMed] [Google Scholar]
- 85.Patel D, Guo X, Ng S, et al. IgG isotype, glycosylation, and EGFR expression determine the induction of antibody-dependent cellular cytotoxicity in vitro by cetuximab. Hum Antibodies. 2010;19:89–99. doi: 10.3233/HAB-2010-0232. [DOI] [PubMed] [Google Scholar]
- 86.Taylor RJ, Chan SL, Wood A, et al. FcgammaRIIIa polymorphisms and cetuximab induced cytotoxicity in squamous cell carcinoma of the head and neck. Cancer Immunol Immunother. 2009;58:997–1006. doi: 10.1007/s00262-008-0613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gan HK, Cvrljevic AN, Johns TG. The epidermal growth factor receptor variant III (EGFRvIII): Where wild things are altered. FEBS J. 2013;280:5350–5370. doi: 10.1111/febs.12393. [DOI] [PubMed] [Google Scholar]
- 88.Hoeben A, Martin D, Clement PM, et al. Role of GRB2-associated binder 1 in epidermal growth factor receptor-induced signaling in head and neck squamous cell carcinoma. Int J Cancer. 2013;132:1042–1050. doi: 10.1002/ijc.27763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vidal L, Gillison ML. Human papillomavirus in HNSCC: Recognition of a distinct disease type. Hematol Oncol Clin North Am. 2008;22:1125–1142, vii. doi: 10.1016/j.hoc.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 90.Hayes DN, Grandis JR, El-Naggar AK. The Cancer Genome Atlas: Integrated analysis of genome alterations in squamous cell carcinoma of the head and neck. J Clin Oncol. 2013;31 (suppl):6009a. [Google Scholar]
- 91.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wong SJ, Harari PM, Garden AS, et al. Longitudinal Oncology Registry of Head and Neck Carcinoma (LORHAN): Analysis of chemoradiation treatment approaches in the United States. Cancer. 2011;117:1679–1686. doi: 10.1002/cncr.25721. [DOI] [PubMed] [Google Scholar]
- 93.Argiris A, Li Y, Forastiere A. Prognostic factors and long-term survivorship in patients with recurrent or metastatic carcinoma of the head and neck. Cancer. 2004;101:2222–2229. doi: 10.1002/cncr.20640. [DOI] [PubMed] [Google Scholar]
- 94.Guigay J, Le Caer H, Mertens C, et al. Elderly Head and Neck Cancer (ELAN) study: Personalized treatment according to geriatric assessment in patients age 70 or older: First prospective trials in patients with squamous cell cancer of the head and neck (SCCHN) unsuitable for surgery. J Clin Oncol. 2014;32:(5S):TPS6099a. [Google Scholar]